Glycemic Variability in Patients with Type 2 Diabetes Mellitus (T2DM): The Role of Melatonin in a Crossover, Double-Blind, Placebo-Controlled, Randomized Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Objectives
2.1.1. Main Objective
2.1.2. Specific Objectives
2.2. Study Design
2.3. Blinding
2.4. Randomization
2.5. Flowchart (Figure 1)
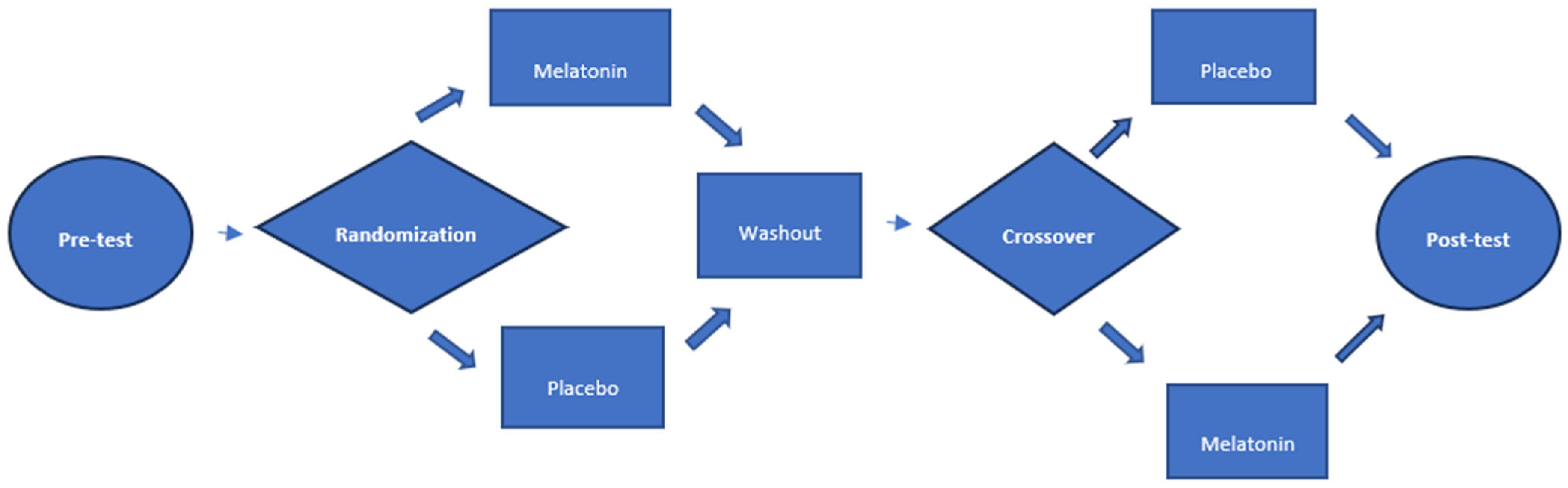
2.6. Sample Size
2.7. Participants
2.7.1. Inclusion Criteria
2.7.2. Exclusion Criteria
2.8. Instruments
2.8.1. Exogenous Melatonin
2.8.2. Assessment of Glycemic Variability
2.8.3. Study Variables
2.8.4. Statistical Analysis
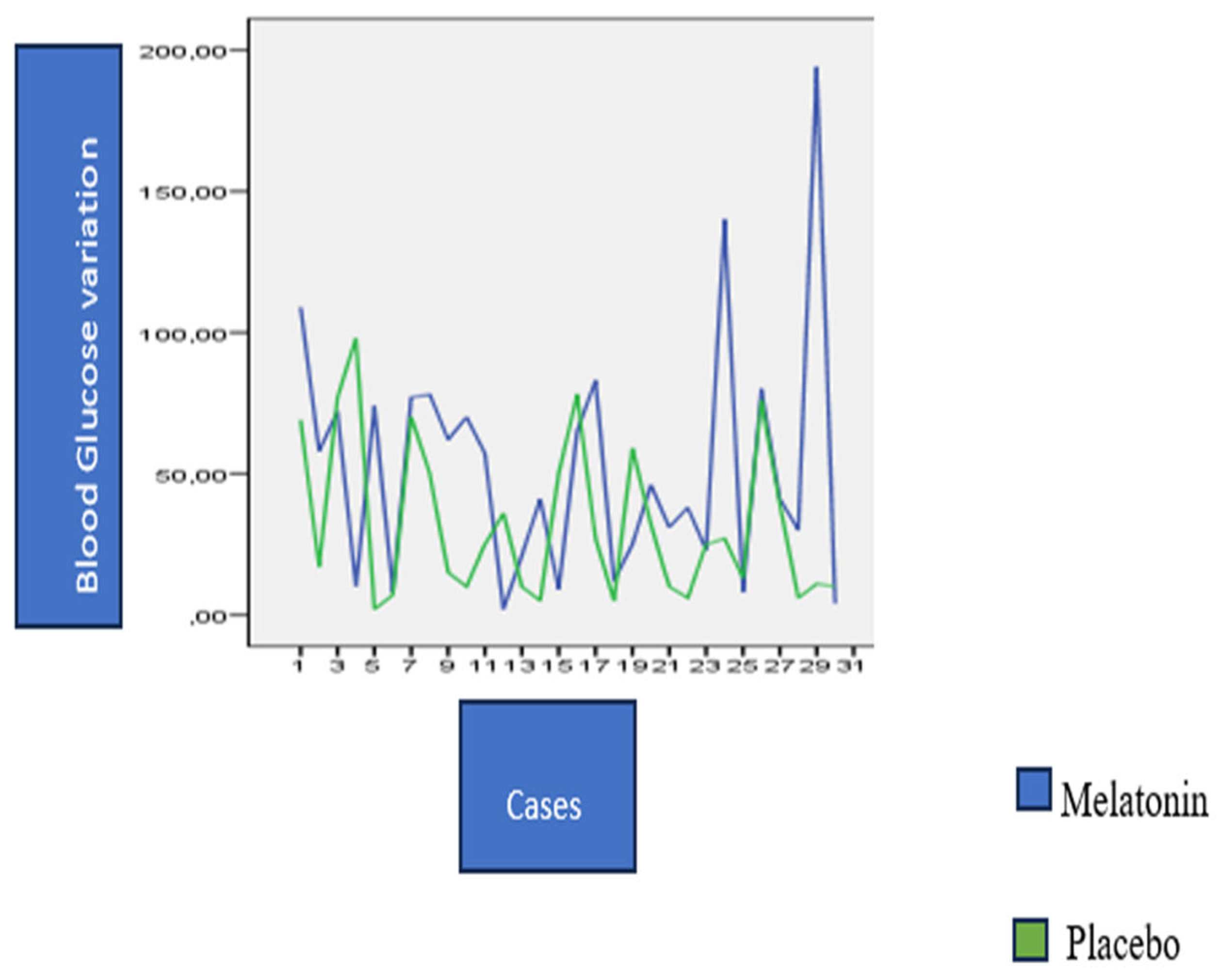
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| T2DM | Type 2 diabetes |
| MNTR1B | melatonina receptor 1B |
| HbA1C | Glycohemoglobin |
| D3 | Third day of glycemic measurement |
| Q1 | quartile 25% |
| Q3 | quartile 75% |
| SD | standard deviation |
References
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 3. Prevention or Delay of Type 2 Diabetes and Associated Comorbidities: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. 1), S41–S48. [Google Scholar] [CrossRef]
- Lorenzo-Medina, M.; de la Iglesia, S.; Ruiz-Garcia, L.; Quintana-Hidalgo, L.; Martin-Alfaro, R.; Herrada, J. Pitfalls of glycated hemoglobin in the glycemic assessment of diabetes patients with hemoglobin louisville: Role of serum fructosamine. J. Diabetes Sci. Technol. 2013, 7, 804–805. [Google Scholar] [CrossRef] [PubMed]
- Battelino, T.; Dovc, K. Glycemic Variability: The Danger of a Physiologically Stable Metric. J. Clin. Endocrinol. Metab. 2020, 105, e3815–e3817. [Google Scholar] [CrossRef]
- Cheng, D.; Fei, Y.; Liu, Y.; Li, J.; Xue, Q.; Wang, X.; Wang, N. HbA1C variability and the risk of renal status progression in Diabetes Mellitus: A meta-analysis. PLoS ONE 2014, 9, e115509. [Google Scholar] [CrossRef] [PubMed]
- Chatziralli, I.P. The Role of Glycemic Control and Variability in Diabetic Retinopathy. Diabetes Ther. 2018, 9, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Firouzabadi, M.D.; Poopak, A.; Sheikhy, A.; Samimi, S.; Nakhaei, P.; Firouzabadi, F.D.; Moosaie, F.; Rabizadeh, S.; Nakhjavani, M.; Esteghamati, A. Glycemic profile variability: An independent risk factor for diabetic neuropathy in patients with type 2 diabetes. Prim. Care Diabetes 2023, 17, 38–42. [Google Scholar] [CrossRef]
- DeVries, J.H. Glucose variability: Where it is important and how to measure it. Diabetes 2013, 62, 1405–1408. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Quantity and quality of sleep and incidence of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2010, 33, 414–420. [Google Scholar] [CrossRef]
- Hein, M.; Lanquart, J.P.; Loas, G.; Hubain, P.; Linkowski, P. Prevalence and risk factors of type 2 diabetes in insomnia sufferers: A study on 1311 individuals referred for sleep examinations. Sleep Med. 2018, 46, 37–45. [Google Scholar] [CrossRef]
- Botros, N.; Concato, J.; Mohsenin, V.; Selim, B.; Doctor, K.; Yaggi, H.K. Obstructive sleep apnea as a risk factor for type 2 diabetes. Am. J. Med. 2009, 122, 1122–1127. [Google Scholar] [CrossRef]
- Pamidi, S.; Aronsohn, R.S.; Tasali, E. Obstructive sleep apnea: Role in the risk and severity of diabetes. Best. Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Martorina, W.; Tavares, A. Real-World Data in Support of Short Sleep Duration with Poor Glycemic Control, in People with Type 2 Diabetes Mellitus. J. Diabetes Res. 2019, 2019, 6297162. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, R.; Yamakawa, T.; Takahashi, K.; Suzuki, J.; Shinoda, M.M.; Sakamaki, K.; Danno, H.; Tsuchiya, H.; Waseda, M.; Takano, T.; et al. Association of usual sleep quality and glycemic control in type 2 diabetes in Japanese: A cross sectional study. Sleep and Food Registry in Kanagawa (SOREKA). PLoS ONE 2018, 13, e0191771. [Google Scholar] [CrossRef] [PubMed]
- Borel, A.L.; Pepin, J.L.; Nasse, L.; Baguet, J.P.; Netter, S.; Benhamou, P.Y. Short sleep duration measured by wrist actimetry is associated with deteriorated glycemic control in type 1 diabetes. Diabetes Care 2013, 36, 2902–2908. [Google Scholar] [CrossRef]
- Low melatonin secretion is a risk factor for type 2 diabetes. BMJ 2013, 346, f2202. [CrossRef]
- Reutrakul, S.; Sumritsopak, R.; Saetung, S.; Chanprasertyothin, S.; Chailurkit, L.O.; Anothaisintawee, T. Lower nocturnal urinary 6-sulfatoxymelatonin is associated with more severe insulin resistance in patients with prediabetes. Neurobiol. Sleep Circadian Rhythm. 2018, 4, 10–16. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, Z.J.; Liu, H.Y.; Cai, J.; Lu, Q.K.; Ji, L.D.; Xu, J. The melatonin receptor 1B gene links circadian rhythms and type 2 diabetes mellitus: An evolutionary story. Ann. Med. 2023, 55, 1262–1286. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, L.; Zhong, M.; Yang, B.; Yang, Q.; Yang, H.; Xie, C. The association between melatonin receptor 1B gene polymorphisms and type 2 diabetes mellitus (T2DM) in Chinese populations: A meta-analysis. Ann. Palliat. Med. 2020, 9, 957–966. [Google Scholar] [CrossRef]
- Zawilska, J.B.; Skene, D.J.; Arendt, J. Physiology and pharmacology of melatonin in relation to biological rhythms. Pharmacol. Rep. 2009, 61, 383–410. [Google Scholar] [CrossRef]
- Doosti-Irani, A.; Ostadmohammadi, V.; Mirhosseini, N.; Mansournia, M.A.; Reiter, R.J.; Kashanian, M.; Rahimi, M.; Razavi, M.; Asemi, Z. The Effects of Melatonin Supplementation on Glycemic Control: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Horm. Metab. Res. 2018, 50, 783–790. [Google Scholar] [CrossRef]
- Xia, A.Y.; Zhu, H.; Zhao, Z.J.; Liu, H.Y.; Wang, P.H.; Ji, L.D.; Xu, J. Molecular Mechanisms of the Melatonin Receptor Pathway Linking Circadian Rhythm to Type 2 Diabetes Mellitus. Nutrients 2023, 15, 1406. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Najafi, M.; Kavyiani, N.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Anti-Inflammatory Activity of Melatonin: A Focus on the Role of NLRP3 Inflammasome. Inflammation 2021, 44, 1207–1222. [Google Scholar] [CrossRef]
- Albreiki, M.S.; Middleton, B.; Hampton, S.M. The effect of melatonin on glucose tolerance, insulin sensitivity and lipid profiles after a late evening meal in healthy young males. J. Pineal Res. 2021, 71, e12770. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Sun, B.; Huang, S.; Zhu, C.; Bian, M. Glycemic variability: Adverse clinical outcomes and how to improve it? Cardiovasc. Diabetol. 2020, 19, 102. [Google Scholar] [CrossRef] [PubMed]
- Dwan, K.; Li, T.; Altman, D.G.; Elbourne, D. CONSORT 2010 statement: Extension to randomised crossover trials. BMJ 2019, 366, l4378. [Google Scholar] [CrossRef] [PubMed]
- Martorina, W.; Tavares, A. Effects of Melatonin on Glycemic Variability in Type 2 Diabetes Mellitus: Protocol for a Crossover, Double-blind, Placebo-controlled Trial. JMIR Res. Protoc. 2023. [Google Scholar] [CrossRef]
- Miot, H.A. Tamanho da amostra em estudos clínicos e experimentais. J. Vasc. Bras. 2011, 10, 275–278. [Google Scholar] [CrossRef]
- Medical Jurisprudence: Physicians-Malpractice-Standard of Care and Skill. Calif. Med. 1948, 68, 188.
- Gooneratne, N.S.; Edwards, A.Y.; Zhou, C.; Cuellar, N.; Grandner, M.A.; Barrett, J.S. Melatonin pharmacokinetics following two different oral surge-sustained release doses in older adults. J. Pineal Res. 2012, 52, 437–445. [Google Scholar] [CrossRef]
- Mun, J.G.; Wang, D.; Doerflein Fulk, D.L.; Fakhary, M.; Gualco, S.J.; Grant, R.W.; Mitmesser, S.H. A Randomized, Double-Blind, Crossover Study to Investigate the Pharmacokinetics of Extended-Release Melatonin Compared to Immediate-Release Melatonin in Healthy Adults. J. Diet. Suppl. 2023, 1–13. [Google Scholar] [CrossRef]
- Harpsoe, N.G.; Andersen, L.P.; Gogenur, I.; Rosenberg, J. Clinical pharmacokinetics of melatonin: A systematic review. Eur. J. Clin. Pharmacol. 2015, 71, 901–909. [Google Scholar] [CrossRef] [PubMed]
- 8th symposium of the Workshop for Neuropsychopharmacology and Pharmacopsychiatry (AGNP) at Nuremberg, 26–29 October 1977. Arzneimittelforschung 1978, 28, 1253–1318.
- Godoy, P.H.; Nucera, A.; Colcher, A.P.; de-Andrade, J.E.; Alves, D. Screening for obstructive sleep apnea in elderly: Performance of the Berlin and STOP-Bang questionnaires and the Epworth Sleepiness Scale using polysomnography as gold standard. Sleep Sci. 2022, 15, 203–208. [Google Scholar] [CrossRef]
- Cipolla-Neto, J.; Amaral, F.G.D. Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr. Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Lopez-Minguez, J.; Dashti, H.S.; Vetter, C.; Hernandez-Martinez, A.M.; Perez-Ayala, M.; Baraza, J.C.; Wang, W.; Florez, J.C.; Scheer, F.; et al. Interplay of Dinner Timing and MTNR1B Type 2 Diabetes Risk Variant on Glucose Tolerance and Insulin Secretion: A Randomized Crossover Trial. Diabetes Care 2022, 45, 512–519. [Google Scholar] [CrossRef]
- Hack, L.M.; Lockley, S.W.; Arendt, J.; Skene, D.J. The effects of low-dose 0.5-mg melatonin on the free-running circadian rhythms of blind subjects. J. Biol. Rhythm. 2003, 18, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Bueno, A.P.R.; Savi, F.M.; Alves, I.A.; Bandeira, V.A.C. Regulatory aspects and evidences of melatonin use for sleep disorders and insomnia: An integrative review. Arq. Neuropsiquiatr. 2021, 79, 732–742. [Google Scholar] [CrossRef]
- Garaulet, M.; Gomez-Abellan, P.; Rubio-Sastre, P.; Madrid, J.A.; Saxena, R.; Scheer, F.A. Common type 2 diabetes risk variant in MTNR1B worsens the deleterious effect of melatonin on glucose tolerance in humans. Metabolism 2015, 64, 1650–1657. [Google Scholar] [CrossRef]
- Morera-Fumero, A.L.; Fernandez-Lopez, L.; Abreu-Gonzalez, P. Melatonin and melatonin agonists as treatments for benzodiazepines and hypnotics withdrawal in patients with primary insomnia. A systematic review. Drug Alcohol. Depend. 2020, 212, 107994. [Google Scholar] [CrossRef]
- Salahub, C.; Wu, P.E.; Burry, L.D.; Soong, C.; Sheehan, K.A.; MacMillan, T.E.; Lapointe-Shaw, L. Melatonin for Insomnia in Medical Inpatients: A Narrative Review. J. Clin. Med. 2022, 12, 256. [Google Scholar] [CrossRef]
- Choi, K.; Lee, Y.J.; Park, S.; Je, N.K.; Suh, H.S. Efficacy of melatonin for chronic insomnia: Systematic reviews and meta-analyses. Sleep Med. Rev. 2022, 66, 101692. [Google Scholar] [CrossRef] [PubMed]
- Kunz, D.; Stotz, S.; Bes, F. Treatment of isolated REM sleep behavior disorder using melatonin as a chronobiotic. J. Pineal Res. 2021, 71, e12759. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Sanabria, F.; Carmassi, C.; Bruno, S.; Bazzani, A.; Carli, M.; Scarselli, M.; Faraguna, U. Melatonin as a Chronobiotic with Sleep-promoting Properties. Curr. Neuropharmacol. 2023, 21, 951–987. [Google Scholar] [CrossRef]
- Tortorolo, F.; Farren, F.; Rada, G. Is melatonin useful for jet lag? Medwave 2015, 15 (Suppl. 3), e6343. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Singh, J.; Pandi-Perumal, S.R.; Brown, G.M.; Spence, D.W.; Cardinali, D.P. Jet lag, circadian rhythm sleep disturbances, and depression: The role of melatonin and its analogs. Adv. Ther. 2010, 27, 796–813. [Google Scholar] [CrossRef] [PubMed]
- Carriedo-Diez, B.; Tosoratto-Venturi, J.L.; Canton-Manzano, C.; Wanden-Berghe, C.; Sanz-Valero, J. The Effects of the Exogenous Melatonin on Shift Work Sleep Disorder in Health Personnel: A Systematic Review. Int. J. Environ. Res. Public. Health 2022, 19, 199. [Google Scholar] [CrossRef]
- Caspi, O. Melatonin for the prevention and treatment of jet lag. Altern. Ther. Health Med. 2004, 10, 74–78. [Google Scholar]

| Variables | Melatonin → Placebo n = 15 | Placebo → Melatonin n = 15 | Total n = 30 | p-Value |
|---|---|---|---|---|
| Age (mean ± SD 5) | 62.27 ± 8.20 | 61.87 ± 10.95 | 62.07 ± 9.51 | 0.911 1 |
| Gender n (%) | ||||
| Feminine | 9 (56.2%) | 7 (43.8%) | 16 (100.0) | |
| Masculine | 6 (42.9%) | 8 (57.1%) | 14 (100.0) | 0.464 3 |
| Education n (%) | ||||
| Elementary | 0 (0.0) | 3 (100.0) | 3 (100.0) | |
| School | 5 (45.5) | 6 (54.4) | 11 (100.0) | |
| faculty | 10 (62.5) | 6 (37.5) | 16 (100.0) | 0.143 4 |
| DMT2 diagnosis time | ||||
| Mean ± SD | 13.20 ± 7.66 | 14.13 ± 7.94 | 13.67 ± 7.68 | 0.745 1 |
| Index of body mass Mean ± SD | 30.06 ± 5.63 | 31.55 ± 6.10 | 30.81 ± 5.81 | 0.494 1 |
| Glycohemoglobin Median (Q1;Q3) | 7.30 (7.10–8.10) | 7.30 (7.00–8.40) | 7.30 (7.10–8.10) | 0.723 2 |
| Insulin use n (%) | ||||
| Yes | 2 (22.2%) | 7 (77.8%) | 9 (100.0) | |
| No | 13 (61.9%) | 8 (31.1%) | 21 (100.0) | 0.109 4 |
| Variation of Analyzed Glucose (Post-/Preprandial Difference) | Placebo | Melatonin | p-Value |
|---|---|---|---|
| General (Median (Q1–Q3)) | 29.16 (11.63–36.66) | 25.88 (15.30–45.19) | 0.805 |
| Breakfast (mean ± standard deviation) | 31.47 ± 35.30 | 45.5 ± 39.85 | 0.058 |
| Lunch (Median (Q1–Q3)) | 25.83 (66.66–52.66) | 22.33 (7.66–42.83) | 0.57 |
| Dinner (mean ± standard deviation) | 24.34 ± 32.22 | 19.41 ± 36.45 | 0.58 |
| Breakfast on D7 (Median (Q1–Q3)) | 26 (−7–58.25) | 37.5 (27.25–64.25) | 0.016 |
| Lunch on D7 (mean ± standard deviation) | 25.0 ± 47.14 | 33.13 ± 50.67 | 0.362 |
| Dinner on D7 (mean ± standard deviation) | 15.16 ± 40 | 15 ± 39.46 | 0.98 |
| General on D7 (mean ± standard deviation) | 20.33(8.0–42.0) | 27.5 (9.0–47.5) | 0.077 |
| Night and day difference (mean ± standard deviation) D6–D7 | 23.7 ± 35.48 | 44.80 ± 50.95 | 0.078 |
| Variation of Analyzed Glucose (Delta) | Placebo | Melatonin | p-Value |
|---|---|---|---|
| General (Median (Q1–Q3)) | 38.22 (29.94–55.75) | 37.94 (29.27–56.16) | 0.829 |
| Breakfast (Median (Q1–Q3)) | 33.66 (21.25–67.58) | 44.5 (28–68) | 0.102 |
| Lunch (Median (Q1–Q3)) | 42.33 (24.83–58.91) | 36.5 (2.83–49.08) | 0.44 |
| Dinner (mean ± standard deviation) | 37.60 ± 22.60 | 36.21 ± 25 | 0.86 |
| Breakfast D7 (Median (Q1–Q3)) | 37 (12.7-63.5) | 39.5 (28–64.25) | 0.051 |
| Lunch D7 (Median (Q1–Q3)) | 34 (20.75–71) | 35 (21.75–70.5) | 0.267 |
| Dinner D7 (Median (Q1–Q3)) | 30.5 (10–50) | 21.5 (9.5–42.25) | 0.558 |
| General D7 (Median (Q1–Q3)) | 37 (23.5–50.4) | 37.3 (26.66–52.16) | 0.29 |
| Night and day difference D6–D7 (mean ± standard deviation) | 32.16 ± 27.80 | 52.26 ± 42.97 | 0.032 |
| Glicemic Variability | Placebo | Melatonin | p-Value |
|---|---|---|---|
| Glycemic variability coefficient (Median (Q1–Q3)) | 22.36% (18–28%) | 24.52% (18.7–28%) | 0.098 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martorina, W.; Tavares, A. Glycemic Variability in Patients with Type 2 Diabetes Mellitus (T2DM): The Role of Melatonin in a Crossover, Double-Blind, Placebo-Controlled, Randomized Study. Nutrients 2023, 15, 3523. https://doi.org/10.3390/nu15163523
Martorina W, Tavares A. Glycemic Variability in Patients with Type 2 Diabetes Mellitus (T2DM): The Role of Melatonin in a Crossover, Double-Blind, Placebo-Controlled, Randomized Study. Nutrients. 2023; 15(16):3523. https://doi.org/10.3390/nu15163523
Chicago/Turabian StyleMartorina, Wagner, and Almir Tavares. 2023. "Glycemic Variability in Patients with Type 2 Diabetes Mellitus (T2DM): The Role of Melatonin in a Crossover, Double-Blind, Placebo-Controlled, Randomized Study" Nutrients 15, no. 16: 3523. https://doi.org/10.3390/nu15163523
APA StyleMartorina, W., & Tavares, A. (2023). Glycemic Variability in Patients with Type 2 Diabetes Mellitus (T2DM): The Role of Melatonin in a Crossover, Double-Blind, Placebo-Controlled, Randomized Study. Nutrients, 15(16), 3523. https://doi.org/10.3390/nu15163523





