Effects of Gestational Diabetes Mellitus and Selenium Deficiency on the Offspring Growth and Blood Glucose Mechanisms of C57BL/6J Mice
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Effects of GDM and Se Deficiency on the Growth and Development of Offspring
3.2. Effect of GDM and Se Deficiency on Blood Glucose in Offspring
3.3. Effects of GDM and Se Deficiency on Oxidative Stress in Offspring
3.4. Effects of GDM and Se Deficiency on the PI3K/Akt Signaling Pathway-Related Proteins in Offspring
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuhl, C. Etiology and pathogenesis of gestational diabetes. Diabetes Care 1998, 21 (Suppl. 2), B19–B26. [Google Scholar]
- Metzger, B.E. Summary and recommendations of the Third International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes 1991, 40 (Suppl. 2), 197–201. [Google Scholar] [CrossRef]
- Laurie, J.G.; McIntyre, H.D. A Review of the Current Status of Gestational Diabetes Mellitus in Australia-The Clinical Impact of Changing Population Demographics and Diagnostic Criteria on Prevalence. Int. J. Environ. Res. Public Health 2020, 17, 9387. [Google Scholar] [CrossRef] [PubMed]
- Badakhsh, M.; Daneshi, F.; Abavisani, M.; Rafiemanesh, H.; Bouya, S.; Sheyback, M.; Rezaie Keikhaie, K.; Balouchi, A. Prevalence of gestational diabetes mellitus in Eastern Mediterranean region: A systematic review and meta-analysis. Endocrine 2019, 65, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Sun, X.; Lu, L.; Liu, F.; Yuan, J. Prevalence of gestational diabetes mellitus in mainland China: A systematic review and meta-analysis. J. Diabetes Investig. 2019, 10, 154–162. [Google Scholar] [CrossRef]
- Chinese Nutrition Society. Dietary Guidelines for Chinese Residents; Chinese Nutrition Society, Ed.; People’s Medical Publishing House: Beijing, China, 2016. [Google Scholar]
- Zachara, B.A. Selenium and selenium-dependent antioxidants in chronic kidney disease. Adv. Clin. Chem. 2015, 68, 131–151. [Google Scholar] [CrossRef]
- Du, W.; Fang, J.Y. Nutrients Impact the Pathogenesis and Development of Colorectal Cancer. Gastrointest. Tumors 2016, 2, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Papp, L.V.; Holmgren, A.; Khanna, K.K. Selenium and selenoproteins in health and disease. Antioxid. Redox Signal. 2010, 12, 793–795. [Google Scholar] [CrossRef]
- Benstoem, C.; Goetzenich, A.; Kraemer, S.; Borosch, S.; Manzanares, W.; Hardy, G.; Stoppe, C. Selenium and its supplementation in cardiovascular disease—What do we know? Nutrients 2015, 7, 3094–3118. [Google Scholar] [CrossRef]
- Guillin, O.M.; Vindry, C.; Ohlmann, T.; Chavatte, L. Selenium, Selenoproteins and Viral Infection. Nutrients 2019, 11, 2101. [Google Scholar] [CrossRef]
- Bastola, M.M.; Locatis, C.; Maisiak, R.; Fontelo, P. Selenium, copper, zinc and hypertension: An analysis of the National Health and Nutrition Examination Survey (2011–2016). BMC Cardiovasc. Disord. 2020, 20, 45. [Google Scholar] [CrossRef] [PubMed]
- Kohler, L.N.; Foote, J.; Kelley, C.P.; Florea, A.; Shelly, C.; Chow, H.S.; Hsu, P.; Batai, K.; Ellis, N.; Saboda, K.; et al. Selenium and Type 2 Diabetes: Systematic Review. Nutrients 2018, 10, 1924. [Google Scholar] [CrossRef]
- Hofstee, P.; James-McAlpine, J.; McKeating, D.R.; Vanderlelie, J.J.; Cuffe, J.S.M.; Perkins, A.V. Low serum selenium in pregnancy is associated with reduced T3 and increased risk of GDM. J. Endocrinol. 2021, 248, 45–57. [Google Scholar] [CrossRef]
- Shao, J.; Yamashita, H.; Qiao, L.; Draznin, B.; Friedman, J.E. Phosphatidylinositol 3-kinase redistribution is associated with skeletal muscle insulin resistance in gestational diabetes mellitus. Diabetes 2002, 51, 19–29. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Habibi, N.; Grieger, J.A.; Bianco-Miotto, T. A Review of the Potential Interaction of Selenium and Iodine on Placental and Child Health. Nutrients 2020, 12, 2678. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.; Zhang, L.; Yao, X.; Zhong, X.; Cheng, G.; Wang, L.; Wan, Q. Spiraeoside protects human cardiomyocytes against high glucose-induced injury, oxidative stress, and apoptosis by activation of PI3K/Akt/Nrf2 pathway. J. Biochem. Mol. Toxicol. 2020, 34, e22548. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wu, H.; Wang, X.; He, J.; He, S.; Yin, Y. Resveratrol Attenuates Oxidative Stress-Induced Intestinal Barrier Injury through PI3K/Akt-Mediated Nrf2 Signaling Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 7591840. [Google Scholar] [CrossRef]
- Yin, M.; Wang, Y. The role of PIP5K1A in cancer development and progression. Med. Oncol. 2022, 39, 151. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Yu, S.; Luo, Z.; Chen, Y.; Liu, Q.; Hua, F.; Xu, G.; Yu, P. Sevoflurane postconditioning protects rat hearts against ischemia-reperfusion injury via the activation of PI3K/AKT/mTOR signaling. Sci. Rep. 2014, 4, 7317. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Z.; Li, Y.; Hu, X.; Shen, P.; Fu, Y.; Cao, Y.; Zhang, N. Selenium Deficiency Deteriorate the Inflammation of S. aureus Infection via Regulating NF-kappaB and PPAR-gamma in Mammary Gland of Mice. Biol. Trace Elem. Res. 2016, 172, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Castro, J.; Ojeda, M.L.; Alferez, M.J.; Lopez-Aliaga, I.; Nestares, T.; Campos, M.S. Se bioavailability and glutathione peroxidase activity in iron deficient rats. J. Trace Elem. Med. Biol. 2011, 25, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Vandyousefi, S.; Whaley, S.E.; Widen, E.M.; Asigbee, F.M.; Landry, M.J.; Ghaddar, R.; Davis, J.N. Association of breastfeeding and early exposure to sugar-sweetened beverages with obesity prevalence in offspring born to mothers with and without gestational diabetes mellitus. Pediatr. Obes. 2019, 14, e12569. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, M.L.; Nogales, F.; Romero-Herrera, I.; Carreras, O. Fetal Programming Is Deeply Related to Maternal Selenium Status and Oxidative Balance; Experimental Offspring Health Repercussions. Nutrients 2021, 13, 2085. [Google Scholar] [CrossRef] [PubMed]
- Regnault, N.; Gillman, M.W.; Rifas-Shiman, S.L.; Eggleston, E.; Oken, E. Sex-specific associations of gestational glucose tolerance with childhood body composition. Diabetes Care 2013, 36, 3045–3053. [Google Scholar] [CrossRef]
- Li, S.; Zhu, Y.; Yeung, E.; Chavarro, J.E.; Yuan, C.; Field, A.E.; Missmer, S.A.; Mills, J.L.; Hu, F.B.; Zhang, C. Offspring risk of obesity in childhood, adolescence and adulthood in relation to gestational diabetes mellitus: A sex-specific association. Int. J. Epidemiol. 2017, 46, 2104. [Google Scholar] [CrossRef]
- Piao, Y.; Liu, Y.; Xie, X. Change trends of organ weight background data in sprague dawley rats at different ages. J. Toxicol. Pathol. 2013, 26, 29–34. [Google Scholar] [CrossRef]
- Boulan, L.; Leopold, P. What determines organ size during development and regeneration? Development 2021, 148, dev196063. [Google Scholar] [CrossRef]
- Krishnaveni, G.V.; Veena, S.R.; Hill, J.C.; Kehoe, S.; Karat, S.C.; Fall, C.H. Intrauterine exposure to maternal diabetes is associated with higher adiposity and insulin resistance and clustering of cardiovascular risk markers in Indian children. Diabetes Care 2010, 33, 402–404. [Google Scholar] [CrossRef]
- Hofstee, P.; McKeating, D.R.; Bartho, L.A.; Anderson, S.T.; Perkins, A.V.; Cuffe, J.S.M. Maternal Selenium Deficiency in Mice Alters Offspring Glucose Metabolism and Thyroid Status in a Sexually Dimorphic Manner. Nutrients 2020, 12, 267. [Google Scholar] [CrossRef]
- Cheng, W.; Zhang, L.; Sa, P.; Luo, J.; Li, M. Transcriptomic analysis reveals the effects of maternal selenium deficiency on placental transport, hormone synthesis, and immune response in mice. Metallomics 2022, 14, mfac062. [Google Scholar] [CrossRef]
- Duan, S.Y.; Chen, S.J.; Liang, W.; Chen, M.Y.; Chen, Y.; Guo, M.Y. Dietary Selenium Deficiency Facilitated Reduced Stomatin and Phosphatidylserine Externalization, Increasing Erythrocyte Osmotic Fragility in Mice. Biol. Trace Elem. Res. 2021, 199, 594–603. [Google Scholar] [CrossRef]
- Xu, Z.J.; Liu, M.; Niu, Q.J.; Huang, Y.X.; Zhao, L.; Lei, X.G.; Sun, L.H. Both selenium deficiency and excess impair male reproductive system via inducing oxidative stress-activated PI3K/AKT-mediated apoptosis and cell proliferation signaling in testis of mice. Free. Radic. Biol. Med. 2023, 197, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sun, X.; Lei, Y.; Liu, X.; Zhang, Y.; Wang, Y.; Lin, H. Roles of selenoprotein K in oxidative stress and endoplasmic reticulum stress under selenium deficiency in chicken liver. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 264, 109504. [Google Scholar] [CrossRef] [PubMed]
- Ajmone-Cat, M.A.; De Simone, R.; Tartaglione, A.M.; Di Biase, A.; Di Benedetto, R.; D’Archivio, M.; Vari, R.; Ricceri, L.; Aureli, F.; Iacoponi, F.; et al. Critical Role of Maternal Selenium Nutrition in Neurodevelopment: Effects on Offspring Behavior and Neuroinflammatory Profile. Nutrients 2022, 14, 1850. [Google Scholar] [CrossRef] [PubMed]
- Roe, N.D.; Ren, J. Akt2 knockout mitigates chronic iNOS inhibition-induced cardiomyocyte atrophy and contractile dysfunction despite persistent insulin resistance. Toxicol. Lett. 2011, 207, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Hofstee, P.; Perkins, A.V.; Cuffe, J.S.M. Selenium Deficiency during Pregnancy in Mice Impairs Exercise Performance and Metabolic Function in Adult Offspring. Nutrients 2022, 14, 1125. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Duntas, L.H.; Rayman, M.P. The role of selenium in type-2 diabetes mellitus and its metabolic comorbidities. Redox Biol. 2022, 50, 102236. [Google Scholar] [CrossRef]
- Habibi, N.; Labrinidis, A.; Leemaqz, S.Y.; Jankovic-Karasoulos, T.; McCullough, D.; Grieger, J.A.; Gilbert, S.; Ricciardelli, C.; Zhou, S.J.; Perkins, A.V.; et al. Effect of Selenium and Iodine on Oxidative Stress in the First Trimester Human Placenta Explants. Nutrients 2021, 13, 800. [Google Scholar] [CrossRef]
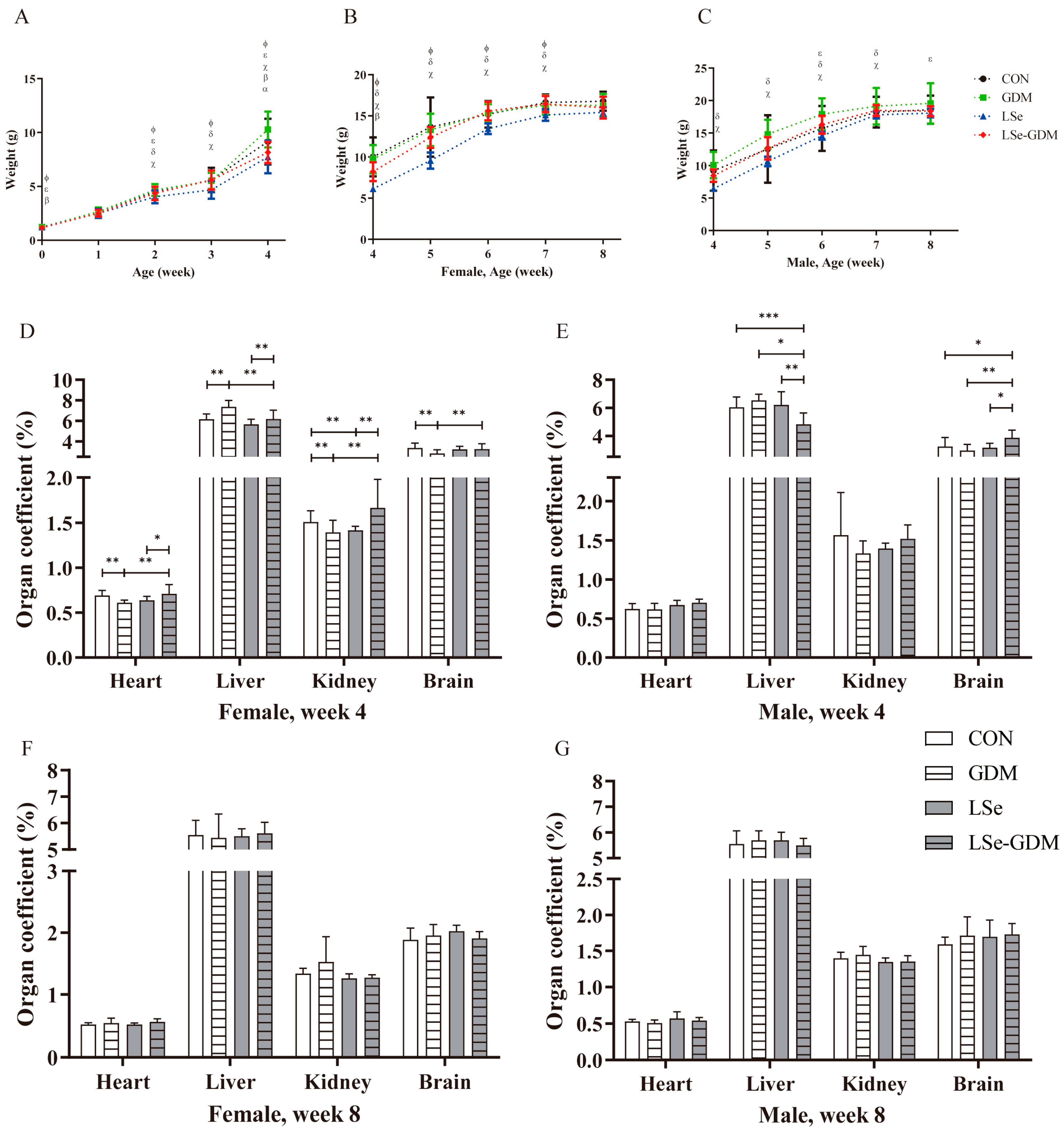

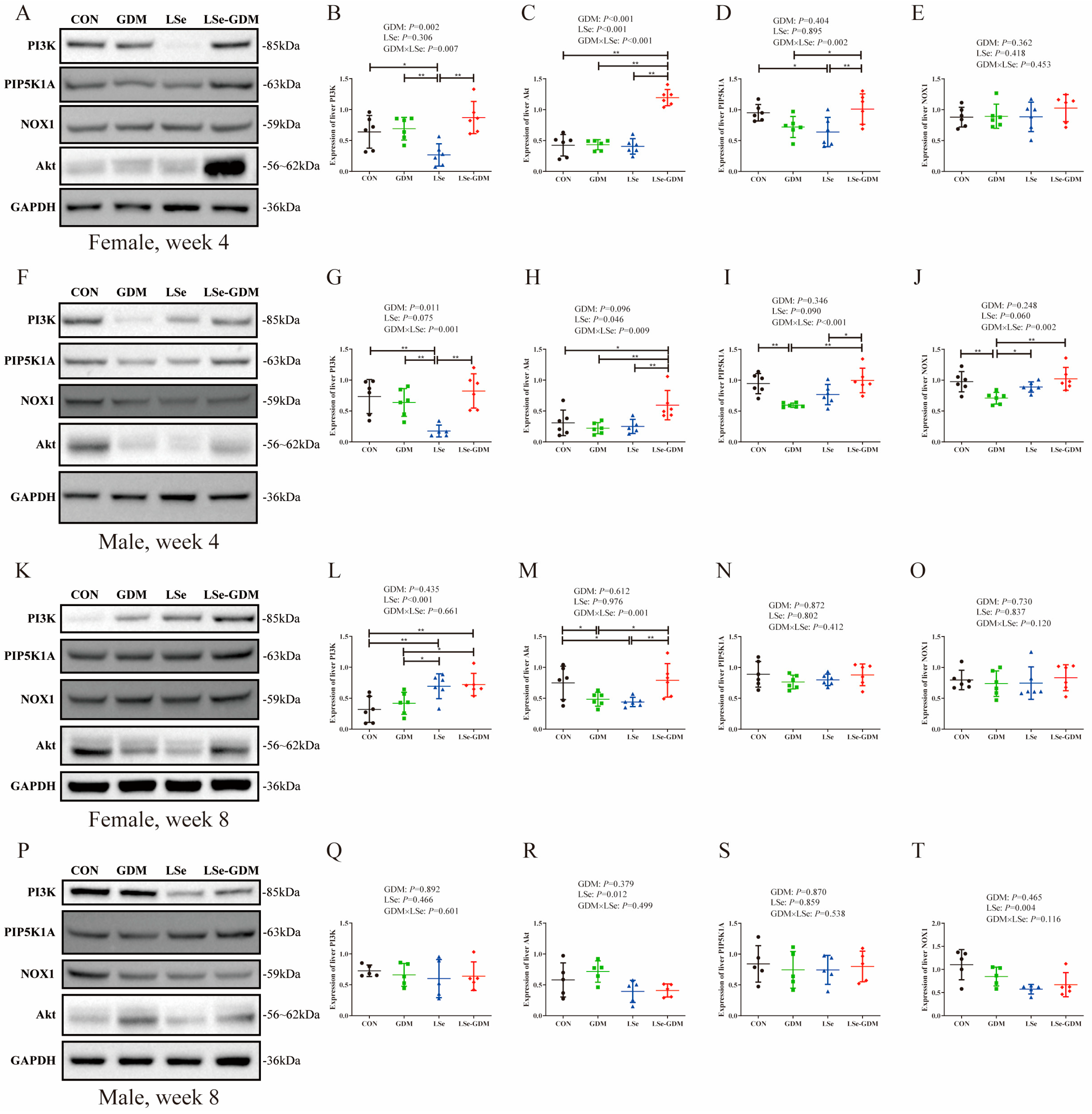
| Week | CON | GDM | LSe | LSe-GDM | p | |
|---|---|---|---|---|---|---|
| Female | ||||||
| 4 | 3.40 ± 1.04 ac | 5.33 ± 1.33 | 3.27 ± 0.53 bd | 5.58 ± 1.31 | 0.001 | |
| 5 | 4.25 ± 1.05 | 5.05 ± 0.65 | 5.58 ± 0.76 | 4.75 ± 0.97 | 0.092 | |
| 6 | 4.50 ± 0.77 | 5.32 ± 0.62 | 5.33 ± 1.26 | 4.65 ± 1.01 | 0.307 | |
| 7 | 4.53 ± 0.70 e | 5.33 ± 1.30 | 6.28 ± 0.47 | 5.30 ± 0.72 | 0.018 | |
| 8 | 3.93 ± 0.40 a | 5.43 ± 1.22 | 4.46 ± 0.95 | 4.77 ± 0.69 | 0.049 | |
| Male | ||||||
| 4 | 5.87 ± 0.88 | 6.07 ± 1.62 | 3.25 ± 1.10 be | 4.68 ± 1.51 | 0.005 | |
| 5 | 5.48 ± 0.79 | 5.43 ± 1.43 | 4.92 ± 1.02 | 5.37 ± 0.58 | 0.754 | |
| 6 | 5.65 ± 0.80 | 5.80 ± 0.83 | 4.93 ± 1.51 | 5.35 ± 0.72 | 0.482 | |
| 7 | 5.68 ± 0.90 | 6.15 ± 1.26 | 5.25 ± 1.09 | 6.23 ± 0.25 | 0.281 | |
| 8 | 5.67 ± 0.74 | 6.07 ± 1.30 | 5.24 ± 1.22 | 5.38 ± 0.67 | 0.543 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Gong, J.; Chen, Y.; Chen, Y.; Chen, S.; Wu, Y.; He, Y.; Li, C.; Yu, H.; Xie, L. Effects of Gestational Diabetes Mellitus and Selenium Deficiency on the Offspring Growth and Blood Glucose Mechanisms of C57BL/6J Mice. Nutrients 2023, 15, 4519. https://doi.org/10.3390/nu15214519
Xu W, Gong J, Chen Y, Chen Y, Chen S, Wu Y, He Y, Li C, Yu H, Xie L. Effects of Gestational Diabetes Mellitus and Selenium Deficiency on the Offspring Growth and Blood Glucose Mechanisms of C57BL/6J Mice. Nutrients. 2023; 15(21):4519. https://doi.org/10.3390/nu15214519
Chicago/Turabian StyleXu, Wenhui, Jiayu Gong, Yifei Chen, Yiru Chen, Shutong Chen, Yanyan Wu, Yuan He, Chenxu Li, Haitao Yu, and Lin Xie. 2023. "Effects of Gestational Diabetes Mellitus and Selenium Deficiency on the Offspring Growth and Blood Glucose Mechanisms of C57BL/6J Mice" Nutrients 15, no. 21: 4519. https://doi.org/10.3390/nu15214519
APA StyleXu, W., Gong, J., Chen, Y., Chen, Y., Chen, S., Wu, Y., He, Y., Li, C., Yu, H., & Xie, L. (2023). Effects of Gestational Diabetes Mellitus and Selenium Deficiency on the Offspring Growth and Blood Glucose Mechanisms of C57BL/6J Mice. Nutrients, 15(21), 4519. https://doi.org/10.3390/nu15214519






