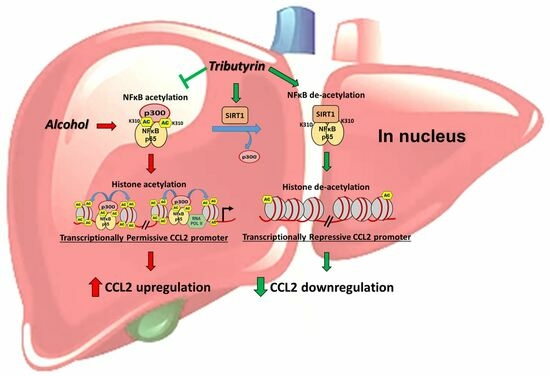
Tributyrin Mitigates Ethanol-Induced Lysine Acetylation of Histone-H3 and p65-NFκB Downregulating CCL2 Expression and Consequent Liver Inflammation and Injury
Abstract
:1. Introduction
2. Materials and Methods
| (Region I) mmCcl2_F1 | CAAGCACCCTGCCTGACT |
| (Region I) mmCcl2_R1 | CTCCCGTCTGGCTCTCTG |
| (Region II) mmCcl2_F2 | TCCCAGGAGTGGCTAGAAAA |
| (Region II) mmCcl2_R2 | TCCGCTGAGTAAGTGCAGAG |
| (Region III) mmCcl2_F3 | CATCTGGAGCTCACATTCCA |
| (Region III) mmCcl2_R3 | GGCAGGTCAGAGGCAGAGTA |
3. Results
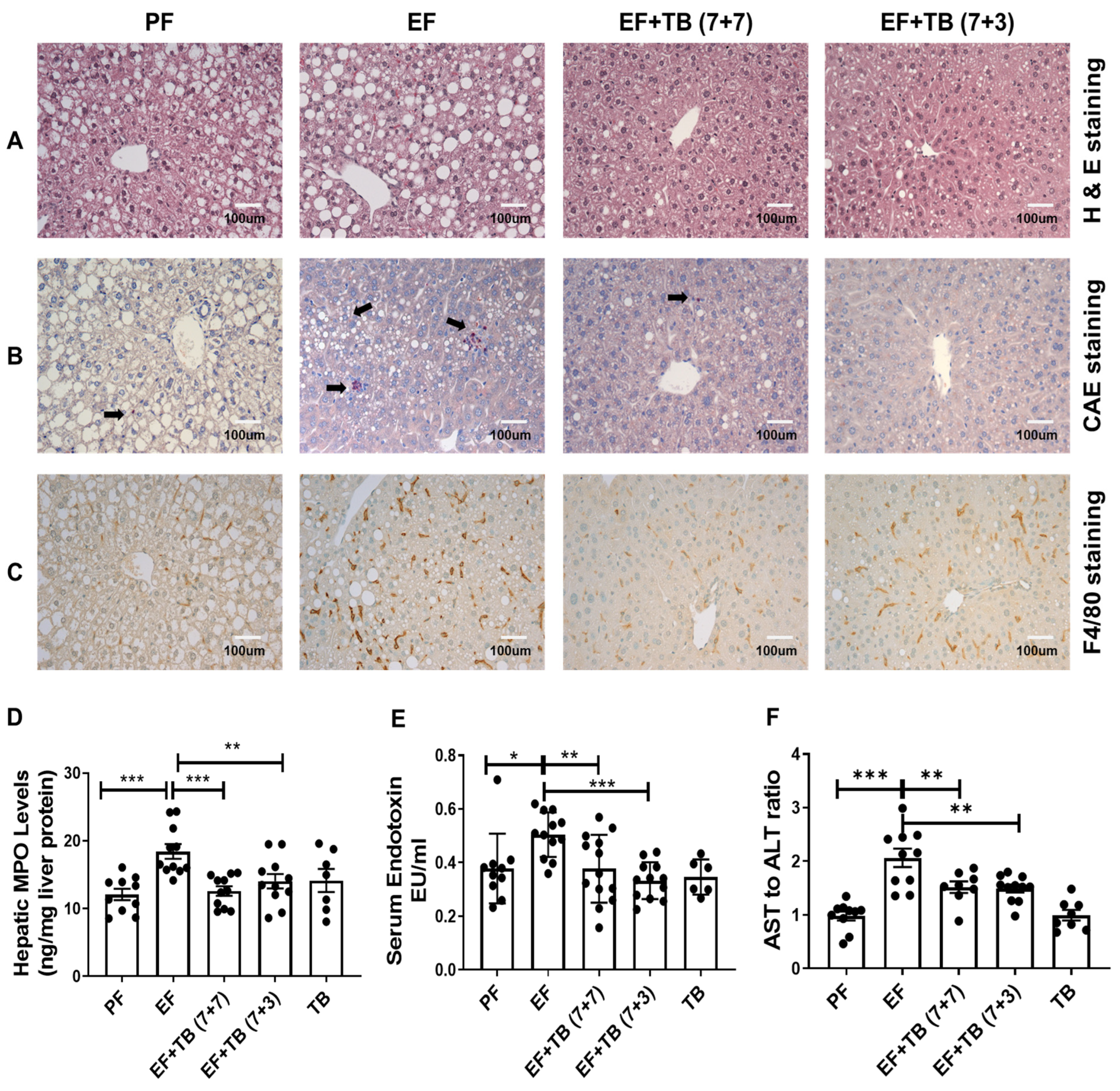
3.1. Oral Administration of Tributyrin Attenuates Alcohol-Induced, Neutrophil Infiltration, Kupffer Cell Activation, Systemic Endotoxemia, and Hepatic Injury
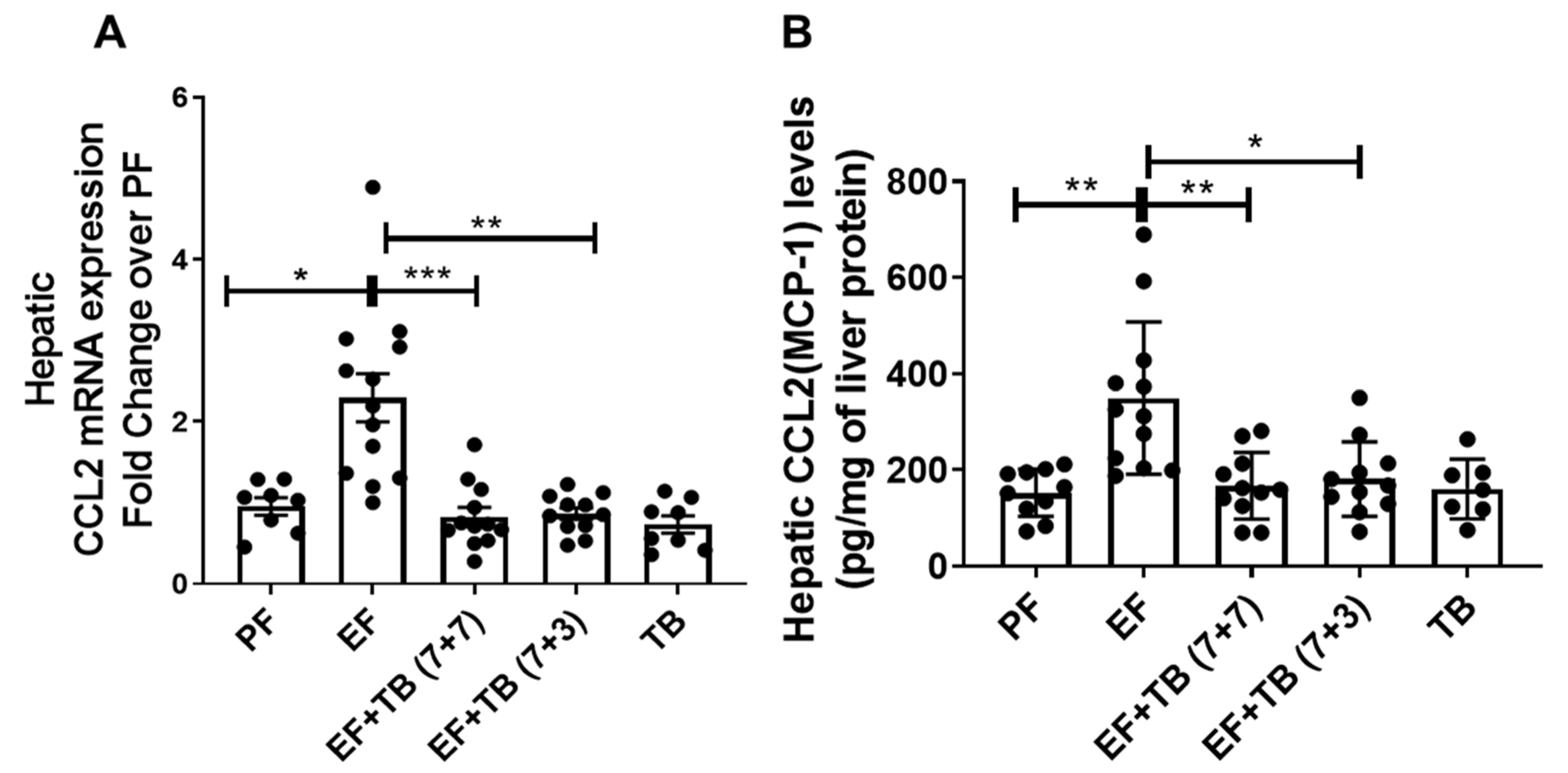
3.2. Tributyrin Mitigates Ethanol-Inducible Increase in Hepatic CCL2 Chemokine Expression
3.3. Tributyrin Prevents Ethanol-Induced Transcriptionally Permissive CCL2 Promoter-Associated Histone Modifications in the Liver
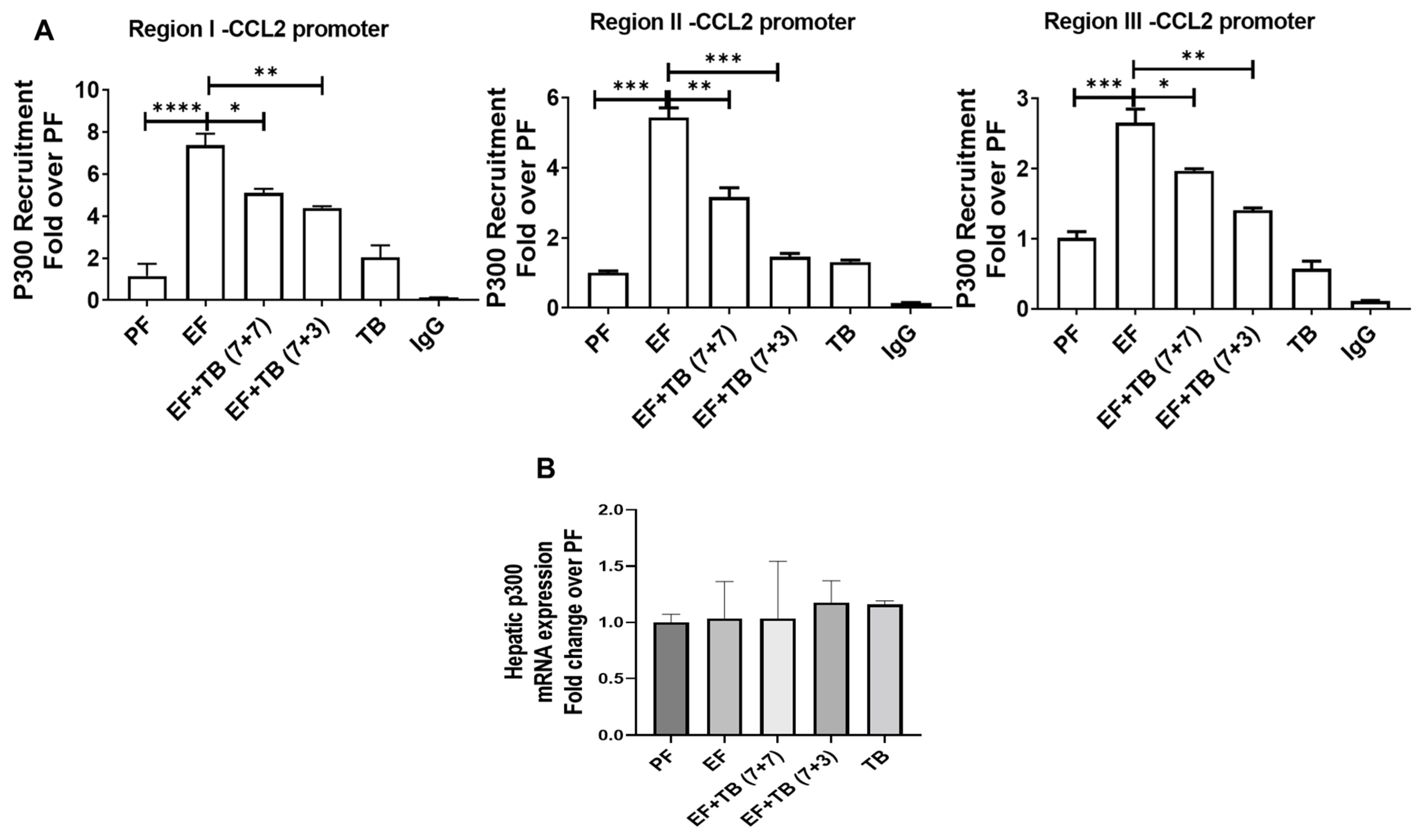
3.4. Tributyrin Impedes Ethanol-Responsive p300 Recruitment to the CCL2 Gene Promoter
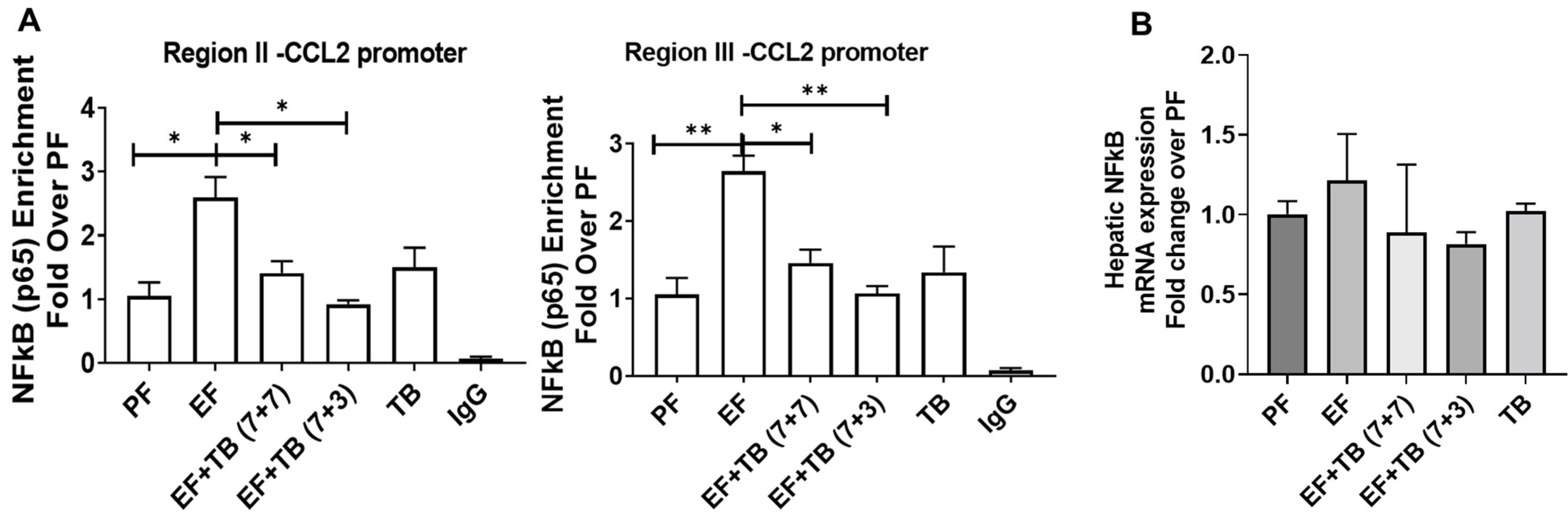
3.5. Tributyrin Attenuates Ethanol-Induced Enhanced Binding of Transcription Factor NFκB at the CCL2 Promoter
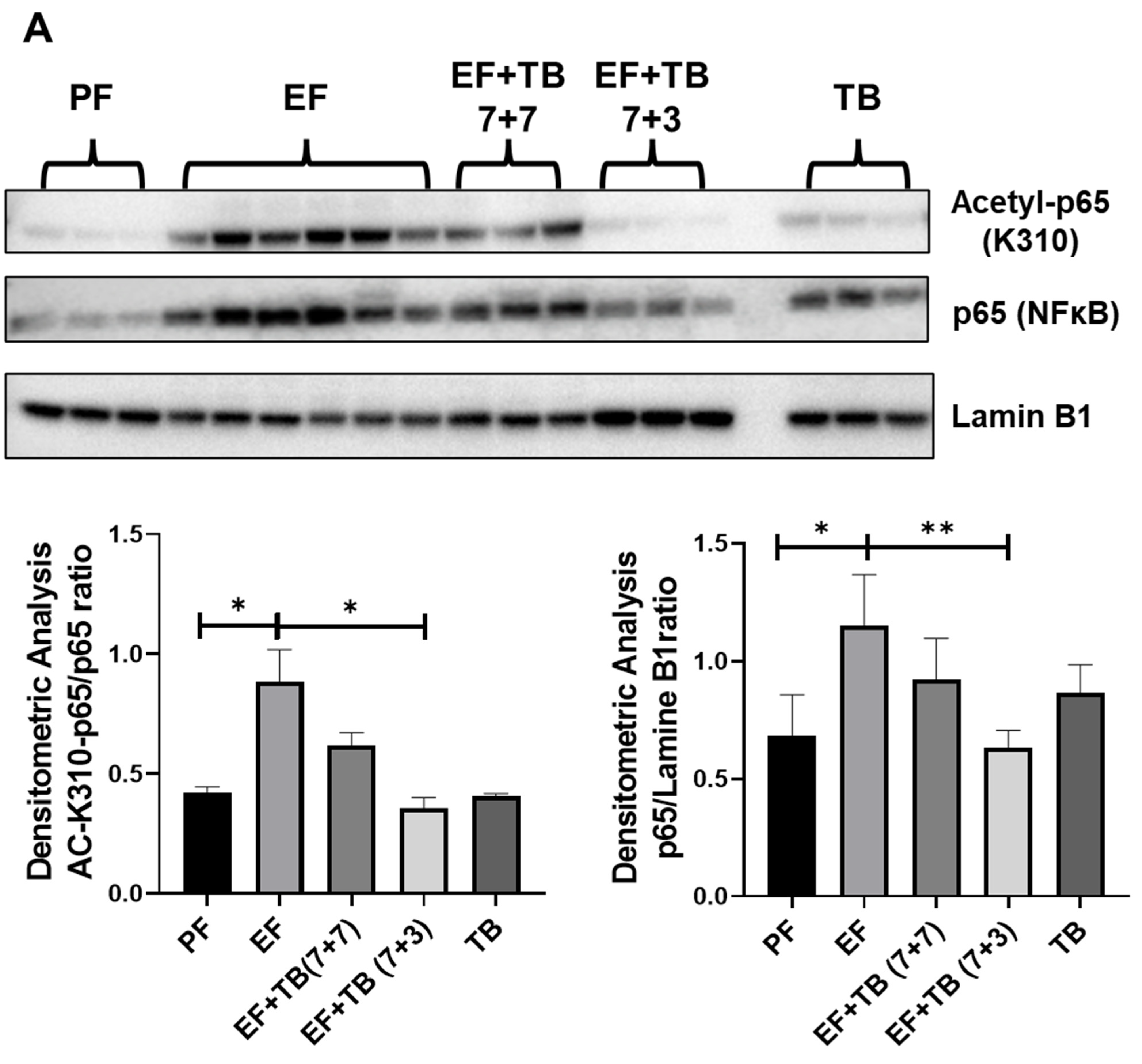
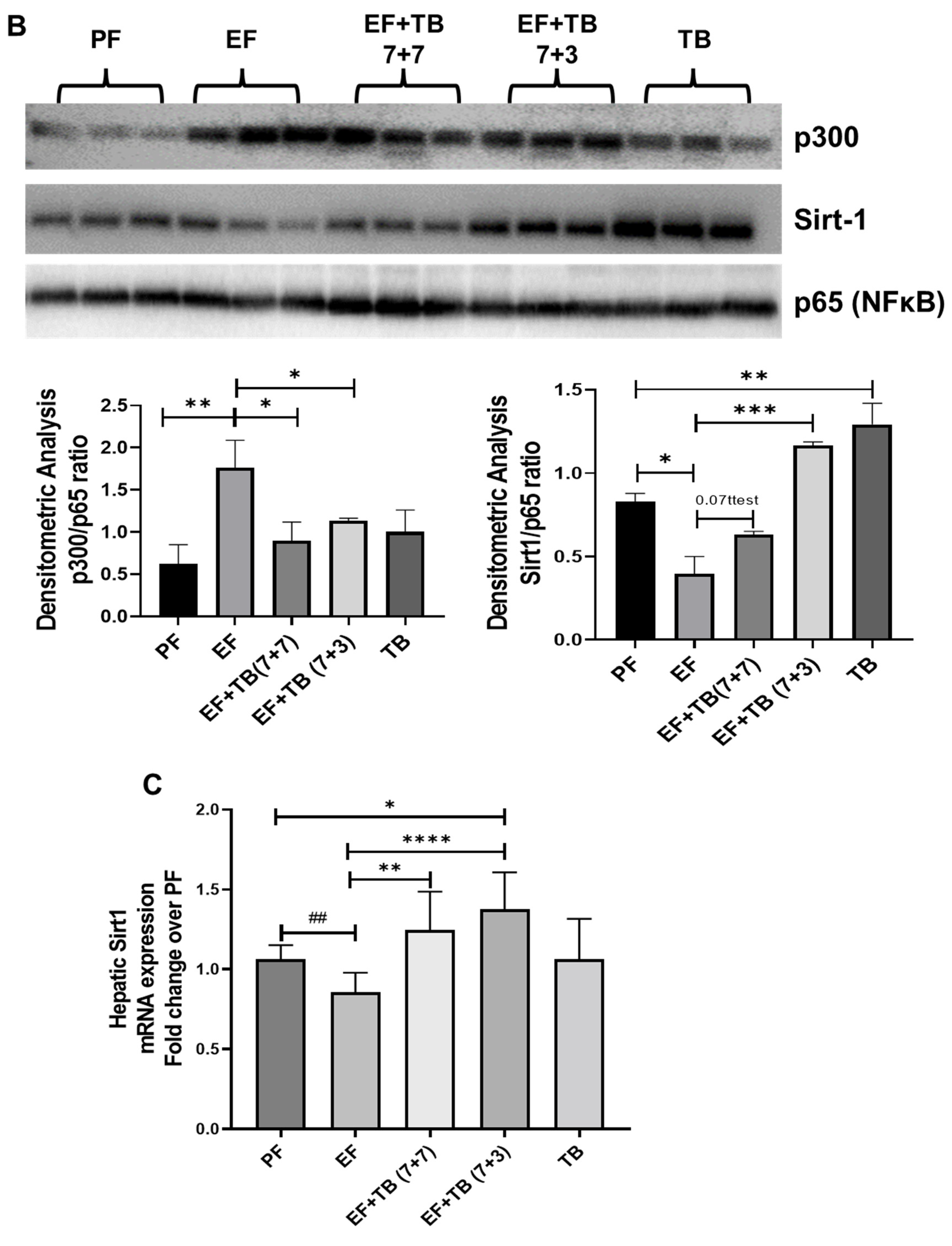
3.6. Tributyrin Modulates the SIRT1-NFκB-p300 Interaction and Transcriptional Activation of NFκB via Decreasing K310-Acetylation of the Nuclear RelA/p65 Subunit of NFκB in Ethanol-Fed Livers
3.7. TB Decreases the Binding of RNA Pol II in Ethanol-Fed Livers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bruha, R.; Dvorak, K.; Petrtyl, J. Alcoholic liver disease. World J. Hepatol. 2012, 4, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Bautista, A.P. Neutrophilic infiltration in alcoholic hepatitis. Alcohol 2002, 27, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Xu, M. Chemokines and alcoholic hepatitis: Are chemokines good therapeutic targets? Gut 2014, 63, 1683–1684. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Gao, B.; Zakhari, S.; Nagy, L.E. Inflammation in alcoholic liver disease. Annu. Rev. Nutr. 2012, 32, 343–368. [Google Scholar] [CrossRef] [PubMed]
- Degre, D.; Lemmers, A.; Gustot, T.; Ouziel, R.; Trepo, E.; Demetter, P.; Verset, L.; Quertinmont, E.; Vercruysse, V.; Le Moine, O.; et al. Hepatic expression of CCL2 in alcoholic liver disease is associated with disease severity and neutrophil infiltrates. Clin. Exp. Immunol. 2012, 169, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.B.; Marsano, L.S.; McClain, C.J. Increased plasma interleukin-8 concentrations in alcoholic hepatitis. Hepatology 1993, 18, 576–580. [Google Scholar] [CrossRef]
- Mandrekar, P.; Ambade, A.; Lim, A.; Szabo, G.; Catalano, D. An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: Regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology 2011, 54, 2185–2197. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Zhong, W.; Zheng, X.; Li, Q.; Qiu, Y.; Li, H.; Chen, H.; Zhou, Z.; Jia, W. Chronic ethanol consumption alters mammalian gastrointestinal content metabolites. J. Proteome Res. 2013, 12, 3297–3306. [Google Scholar] [CrossRef]
- Berni Canani, R.; Di Costanzo, M.; Leone, L. The epigenetic effects of butyrate: Potential therapeutic implications for clinical practice. Clin. Epigenet. 2012, 4, 4. [Google Scholar] [CrossRef]
- Mathew, O.P.; Ranganna, K.; Yatsu, F.M. Butyrate, an HDAC inhibitor, stimulates interplay between different posttranslational modifications of histone H3 and differently alters G1-specific cell cycle proteins in vascular smooth muscle cells. Biomed. Pharmacother. 2010, 64, 733–740. [Google Scholar] [CrossRef]
- Mattis, E.R.; Ostapiuk, S.F. The trends in the management reorganization of medical science under market economy conditions. Vestn. Ross. Akad. Meditsinskikh Nauk. 1992, 11–12, 61–63. [Google Scholar]
- Segain, J.P.; Raingeard de la Bletiere, D.; Bourreille, A.; Leray, V.; Gervois, N.; Rosales, C.; Ferrier, L.; Bonnet, C.; Blottiere, H.M.; Galmiche, J.P. Butyrate inhibits inflammatory responses through NFkappaB inhibition: Implications for Crohn’s disease. Gut 2000, 47, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Inan, M.S.; Rasoulpour, R.J.; Yin, L.; Hubbard, A.K.; Rosenberg, D.W.; Giardina, C. The luminal short-chain fatty acid butyrate modulates NF-kappaB activity in a human colonic epithelial cell line. Gastroenterology 2000, 118, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.; Nikolaus, S.; Hampe, J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut 1998, 42, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Cresci, G.A.; Glueck, B.; McMullen, M.R.; Xin, W.; Allende, D.; Nagy, L.E. Prophylactic tributyrin treatment mitigates chronic-binge ethanol-induced intestinal barrier and liver injury. J. Gastroenterol. Hepatol. 2017, 32, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Donde, H.; Ghare, S.; Joshi-Barve, S.; Zhang, J.; Vadhanam, M.V.; Gobejishvili, L.; Lorkiewicz, P.; Srivastava, S.; McClain, C.J.; Barve, S. Tributyrin Inhibits Ethanol-Induced Epigenetic Repression of CPT-1A and Attenuates Hepatic Steatosis and Injury. Cell Mol. Gastroenterol. Hepatol. 2020, 9, 569–585. [Google Scholar] [CrossRef] [PubMed]
- Shvedunova, M.; Akhtar, A. Modulation of cellular processes by histone and non-histone protein acetylation. Nat. Rev. Mol. Cell Biol. 2022, 23, 329–349. [Google Scholar] [CrossRef] [PubMed]
- Moghe, A.; Joshi-Barve, S.; Ghare, S.; Gobejishvili, L.; Kirpich, I.; McClain, C.J.; Barve, S. Histone modifications and alcohol-induced liver disease: Are altered nutrients the missing link? World J. Gastroenterol. 2011, 17, 2465–2472. [Google Scholar] [CrossRef]
- Eberharter, A.; Becker, P.B. Histone acetylation: A switch between repressive and permissive chromatin. Second in review series on chromatin dynamics. EMBO Rep. 2002, 3, 224–229. [Google Scholar] [CrossRef]
- Kirpich, I.; Ghare, S.; Zhang, J.; Gobejishvili, L.; Kharebava, G.; Barve, S.J.; Barker, D.; Moghe, A.; McClain, C.J.; Barve, S. Binge alcohol-induced microvesicular liver steatosis and injury are associated with down-regulation of hepatic Hdac 1, 7, 9, 10, 11 and up-regulation of Hdac 3. Alcohol. Clin. Exp. Res. 2012, 36, 1578–1586. [Google Scholar] [CrossRef]
- Osna, N.A.; Carter, W.G.; Ganesan, M.; Kirpich, I.A.; McClain, C.J.; Petersen, D.R.; Shearn, C.T.; Tomasi, M.L.; Kharbanda, K.K. Aberrant post-translational protein modifications in the pathogenesis of alcohol-induced liver injury. World J. Gastroenterol. 2016, 22, 6192–6200. [Google Scholar] [CrossRef] [PubMed]
- Park, P.H.; Lim, R.W.; Shukla, S.D. Gene-selective histone H3 acetylation in the absence of increase in global histone acetylation in liver of rats chronically fed alcohol. Alcohol Alcohol. 2012, 47, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Shukla, S.D. Acute in vivo effect of ethanol (binge drinking) on histone H3 modifications in rat tissues. Alcohol Alcohol. 2006, 41, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Park, P.H.; Miller, R.; Shukla, S.D. Acetylation of histone H3 at lysine 9 by ethanol in rat hepatocytes. Biochem. Biophys. Res. Commun. 2003, 306, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Ghare, S.S.; Joshi-Barve, S.; Moghe, A.; Patil, M.; Barker, D.F.; Gobejishvili, L.; Brock, G.N.; Cave, M.; McClain, C.J.; Barve, S.S. Coordinated histone H3 methylation and acetylation regulate physiologic and pathologic fas ligand gene expression in human CD4+ T cells. J. Immunol. 2014, 193, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Ghare, S.; Patil, M.; Hote, P.; Suttles, J.; McClain, C.; Barve, S.; Joshi-Barve, S. Ethanol inhibits lipid raft-mediated TCR signaling and IL-2 expression: Potential mechanism of alcohol-induced immune suppression. Alcohol. Clin. Exp. Res. 2011, 35, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Xu, M.; Yuan, C.; Yin, L.; Chen, X.; Zhou, X.; Li, G.; Fu, Y.; Feghali-Bostwick, C.A.; Pang, L. Transcriptional regulation of increased CCL2 expression in pulmonary fibrosis involves nuclear factor-kappaB and activator protein-1. Int. J. Biochem. Cell Biol. 2013, 45, 1366–1376. [Google Scholar] [CrossRef]
- Hildebrand, D.G.; Alexander, E.; Horber, S.; Lehle, S.; Obermayer, K.; Munck, N.A.; Rothfuss, O.; Frick, J.S.; Morimatsu, M.; Schmitz, I.; et al. IkappaBzeta is a transcriptional key regulator of CCL2/MCP-1. J. Immunol. 2013, 190, 4812–4820. [Google Scholar] [CrossRef]
- Ueda, A.; Ishigatsubo, Y.; Okubo, T.; Yoshimura, T. Transcriptional regulation of the human monocyte chemoattractant protein-1 gene. Cooperation of two NF-kappaB sites and NF-kappaB/Rel subunit specificity. J. Biol. Chem. 1997, 272, 31092–31099. [Google Scholar] [CrossRef]
- Gao, J.; Wei, B.; Liu, M.; Hirsova, P.; Sehrawat, T.S.; Cao, S.; Hu, X.; Xue, F.; Yaqoob, U.; Kang, N.; et al. Endothelial p300 Promotes Portal Hypertension and Hepatic Fibrosis Through C-C Motif Chemokine Ligand 2-Mediated Angiocrine Signaling. Hepatology 2021, 73, 2468–2483. [Google Scholar] [CrossRef]
- Deng, X.; Zhou, X.; Deng, Y.; Liu, F.; Feng, X.; Yin, Q.; Gu, Y.; Shi, S.; Xu, M. Thrombin Induces CCL2 Expression in Human Lung Fibroblasts via p300 Mediated Histone Acetylation and NF-KappaB Activation. J. Cell Biochem. 2017, 118, 4012–4019. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.P.; Behar, M.; Birnbaum, H.A.; Hoffmann, A.; Wright, P.E.; Ghosh, G. Analysis of the RelA:CBP/p300 interaction reveals its involvement in NF-kappaB-driven transcription. PLoS Biol. 2013, 11, e1001647. [Google Scholar] [CrossRef] [PubMed]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-kappaB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.F.; Mu, Y.; Greene, W.C. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. EMBO J. 2002, 21, 6539–6548. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, W.; Pan, H.; Feldser, H.G.; Lainez, E.; Miller, C.; Leung, S.; Zhong, Z.; Zhao, H.; Sweitzer, S.; et al. SIRT1 activators suppress inflammatory responses through promotion of p65 deacetylation and inhibition of NF-kappaB activity. PLoS ONE 2012, 7, e46364. [Google Scholar] [CrossRef]
- Huang, B.; Yang, X.D.; Lamb, A.; Chen, L.F. Posttranslational modifications of NF-kappaB: Another layer of regulation for NF-kappaB signaling pathway. Cell Signal 2010, 22, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 2004, 23, 2369–2380. [Google Scholar] [CrossRef]
- Chacko, K.R.; Reinus, J. Spectrum of Alcoholic Liver Disease. Clin. Liver. Dis. 2016, 20, 419–427. [Google Scholar] [CrossRef]
- Farooq, M.O.; Bataller, R. Pathogenesis and Management of Alcoholic Liver Disease. Dig. Dis. 2016, 34, 347–355. [Google Scholar] [CrossRef]
- Siklos, M.; Kubicek, S. Therapeutic targeting of chromatin: Status and opportunities. FEBS J. 2022, 289, 1276–1301. [Google Scholar] [CrossRef]
- Swygert, S.G.; Peterson, C.L. Chromatin dynamics: Interplay between remodeling enzymes and histone modifications. Biochim. Biophys. Acta 2014, 1839, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Mikula, M.; Majewska, A.; Ledwon, J.K.; Dzwonek, A.; Ostrowski, J. Obesity increases histone H3 lysine 9 and 18 acetylation at Tnfa and Ccl2 genes in mouse liver. Int. J. Mol. Med. 2014, 34, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Park, P.H.; Lim, R.W.; Shukla, S.D. Involvement of histone acetyltransferase (HAT) in ethanol-induced acetylation of histone H3 in hepatocytes: Potential mechanism for gene expression. Am. J. Physiol. Gastrointest. Liver. Physiol. 2005, 289, G1124–G1136. [Google Scholar] [CrossRef] [PubMed]
- Keppler, B.R.; Archer, T.K. Chromatin-modifying enzymes as therapeutic targets—Part 2. Expert Opin. Ther. Targets 2008, 12, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Goto, H.; Inagaki, M.; Dong, Z. Phosphorylation at serine 28 and acetylation at lysine 9 of histone H3 induced by trichostatin A. Oncogene 2003, 22, 5291–5297. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Tao, Y.; Li, M.; Che, T.; Qu, J. Protein acetylation and deacetylation: An important regulatory modification in gene transcription (Review). Exp. Ther. Med. 2020, 20, 2923–2940. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Jo, S.H.; Kim, M.Y.; Kim, T.H.; Ahn, Y.H. Role of transcription factor acetylation in the regulation of metabolic homeostasis. Protein Cell 2015, 6, 804–813. [Google Scholar] [CrossRef]
- Ghizzoni, M.; Haisma, H.J.; Maarsingh, H.; Dekker, F.J. Histone acetyltransferases are crucial regulators in NF-kappaB mediated inflammation. Drug Discov. Today 2011, 16, 504–511. [Google Scholar] [CrossRef]
- Wang, Y.; Rangan, G.K.; Goodwin, B.; Tay, Y.C.; Harris, D.C. Lipopolysaccharide-induced MCP-1 gene expression in rat tubular epithelial cells is nuclear factor-kappaB dependent. Kidney Int. 2000, 57, 2011–2022. [Google Scholar] [CrossRef]
- Chan, H.M.; La Thangue, N.B. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J. Cell Sci. 2001, 114, 2363–2373. [Google Scholar] [CrossRef]
- Ogryzko, V.V.; Schiltz, R.L.; Russanova, V.; Howard, B.H.; Nakatani, Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell 1996, 87, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, M.; Sakaki, H.; Usami, M.; Iizuka, N.; Shuno, K.; Aoyama, M.; Usami, Y. Oral administration of tributyrin increases concentration of butyrate in the portal vein and prevents lipopolysaccharide-induced liver injury in rats. Clin. Nutr. 2011, 30, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.A.; Rodrigues, H.G.; Festuccia, W.T.; Crisma, A.R.; Alves, V.S.; Martins, A.R.; Amaral, C.L.; Fiamoncini, J.; Hirabara, S.M.; Sato, F.T.; et al. Tributyrin attenuates obesity-associated inflammation and insulin resistance in high-fat-fed mice. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E272–E282. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Wang, Z.; Wu, M.; Wang, H. Emerging Roles of SIRT1 in Alcoholic Liver Disease. Int. J. Biol. Sci. 2020, 16, 3174–3183. [Google Scholar] [CrossRef]
- You, M.; Jogasuria, A.; Taylor, C.; Wu, J. Sirtuin 1 signaling and alcoholic fatty liver disease. Hepatobiliary Surg. Nutr. 2015, 4, 88–100. [Google Scholar] [CrossRef]
- Wang, S.; Deng, W.; Li, F.; Xiang, L.; Lv, P.; Chen, Y. Treatment with butyrate alleviates dextran sulfate sodium and Clostridium difficile-induced colitis by preventing activity of Th17 cells via regulation of SIRT1/mTOR in mice. J. Nutr. Biochem. 2023, 111, 109155. [Google Scholar] [CrossRef]
- Schug, T.T.; Xu, Q.; Gao, H.; Peres-da-Silva, A.; Draper, D.W.; Fessler, M.B.; Purushotham, A.; Li, X. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol. Cell Biol. 2010, 30, 4712–4721. [Google Scholar] [CrossRef]
- Shakibaei, M.; Buhrmann, C.; Mobasheri, A. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-kappaB ligand (RANKL) activation of NF-kappaB signaling and inhibit osteoclastogenesis in bone-derived cells. J. Biol. Chem. 2011, 286, 11492–11505. [Google Scholar] [CrossRef]
- Bouras, T.; Fu, M.; Sauve, A.A.; Wang, F.; Quong, A.A.; Perkins, N.D.; Hay, R.T.; Gu, W.; Pestell, R.G. SIRT1 deacetylation and repression of p300 involves lysine residues 1020/1024 within the cell cycle regulatory domain 1. J. Biol. Chem. 2005, 280, 10264–10276. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghare, S.S.; Charpentier, B.T.; Ghooray, D.T.; Zhang, J.; Vadhanam, M.V.; Reddy, S.; Joshi-Barve, S.; McClain, C.J.; Barve, S.S. Tributyrin Mitigates Ethanol-Induced Lysine Acetylation of Histone-H3 and p65-NFκB Downregulating CCL2 Expression and Consequent Liver Inflammation and Injury. Nutrients 2023, 15, 4397. https://doi.org/10.3390/nu15204397
Ghare SS, Charpentier BT, Ghooray DT, Zhang J, Vadhanam MV, Reddy S, Joshi-Barve S, McClain CJ, Barve SS. Tributyrin Mitigates Ethanol-Induced Lysine Acetylation of Histone-H3 and p65-NFκB Downregulating CCL2 Expression and Consequent Liver Inflammation and Injury. Nutrients. 2023; 15(20):4397. https://doi.org/10.3390/nu15204397
Chicago/Turabian StyleGhare, Smita S., Benjamin T. Charpentier, Dushan T. Ghooray, Jingwen Zhang, Manicka V. Vadhanam, Sreelatha Reddy, Swati Joshi-Barve, Craig J. McClain, and Shirish S. Barve. 2023. "Tributyrin Mitigates Ethanol-Induced Lysine Acetylation of Histone-H3 and p65-NFκB Downregulating CCL2 Expression and Consequent Liver Inflammation and Injury" Nutrients 15, no. 20: 4397. https://doi.org/10.3390/nu15204397
APA StyleGhare, S. S., Charpentier, B. T., Ghooray, D. T., Zhang, J., Vadhanam, M. V., Reddy, S., Joshi-Barve, S., McClain, C. J., & Barve, S. S. (2023). Tributyrin Mitigates Ethanol-Induced Lysine Acetylation of Histone-H3 and p65-NFκB Downregulating CCL2 Expression and Consequent Liver Inflammation and Injury. Nutrients, 15(20), 4397. https://doi.org/10.3390/nu15204397