Does the Protective Effect of Zinc on Telomere Length Depend on the Presence of Hypertension or Type 2 Diabetes? Results from the Iwaki Health Promotion Project, Japan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Questionnaire, Biochemical and Anthropometric Measurements
2.3. Serum Zinc Measurement
2.4. Definitions of Hypertension and Type 2 Diabetes Mellitus
2.5. Telomere Length Measurement
2.6. Statistical Analysis
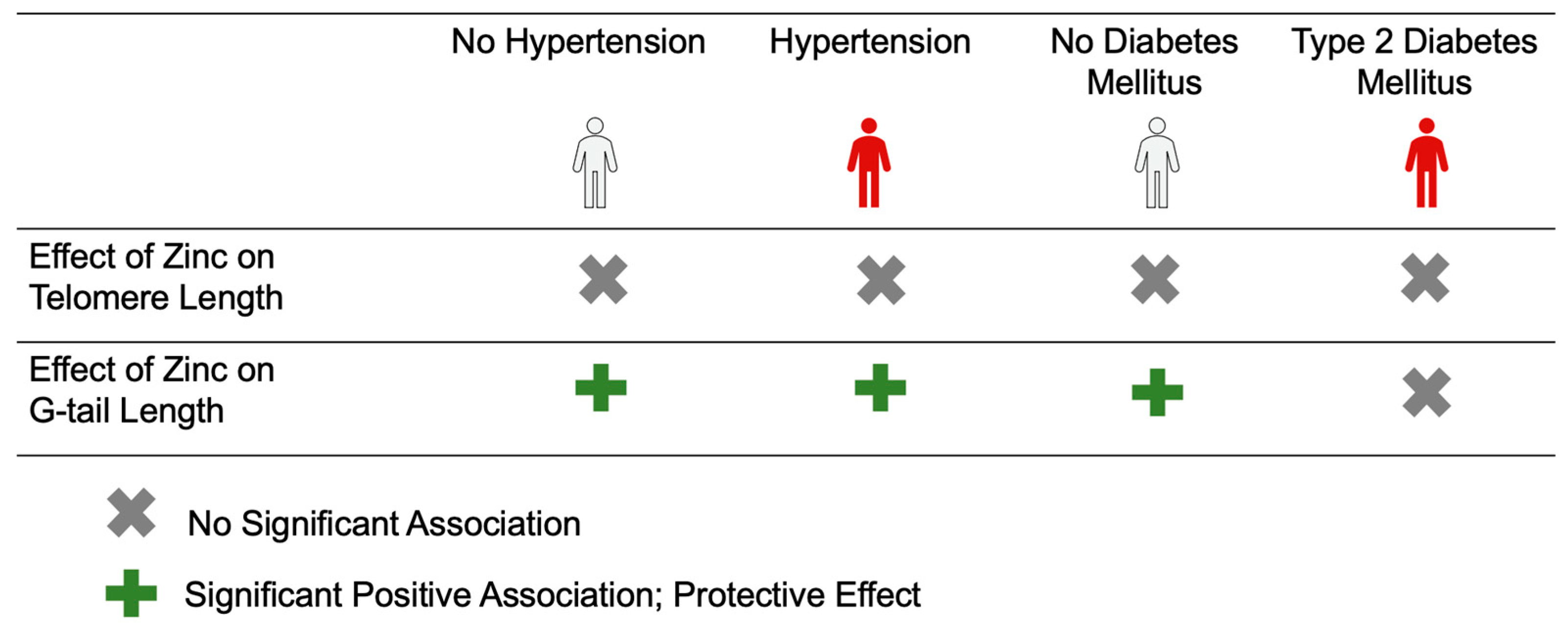
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zakian, V.A. Structure and function of telomeres. Annu. Rev. Genet. 1989, 23, 579–604. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Zhang, Y.; Liu, D.; Songyang, Z.; Wan, M. Telomeres-structure, function, and regulation. Exp. Cell Res. 2013, 319, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Makarov, V.L.; Hirose, Y.; Langmore, J.P. Long G Tails at Both Ends of Human Chromosomes Suggest a C Strand Degradation Mechanism for Telomere Shortening. Cell 1997, 88, 657–666. [Google Scholar] [CrossRef]
- Shammas, M.A. Telomeres, lifestyle, cancer, and aging. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 28–34. [Google Scholar] [CrossRef]
- Von Zglinicki, T. Role of oxidative stress in telomere length regulation and replicative senescence. Ann. N. Y. Acad. Sci. 2000, 908, 99–110. [Google Scholar] [CrossRef]
- De Lange, T. T-loops and the origin of telomeres. Nat. Rev. Mol. Cell. Biol. 2004, 5, 323–329. [Google Scholar] [CrossRef]
- Houben, J.M.; Moonen, H.J.J.; van Schooten, F.J.; Hageman, G.J. Telomere length assessment: Biomarker of chronic oxidative stress? Free. Radic. Biol. Med. 2008, 44, 235–246. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Y.; Ma, L. Telomere length and associated factors in older adults with hypertension. J. Int. Med. Res. 2019, 47, 5465–5474. [Google Scholar] [CrossRef]
- Seimiya, H. Predicting Risk at the End of the End: Telomere G-tail as a Biomarker. EBioMedicine 2015, 2, 804–805. [Google Scholar] [CrossRef]
- Révész, D.; Milaneschi, Y.; Verhoeven, J.E.; Penninx, B.W.J.H. Telomere Length as a Marker of Cellular Aging Is Associated with Prevalence and Progression of Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2014, 99, 4607–4615. [Google Scholar] [CrossRef]
- Wai, K.M.; Kaori, S.; Itoh, K.; Shinya, O.; Uchikawa, Y.; Hayashi, S.; Shiraki, A.; Murashita, K.; Nakaji, S.; Ihara, K. Telomere Length and Arterial Stiffness Reflected by Brachial–Ankle Pulse Wave Velocity: A Population-Based Cross-Sectional Study. J. Pers. Med. 2021, 11, 1278. [Google Scholar] [CrossRef] [PubMed]
- Haycock, P.C.; Heydon, E.E.; Kaptoge, S.; Butterworth, A.S.; Thompson, A.; Willeit, P. Leucocyte telomere length and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2014, 349, g4227. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.-K.; Wang, C.-Y. Telomeres and Telomerase in Cardiovascular Diseases. Genes 2016, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Ortiz, B.; Albarrán-Tamayo, F.; Arenas-Aranda, D.; Benítez-Bribiesca, L.; Malacara-Hernández, J.; Martínez-Garza, S.; Hernández-González, M.; Solorio, S.; Garay-Sevilla, M.; Mora-Villalpando, C. Telomere length and type 2 diabetes in males, a premature aging syndrome. Aging Male 2011, 15, 54–58. [Google Scholar] [CrossRef]
- Tellechea, M.L.; Pirola, C.J. The impact of hypertension on leukocyte telomere length: A systematic review and me-ta-analysis of human studies. J. Hum. Hypertens. 2017, 31, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Bhupatiraju, C.; Saini, D.; Patkar, S.; Deepak, P.; Das, B.; Padma, T. Association of shorter telomere length with essential hypertension in Indian population. Am. J. Hum. Biol. 2012, 24, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Takubo, K.; Aida, J.; Araki, A.; Ito, H. Telomere attrition and diabetes mellitus. Geriatr. Gerontol. Int. 2016, 16 (Suppl. S1), 66–74. [Google Scholar] [CrossRef]
- Zee, R.Y.; Castonguay, A.J.; Barton, N.S.; Germer, S.; Martin, M. Mean leukocyte telomere length shortening and type 2 diabetes mellitus: A case-control study. Transl. Res. 2010, 155, 166–169. [Google Scholar] [CrossRef]
- D’Mello, M.J.J.; Ross, S.A.; Briel, M.; Anand, S.S.; Gerstein, H.; Paré, G. Association between shortened leukocyte telomere length and cardiometabolic outcomes: Systematic review and meta-analysis. Circ. Cardiovasc. Genet. 2015, 8, 82–90. [Google Scholar] [CrossRef]
- Hirashio, S.; Nakashima, A.; Doi, S.; Anno, K.; Aoki, E.; Shimamoto, A.; Yorioka, N.; Kohno, N.; Masaki, T.; Tahara, H. Telomeric G-Tail Length and Hospitalization for Cardiovascular Events in Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 2117–2122. [Google Scholar] [CrossRef]
- Han, J.; Hayashi, S.; Takahashi, R.-U.; Hirohata, R.; Kurokawa, T.; Tashiro, M.; Yamamoto, Y.; Okada, M.; Tahara, H. Leukocyte Telomeric G-Tail Length Shortening Is Associated with Esophageal Cancer Recurrence. J. Clin. Med. 2022, 11, 7385. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Zinc: An overview. Nutrition 1995, 11 (Suppl. S1), 93–99. [Google Scholar] [PubMed]
- Sharif, R.; Thomas, P.; Zalewski, P.; Fenech, M. The role of zinc in genomic stability. Mutat. Res. Mol. Mech. Mutagen. 2012, 733, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Paul, L. Diet, nutrition and telomere length. J. Nutr. Biochem. 2011, 22, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Olechnowicz, J.; Tinkov, A.; Skalny, A.; Suliburska, J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol. Sci. 2017, 68, 19–31. [Google Scholar] [CrossRef]
- Hartwig, A.; Asmuss, M.; Blessing, H.; Hoffmann, S.; Jahnke, G.; Khandelwal, S.; Pelzer, A.; Bürkle, A. Interference by toxic metal ions with zinc-dependent proteins involved in maintaining genomic stability. Food Chem. Toxicol. 2002, 40, 1179–1184. [Google Scholar] [CrossRef]
- Sharif, R.; Thomas, P.; Zalewski, P.; Fenech, M. Zinc supplementation influences genomic stability biomarkers, antioxidant activity, and zinc transporter genes in an elderly Australian population with low zinc status. Mol. Nutr. Food Res. 2015, 59, 1200–1212. [Google Scholar] [CrossRef]
- Bai, Y.; Fu, W.; Guan, X.; Wu, X.; Li, G.; Wei, W.; Feng, Y.; Meng, H.; Li, H.; Li, M.; et al. Co-exposure to multiple metals, TERT-CLPTM1L variants, and their joint influence on leukocyte telomere length. Environ. Int. 2020, 140, 105762. [Google Scholar] [CrossRef]
- Shi, H.; Li, X.; Yu, H.; Shi, W.; Lin, Y.; Zhou, Y. Potential effect of dietary zinc intake on telomere length: A cross-sectional study of US adults. Front. Nutr. 2022, 9, 993425. [Google Scholar] [CrossRef]
- Lee, J.; Shin, C.; Baik, I. Longitudinal associations between micronutrient consumption and leukocyte telomere length. J. Hum. Nutr. Diet. 2016, 30, 236–243. [Google Scholar] [CrossRef]
- Xu, Q.; Parks, C.G.; DeRoo, L.A.; Cawthon, R.M.; Sandler, D.P.; Chen, H. Multivitamin use and telomere length in women. Am. J. Clin. Nutr. 2009, 89, 1857–1863. [Google Scholar] [CrossRef] [PubMed]
- Vahter, M.; Broberg, K.; Harari, F. Placental and Cord Blood Telomere Length in Relation to Maternal Nutritional Status. J. Nutr. 2020, 150, 2646–2655. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ge, X.; Cheng, H.; Bao, Y.; Feng, X.; Zan, G.; Wang, F.; Zou, Y.; Yang, X. Sex-specific associations of exposure to metal mixtures with telomere length change: Results from an 8-year longitudinal study. Sci. Total. Environ. 2022, 811, 151327. [Google Scholar] [CrossRef] [PubMed]
- Nakaji, S.; Ihara, K.; Sawada, K.; Parodi, S.; Umeda, T.; Takahashi, I.; Murashita, K.; Kurauchi, S.; Tokuda, I. Social innovation for life expectancy extension utilizing a platform-centered system used in the Iwaki health promotion project: A protocol paper. SAGE Open Med. 2021, 9, 20503121211002606. [Google Scholar] [CrossRef]
- Marreiro, D.D.N.; Cruz, K.J.C.; Morais, J.B.S.; Beserra, J.B.; Severo, J.S.; De Oliveira, A.R.S. Zinc and oxidative stress: Current mechanisms. Antioxidants 2017, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Eide, D.J. The oxidative stress of zinc deficiency. Metallomics 2011, 3, 1124–1129. [Google Scholar] [CrossRef]
- Cipriano, C.; Tesei, S.; Malavolta, M.; Giacconi, R.; Muti, E.; Costarelli, L.; Piacenza, F.; Pierpaoli, S.; Galeazzi, R.; Blasco, M.; et al. Accumulation of Cells with Short Telomeres Is Associated with Impaired Zinc Homeostasis and Inflammation in Old Hypertensive Participants. J. Gerontol. Ser. A 2009, 64, 745–751. [Google Scholar] [CrossRef]
- Nemoto, K.; Kondo, Y.; Himeno, S.; Suzuki, Y.; Hara, S.; Akimoto, M.; Imura, N. Modulation of telomerase activity by zinc in human prostatic and renal cancer cells. Biochem. Pharmacol. 2000, 59, 401–405. [Google Scholar] [CrossRef]
- Koi, Y.; Tsutani, Y.; Nishiyama, Y.; Kanda, M.; Shiroma, Y.; Yamamoto, Y.; Sasada, S.; Akita, T.; Masumoto, N.; Kadoya, T.; et al. Diagnostic performance of peripheral leukocyte telomere G-tail length for detecting breast cancer. Cancer Sci. 2020, 111, 1856–1861. [Google Scholar] [CrossRef]
- Tahara, H.; Shin-Ya, K.; Seimiya, H.; Yamada, H.; Tsuruo, T.; Ide, T. G-Quadruplex stabilization by telomestatin induces TRF2 protein dissociation from telomeres and anaphase bridge formation accompanied by loss of the 3′ telomeric overhang in cancer cells. Oncogene 2005, 25, 1955–1966. [Google Scholar] [CrossRef]
- Miao, X.; Sun, W.; Fu, Y.; Miao, L.; Cai, L. Zinc homeostasis in the metabolic syndrome and diabetes. Front. Med. 2013, 7, 31–52. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro, M.J.; Krebs, N.; Zubillaga, M.B.; Weill, R.; Postaire, E.; Lysionek, A.E.; Caro, R.A.; De Paoli, T.; Hager, A.; Boccio, J. Zinc and diabetes mellitus: Is there a need of zinc supplementation in diabetes mellitus patients? Biol. Trace Elem. Res. 2001, 81, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Montpetit, A.J.; Alhareeri, A.A.; Montpetit, M.; Starkweather, A.R.; Elmore, L.W.; Filler, K.; Mohanraj, L.; Burton, C.W.; Menzies, V.S.; Lyon, D.E.; et al. Telomere length: A review of methods for measurement. Nurs. Res. 2014, 63, 289–299. [Google Scholar] [CrossRef] [PubMed]
| Variables | Mean | Standard Deviation |
| Age (years) | 52.7 | 15.3 |
| Body Mass Index: BMI (kg/m2) | 23.0 | 3.6 |
| Telomere length (RLU/mg DNA) | 356,478.1 | 50,678.4 |
| G-tail length (RLU/mg DNA) | 31,664.2 | 4744.9 |
| Systolic blood pressure (mmHg) | 120.8 | 16.9 |
| Diastolic blood pressure (mmHg) | 76.9 | 11.4 |
| HbA1c (%) ‡ | 5.6 | 0.4 |
| Serum sugar (mg/dL) ‡ | 93.0 | 133.0 |
| Serum zinc (μg/dL) | 84.8 | 12.9 |
| N | (%) | |
| Sex | ||
| Male | 435 | 40.9 |
| Female | 629 | 59.1 |
| Education | ||
| Junior high school | 112 | 10.5 |
| High school | 577 | 54.2 |
| Junior college | 248 | 23.3 |
| University/college | 117 | 11.0 |
| Others | 6 | 0.6 |
| Smoking status | ||
| No | 673 | 63.3 |
| Current | 182 | 17.1 |
| Past | 199 | 18.7 |
| Drinking status | ||
| No | 503 | 47.3 |
| Current | 504 | 47.4 |
| Past | 43 | 4.0 |
| Physical exercise (Other than winter) | 234 | 22.0 |
| Physical exercise (winter) | 230 | 21.6 |
| Hypertension | 378 | 35.5 |
| Type 2 diabetes mellitus | 71 | 6.7 |
| Dyslipidemia | 399 | 37.5 |
| Currently taking anti-hypertensive drugs | 247 | 23.2 |
| Currently taking anti-diabetic drugs | 49 | 4.6 |
| Currently taking anti-dyslipidemia drugs | 107 | 9.8 |
| Telomere Length | G-Tail Length | |||
| Variables | Pearson’s γ | p-Value | Pearson’s γ | p-Value |
| Age (years) | −0.4 | <0.001 | −0.24 | <0.001 |
| Systolic blood pressure (mmHg) | −0.157 | <0.001 | −0.106 | <0.001 |
| Diastolic blood pressure (mmHg) | −0.072 | 0.019 | −0.036 | 0.243 |
| Body mass index (kg/m2) | −0.113 | <0.001 | −0.006 | 0.854 |
| Serum zinc (μg/dL) | 0.033 | 0.287 | 0.151 | <0.001 |
| G-tail length (RLU/mg DNA) | 0.38 | <0.001 | ||
| Spearman’s Rho | p-Value | Spearman’s Rho | p-Value | |
| HbA1c (%) | −0.257 | <0.001 | −0.125 | <0.001 |
| Serum sugar (mg/dL) | −0.209 | <0.001 | −0.084 | 0.006 |
| Variables | Telomere Length | G-tail Length | ||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |
| β (95%CI) | β (95%CI) | β (95%CI) | β (95%CI) | |
| Serum zinc (μg/dL) | 128.8 (−108.47, 366.06) | 18.4 (−208.57, 245.37) | 55.69 (33.72, 77.66) *** | 48.11 (25.69, 70.54) *** |
| Age (years) | −1321.38 (−1546.27, −1096.3) *** | −76.22 (−98.45, −53.99) *** | ||
| Sex (ref: Male) | ||||
| Female | 3626.97 (−3027.58, 10,281.51) | 17.13 (−640.39, 674.66) | ||
| Education (ref: below junior college) | ||||
| Junior college and above | −1882.05 (−8071.28, 4307.18) | −41.64 (−653.19, 569.90) | ||
| Smoking (ref: Non-smokers) | ||||
| Current smokers | −9761.76 (−18,281.73, −1241.79) * | −59.40 (−901.24, 782.45) | ||
| Past smokers | −3742.33 (−11,615.11, 4130.45) | −472.04 (−1249.94, 305.86) | ||
| Alcohol drinking (ref: Non-drinkers) | ||||
| Current drinkers | −2264.24 (−8646.38, 4117.89) | −88.59 (−719.20, 542.02) | ||
| Past drinkers | 698.24 (−13,979.32, 15,375.81) | 744.92 (−705.34, 2195.19) | ||
| Physical exercise (ref: No) | −183.31 (−7711.99, 7345.38) | −674.80 (−1418.69, 69.10) | ||
| Body mass index (kg/m2) (ref: <18.5 kg/m2) | ||||
| 18.5 or 18.5–25 kg/m2 | −2542.98 (−13,427.81, 8341.86) | 646.28 (−429.23, 1721.80) | ||
| ≥25 kg/m2 | −5138.97 (−17,226.54, 6948.60) | 562.82 (−631.53, 1757.17) | ||
| Hypertension (ref: No) | 2597.17 (−4280.28, 9474.63) | −25.67 (−705.22, 653.88) | ||
| Type 2 diabetes mellitus (ref: No) | −18,999.33 (−30,833.92, −7164.73) ** | 260.66 (−908.70, 1430.01) | ||
| Dyslipidemia (ref: No) | −2419.61 (−8771.77, −3932.55) | 374.69 (−252.96, 1002.34) | ||
| Variables | No Hypertension N = 686 | Hypertension N = 378 | ||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |
| β (95%CI) | β (95%CI) | β (95%CI) | β (95%CI) | |
| Serum zinc (μg/dL) | 78.96 (−218.05, 375.97) | 50.89 (−237.35, 339.13) | 148.10 (−228.89, 525.08) | −46.11 (−428.44, 336.23) |
| Age (years) | −1274.38 (−1553.51, −995.28) ** | −1470.51 (−1893.65, −1047.36) ** | ||
| Sex (ref: Male) | ||||
| Female | 2814.95 (−5621.74, 11251.65) | 6106.05 (−5264.46, 17476.56) | ||
| Education (ref: below junior college) | ||||
| Junior college and above | −173.93 (−7768.76, 7420.91) | −6866.10 (−18,102.29, 4370.10) | ||
| Smoking (ref: Non-smokers) | ||||
| Current smokers | −10,668.75 (−27748.21, −589.3) * | −8811.48 (−25,681.46, 8058.50) | ||
| Past smokers | −5571.46 (−15,543.24, 4400.32) | 684.60 (−12,384.35, 13,753.56) | ||
| Alcohol drinking (ref: Non-drinkers) | ||||
| Current drinkers | −2597.51 (−10,406.51, 5211.49) | −3044.71 (−148,04.48, 8715.06) | ||
| Past drinkers | −4565.16 (−22,919.42, 13,789.10) | 12,006.70 (−13,108.77, 37,122.16) | ||
| Physical exercise (ref: No) | 2258.92 (−7368.96, 11,886.79) | −5096.39 (−17,523.87, 7331.09) | ||
| Body mass index (kg/m2) (ref: <18.5 kg/m2) | ||||
| 18.5 or 18.5–25 kg/m2 | −5041.06 (−17,579.15, 7497.02) | 3200.69 (−20,175.63, 26,577.02) | ||
| ≥25 kg/m2 | −4350.55 (−19,115.25, 10,454.14) | −4023.92 (−28,019.93, 19,972.10) | ||
| Type 2 diabetes mellitus (ref: No) | −24,322.36 (−45,408.30, −3236.42) * | −16,994.83 (−31,321.67, −2667.99) * | ||
| Dyslipidemia (ref: No) | −2248.33 (−10,938.59, 6441.94) | −2814.97 (−12,611.19, 6981.25) | ||
| Variables | No Hypertension N = 686 | Hypertension N = 378 | ||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |
| β (95%CI) | β (95%CI) | β (95%CI) | β (95%CI) | |
| Serum zinc (μg/dL) | 52.90 (24.94, 80.86) *** | 49.47 (20.75, 78.18) ** | 57.04 (22.01, 92.08) *** | 46.84 (9.69, 84.00) * |
| Age (years) | −72.99 (−100.80, −45.18) *** | −88.25 (−129.37, −47.12) *** | ||
| Sex (ref: Male) | ||||
| Female | 257.68 (−582.90, 1098.26) | −494.67 (−1599.65, 610.32) | ||
| Education (ref: below junior college) | ||||
| Junior college and above | −126.64 (−883.34, 630.06) | −38.66 (−1130.60, 1053.27) | ||
| Smoking (ref: Non-smokers) | ||||
| Current smokers | 5.79 (−998.46, 1010.05) | −420.91 (−2060.33, 1218.51) | ||
| Past smokers | −655.54 (−1649.06, 337.99) | −207.28 (−1477.32, 1062.76) | ||
| Alcohol drinking (ref: Non-drinkers) | ||||
| Current drinkers | 79.23 (−698.81, 857.27) | −581.51 (−1724.32, 561.31) | ||
| Past drinkers | 772.30 (−1056.41, 2601.00) | 749.43 (−1691.29, 3190.15) | ||
| Physical exercise (ref: No) | −837.25 (−1796.51, 122.01) | −406.62 (−1614.32, 801.09) | ||
| Body mass index (kg/m2) (ref: <18.5 kg/m2) | ||||
| 18.5 or 18.5–25 kg/m2 | 972.22 (−277.00, 2221.43) | −549.98 (−2821.69, 1721.73) | ||
| ≥25 kg/m2 | 1270.78 (−204.27, 2745.82) | −1096.55 (−3428.48, 1235.38) | ||
| Type 2 diabetes mellitus (ref: No) | −225.87 (−2326.74, 1875.00) | 480.81 (−911.47, 1873.09) | ||
| Dyslipidemia (ref: No) | 294.84 (−571.00, 1160.68) | 315.29 (−636.70, 1267.29) | ||
| Variables | No Diabetes Mellitus N = 993 | Type 2 Diabetes Mellitus N = 71 | ||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |
| β (95%CI) | β (95%CI) | β (95%CI) | β (95%CI) | |
| Serum zinc (μg/dL) | 132.76 (−108.81, 374.34) | 40.29 (−194.71, 275.28) | −389.19 (−1373.16, 594.79) | −355.88 (−1399.54, 687.79) |
| Age (years) | −1291.28 (−1524.56, −1058.00) * | −1792.54 (−2867.96, −717.11) | ||
| Sex (ref: Male) | ||||
| Female | 3287.20 (−3661.98, 10,236.38) | 11,391.46 (−17,304.77, 40,087.68) | ||
| Education (ref: below junior college) | ||||
| Junior college and above | −1297.31 (−7691.85, 5097.23) | −11,415.26 (−38,286.81, 15,456.30) | ||
| Smoking (ref: Non-smokers) | ||||
| Current smokers | −8639.30 (−17462.37, 183.78) | −21,291.34 (−57,760.54, 15,177.87) | ||
| Past smokers | −4541.19 (−12,680.77, 3598.39) | 18,358.79 (−17,623.85, 54,341.43) | ||
| Alcohol drinking (ref: Non-drinkers) | ||||
| Current drinkers | −1980.46 (−8542.66, 4581.74) | −14,222.07 (−43656.04, 15211.89) | ||
| Past drinkers | −1757.56 (−17,387.16, 13,872.03) | 22,346.24 (−29005.18, 73697.66) | ||
| Physical exercise (ref: No) | −2030.63 (−9841.42, 5780.16) | 23,971.98 (−7722.96, 55666.93) | ||
| Body mass index (kg/m2) (ref: <18.5 kg/m2) | ||||
| 18.5 or 18.5–25 kg/m2 | −2987.63 (−13,991.04, 8015.84) | 19,289.75 (−80,950.12, 119,529.60) | ||
| ≥25 kg/m2 | −4208.25 (−16,576.26, 8159.76) | −6057.40 (−107,536.20, 95,421.45) | ||
| Hypertension (ref: No) | 2183.22 (−5036.14, 9402.58) | 8282.28 (−17,365.05, 33,929.60) | ||
| Dyslipidemia (ref: No) | −4333.54 (−10,966.07, 2299.00) | 23,947.59 (678.77, 47,216.41) | ||
| Variables | No Diabetes Mellitus N = 993 | Type 2 Diabetes Mellitus N = 71 | ||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |
| β (95%CI) | β (95%CI) | β (95%CI) | β (95%CI) | |
| Serum zinc (μg/dL) | 57.93 (35.26, 80.59) * | 50.82 (27.54, 74.11) ** | 11.85 (−81.43, 105.13) | 33.63 (−63.62, 130.87) |
| Age (years) | −67.89 (−91.01, −44.77) ** | −197.49 (−297.69, −97.28) ** | ||
| Sex (ref: Male) | ||||
| Female | −26.28 (−714.94, 662.38) | −417.87 (−3091.69, 2255.96) | ||
| Educational level (ref: below junior college) | ||||
| Junior college and above | 60.93 (−572.76, 694.63) | −2141.87 (−4645.67, 361.94) | ||
| Smoking (ref: Non-smokers) | ||||
| Current smokers | −127.21 (−1001.57, 747.15) | 312.27 (−3085.82, 3710.35) | ||
| Past smokers | −677.16 (−1483.79, 129.47) | 2627.06 (−725.69, 5979.81) | ||
| Alcohol drinking (ref: Non-drinkers) | ||||
| Current drinkers | −6.82 (−657.13, 643.49) | −2382.77 (−5125.34, 359.79) | ||
| Past drinkers | 584.48 (−964.40, 2133.36) | 2149.24 (−2635.53, 6934.01) | ||
| Physical exercise (ref: No) | −768.01 (1542.05, 6.04) | 1227.39 (−1725.85, 4180.62) | ||
| Body mass index (kg/m2) (ref: <18.5 kg/m2) | ||||
| 18.5 or 18.5–25 kg/m2 | 589.67 (−500.76, 1680.10) | 4467.88 (−4872.16, 13807.93) | ||
| ≥25 kg/m2 | 488.61 (−737.05, 1714.27) | 3138.96 (−6316.53, 12594.45) | ||
| Hypertension (ref: No) | −153.38 (−868.81, 562.06) | 1298.06 (−1091.68, 3687.80) | ||
| Dyslipidemia (ref: No) | 284.32 (−372.96, 941.70) | 1267.91 (−900.21, 3436.03) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, M.; Wai, K.M.; Itoh, K.; Yang, Y.; Uchikawa, Y.; Ito, Y.; Nakaji, S.; Ihara, K. Does the Protective Effect of Zinc on Telomere Length Depend on the Presence of Hypertension or Type 2 Diabetes? Results from the Iwaki Health Promotion Project, Japan. Nutrients 2023, 15, 4373. https://doi.org/10.3390/nu15204373
Sato M, Wai KM, Itoh K, Yang Y, Uchikawa Y, Ito Y, Nakaji S, Ihara K. Does the Protective Effect of Zinc on Telomere Length Depend on the Presence of Hypertension or Type 2 Diabetes? Results from the Iwaki Health Promotion Project, Japan. Nutrients. 2023; 15(20):4373. https://doi.org/10.3390/nu15204373
Chicago/Turabian StyleSato, Mahiro, Kyi Mar Wai, Ken Itoh, Yichi Yang, Yuka Uchikawa, Yukihiko Ito, Shigeyuki Nakaji, and Kazushige Ihara. 2023. "Does the Protective Effect of Zinc on Telomere Length Depend on the Presence of Hypertension or Type 2 Diabetes? Results from the Iwaki Health Promotion Project, Japan" Nutrients 15, no. 20: 4373. https://doi.org/10.3390/nu15204373
APA StyleSato, M., Wai, K. M., Itoh, K., Yang, Y., Uchikawa, Y., Ito, Y., Nakaji, S., & Ihara, K. (2023). Does the Protective Effect of Zinc on Telomere Length Depend on the Presence of Hypertension or Type 2 Diabetes? Results from the Iwaki Health Promotion Project, Japan. Nutrients, 15(20), 4373. https://doi.org/10.3390/nu15204373






