Exploring the Nutrition Strategies Employed by Ultra-Endurance Athletes to Alleviate Exercise-Induced Gastrointestinal Symptoms—A Systematic Review
Abstract
:1. Introduction
2. Methods
Study Design
3. Results
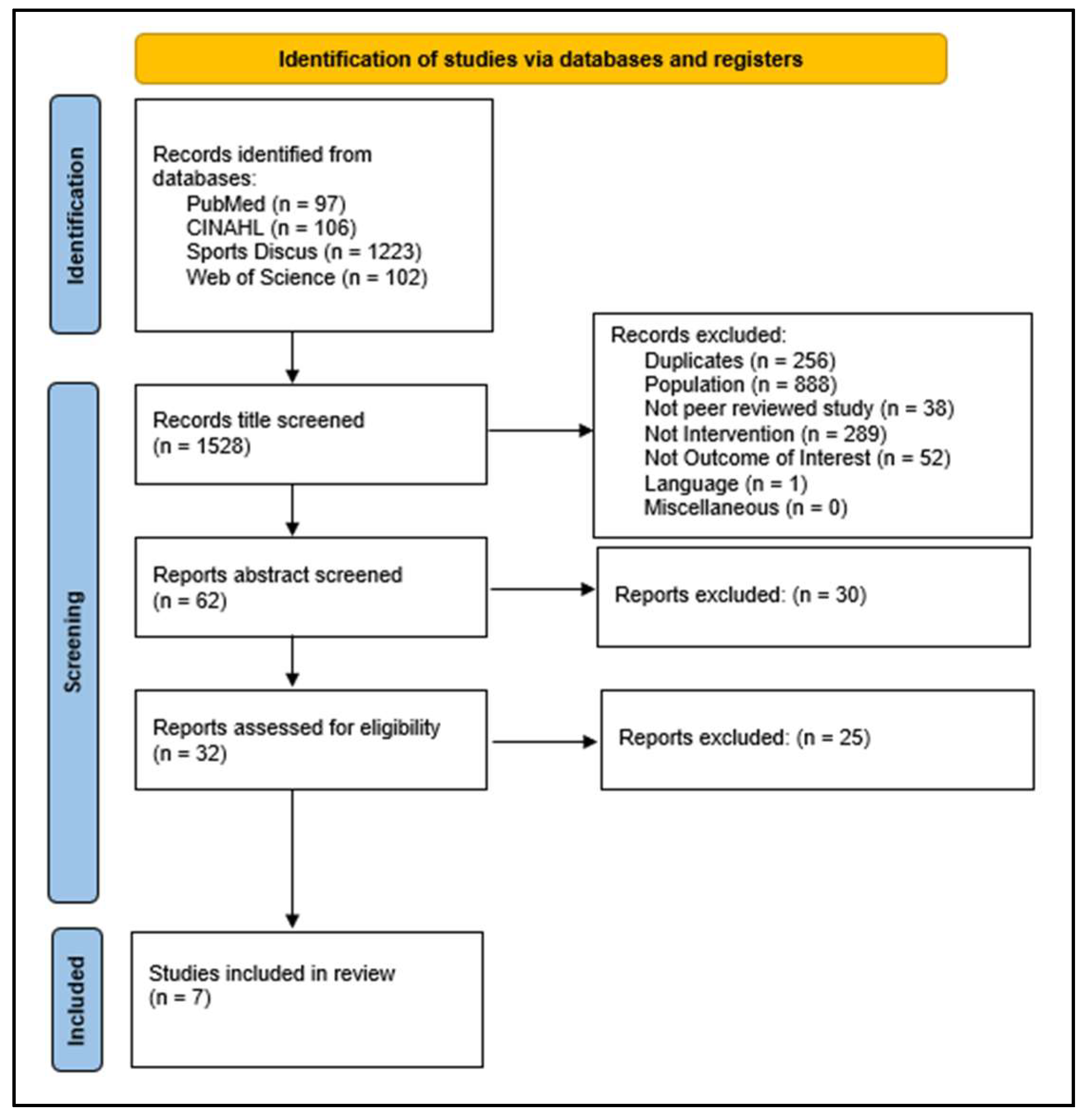
3.1. Flow Diagram of Studies Retrieved for the Review
3.2. Study Selection and Characteristics
4. Synthesized Findings
4.1. Dietary Intervention: Low FODMAP
4.2. Supplementation: Lactose-Free Pre-Exercise Meal
4.3. Supplementation: Medium-Chain Triacylglycerol and Carbohydrates
4.4. Adaption: Gut Training for Carbohydrate Tolerance
4.5. Assessment and Risk of Bias
5. Discussion
5.1. Summary of Main Findings
Low FODMAP
5.2. Lactose-Rich Pre-Exercise Meal
5.3. Medium-Chain Triacylglycerol and Carbohydrates
5.4. Repetitive Gut Challenges
5.5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hawley, J.A.; Hopkins, W.G. Aerobic glycolytic and aerobic lipolytic power systems. A new paradigm with implications for endurance and ultraendurance events. Sports Med. 1995, 19, 240–250. [Google Scholar] [PubMed]
- Kreider, R.B. Physiological considerations of ultraendurance performance. Int. J. Sport Nutr. 1991, 1, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Zaryski, C.; Smith, D.J. Training principles and issues for ultra-endurance athletes. Curr. Sports Med. Rep. 2005, 4, 165–170. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, E.; Nikolaidis, P.T.; Rosemann, T.; Knechtle, B. Different Predictor Variables for Women and Men in Ultra-Marathon Running-The Wellington Urban Ultramarathon 2018. Int. J. Environ. Res. Public Health 2019, 16, 1844. [Google Scholar] [CrossRef]
- Williamson, E. Nutritional implications for ultra-endurance walking and running events. Extrem Physiol Med. 2016, 5, 13. [Google Scholar] [CrossRef]
- Ivy, J.L.; Res, P.T.; Sprague, R.C.; Widzer, M.O. Effect of a carbohydrate-protein supplement on endurance performance during exercise of varying intensity. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 382–395. [Google Scholar] [CrossRef]
- Vereinigung, D.U. Ultra Marathon Statistics. 2018. Available online: https://statistik.d-u-v.org/ (accessed on 12 May 2023).
- Thuany, M.; Gomes, T.N.; Villiger, E.; Weiss, K.; Scheer, V.; Nikolaidis, P.T.; Knechtle, B. Trends in Participation, Sex Differences and Age of Peak Performance in Time-Limited Ultramarathon Events: A Secular Analysis. Medicina 2022, 58, 366. [Google Scholar] [CrossRef]
- Baar, K. Nutrition and the adaptation to endurance training. Sports Med. 2014, 44 (Suppl. 1), S5–S12. [Google Scholar] [CrossRef]
- Scheer, V.; Basset, P.; Giovanelli, N.; Vernillo, G.; Millet, G.P.; Costa, R.J.S. Defining Off-road Running: A Position Statement from the Ultra Sports Science Foundation. Int. J. Sports Med. 2020, 41, 275–284. [Google Scholar] [CrossRef]
- Gill, S.K.; Teixeira, A.; Rama, L.; Prestes, J.; Rosado, F.; Hankey, J.; Scheer, V.; Hemmnigs, K.; Ansley-Robson, P.; Costa, R.J.S. Circulatory endotoxin concentration and cytokine profile in response to exertional-heat stress during a multi-stage ultra-marathon competition. Exerc. Immunol. Rev. 2015, 21, 114–128. [Google Scholar]
- Gill, S.K.; Hankey, J.; Wright, A.; Marzak, S.; Hemming, K.; Allerton, D.M.; Ansley-Robson, P.; Costa, R.J.S. The Impact of a 24-h Ultra-Marathon on Circulatory Endotoxin and Cytokine Profile. Int. J. Sports Med. 2015, 36, 688–695. [Google Scholar] [CrossRef]
- Dempster, S.; Britton, R.; Murray, A.; Costa, R.J.S. Case study: Nutrition and hydration status during 4,254 km of running over 78 consecutive days. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Brouns, F.; Beckers, E. Is the gut an athletic organ? Digestion, absorption and exercise. Sports Med. 1993, 15, 242–257. [Google Scholar] [CrossRef] [PubMed]
- ter Steege, R.W.; Van der Palen, J.; Kolkman, J.J. Prevalence of gastrointestinal complaints in runners competing in a long-distance run: An internet-based observational study in 1281 subjects. Scand J. Gastroenterol. 2008, 43, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.E.; Anglem, N.; Roberts, W.; Anson, J.G.; Palmer, C.D.; Walker, R.J.; Cook, C.J.; Cotter, J.D. Intensity and physiological strain of competitive ultra-endurance exercise in humans. J. Sports Sci. 2008, 26, 477–489. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, E.P.; Burini, R.C. Carbohydrate-dependent, exercise-induced gastrointestinal distress. Nutrients 2014, 6, 4191–4199. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.J.S.; Snipe, R.M.J.; Kitic, C.M.; Gibson, P.R. Systematic review: Exercise-induced gastrointestinal syndrome—Implications for health and intestinal disease. Aliment. Pharmacol. Ther. 2017, 46, 246–265. [Google Scholar] [CrossRef]
- Costa, R.J.S.; Hoffman, M.D.; Stellingwerff, T. Considerations for ultra-endurance activities: Part 1- nutrition. Res. Sports Med. 2019, 27, 166–181. [Google Scholar] [CrossRef]
- Snipe, R.M.J.; Khoo, A.; Kitic, C.M.; Gibson, P.R.; Costa, R.J.S. The Impact of Mild Heat Stress During Prolonged Running On Gastrointestinal Integrity, Gastrointestinal Symptoms, Systemic Endotoxin and Cytokine Profiles. Int. J. Sports Med. 2018, 39, 255–263. [Google Scholar] [CrossRef]
- Hermand, E.; Chabert, C.; Hue, O. Ultra-endurance events in tropical environments and countermeasures to optimize performances and health. Int. J. Hyperth. 2019, 36, 752–759. [Google Scholar] [CrossRef]
- McKenna, Z.J.; Gorini Pereira, F.; Gillum, T.L.; Amorim, F.T.; Deyhle, M.R.; Mermier, C.M. High-altitude exposures and intestinal barrier dysfunction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022, 322, R192–R203. [Google Scholar] [CrossRef]
- de Oliveira, E.P.; Burini, R.C.; Jeukendrup, A. Gastrointestinal Complaints During Exercise: Prevalence, Etiology, and Nutritional Recommendations. Sports Med. 2014, 44, 79–85. [Google Scholar] [CrossRef]
- Moses, F.M. The effect of exercise on the gastrointestinal tract. Sports Med. 1990, 9, 159–172. [Google Scholar] [CrossRef]
- Drossman, D.A.; Dumitrascu, D.L. Rome III: New standard for functional gastrointestinal disorders. J. Gastrointest. Liver Dis. 2006, 15, 237. [Google Scholar]
- Thompson, W.G. The road to Rome. Gastroenterology 2006, 130, 1552–1556. [Google Scholar] [CrossRef] [PubMed]
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Lesley, A.H.; Mearin, F.; Spiller, R.C. Functional bowel disorders. Gastroenterology 2006, 130, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- Tim, V.; Premysl, B.; Guy, B. Understanding neuroimmune interactions in disorders of gut–brain interaction: From functional to immune-mediated disorders. Gut 2023, 72, 787. [Google Scholar]
- Drossman, D.A. Functional gastrointestinal disorders: History, pathophysiology, clinical features, and Rome IV. Gastroenterology 2016, 150, 1262–1279.e2. [Google Scholar] [CrossRef] [PubMed]
- Occhipinti, K.; Smith, J.W. Irritable bowel syndrome: A review and update. Clin. Colon Rectal Surg. 2012, 25, 046–052. [Google Scholar] [CrossRef]
- Dunlop, S.P.; Coleman, N.S.; Blackshaw, E.; Perkins, A.C.; Singh, G.; Marsen, C.A.; Spiller, R.C. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2005, 3, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.A.; Camilleri, M.; Mayer, E.A.; Whitehead, W.E. AGA technical review on irritable bowel syndrome. Gastroenterology 2002, 123, 2108–2131. [Google Scholar] [CrossRef] [PubMed]
- Talley, N.J.; Spiller, R. Irritable bowel syndrome: A little understood organic bowel disease? The Lancet 2002, 360, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett Jr, D.R.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Killian, L.A.; Lee, S.-Y. Irritable bowel syndrome is underdiagnosed and ineffectively managed among endurance athletes. Appl.Physiol. Nutr. Metab. 2019, 44, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, N.R.; Di Marco, N.M.; Langley, S. American College of Sports Medicine position stand. Nutrition and athletic performance. Med. Sci. Sports Exerc. 2009, 41, 709–731. [Google Scholar] [PubMed]
- Costa, M.S.; Toscano, L.T.; Toscano, L.L.T.; Luna, V.R.; Torres, R.A.; Silva, J.S.; Silva, A.S. Ergogenic potential of foods for performance and recovery: A new alternative in sports supplementation? A systematic review. Crit. Rev. Food Sci. Nutr. 2022, 62, 1480–1501. [Google Scholar] [CrossRef]
- Nikolaidis, P.T.; Veniamakis, E.; Rosemann, T.; Knechtle, B. Nutrition in Ultra-Endurance: State of the Art. Nutrients 2018, 10, 1995. [Google Scholar] [CrossRef] [PubMed]
- Scrivin, R.; Costa, R.J.S.; Pelly, F.; Lis, D.; Slater, G. An exploratory study of the management strategies reported by endurance athletes with exercise-associated gastrointestinal symptoms. Front. Nutr. 2022, 9, 1003445. [Google Scholar] [CrossRef]
- Parnell, J.A.; Wagner-Jones, K.; Madden, R.F.; Erdman, K.A. Dietary restrictions in endurance runners to mitigate exercise-induced gastrointestinal symptoms. J. Int. Soc. Sports Nutr. 2020, 17, 32. [Google Scholar] [CrossRef]
- Tiller, N.B.; Roberts, J.D.; Beasley, L.; Chapman, S.; Pinto, J.M.; Smith, L.; Wiffin, M.; Russell, M.; Sparks, S.A.; Duckworth, L.; et al. International Society of Sports Nutrition Position Stand: Nutritional considerations for single-stage ultra-marathon training and racing. J. Int. Soc. Sports Nutr. 2019, 16, 50. [Google Scholar] [CrossRef]
- Costa, R.J.S.; Knechtle, B.; Tarnopolsky, M.; Hoffman, M.D. Nutrition for Ultramarathon Running: Trail, Track, and Road. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 130–140. [Google Scholar] [CrossRef]
- Saturni, L.; Ferretti, G.; Bacchetti, T. The gluten-free diet: Safety and nutritional quality. Nutrients 2010, 2, 16–34. [Google Scholar] [CrossRef]
- Stevens, L.; Rashid, M. Gluten-free and regular foods: A cost comparison. Can. J. Diet Pract. Res. 2008, 69, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Vitale, K.; Getzin, A. Nutrition and Supplement Update for the Endurance Athlete: Review and Recommendations. Nutrients 2019, 11, 1289. [Google Scholar] [CrossRef] [PubMed]
- Lis, D.M.; Stellingwerff, T.; Shing, C.M.; Ahuja, K.D.K.; Fell, J.W. Exploring the popularity, experiences, and beliefs surrounding gluten-free diets in nonceliac athletes. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 37–45. [Google Scholar] [CrossRef]
- Wiffin, M.; Smith, L.; Antonio, J.; Johnstone, J.; Beasley, L.; Roberts, J. Effect of a short-term low fermentable oligiosaccharide, disaccharide, monosaccharide and polyol (FODMAP) diet on exercise-related gastrointestinal symptoms. J. Int. Soc. Sports Nutr. 2019, 16, 1. [Google Scholar] [CrossRef]
- Erdman, K.A.; Jones, K.W.; Madden, R.F.; Gammack, N.; Parnell, J.A. Dietary Patterns in Runners with Gastrointestinal Disorders. Nutrients 2021, 13, 448. [Google Scholar] [CrossRef]
- Saaiq, M.; Ashraf, B. Modifying “Pico” Question into “Picos” Model for More Robust and Reproducible Presentation of the Methodology Employed in A Scientific Study. World J. Plast Surg. 2017, 6, 390–392. [Google Scholar] [PubMed]
- Association, A.D. ADA Quality Criteria Checklist: Primary Research; American Dietetic Association: Chicago, IL, USA, 2008. [Google Scholar]
- Farrah, K.; Young, K.; Tunis, M.C.; Zhao, L. Risk of bias tools in systematic reviews of health interventions: An analysis of PROSPERO-registered protocols. Syst. Rev. 2019, 8, 280. [Google Scholar] [CrossRef]
- Gaskell, S.K.; Costa, R.J.S. Applying a Low-FODMAP Dietary Intervention to a Female Ultraendurance Runner With Irritable Bowel Syndrome during a Multistage Ultramarathon. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 61–67. [Google Scholar] [CrossRef]
- Haakonssen, E.C.; Ross, M.L.; Cato, L.E.; Nana, A.; Knight, E.J.; Jenkins, D.G.; Martin, D.T.; Burke, L.M. Dairy-Based Preexercise Meal Does Not Affect Gut Comfort or Time-Trial Performance in Female Cyclists. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.; Aitchison, T.; Henderson, E.; Zare, S.; McMurray, J.; Dargie, H. A comparison of the reproducibility and the sensitivity to change of visual analogue scales, Borg scales, and Likert scales in normal subjects during submaximal exercise. Chest 1999, 116, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Storhaug, C.L.; Fosse, S.K.; Fadnes, L.T. Country, regional, and global estimates for lactose malabsorption in adults: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2017, 2, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Lis, D.; Ahuja, K.D.; Stellingwerff, T.; Kitic, C.M.; Fell, J. Food avoidance in athletes: FODMAP foods on the list. Appl. Physiol. Nutr. Metab. 2016, 41, 1002–1004. [Google Scholar] [CrossRef]
- Russo, I.; Della Gatta, P.A.; Garnham, A.; Porter, J.; Burke, L.M.; Costa, R.J.S. Does the Nutritional Composition of Dairy Milk Based Recovery Beverages Influence Post-exercise Gastrointestinal and Immune Status, and Subsequent Markers of Recovery Optimisation in Response to High Intensity Interval Exercise? Front. Nutr. 2021, 7, 622270. [Google Scholar] [CrossRef] [PubMed]
- Goedecke, J.H.; Clark, V.R.; Noakes, T.D.; Lambert, E.V. The effects of medium-chain triacylglycerol and carbohydrate ingestion on ultra-endurance exercise performance. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 15–27. [Google Scholar] [CrossRef]
- Costa, R.J.S.; Miall, A.; Khoo, A.; Rauch, C.; Snipe, R.; Camões-Costa, V.; Gibson, P. Gut-training: The impact of two weeks repetitive gut-challenge during exercise on gastrointestinal status, glucose availability, fuel kinetics, and running performance. Appl. Physiol. Nutr. Metab. 2017, 42, 547–557. [Google Scholar] [CrossRef]
- Costa, R.; Snipe, R.; Camões-Costa, V.; Scheer, V.; Murray, A. The Impact of Gastrointestinal Symptoms and Dermatological Injuries on Nutritional Intake and Hydration Status during Ultramarathon Events. Sports Med.-Open 2016, 2, 1–14. [Google Scholar] [CrossRef]
- Miall, A.; Khoo, A.; Rauch, C.; Snipe, R.M.J.; Camões-Costa, V.L.; Gibson, P.R.; Costa, R.J.S. Two weeks of repetitive gut-challenge reduce exercise-associated gastrointestinal symptoms and malabsorption. Scand. J. Med. Sci. Sports 2018, 28, 630–640. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Whelan, K. The low FODMAP diet: Recent advances in understanding its mechanisms and efficacy in IBS. Gut 2017, 66, 1517–1527. [Google Scholar] [CrossRef]
- Gibson, P.R. History of the low FODMAP diet. J. Gastroenterol. Hepatol. 2017, 32, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, T.; Mulligan, K.; Wada, L.; Schumacher, L.; Kretchmer, N. The effect of exercise on fructose absorption. Am. J. Clin. Nutr. 1993, 58, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Varjú, P.; Farkas, N.; Hegyi, P.; Garami, A.; Szabó, I.; Illés, A.; Solymár, M.; Vincze, Á.; Balaskó, M.; Pár, G.; et al. Low fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) diet improves symptoms in adults suffering from irritable bowel syndrome (IBS) compared to standard IBS diet: A meta-analysis of clinical studies. PLoS ONE 2017, 12, e0182942. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, P.; Zhang, L.; Hou, X. A Low-FODMAP Diet Improves the Global Symptoms and Bowel Habits of Adult IBS Patients: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 683191. [Google Scholar] [CrossRef] [PubMed]
- Lis, D.; Ahuja, K.D.; Stellingwerff, T.; Kitic, C.K.; Fell, J. Case Study: Utilizing a Low FODMAP Diet to Combat Exercise-Induced Gastrointestinal Symptoms. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 481–487. [Google Scholar] [CrossRef]
- Lis, D.M.; Stellingwerff, T.; Kitic, C.M.; Fell, J.W.; Ahuja, K.D.K. Low FODMAP: A Preliminary Strategy to Reduce Gastrointestinal Distress in Athletes. Med. Sci. Sports Exerc. 2018, 50, 116–123. [Google Scholar] [CrossRef]
- Gaskell, S.K.; Taylor, B.; Muir, J.; Costa, R.J.S. Impact of 24-h high and low fermentable oligo-, di-, monosaccharide, and polyol diets on markers of exercise-induced gastrointestinal syndrome in response to exertional heat stress. Appl.Physiol. Nutr. Metab. 2020, 45, 569–580. [Google Scholar] [CrossRef]
- King, A.J.; Etxebarria, N.; Ross, M.L.; Garvican-Lewis, L.; Heikura, I.A.; McKay, A.K.A.; Tee, N.; Forbes, S.F.; Beard, N.A.; Saunders, P.U.; et al. Short-Term Very High Carbohydrate Diet and Gut-Training Have Minor Effects on Gastrointestinal Status and Performance in Highly Trained Endurance Athletes. Nutrients 2022, 14, 1929. [Google Scholar] [CrossRef]
- Rozenberg, S.; Body, J.J.; Bruyère, O.; Bergmann, P.; Brandi, M.L.; Cooper, C.; Devogelear, J.; Gilen, E.; Goemaere, S.; Kaufmann, J.; et al. Effects of Dairy Products Consumption on Health: Benefits and Beliefs--A Commentary from the Belgian Bone Club and the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases. Calcif. Tissue Int. 2016, 98, 1–17. [Google Scholar] [CrossRef]
- Périard, J.D.; Eijsvogels, T.M.H.; Daanen, H.A.M. Exercise under heat stress: Thermoregulation, hydration, performance implications, and mitigation strategies. Physiol. Rev. 2021, 101, 1873–1979. [Google Scholar] [CrossRef]
- Akerman, A.P.; Tipton, M.; Minson, C.T.; Cotter, J.D. Heat stress and dehydration in adapting for performance: Good, bad, both, or neither? Temperature 2016, 3, 412–436. [Google Scholar] [CrossRef]
- Passos, B.N.; Lima, M.C.; Sierra, A.P.R.; Oliviera, R.A.; Maciel, J.F.S.; Manoel, R.; Rogante, J.I.; Pesquero, J.B.; Cury-Boaventura, M.F. Association of Daily Dietary Intake and Inflammation Induced by Marathon Race. Mediators Inflamm. 2019, 2019, 1537274. [Google Scholar] [CrossRef]
- Hew-Butler, T.; Loi, V.; Pani, A.; Rosner, M.H. Exercise-Associated Hyponatremia: 2017 Update. Front. Med. 2017, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, B.; Cotterill, A.; Grathwohl, D.; Stellingwerff, T.; Jeukendrup, A.E. The effect of carbohydrate gels on gastrointestinal tolerance during a 16-km run. Int. J. Sport. Nutr. Exerc. Metab. 2009, 19, 485–503. [Google Scholar] [CrossRef] [PubMed]
- Knapik, J.J.; Steelman, R.A.; Hoedebecke, S.S.; Austin, K.G.; Farina, E.K.; Lieberman, H.R. Prevalence of Dietary Supplement Use by Athletes: Systematic Review and Meta-Analysis. Sports Med. 2016, 46, 103–123. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Castell, L.M.; Casa, D.J.; Close, G.L.; Costa, R.J.S.; Desbrow, B.; Halson, S.L.; Lis, D.M.; Melin, A.K.; Peeling, P.; et al. International Association of Athletics Federations Consensus Statement 2019: Nutrition for Athletics. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E.; Thielen, J.J.; Wagenmakers, A.J.; Brouns, F.; Saris, W.H. Effect of medium-chain triacylglycerol and carbohydrate ingestion during exercise on substrate utilization and subsequent cycling performance. Am. J. Clin. Nutr. 1998, 67, 397–404. [Google Scholar] [CrossRef]
- Décombaz, J.; Arnaud, M.J.; Milon, H.; Moesch, H.; Philippossian, G.; Thélin, A.L.; Howald, H. Energy metabolism of medium-chain triglycerides versus carbohydrates during exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1983, 52, 9–14. [Google Scholar] [CrossRef]
- Nosaka, N.; Suzuki, Y.; Suemitsu, H.; Kasai, M.; Kato, K.; Taguchi, M. Medium-chain Triglycerides with Maltodextrin Increase Fat Oxidation during Moderate-intensity Exercise and Extend the Duration of Subsequent High-intensity Exercise. J. Oleo Sci. 2018, 67, 1455–1462. [Google Scholar] [CrossRef]
- Berning, J.R. The role of medium-chain triglycerides in exercise. Int. J. Sport Nutr. 1996, 6, 121–133. [Google Scholar] [CrossRef]
- Jeukendrup, A.E. Carbohydrate and exercise performance: The role of multiple transportable carbohydrates. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Goedecke, J.H.; Elmer-English, R.; Dennis, S.C.; Schloss, I.; Noakes, T.D.; Lambert, E.V. Effects of medium-chain triaclyglycerol ingested with carbohydrate on metabolism and exercise performance. Int. J. Sport Nutr. 1999, 9, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jäger, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E.; et al. ISSN exercise & sports nutrition review update: Research & recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 38. [Google Scholar] [PubMed]
- Jeukendrup, A.E.; McLaughlin, J. Carbohydrate ingestion during exercise: Effects on performance, training adaptations and trainability of the gut. Nestle Nutr. Inst. Workshop Ser. 2011, 69, 1–12. [Google Scholar]
- Jeukendrup, A.E. Training the Gut for Athletes. Sports Med. 2017, 47 (Suppl. 1), 101–110. [Google Scholar] [CrossRef]
- Fairer-Wessels, F.A. Motivation and behaviour of serious leisure participants: The case of the comrades marathon. South Afr. J. Res. Sport Phys. Educ. Recreat. 2013, 35, 83–103. [Google Scholar]
- Heaney, S.; O’Connor, H.; Michael, S.; Gifford, J.; Naughton, G. Nutrition knowledge in athletes: A systematic review. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 248–261. [Google Scholar] [CrossRef]
- Scholz, H.; Sousa, C.V.; Baumgartner, S.; Rosemann, T.; Knechtle, B. Changes in Sex Difference in Time-Limited Ultra-Cycling Races from 6 Hours to 24 Hours. Medicina 2021, 57, 923. [Google Scholar] [CrossRef]
- Knechtle, B.; Knechtle, P.; Lepers, R. Participation and performance trends in ultra-triathlons from 1985 to 2009. Scand. J. Med. Sci. Sports 2011, 21, e82–e90. [Google Scholar] [CrossRef]
- Colditz, G.A. Overview of the Epidemiology Methods and Applications: Strengths and Limitations of Observational Study Designs. Crit. Rev. Food Sci. Nutr. 2010, 50 (Suppl. 1), 10–12. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
| Topic | Criteria | |
|---|---|---|
| P | Population | Humans ≥18 years old are considered “ultra-endurance athletes” by the research authors. |
| I | Intervention | Prescribed nutritional intervention to alter gastrointestinal symptoms. |
| C | Comparators | None/placebo. |
| O | Outcomes | Altered gastrointestinal health (prevalence, duration, and severity of GI symptoms). |
| S | Study design | Prospective randomized controlled trials, crossover trials, case studies. |
| Concept 1: “ultra-endurance” OR “ultra-athlete” OR ironman OR “ultra-endurance training” OR “ultra-distance” OR “ultramarathon” OR “ultra-event” |
| Concept 2: “nutritional intervention” OR nutrition* OR food OR diet OR diets OR “dietary pattern” OR carbohydrate* OR fat OR fats OR “fatty acids” OR “dietary fats” OR protein* OR antioxidant* OR supplement* OR fasting OR hydration OR drink* OR beverage* OR energy OR macronutrient* OR micronutrient* OR keto* OR glucose OR sugar OR calorie* OR prebiotic OR probiotic |
| Concept 3: gastrointestinal OR “gastrointestinal problems” OR “gastrointestinal symptoms” OR “gastrointestinal events” OR “GI” OR “GI problems” OR “GI symptoms” OR “GI events” OR vomiting OR diarrhea OR constipation OR nausea OR “abdominal pain” OR pain OR discomfort OR ache OR stitch OR bloating OR reflux OR cramp* OR abdominal OR digest* OR stomach OR intestinal OR gut OR “gut microbiome” OR microbiota OR “nutritional manipulation” |
| Trial (First Author) | Year | n | Sex | Characteristics | Setting/Condition | Duration of Supplement Use/Dietary Intervention in Days | Diet Labels | Energy | CHO | Protein | Fat | Other | GIS Recordings | Main Findings | ADA Quality Assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Goedecke | 2005 | 9 | M | Competitive ultra-cyclists. | Laboratory | 2 (1 day with each supplement). | Medium-chain triacylglycerol (MCT) | - | - | - | - | Pre: 32 g MCT, In: 200 mL 4.3% MCT, 10% CHO solution every 20 m. | 0–3 scale. | 50% of participants suffered GIS during or after MCT trial (n = 1 mild and n = 3 severe). No GIS reported during control trial. | + |
| Control | - | Pre: 75 g, In: 200 mL 10% CHO solution every 20 m | - | - | - | ||||||||||
| Gaskell | 2019 | 1 | F | Recreational ultrarunner. IBS diagnosed. | Field | 7 | Pre-trial | - | - | - | - | FODMAPs: 3.9 g. | 100 mm VAS at rest and during training. | Successfully implemented low-FODMAP diet. Severe GIS during training but modest GIS (bloating and flatulence) during race. Severe nausea (potentially due to low energy intake). | + |
| During trial | - | - | - | - | FODMAPS (excluding during racing): 5.1 g. | ||||||||||
| Placebo (Water) | - | - | - | - | Water. | ||||||||||
| Gaskell | 2020 | 18 | MF | Endurance and ultra-endurance runners. | Laboratory | 1 day on each diet. | High FODMAP | 2645 ± 747 g | 405 ± 124 g | 102 ± 29 g | 68 ± 18 g | FODMAPs: 46.9 ± 26.2 g. | 10-point modified VAS every 15 mins. | GIS severity (p = 0.056), total (p = 0.014), upper (p = 0.019), and lower (p = 0.006) were higher as a whole in response to the high-FODMAP diet. | + |
| Low FODMAP | 2375 ± 538 g | 355 ± 89 g | 91 ± 23 g | 66 ± 15 g | FODMAPs: 2.0 ± 0.7 g. | ||||||||||
| Haakonssen | 2014 | 25 | F | Competitive cyclists. | Laboratory | 2 (1 day with each supplement). | Dairy | 54 ± 2 kJ·kg−¹ | 2 ± 0 g·kg−¹ | 0.6 ± 0.1 g·kg−¹ | 0.3 ± 0.0 g·kg−¹ | Pre-exercise meal. | 100 mm VAS; 5 different timepoints. | No significantly significant association between pre-trial gut discomfort and meal type (p = 0.15). No statistically significant association between gut comfort delta scores and meal type at 30 min (p = 0.31) or 60 min (p = 0.17) post-meal. Dairy meal may be more palatable. | + |
| Control | 54 ± 2 kJ·kg−¹ | 2 ± 0 g·kg−¹ | 0.2 ± 0.0 g·kg−¹ | 0.4 ± 0.0 g·kg−¹ | Pre-exercise meal. | ||||||||||
| High-CHO, low-frequency | - | 2.4 g/min | - | - | 5 min pre-race, every 15 km in race. | ||||||||||
| Moderate-CHO, high frequency | - | 1.2 g/min | - | - | 5 min pre-race, every 5 km in race. | ||||||||||
| Moderate-CHO, low frequency | - | 1.2 g/min | - | - | 5 min pre-race, every 15 km in race. | ||||||||||
| Russo | 2021 | 9 | MF | Recreationally and competitively trained endurance and ultra-endurance athletes. | Field | 8 | Low FODMAP (24 h pre- and throughout trial) | - | 364 g | 101 g | 32 g | FODMAPS: <2 g per meal. Those with greater BMed were provided with additional meal servings/snacks. | Modified VAS. On day 1: pre-trials, during the final 30 s of cycling, and every 30 min during recovery period. On day 2: on arrival and after performance test. | Greater total gut discomfort on MBSB (p = 0.053). No significant effects or interactions observed for upper-GIS, nausea, or total-GIS. | + |
| Chocolate-flavored dairy milk [54] | 2715 kJ | 92 g | 30 g | 17 g | Served in 3 equal boluses every 10 min, beginning 30 min into recovery. | ||||||||||
| Chocolate-flavored dairy milk-based supplement drink (MBSB) | 4029 kJ | 170 g | 63 g | 2 g | |||||||||||
| Costa | 2017 | 25 | MF | 10F and 15M. Recreationally competitive endurance and ultra-endurance runners. | Field | 14 (2 weeks in 1 of 3 protocols). | CHO-gel disk (CHO-S) | - | 30 g | - | - | While running for 10 days over 2 weeks. | 10-point Likert-type rating scale. | No significant differences in GIS between groups in trial 1 and 2. Significant improvements in gut discomfort (p = 0.018), total GIS (p = 0.003), upper GIS (p = 0.043), lower GIS (p = 0.010), and nausea (p = 0.050) observed in CHO-S and CHO-F compared with placebo. | + |
| CHO-food (CHO-F) | - | 30 g | - | - | While running for 10 days over 2 weeks. | ||||||||||
| Placebo | - | 0 g | - | - | While running for 10 days over 2 weeks. | ||||||||||
| Miall | 2017 | 18 | MF | 10M and 8FM. Recreationally competitive endurance and ultra-endurance runners. | Field | 10 (5 with supplement, 2 resting without supplement, 5 more with supplement, 2 more resting without supplement). | CHO-gel disc (CHO) | - | 30 g | - | - | At 0, 20, and 40 min. | 10-point Likert-type rating scale for feeding tolerance. Every 10 mins during gut trials. | All participants reported at least one GIS during pre-intervention trial. 67% reported at least one of these as severe. GIS more common in CHO (80%) than PLA (50%). Subjects accustomed to CHO during training reported significantly reduced gut discomfort (p = 0.001), total (p = 0.002), upper (p = 0.001) GIS, and nausea (p = 0.026). Significant reduction in gut discomfort on CHO (p < 0.001). No gut discomfort improvements observed on PLA. | + |
| Placebo (PLA) | - | 0 g | - | - | At 0, 20, and 40 min. | ||||||||||
| Gel | - | - | - | - | Glucose/fructose in 2:1 ratio. | ||||||||||
| Drink mix powder | - | - | - | - | Glucose/fructose in 2:1 ratio. | ||||||||||
| Control | - | - | - | - | Glucose/maltodextrin only. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryan, T.; Daly, E.; Ryan, L. Exploring the Nutrition Strategies Employed by Ultra-Endurance Athletes to Alleviate Exercise-Induced Gastrointestinal Symptoms—A Systematic Review. Nutrients 2023, 15, 4330. https://doi.org/10.3390/nu15204330
Ryan T, Daly E, Ryan L. Exploring the Nutrition Strategies Employed by Ultra-Endurance Athletes to Alleviate Exercise-Induced Gastrointestinal Symptoms—A Systematic Review. Nutrients. 2023; 15(20):4330. https://doi.org/10.3390/nu15204330
Chicago/Turabian StyleRyan, Tansy, Ed Daly, and Lisa Ryan. 2023. "Exploring the Nutrition Strategies Employed by Ultra-Endurance Athletes to Alleviate Exercise-Induced Gastrointestinal Symptoms—A Systematic Review" Nutrients 15, no. 20: 4330. https://doi.org/10.3390/nu15204330
APA StyleRyan, T., Daly, E., & Ryan, L. (2023). Exploring the Nutrition Strategies Employed by Ultra-Endurance Athletes to Alleviate Exercise-Induced Gastrointestinal Symptoms—A Systematic Review. Nutrients, 15(20), 4330. https://doi.org/10.3390/nu15204330








