Abstract
We investigated the postpartum mental health of women who had consumed perilla oil or fish oil containing various omega-3 fatty acids for 12 weeks starting in mid-pregnancy. The association between fatty acids in maternal erythrocytes and mental health risk factors was also examined. Healthy Japanese primiparas in mid-pregnancy (gestational weeks 18–25) were randomly divided into two groups and consumed approximately 2.0 g/day of omega-3 fatty acids in either perilla oil (the ALA dose was 2.4 g/day) or fish oil (the EPA + DHA dose was 1.7 g/day) for 12 weeks. Maternal mental health was assessed using the Edinburgh Postnatal Depression Scale (EPDS) as the primary measure and the Mother-to-Infant Bonding Scale (MIBS) as the secondary measure. Data from an observational study were used as a historical control. Maternal blood, cord blood, and colostrum samples were collected for fatty acid composition analysis. In addition, completers of the observational studies were enrolled in a case–control study, wherein logistic regression analysis was performed to examine the association between maternal fatty acids and EPDS score. The proportion of participants with a high EPDS score (≥9) was significantly lower in the perilla oil group (12.0%, p = 0.044) but not in the fish oil group (22.3%, p = 0.882) compared with the historical control (21.6%), while the proportions between the former groups also tended to be lower (p = 0.059). No marked effect of omega-3 fatty acid intake was observed from the MIBS results. In the case–control study of the historical control, high levels of α-linolenic acid in maternal erythrocytes were associated with an EPDS score of <9 (odds ratio of 0.23, 95% confidence interval: 0.06, 0.84, p = 0.018 for trend). The results of this study suggest that consumption of α-linolenic acid during pregnancy may stabilize postpartum mental health.
1. Introduction
For women, pregnancy and childbirth are significant life events accompanied by changes in hormone activity, anxiety about childcare, and changes in life routines and health behaviors. During these events, perinatal mood instability is common, and this can lead to baby blues and postnatal depression. In particular, anxiety factors are increased when mothers are primiparous and/or older [1]. The mental health of pregnant women has a great impact on the quality of childrearing and the establishment of mother–child bonding [2]; in serious cases, parenting may be inadequate, which may negatively influence the child’s development [3]. Moreover, during the perinatal period, mothers tend to avoid taking medication as much as possible in order to avoid adverse effects on their babies. Thus, it would be desirable to stabilize the mental health of expectant mothers through their daily diet and living environment.
Omega-3 fatty acids are important for health; however, they are not synthesized in the body and must be consumed directly from the diet. Major omega-3 fatty acids include α-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). ALA, which is contained in perilla oil and linseed oil, is metabolized in vivo and partially converted to EPA and DHA. In addition, EPA and DHA can be obtained by consuming fish oil [4]. It has been reported that intake of EPA reduces the risk of cardiovascular disease and suppresses inflammation [5,6], while intake of DHA contributes to brain function as well as the development and function of the eyes [7,8]. For these reasons, many studies have focused on seafood that is rich in EPA and DHA. For example, one study reported an inverse relationship between seafood consumption and the risk of postpartum depression [9]. In pregnant women, DHA is supplied from the mother to the fetus for growth and development, so it is thought that expectant mothers are at increased risk of depression due to DHA deficiency [10]. Although many studies on omega-3 fatty acids have assumed that seafood consumption is higher in Japan than in other countries, seafood consumption peaked in Japan in 2001 and has been decreasing every year since, making EPA and DHA deficiency in individuals of reproductive age a cause for concern [11]. Indeed, the beneficial effects of seafood and omega-3 fatty acid intake on perinatal depression was reported in Japan [12,13,14]. In an animal study, female mice raised on a diet deficient in omega-3 fatty acids showed delayed development of maternal behavior, and after giving birth, they tended to neglect the newborn pups. [15]. In another study, dams raised on a diet deficient in omega-3 fatty acids had low brain DHA and oxytocin levels and had difficulties continuing maternal behavior after giving birth [16]. These findings suggest that stabilizing maternal mental health during the perinatal period through nutritional support might help to improve the mental health of pregnant and parturient women and increase the birth rate.
The present study examined the effects of omega-3 fatty acids on perinatal mental health by examining the degree of psychological distress 1 month after giving birth using the Edinburgh Postnatal Depression Scale (EPDS) as well as the degree of attachment with their children using the Mother-to-Infant Bonding Scale (MIBS) in mothers who consumed omega-3 fatty acids in either perilla oil or fish oil. In addition, a case–control study was conducted to evaluate the association between prenatal erythrocyte fatty acid levels and postpartum mental health.
2. Materials and Methods
2.1. Participants
The Perinatal Medical Center, Sagamino Hospital, Japan Community Health Care Organization, recruited the participants for this study, and Azabu University performed tests of the samples and analyzed the data. A total of 321 participants were recruited for the observational study between November 2015 and June 2017 and 250 for the interventional study between July 2017 and April 2019. These were 2 different sets of participants, and none of those in the RCT were included in the observational study (Figure 1).
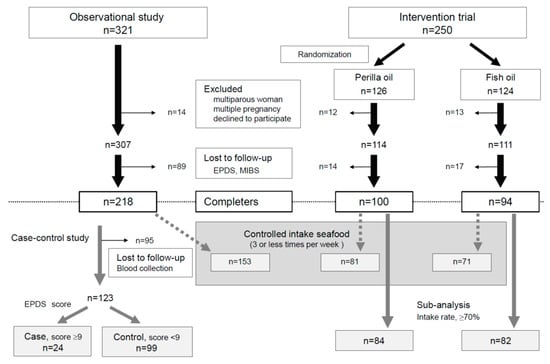
Figure 1.
Flowchart of participant screening and enrollment.
This was a double-blind, parallel-group comparison study wherein participants were randomly assigned to two groups receiving omega-3 fatty acids in either perilla oil or fish oil (the recruited period was 21 months for July 2017 to April 2019), and the two abovementioned groups plus a group of participants in the observational study (historical control; the recruited period was 19 months for November 2015 and June 2017) were compared (Figure 1).
All subjects gave their informed consent for inclusion before they participated in the study. This study was approved by the Human Research Ethics Committee of Azabu University (No. 083, 102) and was registered in the University Hospital Medical Information Network (UMIN) Center Clinical Trial Registry (UMIN000027170).
2.2. Study Design
Participants were healthy primiparous women aged 19–45 years; multiparous women, those with multiple pregnancies, those who declined to participate in the study, and those who did not submit the 1-month postpartum questionnaire were excluded.
Intervention groups received omega-3 fatty acids starting in mid-pregnancy (gestational weeks 18–25) for 12 weeks. The perilla oil group received 4.0 g/day perilla oil containing 2.4 g/day ALA, while the fish oil group received 5.2 g/day sardine oil containing 1.3 g/day EPA and 0.4 g/day DHA. Perilla oil was provided by Ota Oil Co., Ltd. (Okazaki, Japan), and fish oil was provided by Nissui Corporation (Tokyo, Japan); both products were individually packaged without any labels.
The mental health of participants was assessed using the Kessler Psychological Distress Scale (K6) [17] at mid-pregnancy (start of the study) and immediately after giving birth (i.e., within 5 days after giving birth), as well as at 1 month after giving birth, using the EPDS and the MIBS [18,19]. In addition, maternal blood was collected in early pregnancy (around gestational week 12), mid-pregnancy (around gestational week 28), late pregnancy (around gestational week 36), and the day after giving birth, while cord blood and colostrum (milk produced within 5 days after giving birth) were collected for fatty acid composition analysis.
In the case study, data from the observational study were divided into two groups (cases with an EPDS score ≥ 9 and controls with an EPDS score < 9). Controls were divided into tertile groups according to fatty acid level, and logistic regression analysis was performed to obtain odds ratios and 95% confidence intervals.
2.3. Statistical Analysis
Participant characteristics were compared by t-test or Fisher’s Exact test. Comparisons of the primary outcome (EPDS) and a secondary outcome (MIBS) were performed by Fisher’s Exact test. Changes in fatty acids in biological samples collected during the study period were examined by two-way analysis of variance with the Tukey multiple comparison test.
Differences in levels of various types of fatty acids in maternal erythrocytes a day after giving birth in the case–control study were compared by the Mann–Whitney U test. Logistic regression analysis was performed to obtain crude odds ratios and 95% confidence intervals, and then multivariable logistic regression was performed with adjustment for the following five confounding factors: age, pre-pregnancy body mass index, smoking status (never smoker, stopped before pregnancy, stopped upon pregnancy, current smoker), alcohol intake (never drinker, stopped before pregnancy, stopped upon pregnancy, current drinker), and taking supplements (yes/no). Category numbers were assigned to tertiles according to fatty acid level and examined as continuous variables to test for trends.
Data were analyzed using SPSS Statistics 25 (IBM Corp., Armonk, NY, USA). A p-value of 0.05 or lower was considered statistically significant.
2.4. Fatty Acid Analysis
Erythrocytes (obtained by centrifugation of maternal blood and cord blood) and colostrum were stored at −80 °C until analysis. The sample (100 µL) was added to 2 mL methanol:hexane (4:1) solvent containing 50 µg/mL butylhydroxytoluene. The transmethylation method developed by Lepage and Roy was used [20]. Fatty acid methyl esters were analyzed using the methods of Masood et al. [21]. A standard fatty acid mixture (NuChek Prep 462; Elysian, MN, USA) was used to verify the identification and assignment of retention times. Each fatty acid was expressed as % of total fatty acids.
3. Results
3.1. Disposition and Demographics
The recruitment period of the observation study was from November 2015 to June 2017. Among the 321 participants initially enrolled, 14 were excluded because they were multiparous or with multiple pregnancies or declined to participate in the study. Another 89 were excluded because they did not participate in the mental health assessment. The data from the remaining 218 participants were analyzed. The recruitment period of the interventional study was from July 2017 to April 2019. A total of 250 participants were enrolled and then randomly assigned to the perilla oil group (n = 126) or the fish oil group (n = 124); 12 were eliminated from the perilla oil group and 13 from the fish oil group because they were multiparous or with multiple pregnancies or declined to participate in the study. Another 14 and 17 were eliminated from the perilla oil group and the fish oil group, respectively, because they did not participate in the mental health assessment. The data from the remaining 100 participants in the perilla oil group and the remaining 94 in the fish oil group were analyzed (Figure 1). The detailed characteristics of participants in the interventional study are shown in Table 1. There were no differences in characteristics between the excluded participants (historical control; n = 89, perilla oil; n = 14, fish oil; n = 17) and the participants used in the experiment.

Table 1.
Participant characteristics.
In the case–control study, 95 participants without blood samples were eliminated, and the remaining 123 participants were divided into cases (n = 24, EPDS score ≥ 9) and controls (n = 99, EPDS score < 9) (Figure 1). The characteristics of cases and controls were compared (Table 2). There were no differences in characteristics between the excluded participants (n = 95) and the participants used in the experiment.

Table 2.
Case–control participant characteristics.
Because the participants for both the interventional and case–control studies were recruited by the same center, and the recruitments for those studies were carried out in sequence, the basic characteristics of participants in both studies were largely the same.
3.2. Outcomes
Assessment of the degree of psychological distress at the time of recruitment (mid-pregnancy) and immediately after giving birth showed that the proportions of participants with a high K6 score (≥13) were very low (<3%) (Table 3). The proportions of participants with a high EPDS score (≥9) 1 month after giving birth were 21.6% in the historical control group, 12.0% in the perilla oil group, and 22.3% in the fish oil group; the proportion was significantly lower in the perilla oil group compared with the historical control group (p = 0.044), and the proportion with a high EPDS score was nearly significantly lower in the perilla vs. the fish oil group (p = 0.059; Table 4). The proportions of participants with an MIBS score ≥ 3 or ≥5 were 24–30% and 10–14%, respectively and did not differ markedly among the three groups (Table 4). This effect did not change, even after controlling for participants with a high seafood intake (four or more times per week). A high EPDS score in the historical control was 22.2% (n = 153), that in the perilla oil group was 11.1% (n = 81, vs. historical control: p = 0.050), and that in the fish oil group was 22.5% (n = 71, vs. perilla: p = 0.079) (Table A1). A subanalysis was conducted to confirm the influence of each participant’s omega-3 oil intake status on mental health scores. Subanalysis of participants whose self-assessed consumption rate of omega-3 fatty acids over 12 weeks was ≥70% (assessment options: Perfect, ≥90%, ≥70%, <60%, almost none) showed an even lower proportion of those with a high EPDS score (9.5%) in the perilla oil group (n = 84, vs. historical control: p = 0.019), while that in the fish oil group (23.2%, n = 82) was similar (vs. perilla: p = 0.021; Table A2).

Table 3.
Mean (SD) Kessler Psychological Distress Scale score.

Table 4.
Mean Edinburgh Postnatal Depression Scale and Mother-to-Infant Bonding Scale scores.
Analysis of changes in fatty acids in maternal erythrocytes showed increases in ALA levels in the perilla oil group as well as increases in EPA and DHA levels in the fish oil group, confirming that those groups consumed oils containing the corresponding omega-3 fatty acids (Figure 2A–C). The omega-3 index was markedly increased only in the fish oil group (Figure 2D). Table 5 shows the fatty acid compositions in detail. Even though the intervention (i.e., consumption of oils containing omega-3 fatty acids) was completed a month before giving birth, the levels of DHA remained high in the erythrocytes of cord blood samples in the fish oil group (Table A3 and Table A4), suggesting that a preferential supply of omega-3 fatty acids to newborn babies was attributable to the consumption of fish oil during mid-pregnancy.
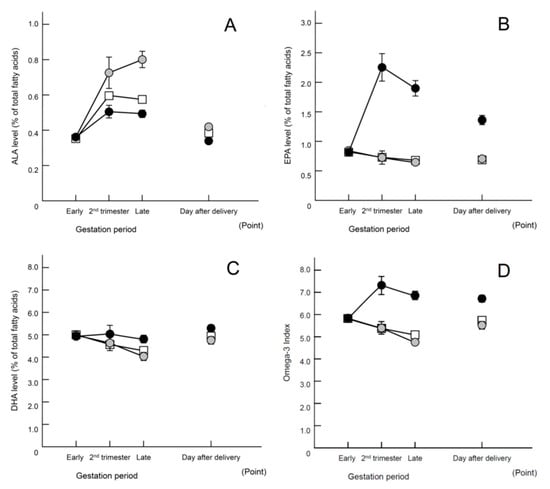
Figure 2.
Changes in fatty acids in maternal erythrocytes (% of total fatty acids). Data are presented as means ± SEM. Open square, historical control; gray circle, perilla oil; closed circle, fish oil. (A) α-Linolenic acid (ALA); (B) eicosapentaenoic acid (EPA); (C) docosahexaenoic acid (DHA); (D) omega-6/omega-3 ratio. Statistical analysis using two-way analysis of variance yielded the following results: (A) Group effects: F (2, 1166) = 34.935, p < 0.01. Tukey’s test indicated the following significant differences between all groups: p < 0.01. Interaction effect: F (6, 1166) = 12.649, p < 0.001. (B) Group effects: F (2, 1166) = 244.447, p < 0.01. Tukey’s test indicated the following significant differences: historical control, p < 0.01; perilla, p < 0.01 compared with fish oil group. Interaction effect: F (6, 1166) = 47.553, p < 0.01. (C) Group effects: F (2, 1166) = 13.016, p < 0.01. Tukey’s test indicated the following significant differences: historical control, p < 0.01; perilla oil, p < 0.01 compared with the fish oil group. Interaction effect: F (6, 1166) = 4.486, p < 0.01. (D) Group effects: F (2, 1166) = 81.651, p < 0.01. Tukey’s test indicated the following significant differences: historical control, p < 0.01; perilla oil, p < 0.01 compared with fish oil group. Interaction effect: F (6, 1166) = 18.405, p < 0.01. Regarding the omega-3 oil intake status at blood collection in the Late, 32% of the participants in perilla oil group and 46% of the participants in fish oil group had completed supplementation.

Table 5.
Fatty acid composition of maternal erythrocytes.
3.3. Case–Control Study (Observational Study)
Fatty acid compositions in maternal erythrocytes the day after giving birth showed significantly lower ALA levels in cases compared with controls (p = 0.039, Table 6). Logistic regression analysis of tertile groups according to fatty acid level was performed to examine the degrees of psychological distress. A significant crude odds ratio was shown only in the highest tertile according to ALA level compared with the lowest tertile (crude odds ratio of 0.29, 95% confidence interval: 0.09, 0.96, p = 0.030 for trend). Similarly, the multivariable model adjusted for five confounding factors (age, pre-pregnancy body mass index, smoking status, alcohol intake, and taking supplements) showed a significant odds ratio in the highest tertile according to ALA level compared with the lowest tertile (a multivariable odds ratio of 0.23, 95% confidence interval: 0.06, 0.84, p = 0.018 for trend, Table 7). Taken together, the results suggest that high maternal erythrocyte ALA levels may help to mitigate psychological distress.

Table 6.
Maternal erythrocyte membrane fatty acid composition of cases and controls the day after giving birth.

Table 7.
Odds ratios (95% confidence intervals) for psychological distress (EPDS score ≥ 9) according to tertile of omega-3 fatty acids of erythrocytes the day after giving birth.
4. Discussion
In a global survey, it was reported that 10–20% of pregnant women experience postpartum depression [22]. This percentage increases in primiparous women because they have many anxiety factors associated with childbirth, childrearing, and other events, all of which are new to them [1]. The present study examined the effects of omega-3 fatty acids on perinatal mental health in primiparous women who are prone to mental instability. There are several reports of trials involving EPA and DHA, but few involving ALA [23]. Although the contribution of ALA is generally thought to be low, we considered it important to investigate whether this is indeed the case.
Of the primiparous women recruited into this study over 3.5 years (between November 2015 and April 2019), approximately 3% had a high K6 score (≥13), indicating that this population contained very few individuals with mental instability during pregnancy. The omega-3 index of maternal erythrocytes around gestational week 12 in this study was as low as in European and North American populations (>4–6%) (Table 5). Schuchardt et al. reported the following omega-3 index values of erythrocytes as long-term biomarkers: desirable, >8%; moderate, >6–8%; low, >4–6%; very low, ≤4% [24]. The proportion of those with a high EPDS score (≥9) in the postpartum period was 21.6% in the observational study (subjects in the first half of recruitment), indicating that this population was a typical primiparous population in Japan. However, analysis of the interventional study (subjects in the second half of recruitment) showed that the proportion of those with a high EPDS score decreased to 12.0% in the perilla oil group (p = 0.044, Table 4). This effect did not change even after controlling for participants with a high seafood intake (four or more times per week) (Table A1). Also, this effect was more prominent in the group having a higher consumption rate (Table A2). It would be a finding of great importance if consumption of ALA during pregnancy stabilizes postpartum mental health.
The possibility that EPA and DHA prevent or mitigate postpartum depression has been demonstrated previously [23]. Such benefits were more likely to be observed in people with severely depressed status (EPDS score ≥ 12 or Hamilton Rating Scale for Depression ≥ 20) [25]. Participants in the present study were healthy pregnant women, judged by K6 score, and this may explain why the effect of fish oil was not observed in this study. This raises the question of why the proportion of those with a high EPDS score decreased only in the perilla oil group. We do not think that this occurred by chance because the case–control study of participants in the observational study showed that maternal erythrocyte ALA level was associated with a high EPDS score. A 10-year follow-up study of middle-aged and older women demonstrated that the risk of depression was not associated with intake of EPA plus DHA but was inversely associated with ALA intake; this association was stronger in women with low linoleic acid intake [26]. An inverse correlation between fear of breast cancer recurrence and blood ALA level has also been reported [27]. It is likely that the results of the present study show the mitigation of a vague anxiety about childbirth and childrearing but not a pathologic mental disorder. Thus, a more detailed assessment of the degree of anxiety using the State–Trait Anxiety Inventory and the Manifest Anxiety Scale is necessary.
The Omega-6/Omega-3 ratio is treated as one of the important items for detecting the effects of PUFA [28,29]. In the present study, fish oil intervention clearly decreased the Omega-6/Omega-3 ratio, whereas perilla oil intervention did not show any change. However, only the perilla oil intervention improved mental health scores, suggesting that changes in the Omega-6/Omega-3 ratio do not affect pregnant women’s emotions. Furthermore, it was suggested that metabolites such as oxylipin, which are unique to perilla oil, may be contributing to the improvement in mental health scores due to perilla oil intervention.
Differences in ALA metabolism [30] are likely to contribute to the effect seen in the perilla oil group in this study. During pregnancy, maternal DHA is preferentially distributed via transport proteins to the fetus for its development [31]. Indeed, levels of EPA, DHA, and docosapentaenoic acid n-3 in erythrocytes of cord blood were high in the fish oil group, suggesting they were supplied to the fetus [32]. However, DHA levels in erythrocytes of cord blood were significantly lower in the perilla oil group than in the other two groups. DHA synthesized from ALA appears in blood at a different time compared with DHA that has been ingested [33]. This difference may have been responsible for the accumulated effects on the mothers themselves rather than the supply of DHA to the fetus. The omega-3 index of erythrocytes indicated that the participants were DHA-deficient. It is considered that DHA metabolized from ALA was preferentially accumulated in the maternal brain instead of the fetus. Therefore, in the future, it will be necessary to measure DHA levels in the brain.
Reduced intake of omega-3 fatty acids is a global phenomenon, and Japan is no exception. In fact, there has been a marked decline in seafood consumption in Japan [11]. Considering that it takes a long time to restore DHA in the body [34], we believe that it is necessary to sound the alarm, even in areas where seafood consumption was previously commonplace. No effect on maternal mental health was found in the fish oil group. However, given the changes observed in fatty acid compositions in maternal blood and cord blood, fish oil is expected to have beneficial effects on newborn babies [35]. Adequate supplementation of EPA and DHA in early pregnancy reduces the risk of preterm birth, and the resulting continuation of pregnancy leads to the prevention of premature births [36]. In the future, we would like to analyze the relationship of maternal intake of omega-3 fatty acids with the growth and development of newborn babies.
Limitations
A placebo control was not included in the interventional study because of humanitarian considerations. The State–Trait Anxiety Inventory and the Manifest Anxiety Scale were not used in addition to EPDS. Socio-economic status was not assessed. The omega-3 fatty acid contents in the consumed oils were not precisely adjusted. Analysis of blood fatty acids alone did not depict a complete picture of the changes in the brain. The results obtained here may be limited to pregnant women in such regions since Japan is a region where the intake of omega-3 fatty acids is generally higher than in other countries.
5. Conclusions
ALA intake during pregnancy may stabilize maternal mental health at 1 month after giving birth. In addition, high levels of maternal blood ALA were suggested to be associated with mitigation of psychological distress.
Author Contributions
Conceptualization, T.M.; Methodology, A.H. and T.M.; Formal Analysis, A.H.; Investigation, A.H., Y.H. and H.Y.; Resources, H.Y.; Writing—Original Draft Preparation, A.H.; Writing—Review and Editing, K.H.; Project Administration, A.H. and T.M.; Funding acquisition, T.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Human Research Ethics Committee of Azabu University (No. 083, 102) and was registered in the University Hospital Medical Information Network (UMIN) Center Clinical Trial Registry (UMIN000027170). This study was conducted in accordance with the principles of the Declaration of Helsinki and its later amendments.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Yoko Arai and Daisuke Nishi for their support with the mental health assessments. The authors would like to thank the participants who volunteered to take part in this study. The authors would also like to thank Ota Oil Co., Ltd. and Nissui Corporation for providing oil materials.
Conflicts of Interest
A.H. and T.M. belong to an endowment course of Ota Oil Co., Ltd. and Nissui Corporation. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Appendix A

Table A1.
Mean Edinburgh Postnatal Depression Scale score of participants who consumed seafood ≤3 times per week (sub-analysis).
Table A1.
Mean Edinburgh Postnatal Depression Scale score of participants who consumed seafood ≤3 times per week (sub-analysis).
| Observational Study | Intervention Trial | a p | b p | c p | ||||
|---|---|---|---|---|---|---|---|---|
| Historical Control | Perilla Oil Group | Fish Oil Group | ||||||
| One month after childbirth | n | 153 | 81 | 71 | ||||
| EPDS | Mean ± SD | 5.7 ± 4.2 | 5.4 ± 3.3 | 5.5 ± 3.7 | ||||
| score ≥ 9 | % (n) | 22.2 (34) | 11.1 (9) | 22.5 (16) | 0.050 | 1.000 | 0.079 | |
p-value: Fisher’s Exact test for categorical variables; a p: Historical control vs. perilla oil; b p: Historical control vs. fish oil; c p: Perilla oil vs. fish oil. EPDS, Edinburgh Postnatal Depression Scale; SD, standard deviation.

Table A2.
Mean Edinburgh Postnatal Depression Scale score of participants who consumed 70% or more of omega-3 fatty-acid oil (sub-analysis).
Table A2.
Mean Edinburgh Postnatal Depression Scale score of participants who consumed 70% or more of omega-3 fatty-acid oil (sub-analysis).
| Observational Study | Intervention Trial | a p | b p | c p | ||||
|---|---|---|---|---|---|---|---|---|
| Historical Control | Perilla Oil Group | Fish Oil Group | ||||||
| One month after childbirth | n | 218 | 84 | 82 | ||||
| EPDS | Mean ± SD | 5.6 ± 4.0 | 5.1 ± 3.4 | 5.3 ± 3.9 | ||||
| score ≥ 9 | % (n) | 21.6 (47) | 9.5 (8) | 23.2 (19) | 0.019 | 0.757 | 0.021 | |
p-value: Fisher’s Exact test for categorical variables; a p: Historical control vs. perilla oil; b p: Historical control vs. fish oil; c p: Perilla oil vs. fish oil. EPDS, Edinburgh Postnatal Depression Scale; MIBS, Mother-to-Infant Bonding Scale; SD, standard deviation.

Table A3.
Fatty acid compositions of erythrocytes of cord blood.
Table A3.
Fatty acid compositions of erythrocytes of cord blood.
| Erythrocytes of Cord Blood | Observational Study | Intervention Trial | ||||
|---|---|---|---|---|---|---|
| Historical Control | Perilla Oil Group | Fish Oil Group | ||||
| Fatty Acids | n = 193 | n = 91 | n = 85 | |||
| Total saturated fatty acids | 49.24 ± 0.06 | 49.84 ± 0.07 | ** | 49.99 ± 0.07 | ** | |
| Total mono-unsaturated fatty acids | 16.3 ± 0.07 | 15.32 ± 0.08 | ** | 15.31 ± 0.08 | ** | |
| ω-6 polyunsaturated fatty acids | ||||||
| Linoleic acid (LA) | 18:2n-6 | 3.94 ± 0.04 | 4.07 ± 0.05 | 4.12 ± 0.05 | * | |
| Arachidonic acid (ARA) | 20:4n-6 | 14.38 ± 0.06 | 14.5 ± 0.08 | 13.64 ± 0.10 | **,## | |
| Docosapentaenoic acid n-6 (DPAn-6) | 22:5n-6 | 1.00 ± 0.01 | 1.03 ± 0.02 | 0.79 ± 0.02 | **,## | |
| Total ω-6 polyunsaturated fatty acids | 24.85 ± 0.08 | 25.27 ± 0.10 | ** | 23.71 ± 0.13 | **,## | |
| ω-3 polyunsaturated fatty acids | ||||||
| α-Linolenic acid (ALA) | 18:3n-3 | ND | ND | ND | ||
| Eicosapentaenoic acid (EPA) | 20:5n-3 | 0.33 ± 0.01 | 0.32 ± 0.01 | 0.63 ± 0.03 | **,## | |
| Docosapentaenoic acid n-3 (DPAn-3) | 22:5n-3 | 0.78 ± 0.01 | 0.85 ± 0.02 | 1.36 ± 0.04 | **,## | |
| Docosahexaenoic acid (DHA) | 22:6n-3 | 6.52 ± 0.06 | 6.26 ± 0.08 | * | 6.91 ± 0.07 | **,## |
| Total ω-3 polyunsaturated fatty acids | 7.64 ± 0.07 | 7.43 ± 0.10 | 8.91 ± 0.12 | **,## | ||
| Omega-3 Index (EPA + DHA) | 6.85 ± 0.06 | 6.58 ± 0.09 | * | 7.54 ± 0.08 | **,## | |
| ARA/EPA | 47.7 ± 1.27 | 46.18 ± 1.50 | 26.43 ± 1.44 | **,## | ||
| ARA/DHA | 2.24 ± 0.02 | 2.36 ± 0.04 | * | 2.00 ± 0.03 | **,## | |
| ω-6/ω-3 | 3.32 ± 0.04 | 3.47 ± 0.06 | 2.72 ± 0.05 | **,## | ||
% of total fatty acids; data are presented as means ± SEM; p-value: one-way analysis of variance followed by Tukey’s test; * p < 0.05, ** p < 0.01 vs. historical control; ## p < 0.01 vs. perilla oil.

Table A4.
Fatty acid compositions of colostrum.
Table A4.
Fatty acid compositions of colostrum.
| Colostrum | Observational Study | Intervention Trial | ||||
|---|---|---|---|---|---|---|
| Historical Control | Perilla Oil Group | Fish Oil Group | ||||
| Fatty Acids | n = 208 | n = 92 | n = 88 | |||
| Total saturated fatty acids | 37.21 ± 0.19 | 37.70 ± 0.28 | 37.49 ± 0.32 | |||
| Total mono-unsaturated fatty acids | 41.40 ± 0.18 | 40.03 ± 0.29 | ** | 40.61 ± 0.30 | ||
| ω-6 polyunsaturated fatty acids | ||||||
| Linoleic acid (LA) | 18:2n-6 | 12.43 ± 0.10 | 13.05 ± 0.15 | ** | 12.68 ± 0.16 | |
| Arachidonic acid (ARA) | 20:4n-6 | 0.93 ± 0.02 | 0.86 ± 0.02 | * | 0.81 ± 0.02 | ** |
| Docosapentaenoic acid n-6 (DPAn-6) | 22:5n-6 | 0.10 ± 0.004 | 0.09 ± 0.004 | 0.09 ± 0.004 | ||
| Total ω-6 polyunsaturated fatty acids | 15.73 ± 0.10 | 16.21 ± 0.15 | * | 15.68 ± 0.16 | # | |
| ω-3 polyunsaturated fatty acids | ||||||
| α-Linolenic acid (ALA) | 18:3n-3 | 1.04 ± 0.02 | 1.21 ± 0.02 | ** | 1.07 ± 0.03 | ## |
| Eicosapentaenoic acid (EPA) | 20:5n-3 | 0.24 ± 0.01 | 0.24 ± 0.01 | 0.28 ± 0.01 | *,# | |
| Docosapentaenoic acid n-3 (DPAn-3) | 22:5n-3 | 0.64 ± 0.01 | 0.59 ± 0.02 | 0.86 ± 0.03 | **,## | |
| Docosahexaenoic acid (DHA) | 22:6n-3 | 1.34 ± 0.03 | 1.24 ± 0.05 | 1.32 ± 0.05 | ||
| Total ω-3 polyunsaturated fatty acids | 3.42 ± 0.06 | 3.45 ± 0.09 | 3.68 ± 0.09 | * | ||
| Omega-3 Index (EPA + DHA) | 1.58 ± 0.04 | 1.48 ± 0.06 | 1.60 ± 0.06 | |||
| ARA/EPA | 4.50 ± 0.16 | 4.56 ± 0.25 | 3.51 ± 0.20 | **,## | ||
| ARA/DHA | 0.76 ± 0.02 | 0.77 ± 0.03 | 0.67 ± 0.02 | *,# | ||
| ω-6/ω-3 | 4.84 ± 0.08 | 4.92 ± 0.11 | 4.44 ± 0.10 | **,## | ||
% of total fatty acids; data are presented as means ± SEM; p-value: one-way analysis of variance followed by Tukey’s test; * p < 0.05, ** p < 0.01 vs. historical control; # p < 0.05, ## p < 0.01 vs. perilla oil.
References
- Brunton, R.; Simpson, N.; Dryer, R. Pregnancy-Related Anxiety, Perceived Parental Self-Efficacy and the Influence of Parity and Age. Int. J. Environ. Res. Public Health 2020, 17, 6709. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka-Maeda, K.; Kuroda, M. Characteristics and related factors of Japanese mothers who have faced difficulties with childrearing. Public Health Nurs. 2017, 34, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Tavormina, M.G.M.; Tavormina, R. Depression in Early Childhood. Psychiatr. Danub. 2022, 34, 64–70. [Google Scholar] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Casula, M.; Olmastroni, E.; Gazzotti, M.; Galimberti, F.; Zambon, A.; Catapano, A.L. Omega-3 polyunsaturated fatty acids supplementation and cardiovascular outcomes: Do formulation, dosage, and baseline cardiovascular risk matter? An updated meta-analysis of randomized controlled trials. Pharmacol. Res. 2020, 160, 105060. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’keefe, J.H. The Importance of Marine Omega-3s for Brain Development and the Prevention and Treatment of Behavior, Mood, and Other Brain Disorders. Nutrients 2020, 12, 2333. [Google Scholar] [CrossRef]
- Shulkin, M.; Pimpin, L.; Bellinger, D.; Kranz, S.; Fawzi, W.; Duggan, C.; Mozaffarian, D. n-3 fatty acid supplementation in mothers, preterm infants, and term infants and childhood psychomotor and visual development: A systematic review and metaanalysis. J. Nutr. 2018, 148, 409–418. [Google Scholar]
- Hibbeln, J.R. Seafood consumption, the DHA content of mothers’ milk and prevalence rates of postpartum depression: A cross-national, ecological analysis. J. Affect. Disord. 2002, 69, 5–29. [Google Scholar]
- Rees, A.-M.; Austin, M.-P.; Parker, G. Role of Omega-3 Fatty Acids as a Treatment for Depression in the Perinatal Period. Aust. N. Z. J. Psychiatry 2005, 39, 274–280. [Google Scholar] [CrossRef]
- The National Health and Nutrition Survey in Japan; Ministry of Health, Labour and Welfare: Tokyo, Japan, 2019; pp. 78–89.
- Miyake, Y.; Tanaka, K.; Okubo, H.; Sasaki, S.; Arakawa, M. Fish and fat intake and prevalence of depressive symptoms dur-ing pregnancy in Japan: Baseline data from the Kyushu Okinawa Maternal and Child Health Study. J. Psychiatr. Res. 2013, 47, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, M.; Matsuzaki, M.; Yatsuki, Y.; Murayama, R.; Severinsson, E.; Haruna, M. Associations of dietary intake and plasma concentrations of eicosapentaenoic and docosahexaenoic acid with prenatal depressive symptoms in Japan. Nurs. Health Sci. 2014, 17, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Hamazaki, K.; Takamori, A.; Tsuchida, A.; Kigawa, M.; Tanaka, T.; Ito, M.; Adachi, Y.; Saito, S.; Origasa, H.; Inadera, H. Japan Environment and Children’s Study (JECS) Group. Dietary intake of fish and n-3 polyunsaturated fatty acids and risks of perinatal depression: The Japan Environment and Children’s Study (JECS). J. Psychiatr. Res. 2018, 98, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Harauma, A.; Sagisaka, T.; Horii, T.; Watanabe, Y.; Moriguchi, T. The influence of n-3 fatty acid to material behavior and monoamine of brain in perinatal period. Prostaglandins Leukot. Essent. Fat. Acids 2016, 107, 1–7. [Google Scholar] [CrossRef]
- Harauma, A.; Nakamura, S.; Wakinaka, N.; Mogi, K.; Moriguchi, T. Influence of ω3 fatty acids on maternal behavior and brain oxytocin in the murine perinatal period. Prostaglandins Leukot. Essent. Fat. Acids 2021, 176, 102386. [Google Scholar] [CrossRef]
- Hamazaki, K.; Harauma, A.; Otaka, Y.; Moriguchi, T.; Inadera, H. Serum n-3 polyunsaturated fatty acids and psychological distress in early pregnancy: Adjunct Study of Japan Environment and Children’s Study. Transl. Psychiatry 2016, 6, e737. [Google Scholar] [CrossRef][Green Version]
- Okano, T.; Murata, M.; Masuji, F.; Tamaki, R. Validation and reliability of Japanese version of EPDS (Edinburgh Postnatal Depres-sion Scale). Arch. Psychiatr. Diagn. Clin. Eval. 1996, 7, 525–533. [Google Scholar]
- Ohoka, H.; Koide, T.; Goto, S.; Murase, S.; Kanai, A.; Masuda, T.; Aleksic, B.; Ishikawa, N.; Furumura, K.; Ozaki, N. Effects of maternal depressive symptomatology during pregnancy and the postpartum period on infant-mother attachment. Psychiatry Clin. Neurosci. 2014, 68, 631–639. [Google Scholar] [CrossRef]
- Lepage, G.; Roy, C.C. Improved recovery of fatty acid through direct transesterification without prior extraction or purification. J. Lipid Res. 1984, 25, 1391–1396. [Google Scholar] [CrossRef]
- Masood, A.; Stark, K.D.; Salem, N., Jr. A simplified and efficient method for the analysis of fatty acid methyl esters suitable for large clinical studies. J. Lipid Res. 2005, 46, 2299–2305. [Google Scholar] [CrossRef]
- Hahn-Holbrook, J.; Cornwell-Hinrichs, T.; Anaya, I. Economic and Health Predictors of National Postpartum Depression Prevalence: A Systematic Review, Meta-analysis, and Meta-Regression of 291 Studies from 56 Countries. Front. Psychiatry 2018, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Mocking, R.J.T.; Steijn, K.; Roos, C.; Assies, J.; Bergink, V.; Ruhé, H.G.; Schene, A.H. Omega-3 Fatty Acid Supplementation for Perinatal Depression: A Meta-Analysis. J. Clin. Psychiatry 2020, 81, 19r13106. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, J.P.; Cerrato, M.; Ceseri, M.; DeFina, L.F.; Delgado, G.E.; Gellert, S.; Hahn, A.; Howard, B.V.; Kadota, A.; Kleber, M.E.; et al. Red blood cell fatty acid patterns from 7 countries: Focus on the Omega-3 index. Prostaglandins Leukot. Essent. Fat. Acids 2022, 179, 102418. [Google Scholar] [CrossRef]
- Su, K.P.; Huang, S.Y.; Chiu, T.H.; Huang, K.C.; Huang, C.L.; Chang, H.C.; Pariante, C.M. Omega-3 fatty acids for major depres-sive disorder during pregnancy: Results from a randomized, double-blind, placebo-controlled trial. J. Clin. Psychiatry 2008, 69, 644–651. [Google Scholar] [CrossRef]
- Lucas, M.; Mirzaei, F.; O’reilly, E.J.; Pan, A.; Willett, W.C.; Kawachi, I.; Koenen, K.; Ascherio, A. Dietary intake of n−3 and n−6 fatty acids and the risk of clinical depression in women: A 10-y prospective follow-up study. Am. J. Clin. Nutr. 2011, 93, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Okubo, R.; Noguchi, H.; Hamazaki, K.; Sekiguchi, M.; Kinoshita, T.; Katsumata, N.; Narisawa, T.; Uezono, Y.; Xiao, J.; Matsuoka, Y.J. Fear of cancer recurrence among breast cancer survivors could be controlled by prudent dietary modification with polyunsaturated fatty acids. J. Affect. Disord. 2018, 245, 1114–1118. [Google Scholar] [CrossRef]
- Hibbeln, J.R.; Nieminen, L.R.; Blasbalg, T.L.; Riggs, J.A.; Lands, W.E. Healthy intakes of n−3 and n–6 fatty acids: Estimations considering worldwide diversity. Am. J. Clin. Nutr. 2006, 83, 1483S–1493S. [Google Scholar] [CrossRef]
- Bibus, D.; Lands, B. Balancing proportions of competing omega-3 and omega-6 highly unsaturated fatty acids (HUFA) in tissue lipids. Prostaglandins Leukot. Essent. Fat. Acids 2015, 99, 19–23. [Google Scholar] [CrossRef]
- Pawlosky, R.J.; Hibbein, J.R.; Novotny, J.A.; Salem, N., Jr. Physiological compartmental analysis of α-linolenic acid metabolism in adult humans. J. Lipid Res. 2001, 42, 1257–1265. [Google Scholar] [CrossRef]
- Duttaroy, A.K.; Basak, S. Maternal dietary fatty acids and their roles in human placental development. Prostaglandins Leukot. Essent. Fat. Acids 2020, 155, 102080. [Google Scholar] [CrossRef]
- Harauma, A.; Salem, N.; Moriguchi, T. Repletion of n-3 Fatty Acid Deficient Dams with α-Linolenic Acid: Effects on Fetal Brain and Liver Fatty Acid Composition. Lipids 2010, 45, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Domenichiello, A.F.; Kitson, A.P.; Bazinet, R.P. Is docosahexaenoic acid synthesis from α-linolenic acid sufficient to supply the adult brain? Prog. Lipid Res. 2015, 59, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, T.; Loewke, J.; Garrison, M.; Catalan, J.N.; Salem, N. Reversal of docosahexaenoic acid deficiency in the rat brain, retina, liver, and serum. J. Lipid Res. 2001, 42, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Nevins, J.E.H.; Donovan, S.M.; Snetselaar, L.; Dewey, K.G.; Novotny, R.; Stang, J.; Taveras, E.M.; Kleinman, R.E.; Bailey, R.L.; Raghavan, R.; et al. Omega-3 Fatty Acid Dietary Supplements Consumed during Pregnancy and Lactation and Child Neurodevelopment: A Systematic Review. J. Nutr. 2021, 151, 3483–3494. [Google Scholar] [CrossRef]
- Best, K.P.; Gibson, R.A.; Makrides, M. ISSFAL statement number 7—Omega-3 fatty acids during pregnancy to reduce preterm birth. Prostaglandins Leukot. Essent. Fat. Acids 2022, 186, 102495. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).