Early Intervention in Cognitive Aging with Strawberry Supplementation
Abstract
:1. Introduction
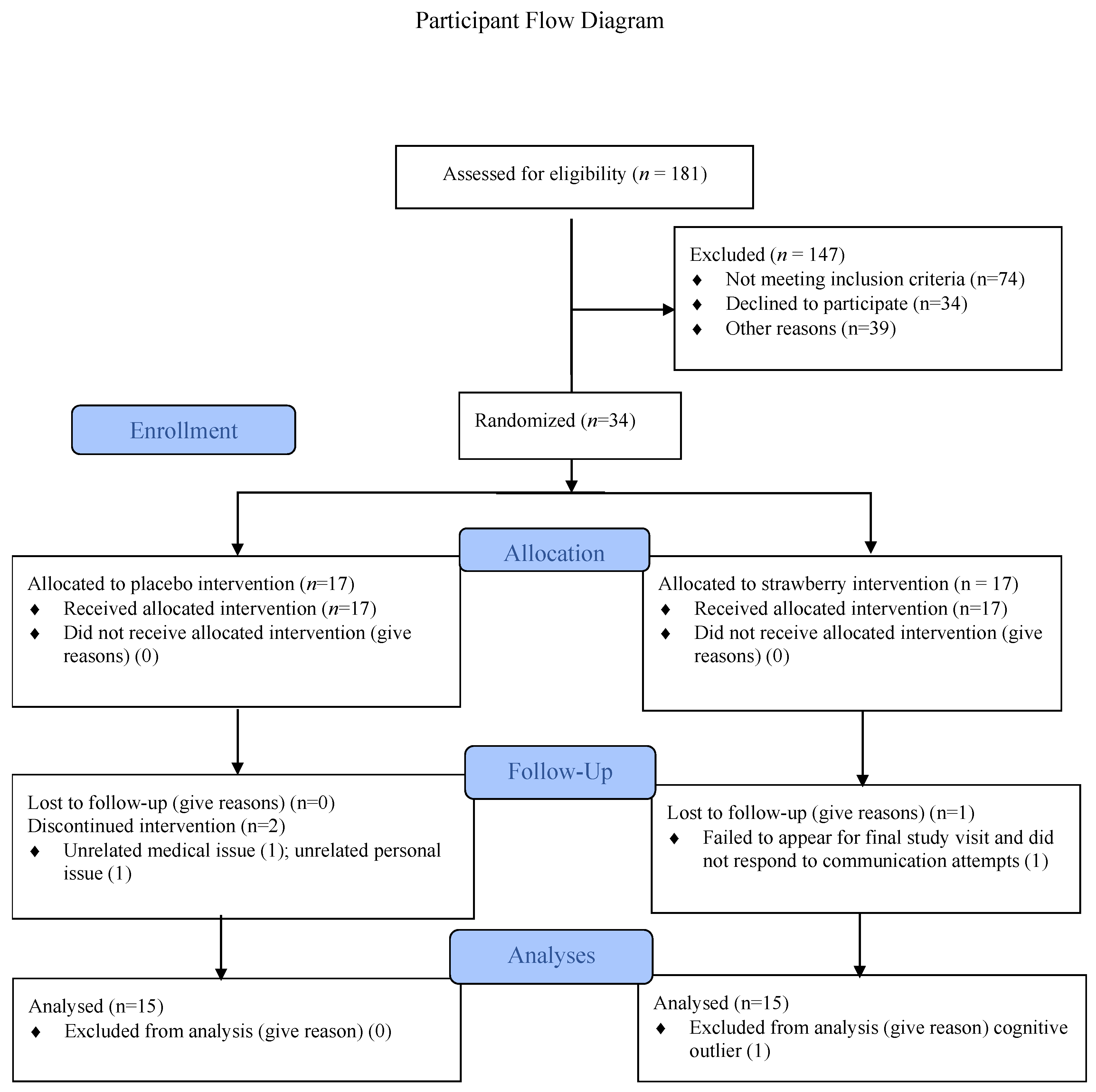
2. Materials & Methods
2.1. Strawberry Powder, Placebo Powder, and Supplement Regimen
2.2. Outcome Measures and Assessment Procedures
2.3. Executive Abilities
2.4. Lexical Access
2.5. Learning and Long-Term Memory
2.6. Mood Symptoms
2.7. Metabolic Parameters
2.8. Anthropometric Measures
2.9. Diet Diaries
2.10. Statistical Analyses and Power Calculations
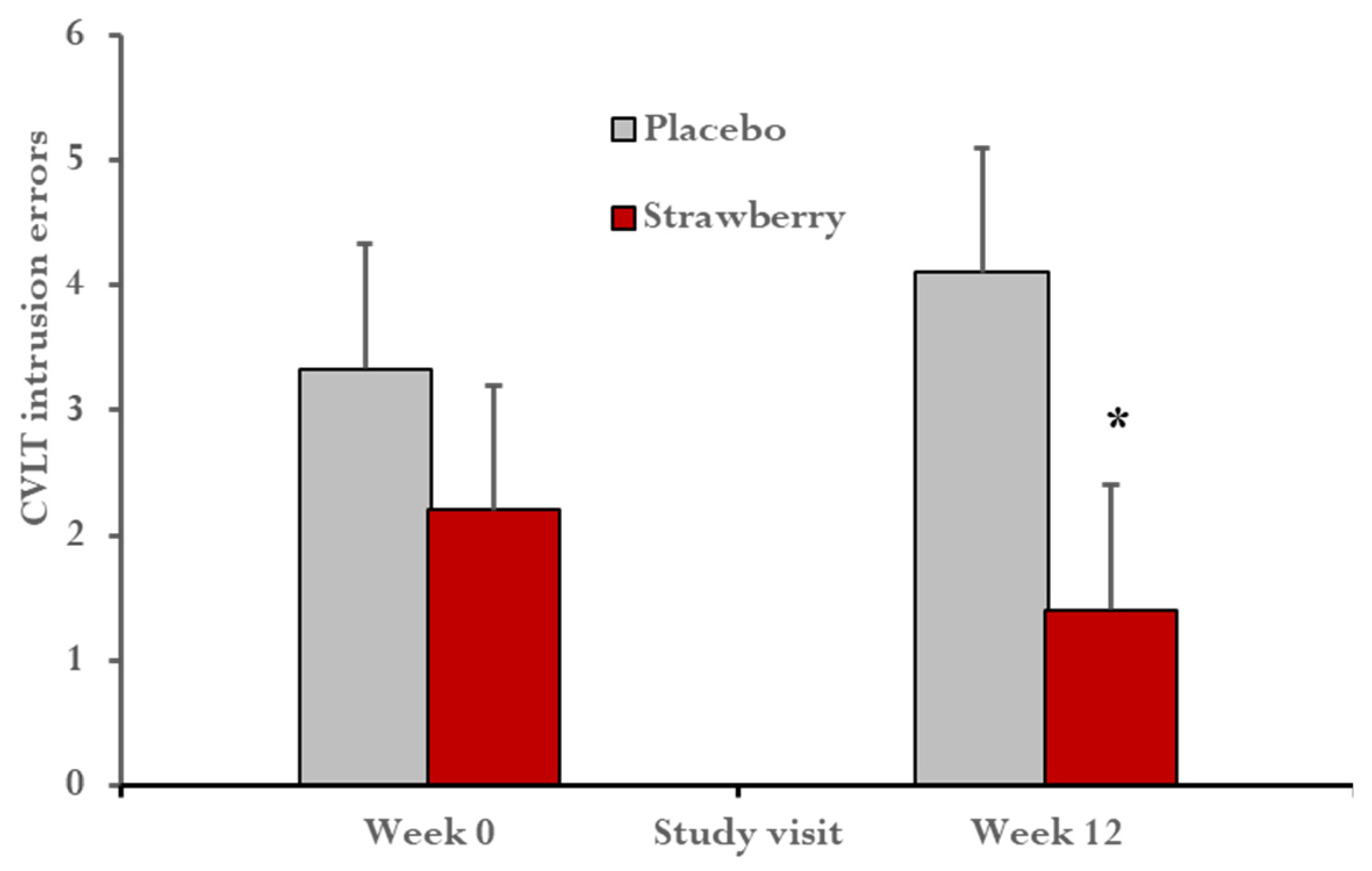
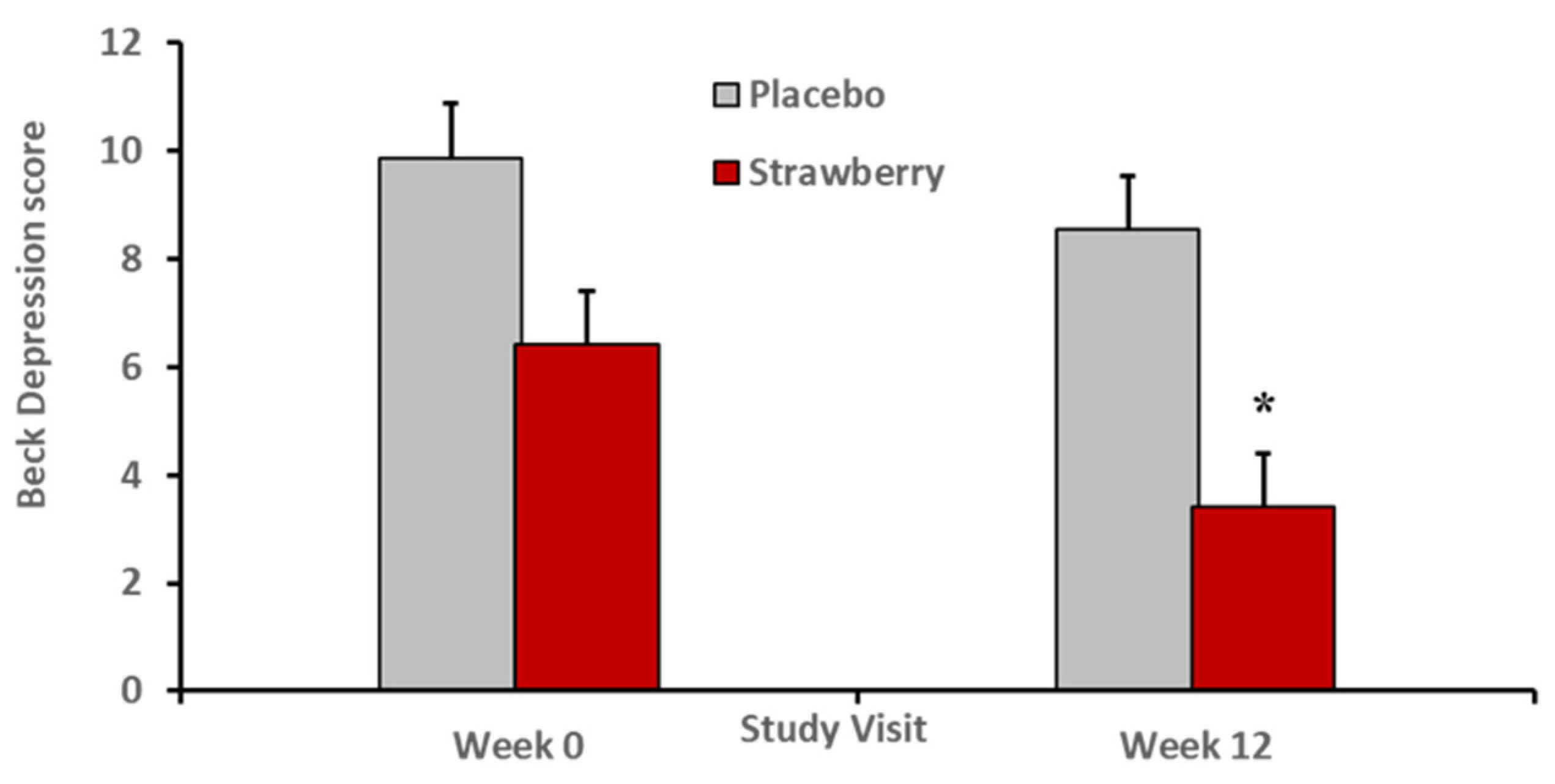
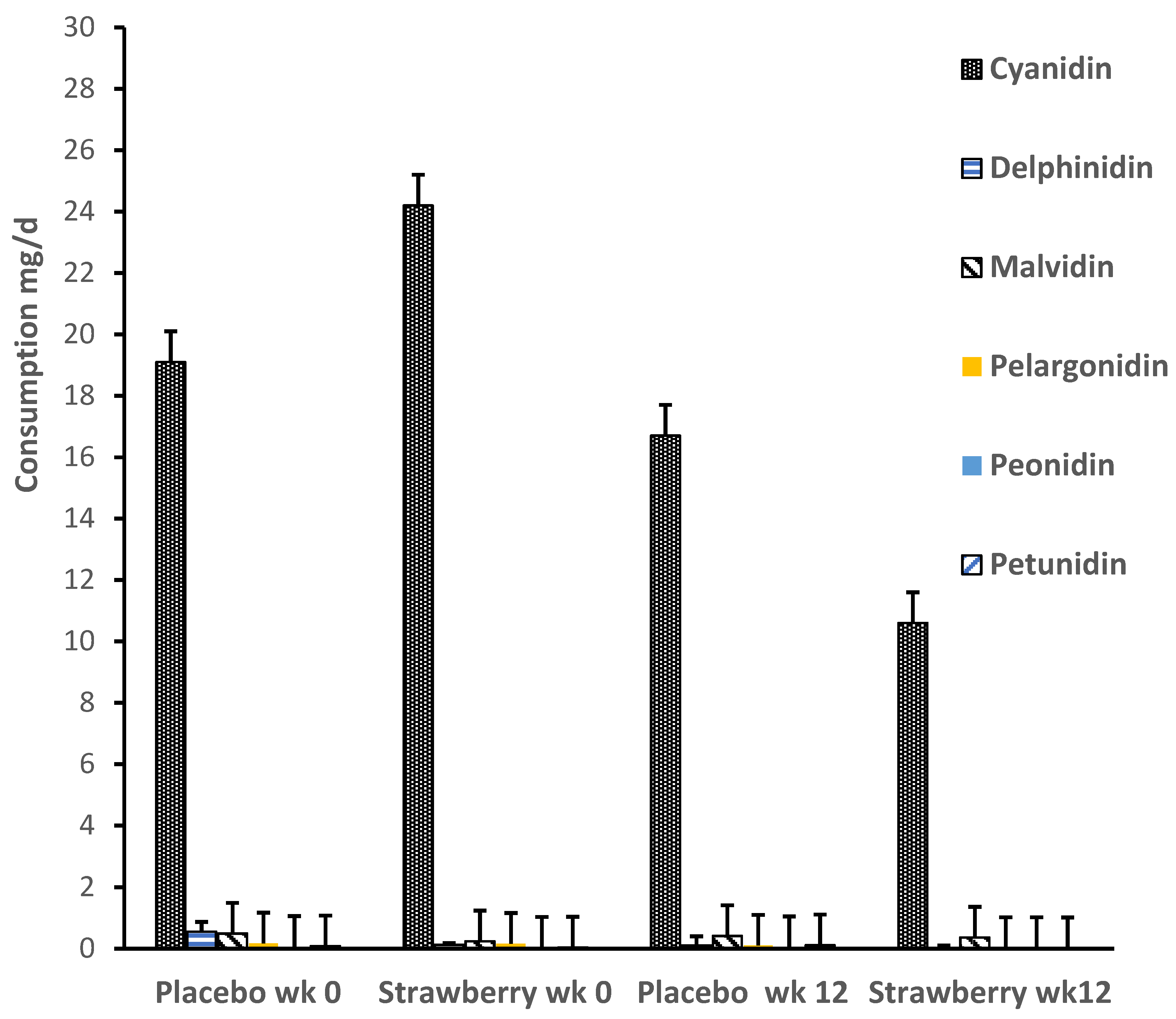
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rajan, K.B.; Weuve, J.; Barnes, L.L.; McAninch, E.A.; Wilson, R.S.; Evans, D.A. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimer’s Dement. 2021, 17, 1966–1975. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: Report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R., Jr.; Kaye, J.; Montine, T.J.; et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2011, 7, 280–292. [Google Scholar] [CrossRef]
- Hirode, G.; Wong, R.J. Trends in the prevalence of metabolic syndrome in the United States, 2011–2016. JAMA Lett. 2020, 323, 2526–2528. [Google Scholar] [CrossRef]
- Jack, C.R.; Knopman, D.S.; Jagust, W.J.; Shaw, L.M.; Aisen, P.S.; Weiner, M.W.; Petersen, R.C.; Trojanowski, J.Q. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010, 9, 119–128. [Google Scholar] [CrossRef]
- Araujo, J.; Cai, J.; Stevens, J. Prevalence of optimal metabolic health in American adults: National Health and Nutrition Examination Survey 2009–2016. Metab. Syndr. Relat. Disord. 2019, 1, 46–52. [Google Scholar] [CrossRef]
- Luchsinger, J.A.; Tang, M.-X.; Shea, S.; Mayeux, R. Hyperinsulinemia and risk of Alzheimer disease. Neurology 2004, 63, 1187–1192. [Google Scholar] [CrossRef]
- Young, S.E.; Mainous, A.G.; Carnemolla, M. Hyperinsulinemia and cognitive decline in a middle-aged cohort. Diabetes Care 2006, 29, 2688–2693. [Google Scholar] [CrossRef]
- Craft, S. The role of metabolic disorders in Alzheimer disease and vascular dementia: Two roads converged. Arch. Neurol. 2009, 66, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Razay, G.; Vreugdenhil, A.; Wilcock, G. The metabolic syndrome and Alzheimer disease. Arch. Neurol. 2007, 64, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Whitmer, R.A.; Gunderson, E.P.; Barrett-Connor, E.; Quesenberry, C.P.; Yaffe, K. Obesity in middle age and future risk of dementia: A 27 year longitudinal population based study. BMJ 2005, 330, 1360. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Koya, J.; Reznik, S.E. Insulin resistance exacerbates Alzheimer disease via multiple mechanisms. Front. Neurosci. 2021, 15, 687157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Henriques, J.F.; Serra, D.; Dinis, T.C.P.; Almeida, L.M. The Anti-Neuroinflammatory Role of Anthocyanins and Their Metabolites for the Prevention and Treatment of Brain Disorders. Int. J. Mol. Sci. 2020, 21, 8653. [Google Scholar] [CrossRef]
- Lail, H.L.; Feresin, R.G.; Hicks, D.; Stone, B.; Price, E.; Wanders, D. Berries as a treatment for obesity-induced inflammation: Evidence from preclinical models. Nutrients 2021, 13, 334. [Google Scholar] [CrossRef]
- Basu, A.; Lyons, T.J. Strawberries, blueberries, and cranberries in the metabolic syndrome: Clinical perspectives. J. Agric. Food Chem. 2011, 60, 5687–5692. [Google Scholar] [CrossRef]
- Burton-Freeman, B. Postprandial metabolic events and fruit-derived phenolics: A review of the science. Br. J. Nutr. 2010, 104, S1–S14. [Google Scholar] [CrossRef]
- Vendrame, S.; Del Bo’, C.; Ciappellano, S.; Riso, P.; Klimis-Zacas, D. Berry fruit consumption and metabolic syndrome. Antioxidants 2016, 5, 34. [Google Scholar] [CrossRef]
- Miller, M.G.; Shukitt-Hale, B. Berry fruit enhances beneficial signaling in the brain. J. Agric. Food Chem. 2012, 60, 5709–5715. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.N.; Bickford, P.C. Anthocyanins and their metabolites as therapeutic agents for neurodegenerative disease. Antioxidants 2019, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.; Fernandes, I.; Meireles, M.; Faria, A.; Spencer, J.P.E.; Mateus, N.; Calhau, C. Gut microbiota modulation accounts for the neuroprotective properties of anthocyanins. Sci. Rep. 2018, 8, 11341. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Izuora, K.; Betts, N.M.; Kinney, J.W.; Salazar, A.M.; Ebersole, J.L.; Scofield, R.H. Dietary strawberries improve cardiometabolic risks in adults with obesity and elevated serum LDL cholesterol in a randomized controlled crossover trial. Nutrients 2021, 13, 1421. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Izuora, K.; Hooyman, A.; Scofield, H.R.; Ebersole, J.L. Dietary strawberries improve serum metabolites of cardiometabolic risks in adults with features of the metabolic syndrome in a randomized controlled crossover trial. Int. J. Mol. Sci. 2023, 24, 2051. [Google Scholar] [CrossRef]
- Cuomo, P.; Capparelli, R.; Iannelli, A.; Iannelli, D. Role of branched-chain amino acid metabolism in type 2 diabetes, obesity, cardiovascular disease and non-alcoholic fatty liver disease. Int. J. Mol. Sci. 2022, 23, 4325. [Google Scholar] [CrossRef]
- Edirisinghe, I.; Banaszewski, K.; Cappozzo, J.; Sandhya, K.; Ellis, C.L.; Tadapaneni, R.; Kappagoda, C.T.; Burton-Freeman, B.M. Strawberry anthocyanin and its association with postprandial inflammation and insulin. Br. J. Nutr. 2011, 106, 913–922. [Google Scholar] [CrossRef]
- Burton-Freeman, B.; Linares, A.; Hyson, D.; Kappagoda, T. Strawberry modulates LDL oxidation and postprandial lipemia in response to high-fat meal in overweight hyperlipidemic men and women. J. Am. Coll. Nutr. 2010, 29, 46–54. [Google Scholar] [CrossRef]
- Huang, L.; Xiao, D.; Zhang, X.; Sandhu, A.K.; Chandra, P.; Kay, C.; Edirisinghe, I.; Burton-Freeman, B. Strawberry consumption, cardiometabolic risk factors, and vascular function: A randomized controlled trial in adults with moderate hypercholesterolemia. J. Nutr. 2021, 151, 1517–1526. [Google Scholar] [CrossRef]
- Joseph, J.; Shukitt-Hale, B.; Denisova, N.A.; Bielinski, D.; Martin, A.; McEwen, J.J.; Bickford, P.C. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J. Neurosci. 1999, 19, 8114–8121. [Google Scholar] [CrossRef]
- Shukitt-Hale, B.; Bielinski, D.F.; Lau, F.C.; Willis, L.M.; Carey, A.N.; Joseph, J.A. The beneficial effects of berries on cognition, motor behaviour and neu-ronal function in ageing. Br. J. Nutr. 2015, 114, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.M.; El Mohsen, M.A.; Vauzour, D.; Rendeiro, C.; Butler, L.T.; Ellis, J.A.; Whiteman, M.; Spencer, J.P. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free. Radic. Biol. Med. 2008, 45, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Devore, E.; Kang, H.; Breteler, M.; Grodstein, F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann. Neurol. 2012, 72, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Holland, T.M.; Wang, Y.; Bennett, D.A.; Morris, M.C. Association of strawberries and anthocyanidin intake with Alzheimer’s dementia risk. Nutrients 2019, 11, 3060. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.G.; Thangthaeng, N.; Rutledge, G.A.; Scott, T.M.; Shukitt-Hale, B. Dietary strawberry improves cognition in a randomised, double-blind, placebo-controlled trial in older adults. Br. J. Nutr. 2021, 126, 320. [Google Scholar] [CrossRef]
- Tsang, M.; Delaney, K.; Kern, M.; Jason, N.; Hong, M.Y.; Liu, C.; Hooshmand, S. The impact of strawberries on cognition and cardiovascular health of older healthy adults: A randomized, crossover, double-blind, placebo controlled clinical trial. Curr. Dev. Nutr. 2023, 7, 100183. [Google Scholar] [CrossRef]
- Krikorian, R.; Zimmerman, M.E.; Fleck, D.E. Inhibitory control in obsessive-compulsive disorder. Brain Cogn. 2004, 54, 257–259. [Google Scholar] [CrossRef]
- Stein, A.L.; Tolle, K.A.; Stover, A.N.; Shidler, M.D.; Krikorian, R. Detecting mild cognitive impairment remotely with the modified memory impairment screen by telephone. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2023, 23, 1–13. [Google Scholar] [CrossRef]
- Taves, D.R. Minimization: A new method of assigning patients to treatment and control groups. Clin. Pharmacol. Ther. 1974, 15, 443–453. [Google Scholar] [CrossRef]
- Porteus, S.D. Porteus Maze Test: Fifty Years’ Application; Pacific Books: Palo Alto, CA, USA, 1965. [Google Scholar]
- Krikorian, R.; Bartok, J.A. Developmental Data for the Porteus Maze Test. Clin. Neuropsychol. 1998, 12, 305–310. [Google Scholar] [CrossRef]
- Reitan, R.M. Trail Making Test: Manual for Administration and Scoring; Reitan Neuropsychology Laboratory: Tucson, AZ, USA, 1992. [Google Scholar]
- Sánchez-Cubillo, I.; Periáñez, J.; Adrover-Roig, D.; Rodríguez-Sánchez, J.; Ríos-Lago, M.; Tirapu, J.; Barceló, F. Construct validity of the Trail Making Test: Role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J. Int. Neuropsychol. Soc. 2009, 15, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Welsh, M.C.; Pennington, B.F.; Groisser, D.B. A normative-developmental study of executive function: A window on prefrontal function in children. Dev. Neuropsychol. 1991, 7, 131–149. [Google Scholar] [CrossRef]
- Shao, Z.; Janse, E.; Visser, K.; Meyer, A.S. What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Front. Psychol. 2014, 5, 772. [Google Scholar] [CrossRef] [PubMed]
- Miceli, G.; Caltagirone, C.; Gainotti, G.; Masullo, C.; Silveri, M.C. Neuropsychological correlates of localized cerebral lesions in non-aphasic brain-damaged patients. J. Clin. Neuropsychol. 1981, 3, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Delis, D.C.; Kramer, J.H.; Kaplan, E.; Ober, B.A. California Verbal Learning Test—Second Edition (CVLT—II); Psychological Corporation: San Antonio, TX, USA, 2000. [Google Scholar] [CrossRef]
- Krikorian, R. Independence of verbal and spatial paired associate learning. Brain Cogn. 1996, 32, 219–223. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Brown, G.K. BDI-II: Beck Depression Inventory Manual, 2nd ed.; Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar]
- Wang, Y.-P.; Gorenstein, C. Psychometric properties of the Beck Depression Inventory-II: A comprehensive review. Rev. Bras. Psiquiatr. 2013, 35, 416–431. [Google Scholar] [CrossRef]
- Steer, R.A.; Ball, R.; Ranieri, W.F.; Beck, A.T. Dimensions of the Beck Depression Inventory-II in clinically depressed outpa-tients. J. Clin. Psychol. 1999, 55, 117–128. [Google Scholar] [CrossRef]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef]
- Gong, R.; Luo, G.; Wang, M.; Ma, L.; Sun, S.; Wei, X. Associations between TG/HDL ratio and insulin resistance in the US population: A cross-sectional study. Endocr. Connect. 2021, 10, 1502–1512. [Google Scholar] [CrossRef]
- Moriyama, K. Associations between the triglyceride to high-density lipoprotein cholesterol ratio and metabolic syndrome, insulin resistance, and lifestyle habits in healthy japanese. Metab. Syndr. Relat. Disord. 2020, 18, 260–266. [Google Scholar] [CrossRef]
- da Luz, P.L.; Favarato, D.; Junior, J.R.F.-N.; Lemos, P.; Chagas, A.C.P. High ratio of triglycerides to HDL-cholesterol predicts extensive coronary disease. Clinics 2008, 63, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, R.; Skelton, M.R.; Summer, S.S.; Shidler, M.D.; Sullivan, P.G. Blueberry supplementation in midlife for dementia risk reduction. Nutrients 2022, 14, 1619. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Mahwak, NJ, USA, 1988. [Google Scholar]
- Riordan, H.J. Constructing composites to optimize cognitive outcomes. J. Clin. Stud. 2017, 9, 42–46. [Google Scholar]
- Sheeber, L.B.; Sorensen, E.D.; Howe, S.R. Data analytic techniques for treatment outcome studies with pretest/posttest measurements: An extensive primer. J. Psychiatr. Res. 1996, 30, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Verkouter, I.; Noordam, R.; le Cessie, S.; van Dam, R.M.; Lamb, H.J.; Rosendaal, F.R.; van Heemst, D.; de Mutsert, R. The Association between Adult Weight Gain and Insulin Resistance at Middle Age: Mediation by Visceral Fat and Liver Fat. J. Clin. Med. 2019, 8, 1559. [Google Scholar] [CrossRef]
- Gutch, M.; Kumar, S.; Razi, S.M.; Gupta, K.K.; Gupta, A. Assessment of insulin sensitivity/resistance. Indian J. Endocrinol. Metab. 2015, 19, 160–164. [Google Scholar] [CrossRef]
- Qaseem, A.; Wilt, T.J.; Kansagara, D.; Horwitch, C.; Barry, M.J.; Forciea, M.A.; Clinical Guidelines Committee of the American College of Physicians. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: A guidance statement update from the American college of physicians. Ann. Intern. Med. 2018, 168, 569–576. [Google Scholar] [CrossRef]
- Babic, N.; Valjevac, A.; Zaciragic, A.; Avdagic, N.; Zukic, S.; Hasic, S. The triglyceride/HDL ratio and triglyceride glucose index as predictors of glycemic control in patients with diabetes mellitus type. Med. Arch. 2019, 73, 163–168. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.D.R.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Álvarez-Parrilla, E.; González-Aguilar, G.A.; De la Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-glucoside: Physical-Chemistry, Foodomics and Health Effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef]
- Atkins, A.S.; Berman, M.G.; Reuter-Lorenz, P.A.; Lewis, R.L.; Jonides, J. Resolving semantic and proactive interference in memory over the short-term. Mem. Cogn. 2011, 39, 806–817. [Google Scholar] [CrossRef] [PubMed]
- Wahlheim, C.N. Proactive effects of memory in young and older adults: The role of change recollection. Mem. Cogn. 2014, 42, 950–964. [Google Scholar] [CrossRef] [PubMed]
- Loewenstein, D.A.; Curiel, R.E.; Wright, C.; Sun, X.; Alperin, N.; Crocco, E.; Czaja, S.J.; Raffo, A.; Penate, A.; Melo, J.; et al. Recovery from Proactive Semantic Interference in Mild Cognitive Impairment and Normal Aging: Relationship to Atrophy in Brain Regions Vulnerable to Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 56, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Kato, T. Coping with stress, executive functions, and depressive symptoms: Focusing on flexible responses to stress. J. Clin. Med. 2021, 10, 3122. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Evans, L.D.; Rao, U.; Garber, J. Executive function moderates the relation between coping and depressive symptoms. Anxiety Stress Coping 2014, 28, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, H.J.; Brunsdon, V.E.A.; Bradford, E.E.F. The developmental trajectories of executive function from adolescence to old age. Sci. Rep. 2021, 11, 1382. [Google Scholar] [CrossRef]
- Yang, Y.; Shields, G.S.; Guo, C.; Liu, Y. Executive function performance in obesity and overweight individuals: A me-ta-analysis and review. Neurosci. Biobehav. Rev. 2018, 84, 225–244. [Google Scholar] [CrossRef]
- Lutski, M.; Weinstein, G.; Goldbourt, U.; Tanne, D. Insulin resistance and future cognitive performance and cognitive decline in elderly patients with cardiovascular disease. J. Alzheimer’s Dis. 2017, 57, 633–643. [Google Scholar] [CrossRef]
- Morys, F.; Potvin, O.; Zeighami, Y.; Vogel, J.; Lamontagne-Caron, R.; Duchesne, S.; Dagher, A.; Initiative, F.T.A.D.N. Obesity-Associated Neurodegeneration Pattern Mimics Alzheimer’s Disease in an Observational Cohort Study. J. Alzheimer’s Dis. 2023, 91, 1059–1071. [Google Scholar] [CrossRef]
- Willmann, C.; Brockmann, K.; Wagner, R.; Kullmann, S.; Preissl, H.; Schnauder, G.; Maetzler, W.; Gasser, T.; Berg, D.; Eschweiler, G.W.; et al. Insulin sensitivity predicts cognitive decline in individuals with prediabetes. BMJ Open Diabetes Res. Care 2020, 8, e001741. [Google Scholar] [CrossRef]
- Raji, C.A.; Meysami, S.; Hashemi, S.; Garg, S.; Akbari, N.; Gouda, A.; Chodakiewitz, Y.G.; Nguyen, T.D.; Niotis, K.; Merrill, D.A.; et al. Visceral and subcutaneous abdominal fat predict brain volume loss at midlife in 10,001 individuals. Aging Dis. 2023. [Google Scholar] [CrossRef]
- Schmoller, A.; Hass, T.; Strugovshchikova, O.; Melchert, U.H.; Scholand-Engler, H.G.; Peters, A.; Schweiger, U.; Hohagen, F.; Oltmanns, K.M. Evidence for a relationship between body mass and energy metabolism in the human brain. J. Cereb. Blood Flow Metab. 2010, 30, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Sharifi-Rad, J.; Cappellini, F.; Reiner, Ž.; Zorzan, D.; Imran, M.; Sener, B.; Kilic, M.; El-Shazly, M.; Fahmy, N.M.; et al. The therapeutic potential of anthocyanins: Current approaches based on their molecular mechanism of action. Front. Pharmacol. 2020, 11, 1300. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Micke, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Andres-Lacueva, C.; Shukitt-Hale, B.; Galli, R.L.; Jauregui, O.; Lamuela-Raventos, R.M.; Joseph, J.A. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr. Neurosci. 2005, 8, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Rendeiro, C.; Vauzour, D.; Rattray, M.; Waffo-Téguo, P.; Mérillon, J.M.; Butler, L.T.; Williams, C.M.; Spencer, J.P.E. Dietary levels of pure flavonoids improve spatial memory performance and increase hippocampal brain-derived neurotrophic factor. PLoS ONE 2013, 8, e63535. [Google Scholar] [CrossRef]
- Poulose, S.M.; Rabin, B.M.; Bielinski, D.F.; Kelly, M.E.; Miller, M.G.; Thanthaeng, N.; Shukitt-Hale, B. Neurochemical differences in learning and memory paradigms among rats supplemented with anthocyanin-rich blueberry diets and exposed to acute doses of 56Fe particles. Life Sci. Space Res. 2017, 12, 16–23. [Google Scholar] [CrossRef]
- Shukitt-Hale, B.; Carey, A.N.; Jenkins, D.; Rabin, B.M.; Joseph, J.A. Beneficial effects of fruit extracts on neuronal function and behavior in a rodent model of accelerated aging. Neurobiol. Aging 2007, 28, 1187–1194. [Google Scholar] [CrossRef]
- Carey, A.N.; Gomes, S.M.; Shukitt-Hale, B. Blueberry supplementation improves memory in middle-aged mice fed a high-fat diet. J. Agric. Food Chem. 2014, 62, 3972–3978. [Google Scholar] [CrossRef]
- Krikorian, R.; Kalt, W.; McDonald, J.E.; Shidler, M.D.; Summer, S.S.; Stein, A.L. Cognitive performance in relation to urinary anthocyanins and their flavonoid-based products following blueberry supplementation in older adults at risk for dementia. J. Funct. Foods 2019, 64, 103667. [Google Scholar] [CrossRef]
| Placebo (n = 15) | Strawberry (n = 15) | t; p | |
|---|---|---|---|
| Age, y | 57.0 (3.9) | 55.9 (4.7) | 0.70; 0.48 |
| Education, y | 16.2 (1.7) | 15.4 (1.9) | 1.08; 0.28 |
| mMIS | 12.2 (1.3) | 12.4 (2.0) | 0.27; 0.78 |
| Body weight, kg | 101.1 (15.4) | 99.2 (24.3) | 0.25; 0.79 |
| BMI | 37.2 (6.2) | 35.1 (8.6) | 0.78; 0.44 |
| Waist circumference, cm | 112.6 (13.4) | 115.5 (22.0) | 0.43; 0.67 |
| Fasting glucose, mg/dL | 113.2 (14.3) | 101.8 (12.2) | 2.32; 0.02 |
| Fasting insulin, µU/mL | 12.8 (9.6) | 11.4 (5.2) | 0.50; 0.62 |
| HOMA2-IR | 3.6 (2.8) | 2.9 (1.5) | 0.82; 0.41 |
| HbA1c, % | 5.8 (0.52) | 5.6 (0.27) | 1.27; 0.26 |
| TG/HDL-C ratio | 1.91 (0.99) | 1.89 (1.02) | 1.04; 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krikorian, R.; Shidler, M.D.; Summer, S.S. Early Intervention in Cognitive Aging with Strawberry Supplementation. Nutrients 2023, 15, 4431. https://doi.org/10.3390/nu15204431
Krikorian R, Shidler MD, Summer SS. Early Intervention in Cognitive Aging with Strawberry Supplementation. Nutrients. 2023; 15(20):4431. https://doi.org/10.3390/nu15204431
Chicago/Turabian StyleKrikorian, Robert, Marcelle D. Shidler, and Suzanne S. Summer. 2023. "Early Intervention in Cognitive Aging with Strawberry Supplementation" Nutrients 15, no. 20: 4431. https://doi.org/10.3390/nu15204431
APA StyleKrikorian, R., Shidler, M. D., & Summer, S. S. (2023). Early Intervention in Cognitive Aging with Strawberry Supplementation. Nutrients, 15(20), 4431. https://doi.org/10.3390/nu15204431





