The Relationship between Cadmium Exposure and Mortality in Postmenopausal Females: A Cohort Study of 2001–2018 NHANES
Abstract
:1. Introduction
2. Materials and Methods
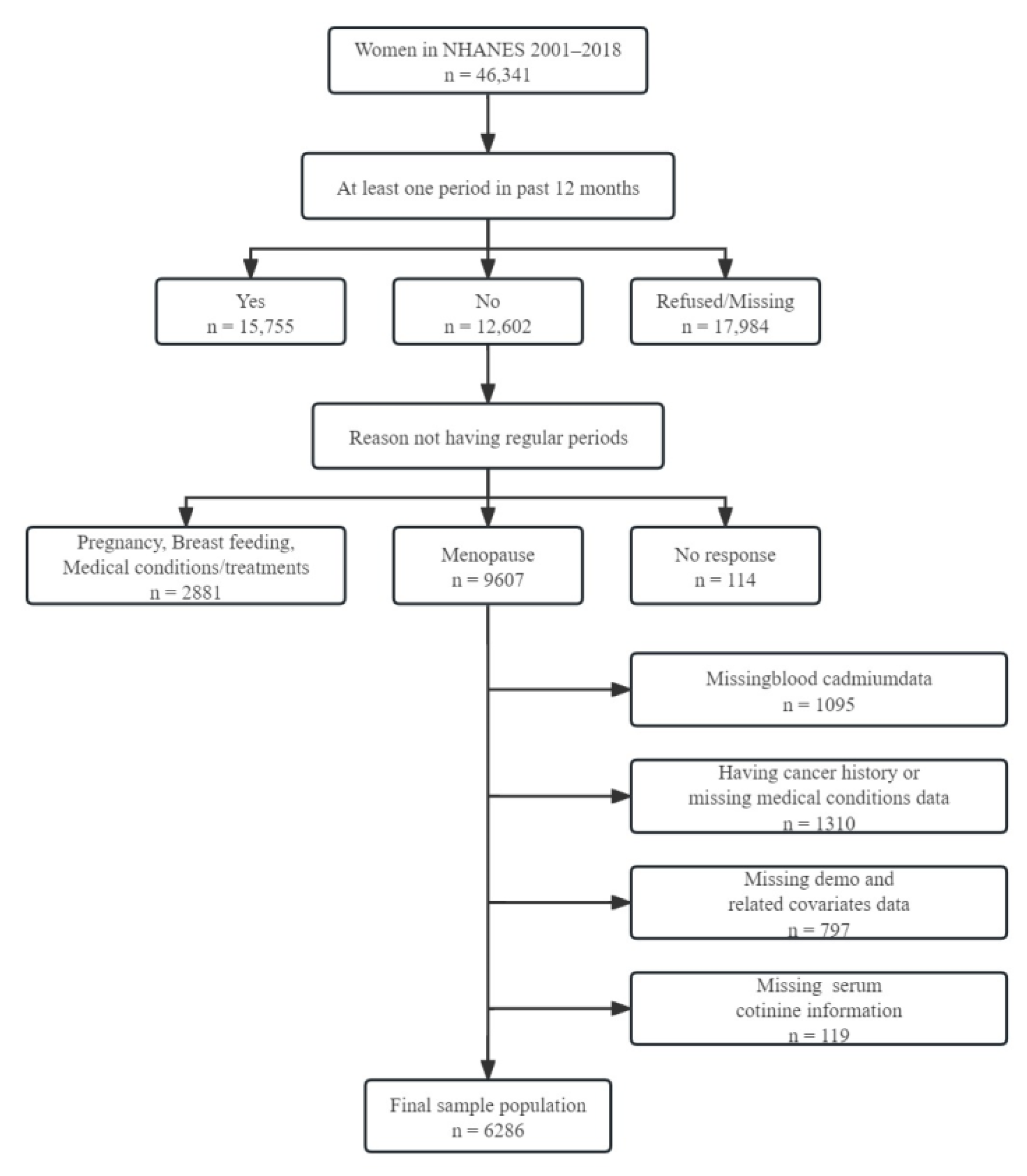
2.1. Study Population
2.2. Cadmium Exposure
2.3. Determination of Mortality Outcomes
2.4. Other Variables
2.5. Statistical Analyses
3. Results
3.1. Characteristics of Participants
3.2. Association of Exposure to Cadmium with Mortality
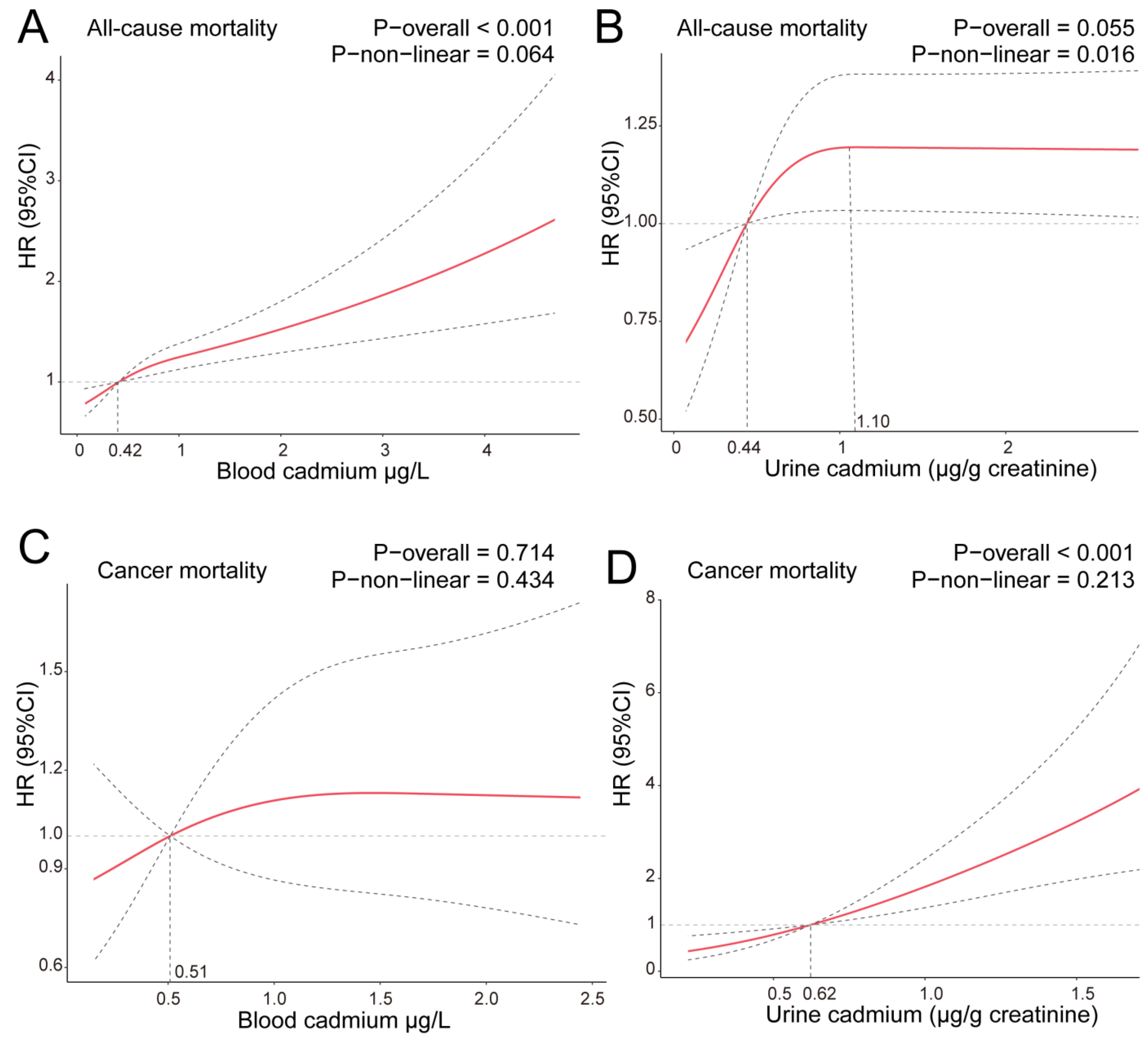
3.3. Results of Curvilinear Relationship of Cadmium Concentration and Mortality
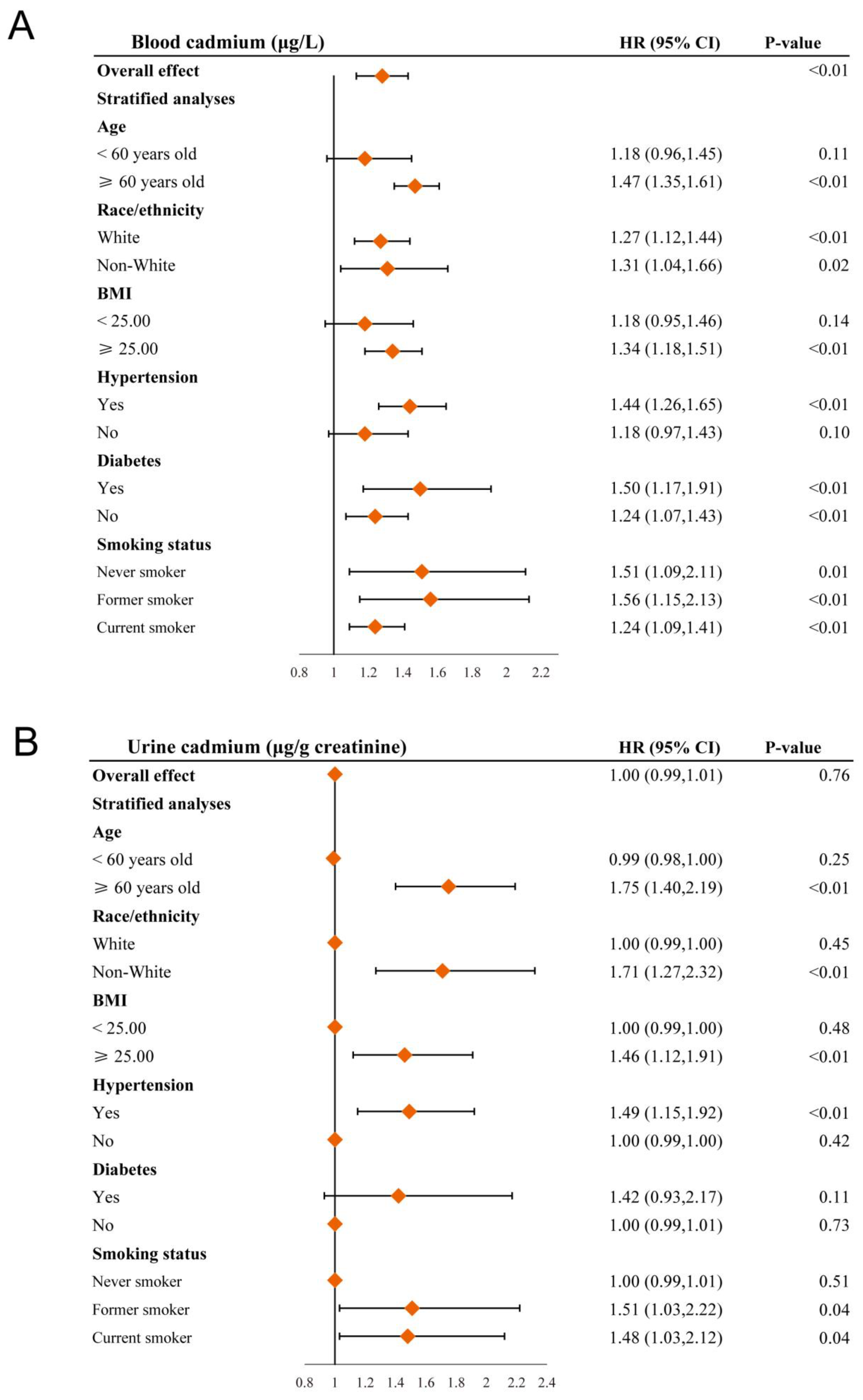
3.4. Stratified Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, S.R.; Lambrinoudaki, I.; Lumsden, M.; Mishra, G.D.; Pal, L.; Rees, M.; Santoro, N.; Simoncini, T. Menopause. Nat. Rev. Dis. Primers. 2015, 1, 15004. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.D. Menopause. Lancet 2008, 371, 760–770. [Google Scholar] [CrossRef]
- Alblooshi, S.; Taylor, M.; Gill, N. Does menopause elevate the risk for developing depression and anxiety? Results from a systematic review. Australas Psychiatry 2023, 31, 165–173. [Google Scholar] [CrossRef] [PubMed]
- American Association of Neurological Surgeons (AANS); American Society of Neuroradiology (ASNR); Cardiovascular and Interventional Radiology Society of Europe (CIRSE); Canadian Interventional Radiology Association (CIRA); Congress of Neurological Surgeons (CNS); European Society of Minimally Invasive Neurological Therapy (ESMINT); European Society of Neuroradiology (ESNR); European Stroke Organization (ESO); Society for Cardiovascular Angiography and Interventions (SCAI); Society of Interventional Radiology (SIR); et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar]
- Utian, W.H. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: A comprehensive review. Health Qual. Life Outcomes 2005, 3, 47. [Google Scholar] [CrossRef] [PubMed]
- Gold, E.B.; Bromberger, J.; Crawford, S.; Samuels, S.; Greendale, G.A.; Harlow, S.D.; Skurnick, J. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am. J. Epidemiol. 2001, 153, 865–874. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Cullen, J.T.; Maldonado, M.T. Biogeochemistry of cadmium and its release to the environment. Cadmium Toxic. Essentiality 2013, 11, 31–62. [Google Scholar]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Current health risk assessment practice for dietary cadmium: Data from different countries. Food Chem. Toxicol. 2017, 106, 430–445. [Google Scholar] [CrossRef]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Health Risk Assessment of Dietary Cadmium Intake: Do Current Guidelines Indicate How Much is Safe? Environ. Health Perspect. 2017, 125, 284–288. [Google Scholar] [CrossRef]
- Sun, T.; Hu, Y.; Wang, Z.; Xia, W.; Lv, Q.; Wang, Y.; Fang, P.; Xu, P. A tissue atlas of cadmium accumulation and the correlation with thiol-containing chelates in zucchini provide insights into cadmium partitioning and food safety. J. Hazard. Mater. 2022, 421, 126756. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Khan, S.; Khan, A.; Alam, M. Soil contamination with cadmium, consequences and remediation using organic amendments. Sci. Total Environ. 2017, 601–602, 1591–1605. [Google Scholar] [CrossRef]
- Kim, K.; Melough, M.M.; Vance, T.M.; Noh, H.; Koo, S.I.; Chun, O.K. Dietary Cadmium Intake and Sources in the US. Nutrients 2018, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Faroon, O.; Ashizawa, A.; Wright, S.; Tucker, P.; Jenkins, K.; Ingerman, L.; Rudisill, C. Toxicological Profile for Cadmium; Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA, 2012. [Google Scholar]
- IARC. Monographs Volume 100C Cadmium and Cadmium Compounds—IARC. 2012. Available online: https://monographs.iarc.fr/iarc-monographs-volume-100c-cadmium-and-cadmium-compounds/ (accessed on 25 June 2023).
- Larsson, S.C.; Wolk, A. Urinary cadmium and mortality from all causes, cancer and cardiovascular disease in the general population: Systematic review and meta-analysis of cohort studies. Int. J. Epidemiol. 2016, 45, 782–791. [Google Scholar] [CrossRef]
- Tellez-Plaza, M.; Navas-Acien, A.; Menke, A.; Crainiceanu, C.M.; Pastor-Barriuso, R.; Guallar, E. Cadmium exposure and all-cause and cardiovascular mortality in the U.S. general population. Environ. Health Perspect. 2012, 120, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 5th ed.; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- CDC. NHANES Laboratory/Medical Technologists Procedures Manual. Atlanta, GA, CDC. 2001. Available online: https://www.cdc.gov/nchs/nhanes/index.htm (accessed on 8 January 2022).
- Nakagawa, H.; Nishijo, M.; Morikawa, Y.; Miura, K.; Tawara, K.; Kuriwaki, J.; Kido, T.; Ikawa, A.; Kobayashi, E.; Nogawa, K. Urinary cadmium and mortality among inhabitants of a cadmium-polluted area in Japan. Environ. Res. 2006, 100, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, T.S.; Van Hecke, E.; Thijs, L.; Richart, T.; Kuznetsova, T.; Jin, Y.; Vangronsveld, J.; Roels, H.A.; Staessen, J.A. Cadmium-related mortality and long-term secular trends in the cadmium body burden of an environmentally exposed population. Environ. Health Perspect. 2008, 116, 1620–1628. [Google Scholar] [CrossRef]
- Menke, A.; Muntner, P.; Silbergeld, E.K.; Platz, E.A.; Guallar, E. Cadmium levels in urine and mortality among U.S. adults. Environ. Health Perspect. 2009, 117, 190–196. [Google Scholar] [CrossRef]
- Nguyen, H.D. Cadmium, lead, and mercury interactions on obstructive lung function in pre- and postmenopausal women. Environ. Sci. Pollut. Res. 2023, 30, 73485–73496. [Google Scholar] [CrossRef]
- Kunioka, C.T.; Manso, M.C.; Carvalho, M. Association between Environmental Cadmium Exposure and Osteoporosis Risk in Postmenopausal Women: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 20, 485. [Google Scholar] [CrossRef]
- Van Maele-Fabry, G.; Lombaert, N.; Lison, D. Dietary exposure to cadmium and risk of breast cancer in postmenopausal women: A systematic review and meta-analysis. Environ. Int. 2016, 86, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.M.M.; Copes, R.M.; Dal Osto, L.C.; Flores, C.; Comim, F.V.; Premaor, M.O. Factors related with osteoporosis treatment in postmenopausal women. Medicine 2018, 97, e11524. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.C. A woman’s journey through the reproductive, transitional and postmenopausal periods of life: Impact on cardiovascular and musculo-skeletal risk and the role of estrogen replacement. Maturitas 2011, 70, 197–205. [Google Scholar] [CrossRef] [PubMed]
- James, K.A.; Meliker, J.R. Environmental cadmium exposure and osteoporosis: A review. Int. J. Public Health 2013, 58, 737–745. [Google Scholar] [CrossRef]
- Luo, H.; Gu, R.; Ouyang, H.; Wang, L.; Shi, S.; Ji, Y.; Bao, B.; Liao, G.; Xu, B. Cadmium exposure induces osteoporosis through cellular senescence, associated with activation of NF-kappaB pathway and mitochondrial dysfunction. Environ. Pollut. 2021, 290, 118043. [Google Scholar] [CrossRef]
- Ma, Y.; Ran, D.; Shi, X.; Zhao, H.; Liu, Z. Cadmium toxicity: A role in bone cell function and teeth development. Sci. Total Environ. 2021, 769, 144646. [Google Scholar] [CrossRef]
- Brama, M.; Gnessi, L.; Basciani, S.; Cerulli, N.; Politi, L.; Spera, G.; Mariani, S.; Cherubini, S.; Scotto d’Abusco, A.; Scandurra, R.; et al. Cadmium induces mitogenic signaling in breast cancer cell by an ERalpha-dependent mechanism. Mol. Cell. Endocrinol. 2007, 264, 102–108. [Google Scholar] [CrossRef]
- Huff, M.O.; Todd, S.L.; Smith, A.L.; Elpers, J.T.; Smith, A.P.; Murphy, R.D.; Bleser-Shartzer, A.S.; Hoerter, J.E.; Radde, B.N.; Klinge, C.M. Arsenite and Cadmium Activate MAPK/ERK via Membrane Estrogen Receptors and G-Protein Coupled Estrogen Receptor Signaling in Human Lung Adenocarcinoma Cells. Toxicol Sci. 2016, 152, 62–71. [Google Scholar] [CrossRef]
- Safe, S. Cadmium’s disguise dupes the estrogen receptor. Nat. Med. 2003, 9, 1000–1001. [Google Scholar] [CrossRef]
- Dworatzek, E.; Mahmoodzadeh, S. Targeted basic research to highlight the role of estrogen and estrogen receptors in the cardiovascular system. Pharmacol. Res. 2017, 119, 27–35. [Google Scholar] [CrossRef]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex. Differ. 2017, 8, 33. [Google Scholar] [CrossRef]
- Pelzer, T.; Jazbutyte, V.; Hu, K.; Segerer, S.; Nahrendorf, M.; Nordbeck, P.; Bonz, A.W.; Muck, J.; Fritzemeier, K.H.; Hegele-Hartung, C.; et al. The estrogen receptor-alpha agonist 16alpha-LE2 inhibits cardiac hypertrophy and improves hemodynamic function in estrogen-deficient spontaneously hypertensive rats. Cardiovasc. Res. 2005, 67, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, H.; Mao, X.; Qi, H.; Baker, P.N.; Zhang, H. G-protein-coupled receptor 30 mediates the effects of estrogen on endothelial cell tube formation in vitro. Int. J. Mol. Med. 2017, 39, 1461–1467. [Google Scholar] [CrossRef]
- Zhu, X.; Tang, Z.; Cong, B.; Du, J.; Wang, C.; Wang, L.; Ni, X.; Lu, J. Estrogens increase cystathionine-gamma-lyase expression and decrease inflammation and oxidative stress in the myocardium of ovariectomized rats. Menopause 2013, 20, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health. 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Tarhonska, K.; Lesicka, M.; Janasik, B.; Roszak, J.; Reszka, E.; Braun, M.; Kolacinska-Wow, A.; Jablonska, E. Cadmium and breast cancer—Current state and research gaps in the underlying mechanisms. Toxicol. Lett. 2022, 361, 29–42. [Google Scholar] [CrossRef]
- Souza-Arroyo, V.; Fabian, J.J.; Bucio-Ortiz, L.; Miranda-Labra, R.U.; Gomez-Quiroz, L.E.; Gutierrez-Ruiz, M.C. The mechanism of the cadmium-induced toxicity and cellular response in the liver. Toxicology 2022, 480, 153339. [Google Scholar] [CrossRef]
- Pizzino, G.; Bitto, A.; Interdonato, M.; Galfo, F.; Irrera, N.; Mecchio, A.; Pallio, G.; Ramistella, V.; De Luca, F.; Minutoli, L.; et al. Oxidative stress and DNA repair and detoxification gene expression in adolescents exposed to heavy metals living in the Milazzo-Valle del Mela area (Sicily, Italy). Redox. Biol. 2014, 2, 686–693. [Google Scholar] [CrossRef]
- Xiao, C.; Liu, Y.; Xie, C.; Tu, W.; Xia, Y.; Costa, M.; Zhou, X. Cadmium induces histone H3 lysine methylation by inhibiting histone demethylase activity. Toxicol. Sci. 2015, 145, 80–89. [Google Scholar] [CrossRef]
- Lubovac-Pilav, Z.; Borras, D.M.; Ponce, E.; Louie, M.C. Using expression profiling to understand the effects of chronic cadmium exposure on MCF-7 breast cancer cells. PLoS ONE 2013, 8, e84646. [Google Scholar] [CrossRef]
- Ponce, E.; Aquino, N.B.; Louie, M.C. Chronic cadmium exposure stimulates SDF-1 expression in an ERalpha dependent manner. PLoS ONE 2013, 8, e72639. [Google Scholar] [CrossRef]
- Aoki, Y.; Yee, J.; Mortensen, M.E. Blood cadmium by race/hispanic origin: The role of smoking. Environ. Res. 2017, 155, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Song, H.; Lee, J.; Kim, Y.J.; Chung, H.S.; Yu, J.M.; Jang, G.; Park, R.; Chung, W.; Oh, C.M.; et al. Smoking and passive smoking increases mortality through mediation effect of cadmium exposure in the United States. Sci. Rep. 2023, 13, 3878. [Google Scholar] [CrossRef] [PubMed]
- Tellez-Plaza, M.; Navas-Acien, A.; Caldwell, K.L.; Menke, A.; Muntner, P.; Guallar, E. Reduction in cadmium exposure in the United States population, 1988–2008: The contribution of declining smoking rates. Environ. Health Perspect. 2012, 120, 204–209. [Google Scholar] [CrossRef] [PubMed]
| Blood Cadmium (μg/L) | ||||||
|---|---|---|---|---|---|---|
| Characteristics | Total | Quartile 1 <0.29 | Quartile 2 0.29–0.41 | Quartile 3 0.41–0.66 | Quartile 4 >0.66 | p Value |
| Participants, n | 6286 (100.00) | 1609 (25.60) | 1557 (24.77) | 1570 (24.98) | 1550 (24.66) | |
| Age (years) | 61.00 ± 10.98 | 59.00 ± 10.06 | 61.00 ± 10.69 | 63.00 ± 11.47 | 60.00 ± 11.47 | <0.01 |
| Race | <0.01 | |||||
| Mexican American | 979 (15.57) | 298 (18.52) | 273 (17.53) | 238 (15.16) | 170 (10.97) | |
| Non-Hispanic White | 3038 (48.33) | 708 (44.00) | 768 (49.33) | 766 (48.79) | 796 (51.35) | |
| Non-Hispanic Black | 1323 (21.05) | 366 (22.75) | 328 (21.07) | 304 (19.36) | 325 (20.97) | |
| Other Hispanic | 513 (8.16) | 190 (11.81) | 121 (7.77) | 120 (7.64) | 82 (5.29) | |
| Other race | 433 (6.89) | 47 (2.92) | 67 (4.30) | 142 (9.04) | 177 (11.42) | |
| Education | <0.01 | |||||
| Less than 9th grade | 923 (14.68) | 261 (16.22) | 243 (15.61) | 231 (14.71) | 188 (12.13) | |
| 9–11th grade | 944 (15.02) | 176 (10.94) | 212 (13.62) | 220 (14.01) | 336 (21.68) | |
| High school graduate/GED | 1625 (25.85) | 405 (25.17) | 380 (24.41) | 410 (26.11) | 430 (27.74) | |
| Some college or AA | 1671 (26.58) | 451 (28.03) | 415 (26.65) | 411 (26.18) | 394 (25.42) | |
| College graduate or above | 1116 (17.75) | 313 (19.45) | 306 (19.65) | 297 (18.91) | 200 (12.90) | |
| Not recorded | 7 (0.11) | 3 (0.19) | 1 (0.06) | 1 (0.06) | 2 (0.13) | |
| PIR | 3.03 ± 1.61 | 3.56 ± 1.58 | 3.41 ± 1.60 | 2.94 ± 1.58 | 2.29 ± 1.60 | <0.01 |
| BMI | <0.01 | |||||
| Underweight (<18.5) | 84 (1.34) | 9 (0.56) | 8 (0.51) | 20 (1.27) | 47 (3.03) | |
| Normal (18.5 to <25) | 1574 (25.04) | 301 (18.71) | 343 (22.03) | 434 (27.64) | 496 (32.00) | |
| Overweight (25 to <30) | 1998 (31.78) | 487 (30.27) | 505 (32.43) | 516 (32.87) | 490 (31.61) | |
| Obese (30 or greater) | 2630 (41.84) | 812 (50.47) | 701 (45.02) | 600 (38.22) | 517 (33.35) | |
| Alcohol intake | <0.01 | |||||
| Non-drinker | 2708 (43.08) | 761 (47.30) | 710 (45.60) | 673 (42.87) | 564 (36.39) | |
| 1 to <5 drinks/month | 2310 (36.75) | 512 (31.82) | 587 (37.70) | 562 (35.80) | 649 (41.87) | |
| 5 to <10 drinks/month | 201 (3.20) | 48 (2.98) | 57 (3.66) | 48 (3.06) | 48 (3.10) | |
| 10+ drinks/month | 500 (7.95) | 111 (6.90) | 94 (6.04) | 130 (8.28) | 165 (10.65) | |
| Not recorded | 567 (9.02) | 177 (11.00) | 109 (7.00) | 157 (10.00) | 124 (8.00) | |
| Smoking status | <0.01 | |||||
| Never smoker | 3783 (60.18) | 1308 (81.29) | 1102 (70.78) | 939 (59.81) | 434 (28.00) | |
| Former smoker | 1591 (25.31) | 285 (17.71) | 413 (26.53) | 527 (33.57) | 366 (23.61) | |
| Current smoker | 908 (14.44) | 16 (0.99) | 41 (2.63) | 103 (6.56) | 748 (48.26) | |
| Not recorded | 4 (0.06) | 0 (0.00) | 1 (0.06) | 1 (0.06) | 2 (0.13) | |
| Serum cotinine | 0.140 | 0.030 | 0.033 | 0.072 | 6.909 | <0.01 |
| (ng/mL; GM (95% CI)) | (0.12, 0.16) | (0.027, 0.033) | (0.033, 0.043) | (0.059, 0.088) | (4.940, 9.664) | |
| Hypertension | 3381 (53.79) | 874 (54.32) | 828 (53.18) | 851 (54.20) | 828 (53.42) | 0.30 |
| Diabetes | 1493 (23.75) | 447 (27.78) | 394 (25.31) | 330 (21.02) | 322 (20.77) | <0.01 |
| Urine Cadmium (μg/g Creatinine) | ||||||
|---|---|---|---|---|---|---|
| Characteristics | Total | Quartile 1 <0.28 | Quartile 2 0.28–0.44 | Quartile 3 0.44–0.72 | Quartile 4 >0.72 | p Value |
| Participants, n | 1878 (100.00) | 470 (25.03) | 469 (24.97) | 469 (24.97) | 470 (25.03) | |
| Age (years) | 60.00 ± 11.25 | 57.00 ± 11.01 | 61.00 ± 11.52 | 61.00 ± 10.81 | 62.00 ± 10.83 | <0.01 |
| Race | <0.01 | |||||
| Mexican American | 300 (15.97) | 68 (14.47) | 71 (15.14) | 92 (19.62) | 69 (14.68) | |
| Non-Hispanic White | 940 (50.05) | 234 (49.79) | 246 (52.45) | 227 (48.40) | 233 (49.57) | |
| Non-Hispanic Black | 382 (20.34) | 117 (24.89) | 98 (20.90) | 89 (18.98) | 78 (16.60) | |
| Other Hispanic | 151 (8.04) | 37 (7.87) | 35 (7.46) | 42 (8.96) | 37 (7.87) | |
| Other race | 105 (5.59) | 14 (2.98) | 19 (4.05) | 19 (4.05) | 53 (11.28) | |
| Education | <0.01 | |||||
| Less than 9th grade | 285 (15.18) | 71 (15.11) | 73 (15.57) | 76 (16.20) | 65 (13.83) | |
| 9–11th grade | 274 (14.59) | 51 (10.85) | 53 (11.30) | 74 (15.78) | 96 (20.43) | |
| High school graduate/GED | 500 (26.62) | 122 (25.96) | 131 (27.93) | 125 (26.65) | 122 (25.96) | |
| Some college or AA | 496 (26.41) | 127 (27.02) | 120 (25.59) | 118 (25.16) | 131 (27.87) | |
| College graduate or above | 321 (17.09) | 98 (20.85) | 91 (19.40) | 76 (16.20) | 56 (11.91) | |
| Not recorded | 2 (0.11) | 1 (0.21) | 1 (0.21) | 0 (0.00) | 0 (0.00) | |
| PIR | 2.96 ± 1.59 | 3.76 ± 1.60 | 2.89 ± 1.54 | 2.75 ± 1.57 | 2.38 ± 1.55 | <0.01 |
| BMI | <0.01 | |||||
| Underweight (<18.5) | 21 (1.12) | 2 (0.43) | 3 (0.64) | 4 (0.85) | 12 (2.55) | |
| Normal (18.5 to <25) | 483 (25.72) | 84 (17.87) | 122 (26.01) | 115 (24.52) | 162 (34.47) | |
| Overweight (25 to <30) | 607 (32.32) | 128 (27.23) | 153 (32.62) | 166 (35.39) | 160 (34.04) | |
| Obese (30 or greater) | 767 (40.84) | 256 (54.47) | 191 (40.72) | 184 (39.23) | 136 (28.94) | |
| Alcohol intake | 0.7 | |||||
| Non-drinker | 888 (47.28) | 226 (48.09) | 223 (47.55) | 226 (48.19) | 213 (45.32) | |
| 1 to <5 drinks/month | 735 (39.14) | 181 (38.51) | 177 (37.74) | 190 (40.51) | 187 (39.79) | |
| 5 to <10 drinks/month | 69 (3.67) | 22 (4.68) | 23 (4.90) | 9 (1.92) | 15 (3.19) | |
| 10+ drinks/month | 184 (9.80) | 40 (8.51) | 46 (9.81) | 43 (9.17) | 55 (11.70) | |
| Not recorded | 2 (0.11) | 1 (0.21) | 0 (0.00) | 1 (0.21) | 0 (0.00) | |
| Smoking status | <0.01 | |||||
| Never smoker | 1138 (60.60) | 370 (78.72) | 318 (67.80) | 274 (58.42) | 176 (37.45) | |
| Former smoker | 477 (25.40) | 80 (17.02) | 112 (23.88) | 131 (27.93) | 154 (32.77) | |
| Current smoker | 263 (14.00) | 20 (4.26) | 39 (8.32) | 64 (13.65) | 140 (29.79) | |
| Not recorded | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.10) | |
| Serum cotinine | 0.146 | 0.051 | 0.068 | 0.202 | 0.935 | <0.01 |
| (ng/mL; GM (95% CI)) | (0.115, 0.185) | (0.040, 0.064) | (0.050, 0.095) | (0.121, 0.337) | (0.516, 1.693) | |
| Hypertension | 1023 (54.47) | 258 (54.89) | 259 (55.22) | 256 (54.58) | 250 (53.19) | 0.7 |
| Diabetes | 444 (23.64) | 114 (24.26) | 109 (23.24) | 109 (23.24) | 112 (23.83) | >0.9 |
| Blood Cadmium (μg/L) | |||||
|---|---|---|---|---|---|
| <0.29 | 0.29–0.41 | 0.41–0.66 | >0.66 | p Value | |
| Glycohemoglobin (n = 6269) (%) | 5.82 ± 0.99 | 5.81 ± 0.97 | 5.81 ± 0.97 | 5.76 ± 0.86 | 0.7 |
| Glucose (n = 3096) (mg/dL) | 109.75 ± 28.14 | 109.46 ± 34.02 | 107.49 ± 27.85 | 106.81 ± 31.89 | <0.01 |
| Cholesterol (n = 6266) (mg/dL) | 208.67 ± 39.91 | 212.82 ± 40.82 | 212.22 ± 42.09 | 211.22 ± 41.58 | 0.11 |
| LDL (n = 3020) (mg/dL) | 119.74 ± 35.45 | 122.17 ± 36.82 | 124.25 ± 35.66 | 123.04 ± 37.81 | 0.14 |
| HDL (n = 6265) ((mg/dL)) | 58.88 ± 16.19 | 60.95 ± 17.64 | 61.76 ± 18.39 | 58.49 ± 17.04 | <0.01 |
| Triglycerides (n = 3083) (mg/dL) | 135.07 ± 113.59 | 132.61 ± 73.12 | 126.18 ± 67.48 | 145.51 ± 104.99 | <0.01 |
| Urinecadmium (μg/g creatinine) | |||||
| <0.28 | 0.28–0.44 | 0.44–0.72 | >0.72 | ||
| Glycohemoglobin (n = 1874) (%) | 5.72 ± 0.76 | 5.76 ± 0.97 | 5.75 ± 0.85 | 5.77 ± 0.86 | 0.5 |
| Glucose (n = 925) (mg/dL) | 104.55 ± 20.28 | 107.24 ± 30.57 | 109.25 ± 31.70 | 104.25 ± 25.98 | 0.5 |
| Cholesterol (n = 1875) (mg/dL) | 212.94 ± 42.76 | 210.77 ± 40.36 | 214.51 ± 41.10 | 210.88 ± 43.23 | 0.8 |
| LDL (n = 902) (mg/dL) | 115.54 ± 40.01 | 123.88 ± 32.68 | 124.90 ± 35.40 | 122.73 ± 36.56 | 0.11 |
| HDL (n = 1874) ((mg/dL)) | 60.09 ± 18.16 | 61.05 ± 16.93 | 59.94 ± 15.02 | 60.90 ± 17.46 | 0.8 |
| Triglycerides (n = 924) (mg/dL) | 128.46 ± 90.66 | 132.28 ± 79.95 | 146.79 ± 85.42 | 129.31 ± 67.16 | 0.08 |
| Blood Cadmium (μg/L) | p Value | ||||
|---|---|---|---|---|---|
| <0.29 | 0.29–0.41 | 0.41–2.19 | >2.19 | ||
| All-cause mortality | |||||
| Number of deaths (%) | 210 (13.05) | 337 (21.64) | 398 (25.35) | 471 (30.39) | |
| Model 1 | 1.00 | 1.30 (1.06, 1.60) 0.01 | 1.82 (1.50, 2.22) < 0.001 | 2.19 (1.81, 2.64) <0.001 | <0.001 |
| HR (95% CI) p-value | |||||
| Model 2 | 1.00 | 1.03 (0.86, 1.23) 0.74 | 1.24 (1.03, 1.49) 0.02 | 1.83 (1.50, 2.22) <0.001 | <0.001 |
| HR (95% CI) p-value | |||||
| Model 3 | 1.00 | 0.99 (0.83, 1.18) 0.94 | 1.13 (0.94, 1.36) 0.18 | 1.39 (1.13, 1.72) 0.002 | <0.001 |
| HR (95% CI) p-value | |||||
| CVD mortality | |||||
| Number of deaths (%) | 60 (3.73) | 88 (5.65) | 110 (7.00) | 123 (7.94) | |
| Model 1 | 1.00 | 0.88 (0.61, 1.26) 0.48 | 1.03 (0.72, 1.47) 0.86 | 0.879 (0.56, 1.11) 0.18 | 0.33 |
| HR (95% CI) p-value | |||||
| Model 2 | 1.00 | 0.86 (0.61, 1.21) 0.38 | 0.94 (0.67, 1.32) 0.72 | 0.90 (0.63, 1.27) 0.53 | 0.68 |
| HR (95% CI) p-value | |||||
| Model 3 | 1.00 | 0.86 (0.62, 1.21) 0.40 | 0.95 (0.68, 1.33) 0.76 | 0.93 (0.65, 1.32) 0.68 | 0.83 |
| HR (95% CI) p-value | |||||
| Cancer mortality | |||||
| Number of deaths (%) | 38 (2.36) | 57 (3.66) | 71 (4.52) | 95 (6.13) | |
| Model 1 | 1.00 | 0.79 (0.47, 1.35) 0.40 | 0.88 (0.56, 1.41) 0.61 | 1.10 (0.73, 1.65) 0.64 | 0.56 |
| HR (95% CI) p-value | |||||
| Model 2 | 1.00 | 0.73 (0.44, 1.20) 0.21 | 0.88 (0.58, 1.34) 0.56 | 1.19 (0.80, 1.77) 0.40 | 0.31 |
| HR (95% CI) p-value | |||||
| Model 3 | 1.00 | 0.67 (0.41, 1.11) 0.12 | 0.83 (0.56, 1.23) 0.35 | 1.11 (0.70, 1.77) 0.65 | 0.50 |
| HR (95% CI) p-value | |||||
| Urine Cadmium (μg/g Creatinine) | p Trend | ||||
|---|---|---|---|---|---|
| <0.28 | 0.28–0.44 | 0.44–0.72 | >0.72 | ||
| All-cause mortality | |||||
| Number of deaths (%) | 67 (14.26) | 107 (22.81) | 100 (21.32) | 160 (34.04) | |
| Model 1 | 1.00 | 1.47 (1.03, 2.09) 0.03 | 1.48 (0.99, 2.20) 0.05 | 2.76 (1.96, 3.90) <0.001 | <0.001 |
| HR (95% CI) p-value | |||||
| Model 2 | 1.00 | 1.06 (0.74, 1.51) 0.76 | 1.03 (0.69, 1.56) 0.88 | 1.69 (1.18, 2.43) 0.004 | 0.01 |
| HR (95% CI) p-value | |||||
| Model 3 | 1.00 | 1.04 (0.72, 1.50) 0.84 | 0.97 (0.64, 1.47) 0.89 | 1.50 (1.01, 2.23) 0.045 | 0.08 |
| HR (95% CI) p-value | |||||
| CVD mortality | |||||
| Number of deaths (%) | 20 (4.26) | 36 (7.68) | 22 (4.69) | 37 (7.87) | |
| Model 1 | 1.00 | 0.76 (0.38, 1.50) 0.42 | 0.81 (0.40, 1.65) 0.56 | 0.50 (0.22, 1.13) 0.09 | 0.10 |
| HR (95% CI) p-value | |||||
| Model 2 | 1.00 | 0.76 (0.38, 1.50) 0.43 | 0.91 (0.43, 1.95) 0.82 | 0.48 (0.18, 1.26) 0.14 | 0.17 |
| HR (95% CI) p-value | |||||
| Model 3 | 1.00 | 0.80 (0.40, 1.59) 0.53 | 0.95 (0.45, 2.02) 0.89 | 0.51 (0.19, 1.39) 0.19 | 0.24 |
| HR (95% CI) p-value | |||||
| Cancer mortality | |||||
| Number of deaths (%) | 13 (2.77) | 16 (3.41) | 16 (3.41) | 39 (8.30) | |
| Model 1 | 1.00 | 1.58 (0.68, 3.66) 0.28 | 1.61 (0.52, 5.02) 0.41 | 1.36 (0.62, 2.99) 0.45 | 0.50 |
| HR (95% CI) p-value | |||||
| Model 2 | 1.00 | 3.40 (1.08, 10.71) 0.04 | 3.12 (0.90, 10.85) 0.07 | 5.62 (2.04, 15.47) <0.001 | 0.002 |
| HR (95% CI) p-value | |||||
| Model 3 | 1.00 | 3.06 (0.91, 10.35) 0.07 | 3.30 (1.00, 10.88) 0.0499 | 5.70 (1.91, 16.95) 0.002 | 0.002 |
| HR (95% CI) p-value | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.-W.; Fan, D.-X.; Li, M.-Q. The Relationship between Cadmium Exposure and Mortality in Postmenopausal Females: A Cohort Study of 2001–2018 NHANES. Nutrients 2023, 15, 4604. https://doi.org/10.3390/nu15214604
Shi J-W, Fan D-X, Li M-Q. The Relationship between Cadmium Exposure and Mortality in Postmenopausal Females: A Cohort Study of 2001–2018 NHANES. Nutrients. 2023; 15(21):4604. https://doi.org/10.3390/nu15214604
Chicago/Turabian StyleShi, Jia-Wei, Deng-Xuan Fan, and Ming-Qing Li. 2023. "The Relationship between Cadmium Exposure and Mortality in Postmenopausal Females: A Cohort Study of 2001–2018 NHANES" Nutrients 15, no. 21: 4604. https://doi.org/10.3390/nu15214604
APA StyleShi, J.-W., Fan, D.-X., & Li, M.-Q. (2023). The Relationship between Cadmium Exposure and Mortality in Postmenopausal Females: A Cohort Study of 2001–2018 NHANES. Nutrients, 15(21), 4604. https://doi.org/10.3390/nu15214604






