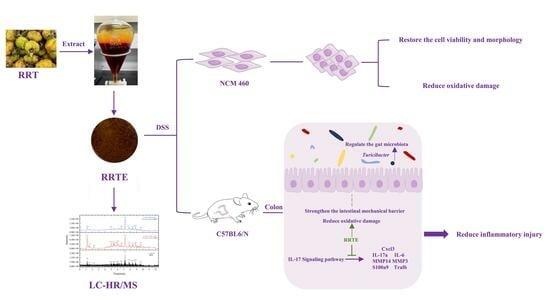
Rosa Roxburghii Tratt Fruit Extract Prevents Dss-Induced Ulcerative Colitis in Mice by Modulating the Gut Microbiota and the IL-17 Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation, Composition, and Antioxidant Ability of RRTE
2.2.1. Preparation of RRTE
2.2.2. Component Analysis of the RRTE
2.2.3. Determination of the Antioxidant Capacity of RRTE
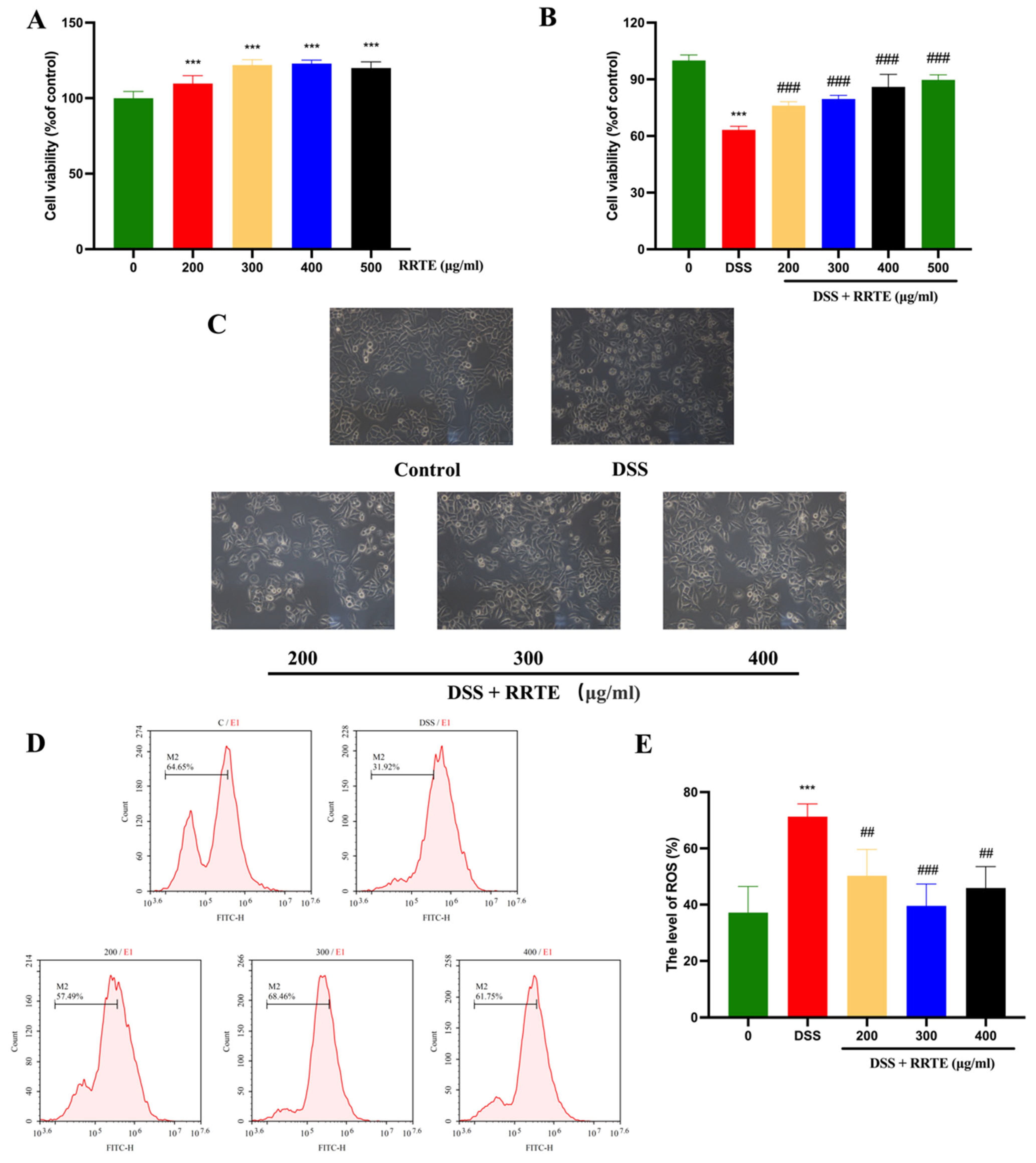
2.3. Protective Effect of RRTE on Damaged NCM460 Cells
2.3.1. Cell Culture
2.3.2. Cell Viability Assay
2.3.3. Cell Morphology Observation
2.3.4. Intracellular Reactive Oxygen Species (ROS) Expression Assay
2.4. The Effect and Mechanism of RRT in Alleviating Ulcerative Colitis In Vivo
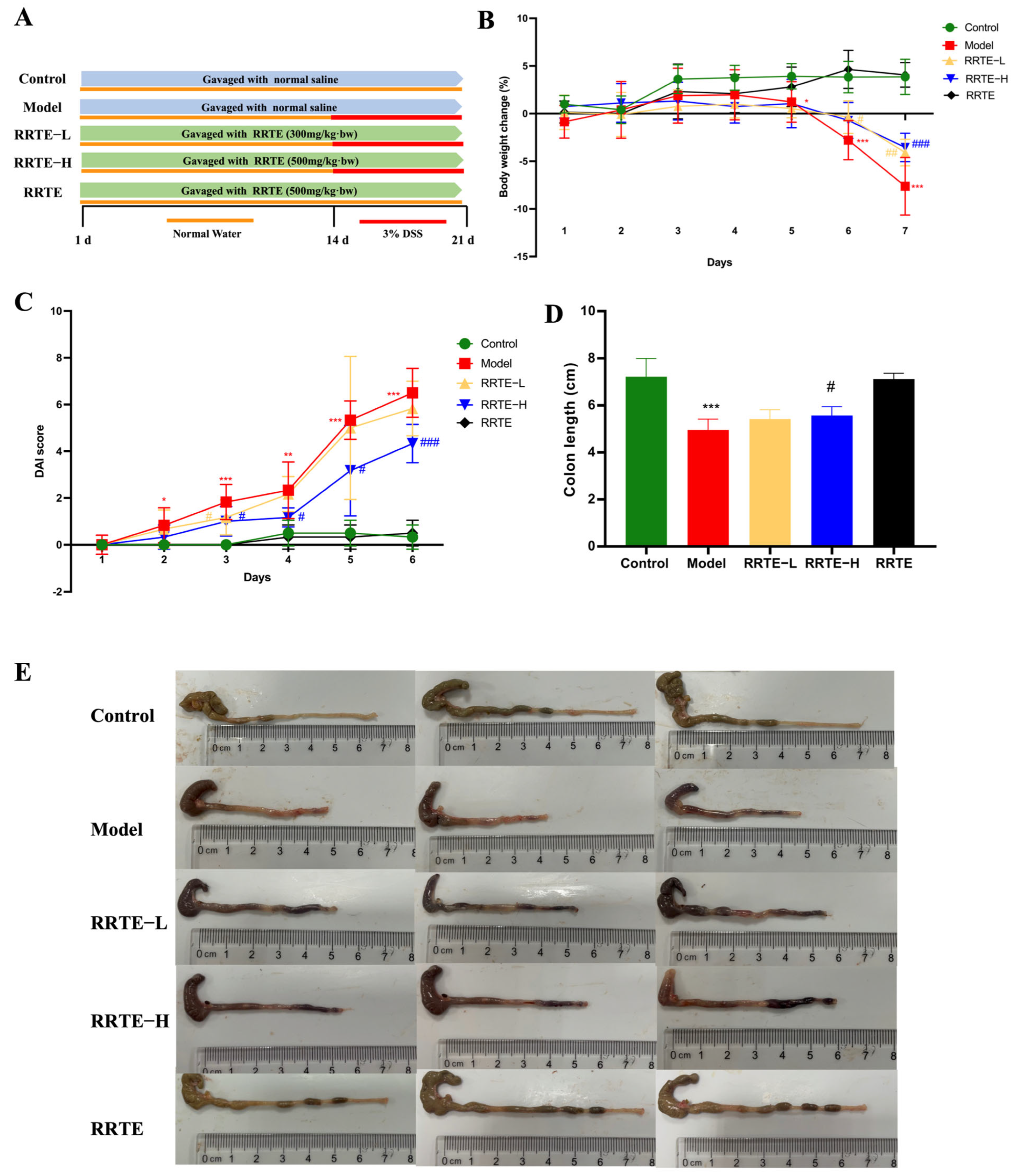
2.4.1. Establishment of the Animal Model
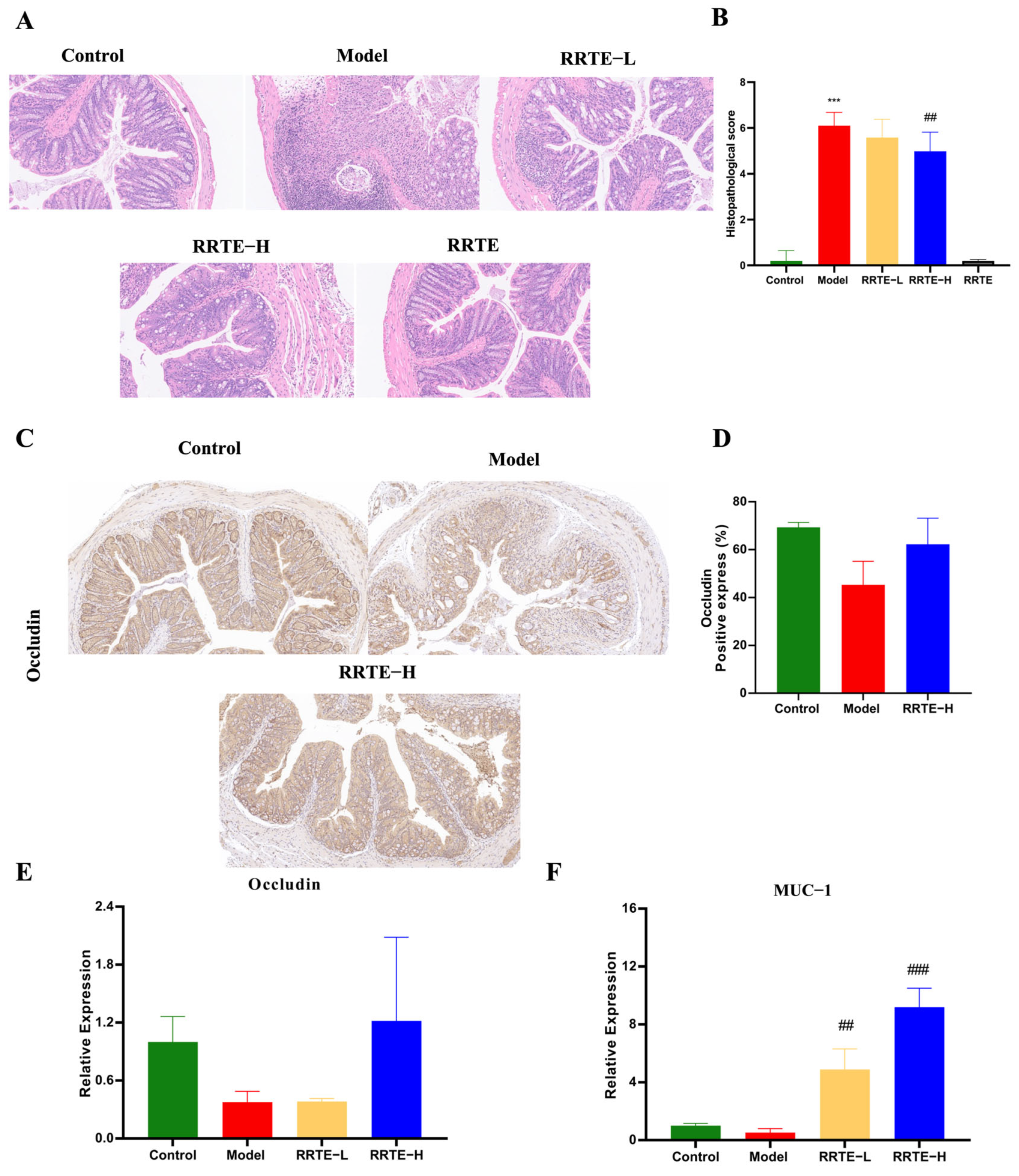
2.4.2. Evaluation of Colonic Mechanical Barrier Damage and SYMPTOMs of Ulcerative Colitis in Mice
2.4.3. qPCR Analysis
2.4.4. Gut Microbiota Analysis
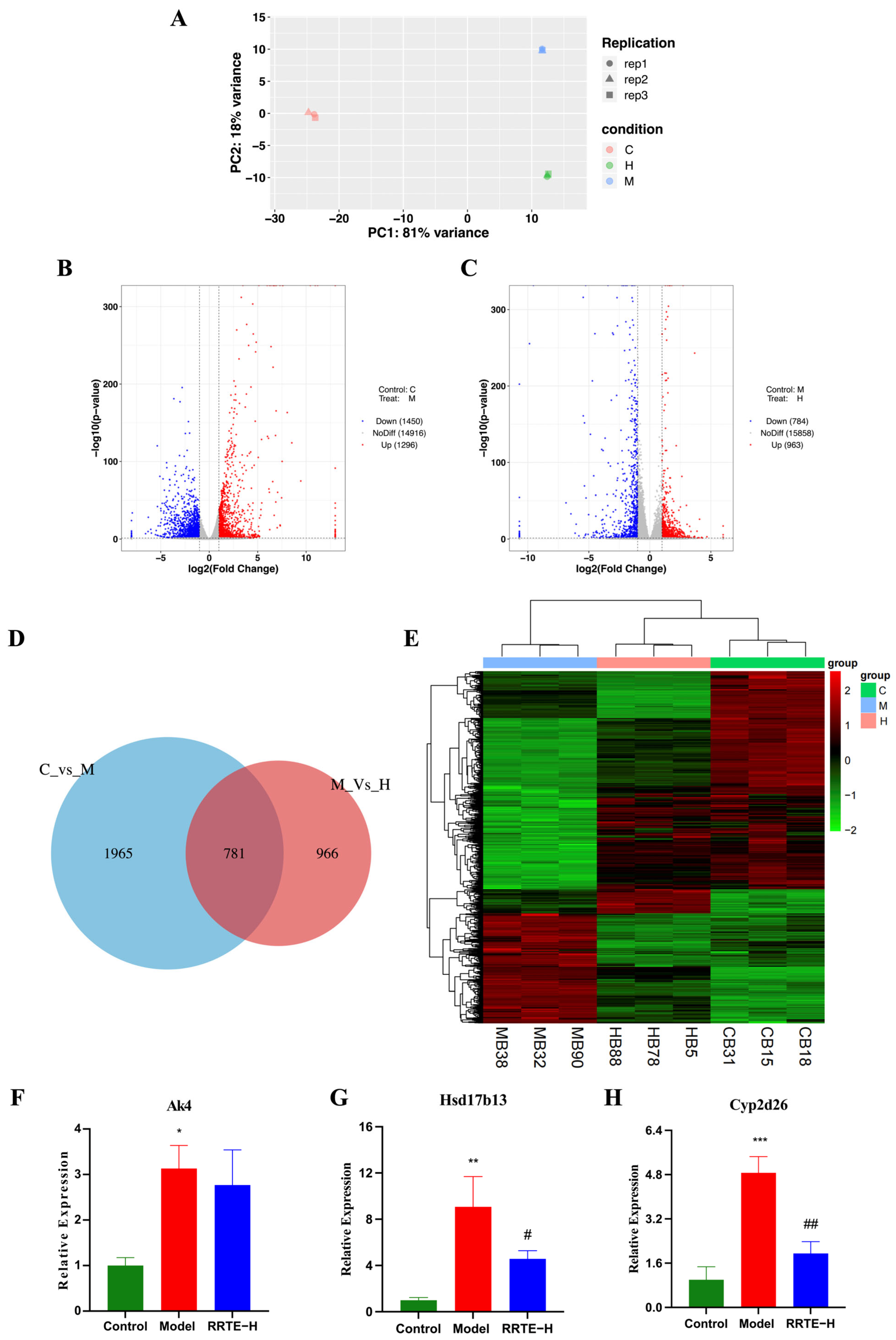
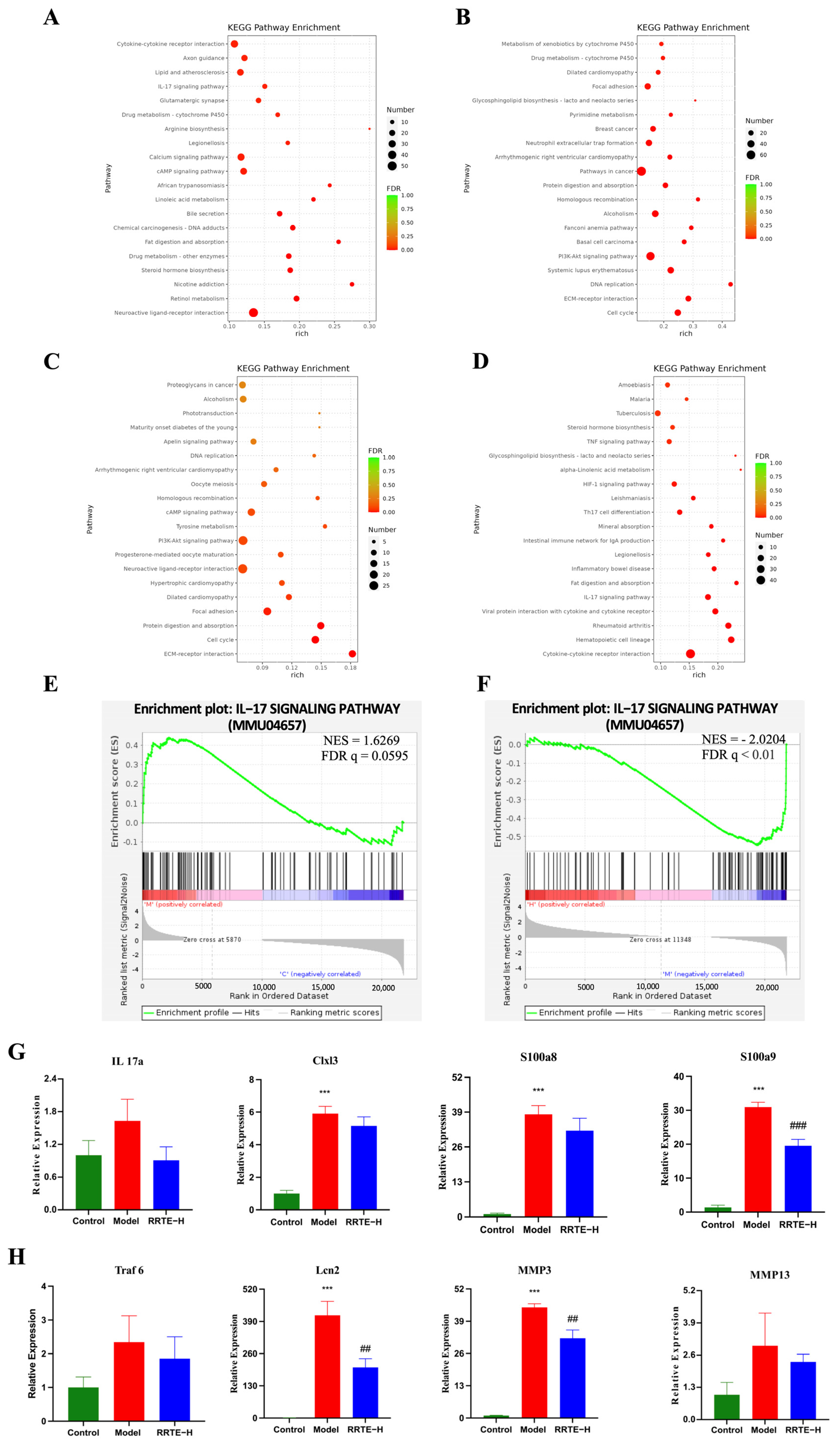
2.4.5. Transcriptomics Sequencing Analysis
2.5. Data Statistics and Analysis
3. Results
3.1. RRTE Compositional Analysis and Antioxidant Capacity Determination
3.2. RRTE Could Prevent NCM460 Cells from DSS-Induced Injury
3.3. RRTE Relief DSS-Induced Ulcerative Colitis in Mice
3.3.1. RRTE Alleviates Colitis Symptoms in UC Mice
3.3.2. RRTE Protects the Colon Mucosa Mechanical Barrier
3.3.3. RRTE Reduces Colonic Mucosal Inflammation
3.3.4. RRTE Reduces the Oxidative Stress of the Colon Mucosa
3.3.5. RRTE Can Regulate the Gut Microbiota of UCMice
3.3.6. Data Analysis on the Mouse Colon Transcriptome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feuerstein, J.D.; Moss, A.C.; Farraye, F.A. Ulcerative Colitis. Mayo Clin. Proc. 2019, 94, 1357–1373. [Google Scholar] [CrossRef]
- Engel, F.; Berens, S.; Gauss, A.; Schaefert, R.; Eich, W.; Tesarz, J. Higher Levels of Psychological Burden and Alterations in Personality Functioning in Crohn’s Disease and Ulcerative Colitis. Front. Psychol. 2021, 12, 671493. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.-C.; Sollano, J.; Hui, Y.T.; Yu, W.; Estrella, P.V.S.; Llamado, L.J.Q.; Koram, N. Epidemiology, burden of disease, and unmet needs in the treatment of ulcerative colitis in Asia. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Aydın, M.F.; Taşdemir, H. Ulcerative Colitis in a COVID-19 Patient: A Case Report. Turk. J. Gastroenterol. 2021, 32, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef] [PubMed]
- Kawalec, P. Indirect costs of inflammatory bowel diseases: Crohn’s disease and ulcerative colitis. A systematic review. Arch. Med. Sci. 2016, 12, 295–302. [Google Scholar] [CrossRef]
- Wang, S.; Dong, G.; Sheng, C. Structural Simplification of Natural Products. Chem. Rev. 2019, 119, 4180–4220. [Google Scholar] [CrossRef]
- Chopra, B.; Dhingra, A.K. Natural products: A lead for drug discovery and development. Phytother. Res 2021, 35, 4660–4702. [Google Scholar] [CrossRef]
- Berdigaliyev, N.; Aljofan, M. An overview of drug discovery and development. Future Med. Chem. 2020, 12, 939–947. [Google Scholar] [CrossRef]
- Huang, X.; Li, X.; Deng, Y.; Zhou, T.; Chen, T.; Wu, S.; Xia, R.; Kang, Y.; Yin, W. The flavonoids extract from Okra flowers protects against DSS-induced colitis via regulating NF-κB signaling pathway and gut microbiota. J. Funct. Foods 2022, 99, 105335. [Google Scholar] [CrossRef]
- Li, F.; Yan, H.; Jiang, L.; Zhao, J.; Lei, X.; Ming, J. Cherry Polyphenol Extract Ameliorated Dextran Sodium Sulfate-Induced Ulcerative Colitis in Mice by Suppressing Wnt/beta-Catenin Signaling Pathway. Foods 2021, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, Z.; Liu, D.; Dong, H.; Zhou, J.; Wu, J. Therapeutic effects of Zhuling Jianpi capsule on experimental ulcerative colitis and characterization of its chemical constituents and metabolomics using UHPLC-Q-TOF-MS. Heliyon 2023, 9, e16553. [Google Scholar] [CrossRef]
- Duan, L.; Cheng, S.; Li, L.; Liu, Y.; Wang, D.; Liu, G. Natural Anti-Inflammatory Compounds as Drug Candidates for Inflammatory Bowel Disease. Front. Pharmacol. 2021, 12, 684486. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wei, T.; Zheng, L.; Jiang, F.; Ma, W.; Lu, M.; Wu, X.A. Recent Advances on Main Active Ingredients, Pharmacological Activities of Rosa roxbughii and Its Development and Utilization. Foods 2023, 12, 1051. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.T.; Lv, M.J.; An, J.Y.; Fan, X.H.; Dong, M.Z.; Zhang, S.D.; Wang, J.D.; Wang, Y.Q.; Cai, Z.H.; Fu, Y.J. Botanical characteristics, phytochemistry and related biological activities of Rosa roxburghii Tratt fruit, and its potential use in functional foods: A review. Food Funct. 2021, 12, 1432–1451. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhang, S.; Zhang, M.; Wang, P.J.; Liang, G.Y.; Gao, X.L. Integrated Proteomics and Metabolomics Analysis to Explore the Amelioration Mechanisms of Rosa roxburghii Tratt Fruit Polyphenols on Lipopolysaccharide-Induced Acute Lung Injury Mice. J. Agric. Food Chem. 2023, 71, 3079–3092. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Zhang, Y.; Shi, Y.; Feng, D.; Zuo, Y.; Hu, P. Effect and Correlation of Rosa roxburghii Tratt Fruit Vinegar on Obesity, Dyslipidemia and Intestinal Microbiota Disorder in High-Fat Diet Mice. Foods 2022, 11, 4108. [Google Scholar] [CrossRef]
- Kang, Y.H.; Zhou, T.; Wu, S.X.; Li, X.J.; Huang, X.Y.; Xia, R.; Ling, Y.H.; Zhou, H.T.; Zhang, S.W.; Yin, W.Y. Effects of Rosa roxburghii Tratt on Ulcerative Colitis: An Integrated Analysis of Network Pharmacology and Experimental Validation. Am. J. Chin. Med. 2023, 51, 1477–1499. [Google Scholar] [CrossRef]
- Farid, A.; Sheibani, M.; Shojaii, A.; Noori, M.; Motevalian, M. Evaluation of anti-inflammatory effects of leaf and seed extracts of Plantago major on acetic acid-induced ulcerative colitis in rats. J. Ethnopharmacol. 2022, 298, 115595. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Zhang, W.H.; Zhu, Q.M.; Ning, J.; Huo, X.K.; Xiao, H.T.; Sun, C.P. Total terpenoids of Inula japonica activated the Nrf2 receptor to alleviate the inflammation and oxidative stress in LPS-induced acute lung injury. Phytomedicine 2022, 107, 154377. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Xu, H.; Han, Y. Extraction of Saponin fromCamellia oleiferaAbel Cake by a Combination Method of Alkali Solution and Acid Isolation. J. Chem. 2016, 2016, 6903524. [Google Scholar] [CrossRef][Green Version]
- Jiang, J.; Dong, H. Preparation of high-purity baicalein from Scutellaria baicalensis Georgi. Nat. Prod. Res. 2008, 22, 1410–1412. [Google Scholar]
- Liu, Y.X.Y.; Yuan, X.H. Study on technology extracting rosolic acid of Rosa roxbunghii Tratt. pomace. Hubei Agric. Sci. 2021, 60, 103–107. [Google Scholar]
- Xia, R.; Wang, L.; Zhou, T.; Zeng, Y.; Li, X.; Wu, S.; Huang, X.; Kang, Y.; Yin, W. Pomegranate juice ameliorates pulmonary fibrosis by regulating inflammatory response and epithelial mesenchymal transformation. J. Funct. Foods 2022, 94, 105113. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Xia, R.; Zheng, X.; Li, X.; Wu, S.; Zhang, Q.; Li, S.; Deng, Y.; Yao, Y.; et al. Component analysis and anti-pulmonary fibrosis effects of Rosa sterilis juice. Food Funct. 2022, 13, 12915–12924. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, T.; Zhou, B.; Gao, W.; Cao, J.; Huang, L. Chemical composition and antioxidant and anti-inflammatory potential of peels and flesh from 10 different pear varieties (Pyrus spp.). Food Chem. 2014, 152, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, S.; Popp, V.; Kindermann, M.; Gerlach, K.; Weigmann, B.; Fichtner-Feigl, S.; Neurath, M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017, 12, 1295–1309. [Google Scholar] [CrossRef]
- Wang, H.; Liu, N.; Yang, Z.; Zhao, K.; Pang, H.; Shao, K.; Zhou, Z.; Li, S.; He, N. Preventive effect of pectic oligosaccharides on acute colitis model mice: Modulating epithelial barrier, gut microbiota and Treg/Th17 balance. Food Funct. 2022, 13, 9999–10012. [Google Scholar] [CrossRef]
- Ji, K.L.; Wu, M.Z.; Huang, C.Y.; GongPan, P.C.; Sun, P.; Sun, Y.L.; Li, J.; Xiao, C.F.; Xu, Y.K.; Fan, Q.F.; et al. Alpinia hainanensis Rhizome Extract Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis: Active Ingredient Investigation and Evaluation. J. Agric. Food Chem. 2022, 70, 3989–3999. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, L.; Shen, Y.; Zhang, C.; Hou, B.; Zhou, Y. Transcription factor paired related homeobox 1 (PRRX1) activates matrix metalloproteinases (MMP)13, which promotes the dextran sulfate sodium-induced inflammation and barrier dysfunction of NCM460 cells. Bioengineered 2022, 13, 645–654. [Google Scholar] [CrossRef]
- Ding, A.; Wen, X. Dandelion root extract protects NCM460 colonic cells and relieves experimental mouse colitis. J. Nat. Med. 2018, 72, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Krugliak Cleveland, N.; Torres, J.; Rubin, D.T. What Does Disease Progression Look Like in Ulcerative Colitis, and How Might It Be Prevented? Gastroenterology 2022, 162, 1396–1408. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wei, C.; Yang, Y.; Liu, M.; Luo, A.; Song, H.; Wang, Y.; Duan, X. New discovery of anti-ulcerative colitis active ingredients of Nostoc commune: P-Hydroxy benzaldehyde. J. Funct. Foods 2021, 77, 104327. [Google Scholar] [CrossRef]
- Wang, X.J.; Zhang, D.; Yang, Y.T.; Li, X.Y.; Li, H.N.; Zhang, X.P.; Long, J.Y.; Lu, Y.Q.; Liu, L.; Yang, G.; et al. Suppression of microRNA-222-3p ameliorates ulcerative colitis and colitis-associated colorectal cancer to protect against oxidative stress via targeting BRG1 to activate Nrf2/HO-1 signaling pathway. Front. Immunol. 2023, 14, 1089809. [Google Scholar] [CrossRef] [PubMed]
- Reinoso Webb, C.; Koboziev, I.; Furr, K.L.; Grisham, M.B. Protective and pro-inflammatory roles of intestinal bacteria. Pathophysiology 2016, 23, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiong, S.; Jin, F.; Zhou, F.; Zhou, H.; Guo, J.; Wen, C.; Huang, B. Global trends in intestinal flora and ulcerative colitis research during the past 10 years: A bibliometric analysis. Front. Microbiol. 2022, 13, 1003905. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Liu, X.J.; Hao, J.Y. Gut microbiota in ulcerative colitis: Insights on pathogenesis and treatment. J. Dig. Dis. 2020, 21, 147–159. [Google Scholar] [CrossRef]
- Dai, W.; Zhan, X.; Peng, W.; Liu, X.; Peng, W.; Mei, Q.; Hu, X. Ficus pandurata Hance Inhibits Ulcerative Colitis and Colitis-Associated Secondary Liver Damage of Mice by Enhancing Antioxidation Activity. Oxidative Med. Cell. Longev. 2021, 2021, 2617881. [Google Scholar] [CrossRef]
- Sun, W.; Lirio, R.A.; Schneider, J.; Aubrecht, J.; Kadali, H.; Baratta, M.; Gulati, P.; Suri, A.; Lin, T.; Vasudevan, R.; et al. Assessment of Vedolizumab Disease-Drug-Drug Interaction Potential in Patients with Inflammatory Bowel Diseases. Clin. Pharmacol. Drug Dev. 2021, 10, 734–747. [Google Scholar] [CrossRef]
- Klapp, V.; Alvarez-Abril, B.; Leuzzi, G.; Kroemer, G.; Ciccia, A.; Galluzzi, L. The DNA Damage Response and Inflammation in Cancer. Cancer Discov. 2023, 13, 1521–1545. [Google Scholar] [CrossRef]
- Fan, W.G.; Zhou, Y.J. Polyphenol and triterpenoid components, content, and antioxidant properties of Rosa roxburghii leaves, petals, and fruits. J. Guizhou Univ. 2022, 39, e72722. [Google Scholar]
- Kotla, N.G.; Isa, I.L.M.; Rasala, S.; Demir, S.; Singh, R.; Baby, B.V.; Swamy, S.K.; Dockery, P.; Jala, V.R.; Rochev, Y.; et al. Modulation of Gut Barrier Functions in Ulcerative Colitis by Hyaluronic Acid System. Adv. Sci. 2022, 9, e2103189. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, A.; Liu, H.; Zhang, Z.; Zhang, C.; Ma, C.; Zhang, L.; Zhang, J. Lactobacillus plantarum HNU082 alleviates dextran sulfate sodium-induced ulcerative colitis in mice through regulating gut microbiome. Food Funct. 2022, 13, 10171–10185. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Du, P.; Xie, Q.; Wang, N.; Li, H.; Smith, E.E.; Li, C.; Liu, F.; Huo, G.; Li, B. Protective effects of tryptophan-catabolizing Lactobacillus plantarum KLDS 1.0386 against dextran sodium sulfate-induced colitis in mice. Food Funct. 2020, 11, 10736–10747. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.; Huang, J.; Ma, X.; Huang, P.; Liu, Y.; Zeng, J. Protopine Alleviates Dextran Sodium Sulfate-Induced Ulcerative Colitis by Improving Intestinal Barrier Function and Regulating Intestinal Microbiota. Molecules 2023, 28, 5277. [Google Scholar] [CrossRef]
- Sun, T.; Kwong, C.H.T.; Gao, C.; Wei, J.; Yue, L.; Zhang, J.; Ye, R.D.; Wang, R. Amelioration of ulcerative colitis via inflammatory regulation by macrophage-biomimetic nanomedicine. Theranostics 2020, 10, 10106–10119. [Google Scholar] [CrossRef]
- Liu, C.; Wang, R.; Jiao, X.; Zhang, J.; Zhang, C.; Wang, Z. Oxysophocarpine suppresses TRAF6 level to ameliorate oxidative stress and inflammatory factors secretion in mice with dextran sulphate sodium (DSS) induced-ulcerative colitis. Microb. Pathog. 2023, 182, 106244. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Wang, P.; Wei, H.; Jia, R.; Zhen, M.; Li, Q.; Xue, C.; Li, J. Treatment of ulcerative colitis with Wu-Mei-Wan by inhibiting intestinal inflammatory response and repairing damaged intestinal mucosa. Phytomedicine 2022, 105, 154362. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, H.; Jie, H.; Ding, H.; Sun, H. Arbutin ameliorated ulcerative colitis of mice induced by dextran sodium sulfate (DSS). Bioengineered 2021, 12, 11707–11715. [Google Scholar] [CrossRef]
- Yao, D.; Dong, M.; Dai, C.; Wu, S. Inflammation and Inflammatory Cytokine Contribute to the Initiation and Development of Ulcerative Colitis and Its Associated Cancer. Inflamm. Bowel Dis. 2019, 25, 1595–1602. [Google Scholar] [CrossRef]
- Zhou, H.F.; Yang, C.; Li, J.Y.; He, Y.Y.; Huang, Y.; Qin, R.J.; Zhou, Q.L.; Sun, F.; Hu, D.S.; Yang, J. Quercetin serves as the major component of Xiang-lian Pill to ameliorate ulcerative colitis via tipping the balance of STAT1/PPARγ and dictating the alternative activation of macrophage. J. Ethnopharmacol. 2023, 313, 116557. [Google Scholar] [CrossRef] [PubMed]
- Cevallos, S.A.; Lee, J.Y.; Velazquez, E.M.; Foegeding, N.J.; Shelton, C.D.; Tiffany, C.R.; Parry, B.H.; Stull-Lane, A.R.; Olsan, E.E.; Savage, H.P.; et al. 5-Aminosalicylic Acid Ameliorates Colitis and Checks Dysbiotic Escherichia coli Expansion by Activating PPAR-gamma Signaling in the Intestinal Epithelium. mBio 2021, 12, e03227-20. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Su, L.; Zhang, L.; Zeng, J.; Chen, Q.; Deng, R.; Wang, Z.; Kuang, W.; Jin, X.; Gui, S.; et al. Spirulina platensis aqueous extracts ameliorate colonic mucosal damage and modulate gut microbiota disorder in mice with ulcerative colitis by inhibiting inflammation and oxidative stress. J. Zhejiang Univ. Sci. B 2022, 23, 481–501. [Google Scholar] [CrossRef] [PubMed]
- Patergnani, S.; Bouhamida, E.; Leo, S.; Pinton, P.; Rimessi, A. Mitochondrial Oxidative Stress and “Mito-Inflammation”: Actors in the Diseases. Biomedicines 2021, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Grácio, D.; Teixeira, J.P.; Magro, F. Oxidative Stress and DNA Damage: Implications in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 2403–2417. [Google Scholar] [CrossRef] [PubMed]
- Tatiya-aphiradee, N.; Chatuphonprasert, W.; Jarukamjorn, K. Oxidative stress exacerbates dextran sulfate sodium-induced ulcerative colitis in ICR mice. Biologia 2020, 75, 2063–2071. [Google Scholar] [CrossRef]
- Tahvilian, N.; Masoodi, M.; Kashani, A.F.; Vafa, M.; Aryaeian, N.; Heydarian, A.; Hosseini, A.; Moradi, N.; Farsi, F. Effects of saffron supplementation on oxidative/antioxidant status and severity of disease in ulcerative colitis patients: A randomized, double-blind, placebo-controlled study. Phytother. Res. 2021, 35, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Zhang, B.; Lu, M.; Ma, J.; Liu, Z.; Huang, J.; Ma, J.; Yang, X.; Wang, F.; et al. Modified Gegen Qinlian decoction ameliorated ulcerative colitis by attenuating inflammation and oxidative stress and enhancing intestinal barrier function in vivo and in vitro. J. Ethnopharmacol. 2023, 313, 116538. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Man, A.W.C.; Zhou, Y.; Xia, N.; Li, H. Involvement of Gut Microbiota, Microbial Metabolites and Interaction with Polyphenol in Host Immunometabolism. Nutrients 2020, 12, 3054. [Google Scholar] [CrossRef]
- Shu, L.Z.; Ding, Y.D.; Xue, Q.M.; Cai, W.; Deng, H. Direct and indirect effects of pathogenic bacteria on the integrity of intestinal barrier. Ther. Adv. Gastroenterol. 2023, 16, 17562848231176427. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Wang, L.; Meng, Y.; Xue, W.; Liang, J.; Peng, Z.; Meng, J.; Zhang, M. Apigenin remodels the gut microbiota to ameliorate ulcerative colitis. Front. Nutr. 2022, 9, 1062961. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, L.; Sun, S. Effect of Intestinal Microbiota Transplantation on Intestinal Flora and Inflammatory Factor Levels in Patients with Ulcerative Colitis. Infect. Drug Resist. 2023, 16, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, C.; Recinella, L.; Ronci, M.; Orlando, G.; Di Simone, S.; Brunetti, L.; Chiavaroli, A.; Leone, S.; Politi, M.; Tirillini, B.; et al. Protective effects induced by alcoholic Phlomis fruticosa and Phlomis herba-venti extracts in isolated rat colon: Focus on antioxidant, anti-inflammatory, and antimicrobial activities in vitro. Phytother. Res. 2019, 33, 2387–2400. [Google Scholar] [CrossRef]
- Pang, B.; Jin, H.; Liao, N.; Li, J.; Jiang, C.; Shao, D.; Shi, J. Lactobacillus rhamnosus from human breast milk ameliorates ulcerative colitis in mice via gut microbiota modulation. Food Funct. 2021, 12, 5171–5186. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, N.; Liu, J.; Wang, T.; Dong, R.; Ge, D.; Peng, G. Gegen Qinlian Decoction Alleviates Experimental Colitis and Concurrent Lung Inflammation by Inhibiting the Recruitment of Inflammatory Myeloid Cells and Restoring Microbial Balance. J. Inflamm. Res. 2022, 15, 1273–1291. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Li, W.; Zhang, B.; Yin, J.; Liuqi, S.; Wang, J.; Peng, B.; Wang, S. Fucoidan Ameliorated Dextran Sulfate Sodium-Induced Ulcerative Colitis by Modulating Gut Microbiota and Bile Acid Metabolism. J. Agric. Food Chem. 2022, 70, 14864–14876. [Google Scholar] [CrossRef]
- Boshagh, M.A.; Foroutan, P.; Moloudi, M.R.; Fakhari, S.; Malakouti, P.; Nikkhoo, B.; Jalili, A. ELR positive CXCL chemokines are highly expressed in an animal model of ulcerative colitis. J. Inflamm. Res. 2019, 12, 167–174. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, L.; Wang, J.; Qin, Z.; Wang, J.; Lu, Y.; Zheng, X.; Peng, Q.; Ye, Q.; Ai, F.; et al. Suppression Colitis and Colitis-Associated Colon Cancer by Anti-S100a9 Antibody in Mice. Front. Immunol. 2017, 8, 1774. [Google Scholar] [CrossRef]
- Kou, F.; Cheng, Y.; Shi, L.; Liu, J.; Liu, Y.; Shi, R.; Peng, G.; Li, J. LCN2 as a Potential Diagnostic Biomarker for Ulcerative Colitis-Associated Carcinogenesis Related to Disease Duration. Front. Oncol. 2021, 11, 793760. [Google Scholar] [CrossRef]
- Deng, L.; He, S.; Li, Y.; Ding, R.; Li, X.; Guo, N.; Luo, L. Identification of Lipocalin 2 as a Potential Ferroptosis-related Gene in Ulcerative Colitis. Inflamm. Bowel Dis. 2023, 29, 1446–1457. [Google Scholar] [CrossRef] [PubMed]
- Bufu, T.; Di, X.; Yilin, Z.; Gege, L.; Xi, C.; Ling, W. Celastrol inhibits colorectal cancer cell proliferation and migration through suppression of MMP3 and MMP7 by the PI3K/AKT signaling pathway. Anticancer Drugs 2018, 29, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Claesson-Welsh, L. How the matrix metalloproteinase MMP14 contributes to the progression of colorectal cancer. J. Clin. Investig. 2020, 130, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Quan, S.; Li, J.; Liu, Y.; Sun, H.; Zhang, J.; Liu, D. Protective Effects of Grape Seed Proanthocyanidin Extract in Preventing DSS Induced Ulcerative Colitis Based on Pharmacodynamic, Pharmacokinetic and Tissue Distribution. Curr. Drug Metab. 2022, 23, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Q.; Li, F.; Chen, G.; Li, J.; Li, J.; Wang, Y.; Lu, Y.; Li, Q.; Li, M.; Chai, K. Ursolic Acid Regulates Intestinal Microbiota and Inflammatory Cell Infiltration to Prevent Ulcerative Colitis. J. Immunol. Res. 2021, 2021, 6679316. [Google Scholar] [CrossRef]
- Gao, W.; Wang, C.; Yu, L.; Sheng, T.; Wu, Z.; Wang, X.; Zhang, D.; Lin, Y.; Gong, Y. Chlorogenic Acid Attenuates Dextran Sodium Sulfate-Induced Ulcerative Colitis in Mice through MAPK/ERK/JNK Pathway. BioMed Res. Int. 2019, 2019, 6769789. [Google Scholar] [CrossRef] [PubMed]
- Maslin, L.A.; Weeks, B.R.; Carroll, R.J.; Byrne, D.H.; Turner, N.D. Chlorogenic Acid and Quercetin in a Diet with Fermentable Fiber Influence Multiple Processes Involved in DSS-Induced Ulcerative Colitis but Do Not Reduce Injury. Nutrients 2022, 14, 3706. [Google Scholar] [CrossRef]
- Yu, T.Y.; Feng, Y.M.; Kong, W.S.; Li, S.N.; Sun, X.J.; Zhou, G.; Xie, R.F.; Zhou, X. Gallic acid ameliorates dextran sulfate sodium-induced ulcerative colitis in mice via inhibiting NLRP3 inflammasome. Front. Pharmacol. 2023, 14, 1095721. [Google Scholar] [CrossRef]
- Sen, A. Prophylactic and therapeutic roles of oleanolic acid and its derivatives in several diseases. World J. Clin. Cases 2020, 8, 1767–1792. [Google Scholar] [CrossRef]
- Al-Rejaie, S.S.; Abuohashish, H.M.; Al-Enazi, M.M.; Al-Assaf, A.H.; Parmar, M.Y.; Ahmed, M.M. Protective effect of naringenin on acetic acid-induced ulcerative colitis in rats. World J. Gastroenterol. 2013, 19, 5633–5644. [Google Scholar] [CrossRef]


| Total Polyphenols mg/g | Total Flavonoids mg/g | Total Triterpenoids mg/g | DPPH IC50 mg/mL | ABTS IC50 mg/mL |
|---|---|---|---|---|
| 171.99 ± 9.02 | 453.69 ± 6.66 | 237.05 ± 21.46 | 0.43 ± 0.018 | 0.078 ± 0.0055 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Ling, Y.; Huang, X.; Zhou, T.; Wu, S.; Zhang, S.; Zhou, H.; Kang, Y.; Wang, L.; Wang, X.; et al. Rosa Roxburghii Tratt Fruit Extract Prevents Dss-Induced Ulcerative Colitis in Mice by Modulating the Gut Microbiota and the IL-17 Signaling Pathway. Nutrients 2023, 15, 4560. https://doi.org/10.3390/nu15214560
Li X, Ling Y, Huang X, Zhou T, Wu S, Zhang S, Zhou H, Kang Y, Wang L, Wang X, et al. Rosa Roxburghii Tratt Fruit Extract Prevents Dss-Induced Ulcerative Colitis in Mice by Modulating the Gut Microbiota and the IL-17 Signaling Pathway. Nutrients. 2023; 15(21):4560. https://doi.org/10.3390/nu15214560
Chicago/Turabian StyleLi, Xingjie, Yihan Ling, Xiaoyi Huang, Ting Zhou, Shouxun Wu, Shuwen Zhang, Heting Zhou, Yuhong Kang, Liqun Wang, Xiaomeng Wang, and et al. 2023. "Rosa Roxburghii Tratt Fruit Extract Prevents Dss-Induced Ulcerative Colitis in Mice by Modulating the Gut Microbiota and the IL-17 Signaling Pathway" Nutrients 15, no. 21: 4560. https://doi.org/10.3390/nu15214560
APA StyleLi, X., Ling, Y., Huang, X., Zhou, T., Wu, S., Zhang, S., Zhou, H., Kang, Y., Wang, L., Wang, X., & Yin, W. (2023). Rosa Roxburghii Tratt Fruit Extract Prevents Dss-Induced Ulcerative Colitis in Mice by Modulating the Gut Microbiota and the IL-17 Signaling Pathway. Nutrients, 15(21), 4560. https://doi.org/10.3390/nu15214560