Effect of Nutritional Deprivation after Sleeve Gastrectomy on Bone Mass, Periostin, Sclerostin and Semaphorin 4D: A Two-Year Longitudinal Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Method
2.1.1. Subjects
2.1.2. Methods
2.1.3. Comorbidities Were Defined According to the Usual Definitions
2.1.4. Assays
2.1.5. Areal Bone Mineral Density
Osteopenia and Osteoporosis Definitions
2.1.6. Statistical Analysis
3. Results
3.1. Anthropometric Parameters
3.2. Biological Parameters
3.3. Areal Bone Mineral Density
3.4. Predictors of aBMD Variation
4. Discussion
4.1. aBMD
4.2. Bone Markers
4.3. Factors Influencing aBMD Loss
4.4. Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andersen, S.; Frederiksen, K.D.; Hansen, S.; Brixen, K.; Gram, J.; Stoving, R.K. Bone structure and estimated bone strength in obese patients evaluated by high-resolution peripheral quantitative computed tomography. Calcif. Tissue Int. 2014, 95, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Bredella, M.A.; Torriani, M.; Ghomi, R.H.; Thomas, B.J.; Brick, D.J.; Gerweck, A.V.; Harrington, L.M.; Breggia, A.; Rosen, C.J.; Miller, K.K. Determinants of bone mineral density in obese premenopausal women. Bone 2011, 48, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Sornay-Rendu, E.; Boutroy, S.; Vilayphiou, N.; Claustrat, B.; Chapurlat, R.D. In obese postmenopausal women, bone microarchitecture and strength are not commensurate to greater body weight: The Os des Femmes de Lyon (OFELY) study. J. Bone Miner. Res. 2013, 28, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.L.; Paggiosi, M.A.; Eastell, R.; Walsh, J.S. Bone Density, Microstructure and Strength in Obese and Normal Weight Men and Women in Younger and Older Adulthood. J. Bone Miner. Res. 2015, 30, 920–928. [Google Scholar] [CrossRef]
- Beck, T.J.; Petit, M.A.; Wu, G.; LeBoff, M.S.; Cauley, J.A.; Chen, Z. Does obesity really make the femur stronger? BMD, geometry, and fracture incidence in the women’s health initiative-observational study. J. Bone Miner. Res. 2009, 24, 1369–1379. [Google Scholar] [CrossRef]
- Maimoun, L.; Mura, T.; Leprieur, E.; Avignon, A.; Mariano-Goulart, D.; Sultan, A. Impact of obesity on bone mass throughout adult life: Influence of gender and severity of obesity. Bone 2016, 90, 23–30. [Google Scholar] [CrossRef]
- Maimoun, L.; Mura, T.; Attalin, V.; Dupuy, A.M.; Cristol, J.P.; Avignon, A.; Mariano-Goulart, D.; Sultan, A. Modification of Muscle-Related Hormones in Women with Obesity: Potential Impact on Bone Metabolism. J. Clin. Med. 2020, 9, 1150. [Google Scholar] [CrossRef]
- Frost, H.M. The role of changes in mechanical usage set points in the pathogenesis of osteoporosis. J. Bone Miner. Res. 1992, 7, 253–261. [Google Scholar] [CrossRef]
- Kontogianni, M.D.; Dafni, U.G.; Routsias, J.G.; Skopouli, F.N. Blood leptin and adiponectin as possible mediators of the relation between fat mass and BMD in perimenopausal women. J. Bone Miner. Res. 2004, 19, 546–551. [Google Scholar] [CrossRef]
- Pasco, J.A.; Henry, M.J.; Kotowicz, M.A.; Collier, G.R.; Ball, M.J.; Ugoni, A.M.; Nicholson, G.C. Serum leptin levels are associated with bone mass in nonobese women. J. Clin. Endocrinol. Metab. 2001, 86, 1884–1887. [Google Scholar] [CrossRef]
- Reid, I.R. Relationships among body mass, its components, and bone. Bone 2002, 31, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Bredella, M.A.; Lin, E.; Gerweck, A.V.; Landa, M.G.; Thomas, B.J.; Torriani, M.; Bouxsein, M.L.; Miller, K.K. Determinants of bone microarchitecture and mechanical properties in obese men. J. Clin. Endocrinol. Metab. 2012, 97, 4115–4122. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, M.W.; McPherron, A.C.; Lovejoy, C.O. Bone mineral content and density in the humerus of adult myostatin-deficient mice. Calcif. Tissue Int. 2002, 71, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Paccou, J.; Tsourdi, E.; Meier, C.; Palermo, A.; Pepe, J.; Body, J.J.; Zillikens, M.C. Bariatric surgery and skeletal health: A narrative review and position statement for management by the European Calcified Tissue Society (ECTS). Bone 2022, 154, 116236. [Google Scholar] [CrossRef]
- Shanbhogue, V.V.; Stoving, R.K.; Frederiksen, K.H.; Hanson, S.; Brixen, K.; Gram, J.; Jorgensen, N.R.; Hansen, S. Bone structural changes after gastric bypass surgery evaluated by HR-pQCT: A two-year longitudinal study. Eur. J. Endocrinol. 2017, 176, 685–693. [Google Scholar] [CrossRef]
- Hofso, D.; Hillestad, T.O.W.; Halvorsen, E.; Fatima, F.; Johnson, L.K.; Lindberg, M.; Svanevik, M.; Sandbu, R.; Hjelmesaeth, J. Bone Mineral Density and Turnover After Sleeve Gastrectomy and Gastric Bypass: A Randomized Controlled Trial (Oseberg). J. Clin. Endocrinol. Metab. 2021, 106, 501–511. [Google Scholar] [CrossRef]
- Paccou, J.; Thuillier, D.; Courtalin, M.; Pigny, P.; Labreuche, J.; Cortet, B.; Pattou, F. A comparison of changes in bone turnover markers after gastric bypass and sleeve gastrectomy, and their association with markers of interest. Surg. Obes. Relat. Dis. 2022, 18, 373–383. [Google Scholar] [CrossRef]
- Paccou, J.; Martignene, N.; Lespessailles, E.; Babykina, E.; Pattou, F.; Cortet, B.; Ficheur, G. Gastric Bypass But Not Sleeve Gastrectomy Increases Risk of Major Osteoporotic Fracture: French Population-Based Cohort Study. J. Bone Miner. Res. 2020, 35, 1415–1423. [Google Scholar] [CrossRef]
- Gagnon, C.; Schafer, A.L. Bone Health After Bariatric Surgery. JBMR Plus 2018, 2, 121–133. [Google Scholar] [CrossRef]
- Fleischer, J.; Stein, E.M.; Bessler, M.; Della Badia, M.; Restuccia, N.; Olivero-Rivera, L.; McMahon, D.J.; Silverberg, S.J. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J. Clin. Endocrinol. Metab. 2008, 93, 3735–3740. [Google Scholar] [CrossRef][Green Version]
- Muschitz, C.; Kocijan, R.; MArterer, C.; Nia, A.R.; Muschitz, G.K.; Resch, H.; Pietschmann, P. Sclerostin levels and changes in bone metabolism after bariatric surgery. J. Clin. Endocrinol. Metab. 2015, 100, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Barbe, M.F.; Barr, A.E.; Litvin, J. Periostin-like-factor and Periostin in an animal model of work-related musculoskeletal disorder. Bone 2009, 44, 502–512. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bonnet, N.; Standley, K.N.; Bianchi, E.N.; Stadelmann, V.; Foti, M.; Conway, S.J.; Ferrari, S.L. The matricellular protein periostin is required for sost inhibition and the anabolic response to mechanical loading and physical activity. J. Biol. Chem. 2009, 284, 35939–35950. [Google Scholar] [CrossRef]
- Zhu, S.; Barbe, M.F.; Liu, C.; Hadjiargyrou, M.; Popoff, S.N.; Rani, S.; Safadi, F.F.; Litvin, J. Periostin-like-factor in osteogenesis. J. Cell. Physiol. 2009, 218, 584–592. [Google Scholar] [CrossRef]
- Maimoun, L.; Garnero, P.; Mura, T.; Nocca, D.; Lefebvre, P.; Philibert, P.; Seneque, M.; Gaspari, L.; Vauchot, F.; Courtet, P.; et al. Specific Effects of Anorexia Nervosa and Obesity on Bone Mineral Density and Bone Turnover in Young Women. J. Clin. Endocrinol. Metab. 2020, 105, e1536–e1548. [Google Scholar] [CrossRef]
- Caswell-Smith, R.; Hosking, A.; Cripps, T.; Holweg, C.; Matthews, J.; Holliday, M.; Maillot, C.; Fingleton, J.; Weatherall, M.; Braithwaite, I.; et al. Reference ranges for serum periostin in a population without asthma or chronic obstructive pulmonary disease. Clin. Exp. Allergy 2016, 46, 1303–1314. [Google Scholar] [CrossRef] [PubMed]
- Adabimohazab, R.; Garfinkel, A.; Milam, E.C.; Frosch, O.; Mangone, A.; Convit, A. Does Inflammation Mediate the Association Between Obesity and Insulin Resistance? Inflammation 2016, 39, 994–1003. [Google Scholar] [CrossRef]
- Varughese, R.; Semprini, R.; Munro, C.; Fingleton, J.; Holweg, C.; Weatherall, M.; Beasley, R.; Braithwaite, I. Serum periostin levels following small bone fractures, long bone fractures and joint replacements: An observational study. Allergy Asthma Clin. Immunol. 2018, 14, 30. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, E.; Xu, Y.; Wang, W.; Zhang, T.; Xiao, L.; Chen, R.; Lin, Y.; Chen, D.; Lin, L.; et al. Serum Sema4D levels are associated with lumbar spine bone mineral density and bone turnover markers in patients with postmenopausal osteoporosis. Int. J. Clin. Exp. Med. 2015, 8, 16352–16357. [Google Scholar]
- Negishi-Koga, T.; Shinohara, M.; Komatsu, N.; Bito, H.; Kodama, T.; Friedel, R.H.; Takayanagi, H. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat. Med. 2011, 17, 1473–1480. [Google Scholar] [CrossRef]
- Ray, K.K.; Colhoun, H.M.; Szarek, M.; Baccara-Dinet, M.; Bhatt, D.L.; Bittner, V.A.; Budaj, A.J.; Diaz, R.; Goodman, S.G.; Hanotin, C.; et al. Effects of alirocumab on cardiovascular and metabolic outcomes after acute coronary syndrome in patients with or without diabetes: A prespecified analysis of the ODYSSEY OUTCOMES randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Geldsetzer, P.; Manne-Goehler, J.; Marcus, M.E.; Ebert, C.; Zhumadilov, Z.; Wesseh, C.S.; Tsabedze, L.; Supiyev, A.; Sturua, L.; Bahendeka, S.K.; et al. The state of hypertension care in 44 low-income and middle-income countries: A cross-sectional study of nationally representative individual-level data from 1.1 million adults. Lancet 2019, 394, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Souberbielle, J.C.; Cormier, C.; Cavalier, E.; Breuil, V.; Debiais, F.; Fardellone, P.; Guggenbuhl, P.; Javier, R.M.; Legrand, E.; Lespessailles, E.; et al. Vitamin D Supplementation in France in patients with or at risk for osteoporosis: Recent data and new practices. Jt. Bone Spine 2020, 87, 25–29. [Google Scholar] [CrossRef]
- Reitshamer, E.; Barrett, K.; Shea, K.; Dawson-Hughes, B. Cross-Calibration of Prodigy and Horizon A Densitometers and Precision of the Horizon A Densitometer. J. Clin. Densitom. 2021, 24, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Melton, L.J., 3rd; Christiansen, C.; Johnston, C.C.; Khaltaev, N. The diagnosis of osteoporosis. J. Bone Miner. Res. 1994, 9, 1137–1141. [Google Scholar] [CrossRef]
- Matos, O.; Ruthes, E.M.P.; Malinowski, A.K.C.; Lima, A.L.; Veiga, M.S.; Krause, M.P.; Farah, L.; Souza, C.J.F.; Lass, A.D.; Castelo-Branco, C. Changes in bone mass and body composition after bariatric surgery. Gynecol. Endocrinol. 2020, 36, 578–581. [Google Scholar] [CrossRef]
- Ou, X.; Chen, M.; Xu, L.; Lin, W.; Huang, H.; Chen, G.; Wen, J. Changes in bone mineral density after bariatric surgery in patients of different ages or patients with different postoperative periods: A systematic review and meta-analysis. Eur. J. Med. Res. 2022, 27, 144. [Google Scholar] [CrossRef]
- Hernandez-Martinez, A.; Veras, L.; Boppre, G.; Soriano-Maldonado, A.; Oliveira, J.; Diniz-Sousa, F.; Fonseca, H. Changes in volumetric bone mineral density and bone quality after Roux-en-Y gastric bypass: A meta-analysis with meta-regression. Obes. Rev. 2022, 23, e13479. [Google Scholar] [CrossRef]
- Nimmala, S.; Kaur, S.; Singhal, V.; Mitchell, D.M.; Stanford, F.C.; Bouxsein, M.L.; Lauze, M.; Huynh, C.; Pedreira, C.C.; Lee, H.; et al. Changes in Sex Steroids and Enteric Peptides After Sleeve Gastrectomy in Youth in Relation to Changes in Bone Parameters. J. Clin. Endocrinol. Metab. 2022, 107, e3747–e3758. [Google Scholar] [CrossRef]
- Castro, M.J.; Jimenez, J.M.; Lopez, M.; Cao, M.J.; Gonzalez-Ramirez, G.; de Lourdes Bolanos-Munoz, M.; Ruiz-Tovar, J. Changes in the Bone Mineral Density after Sleeve Gastrectomy vs. Roux-En-Y Gastric Bypass 2 Years after Surgery. Nutrients 2022, 14, 3056. [Google Scholar] [CrossRef]
- Lindeman, K.G.; Rushin, C.C.; Cheney, M.C.; Bouxsein, M.L.; Hutter, M.M.; Yu, E.W. Bone Density and Trabecular Morphology at Least 10 Years after Gastric Bypass and Gastric Banding. J. Bone Miner. Res. 2020, 35, 2132–2142. [Google Scholar] [CrossRef] [PubMed]
- Ben-Porat, T.; Elazary, R.; Sherf-Dagan, S.; Goldenshluger, A.; Brodie, R.; Mintz, Y.; Weiss, R. Bone Health following Bariatric Surgery: Implications for Management Strategies to Attenuate Bone Loss. Adv. Nutr. 2018, 9, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.A.; Salman, A.; Elewa, A.; Rabiee, A.; Tourky, M.; Shaaban, H.E.; Issa, M.; AbdAlla, A.; Khattab, M.; Refaat, A.; et al. Secondary Hyperparathyroidism before and after Bariatric Surgery: A Prospective Study with 2-Year Follow-Up. Obes. Surg. 2022, 32, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Aarts, E.O.; Janssen, I.M.; Berends, F.J. The gastric sleeve: Losing weight as fast as micronutrients? Obes. Surg. 2011, 21, 207–211. [Google Scholar] [CrossRef][Green Version]
- Armamento-Villareal, R.; Sadler, C.; Napoli, N.; Shah, K.; Chode, S.; Sinacore, D.R.; Qualls, C.; Villareal, D.T. Weight loss in obese older adults increases serum sclerostin and impairs hip geometry but both are prevented by exercise training. J. Bone Miner. Res. 2012, 27, 1215–1221. [Google Scholar] [CrossRef]
- Robling, A.G.; Niziolek, P.J.; Baldridge, L.A.; Condon, K.W.; Allen, M.R.; Alam, I.; Mantila, S.M.; Gluhak-Heinrich, J.; Bellido, T.M.; Harris, S.E.; et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J. Biol. Chem. 2008, 283, 5866–5875. [Google Scholar] [CrossRef]
- Krishnan, V.; Bryant, H.U.; Macdougald, O.A. Regulation of bone mass by Wnt signaling. J. Clin. Investig. 2006, 116, 1202–1209. [Google Scholar] [CrossRef]
- Gerbaix, M.; Vico, L.; Ferrari, S.L.; Bonnet, N. Periostin expression contributes to cortical bone loss during unloading. Bone 2015, 71, 94–100. [Google Scholar] [CrossRef]
- Maimoun, L.; Ben Bouallegue, F.; Gelis, A.; Aouinti, S.; Mura, T.; Philibert, P.; Souberbielle, J.C.; Piketty, M.; Garnero, P.; Mariano-Goulart, D.; et al. Periostin and sclerostin levels in individuals with spinal cord injury and their relationship with bone mass, bone turnover, fracture and osteoporosis status. Bone 2019, 127, 612–619. [Google Scholar] [CrossRef]
- Vico, L.; Rietbergen, B.V.; Vilayphiou, N.; Linossier, M.T.; Locrelle, H.; Normand, M.; Zouch, M.; Gerbaix, M.; Bonnet, N.; Novikov, V.; et al. Cortical and trabecular bone microstructure did not recover at weight-bearing skeletal sites and progressively deteriored at non-weight bearing sites during the year following internartional space station missions. J. Bone Miner. Res. 2017, 32, 2010–2021. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Rhee, Y.; Kim, C.H.; Baek, K.H.; Min, Y.K.; Kim, D.Y.; Ahn, S.H.; Kim, H.; Lee, S.H.; Lee, S.Y.; et al. Plasma periostin associates significantly with non-vertebral but not vertebral fractures in postmenopausal women: Clinical evidence for the different effects of periostin depending on the skeletal site. Bone 2015, 81, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Li, J.; Gao, Q.; Ye, F. The role of Sema4D/CD100 as a therapeutic target for tumor microenvironments and for autoimmune, neuroimmune and bone diseases. Expert. Opin. Ther. Targets 2016, 20, 885–901. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Polyzos, S.A.; Makras, P.; Gkiomisi, A.; Sakellariou, G.; Savvidis, M.; Papatheodorou, A.; Kokkoris, P.; Terpos, E. Circulating semaphorin-4D and plexin-B1 levels in postmenopausal women with low bone mass: The 3-month effect of zoledronic acid, denosumab or teriparatide treatment. Expert. Opin. Ther. Targets 2015, 19, 299–306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cadart, O.; Degrandi, O.; Barnetche, T.; Mehsen-Cetre, N.; Monsaingeon-Henry, M.; Pupier, E.; Bosc, L.; Collet, D.; Gronnier, C.; Tremollieres, F.; et al. Long-Term Effects of Roux-en-Y Gastric Bypass and Sleeve Gastrectomy on Bone Mineral Density: A 4-Year Longitudinal Study. Obes. Surg. 2020, 30, 3317–3325. [Google Scholar] [CrossRef] [PubMed]
- Bredella, M.A.; Greenblatt, L.B.; Eajazi, A.; Torriani, M.; Yu, E.W. Effects of Roux-en-Y gastric bypass and sleeve gastrectomy on bone mineral density and marrow adipose tissue. Bone 2017, 95, 85–90. [Google Scholar] [CrossRef]
- Maghrabi, A.H.; Wolski, K.; Abood, B.; Licata, A.; Pothier, C.; Bhatt, D.L.; Nissen, S.; Brethauer, S.A.; Kirwan, J.P.; Schauer, P.R.; et al. Two-year outcomes on bone density and fracture incidence in patients with T2DM randomized to bariatric surgery versus intensive medical therapy. Obesity 2015, 23, 2344–2348. [Google Scholar] [CrossRef]
- Campanha-Versiani, L.; Pereira, D.A.G.; Ribeiro-Samora, G.A.; Ramos, A.V.; de Sander Diniz, M.F.H.; De Marco, L.A.; Soares, M.M.S. The Effect of a Muscle Weight-Bearing and Aerobic Exercise Program on the Body Composition, Muscular Strength, Biochemical Markers, and Bone Mass of Obese Patients Who Have Undergone Gastric Bypass Surgery. Obes. Surg. 2017, 27, 2129–2137. [Google Scholar] [CrossRef]
- Muschitz, C.; Kocijan, R.; Haschka, J.; Zendeli, A.; Pirker, T.; Geiger, C.; Muller, A.; Tschinder, B.; Kocijan, A.; Marterer, C.; et al. The Impact of Vitamin D, Calcium, Protein Supplementation, and Physical Exercise on Bone Metabolism after Bariatric Surgery: The BABS Study. J. Bone Miner. Res. 2016, 31, 672–682. [Google Scholar] [CrossRef]
- Jassil, F.C.; Carnemolla, A.; Kingett, H.; Doyle, J.; Kirk, A.; Lewis, N.; Montagut, G.; Marvasti, P.; Boniface, D.; Brown, A.; et al. Impact of nutritional-behavioral and supervised exercise intervention following bariatric surgery: The BARI-LIFESTYLE randomized controlled trial. Obesity 2023, 31, 2031–2042. [Google Scholar] [CrossRef]
- Chirurgie de L’obésité: 20 Fois Plus D’interventions Depuis. 1997. Available online: https://drees.solidarites-sante.gouv.fr/publications/etudes-et-resultats/chirurgie-de-lobesite-20-fois-plus-dinterventions-depuis-1997 (accessed on 12 July 2023).
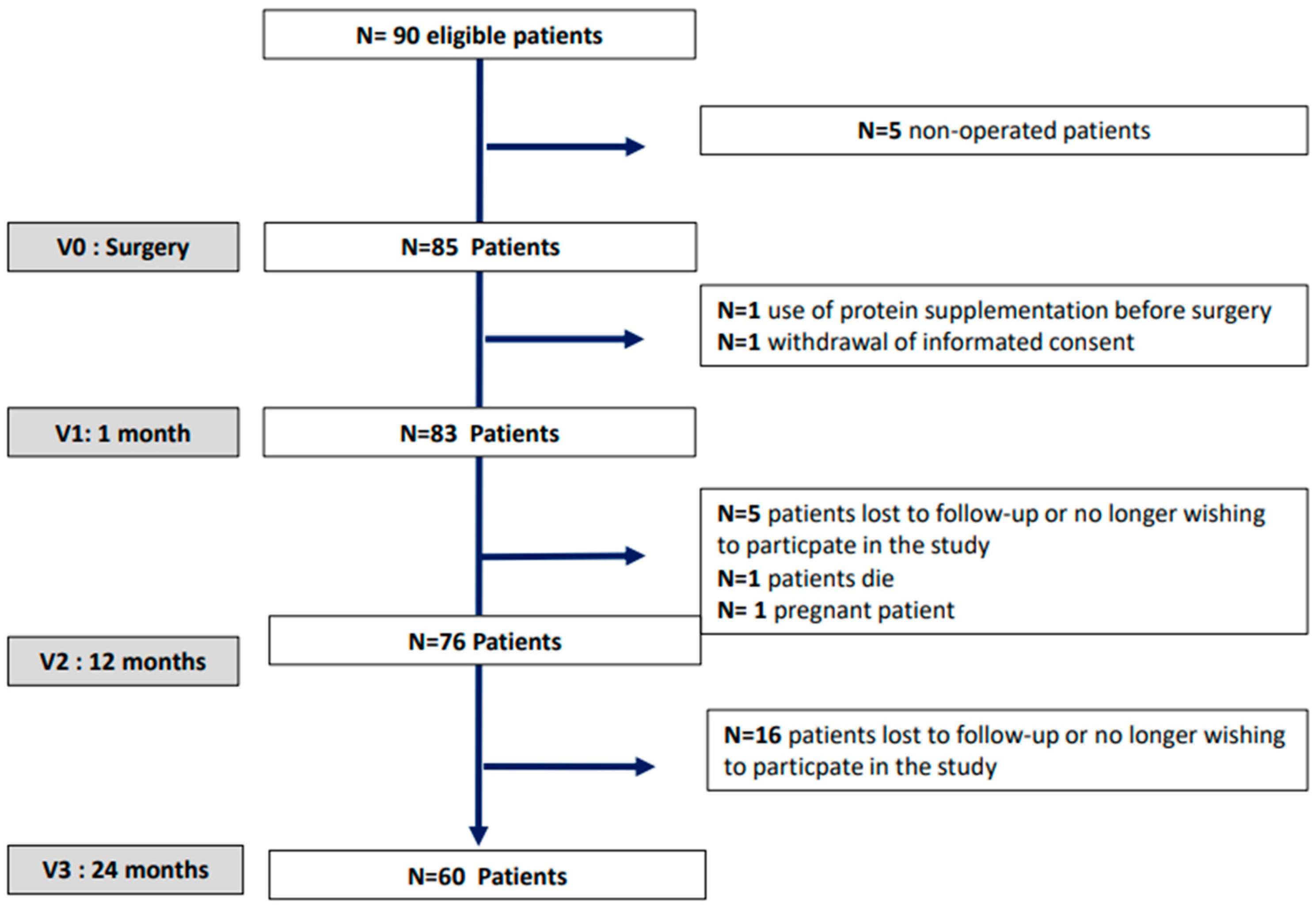
| Baseline (n = 83) | 1-Month (n = 83) | 12-Months (n = 76) | 24-Months (n = 60) | Relative % Variation (Δ 1m-Baseline/Baseline) | Relative % Variation (Δ 12m-Baseline/Baseline) | Relative % Variation (Δ 24m-Baseline/Baseline) | |
|---|---|---|---|---|---|---|---|
| Weight (kg) | 110.9 ± 13.0 | 100.9 ± 12.3 | 79.1 ± 14.2 | 81.9 ± 14.0 | −9.1 ± 2.1 *** | −29.4 ± 8.4 *** | −27.5 ± 9.6 *** |
| BMI (kg/m2) | 40.7 ± 4.2 | 37.1 ± 4.2 | 28.9 ± 4.3 | 29.8 ± 4.5 | −9.1 ± 2.1 *** | −29.4 ± 8.4 *** | −27.6 ± 9.8 *** |
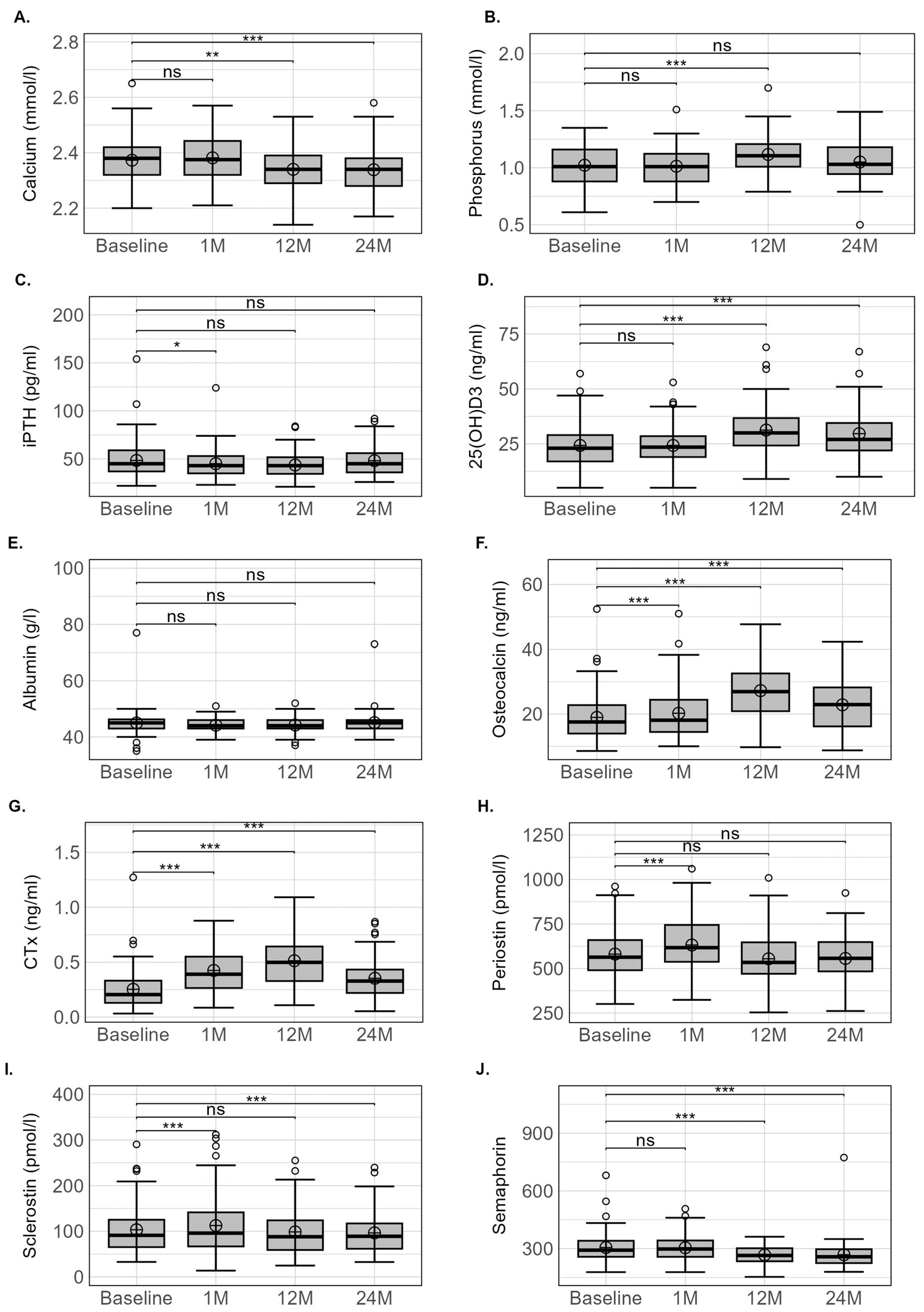
| Calcium homeostasis | |||||||
| Calcium (mmol/L) | 2.37 ± 0.08 | 2.38 ± 0.08 | 2.34 ± 0.08 | 2.34 ± 0.08 | 0.5 ± 3.5 | −0.9 ± 3.0 ** | −1.4 ± 3.2 *** |
| Phosphorus (mmol/L) | 1.03 ± 0.16 | 1.02 ± 0.15 | 1.12 ± 0.15 | 1.05 ± 0.16 | 0.5 ± 15.2 | 11.3 ± 18.7 *** | 3.3 ± 18.5 |
| iPTH (pg/mL) | 48.2 ± 20.0 | 44.0 ± 16.3 | 43.7 ± 13.1 | 47.7 ± 16.2 | −3.8 ± 31.2 * | −3.1 ± 31.2 | 9.1 ± 48.2 |
| 25(OH)D3 (ng/mL) | 24.1 ± 10.1 | 24.3 ± 9.6 | 31.3 ± 11.4 | 29.7 ± 11.1 | 3.3 ± 19.0 | 34.4 ± 39.8 *** | 34.0 ± 57.9 *** |
| Hypovitaminosis D n (%) | |||||||
| Deficiency | 33 (39.8) | 30 (37.5) | 11 (14.9) | 11 (18.3) | |||
| Insufficiency | 30 (36.1) | 30 (37.5) | 24 (32.4) | 23 (38.3) | |||
| Normal | 20 (24.1) | 20 (25.0) | 39 (52.7) | 26 (43.3) | |||
| Albumin (g/L) | 45.0 ± 4.6 | 44.1 ± 2.5 | 44.2 ± 2.8 | 45.1 ± 4.5 | −1.1 ± 7.2 | −0.8 ± 7.2 | 1.2 ± 8.5 |
| Bone markers | |||||||
| Osteocalcin (ng/mL) | 19.0 ± 7.5 | 20.2 ± 7.9 | 27.2 ± 8.4 | 22.8 ± 8.1 | 10.3 ± 23.8 *** | 59.8 ± 61.5 *** | 37.2 ± 56.8 *** |
| CTX (ng/mL) | 0.25 ± 0.19 | 0.43 ± 0.20 | 0.51 ± 0.23 | 0.35 ± 0.19 | 112.8 ± 100.1 *** | 194.4 ± 223.5 *** | 108.8 ± 182.4 *** |
| Periostin (pmol/L) | 577.1 ± 143.7 | 632.7 ± 153.9 | 554.7 ± 144.3 | 556.5 ± 136.0 | 12.0 ± 21.0 *** | −0.7 ± 20.7 | −1.0 ± 22.7 |
| Sclerostin (pmol/L) | 103.3 ± 55.3 | 111.9 ± 64.6 | 98.6 ± 50.9 | 96.5 ± 47.7 | 12.1 ± 24.6 *** | −1.5 ± 19.4 | −11.2 ± 17.1 *** |
| Semaphorin (pmol/L) | 315.0 ± 91.6 | 307.5 ± 68.1 | 260.9 ± 47.1 | 251.8 ± 42.9 | 2.6 ± 18.9 | −10.5 ± 16.2 *** | −11.8 ± 28.8 *** |
| Baseline (n = 83) | 12 Months (n = 76) | 24 Months (n = 60) | Relative % Variation (Δ 12m-Baseline/Baseline) | Relative % Variation (Δ 24m-Baseline/Baseline) | |
|---|---|---|---|---|---|
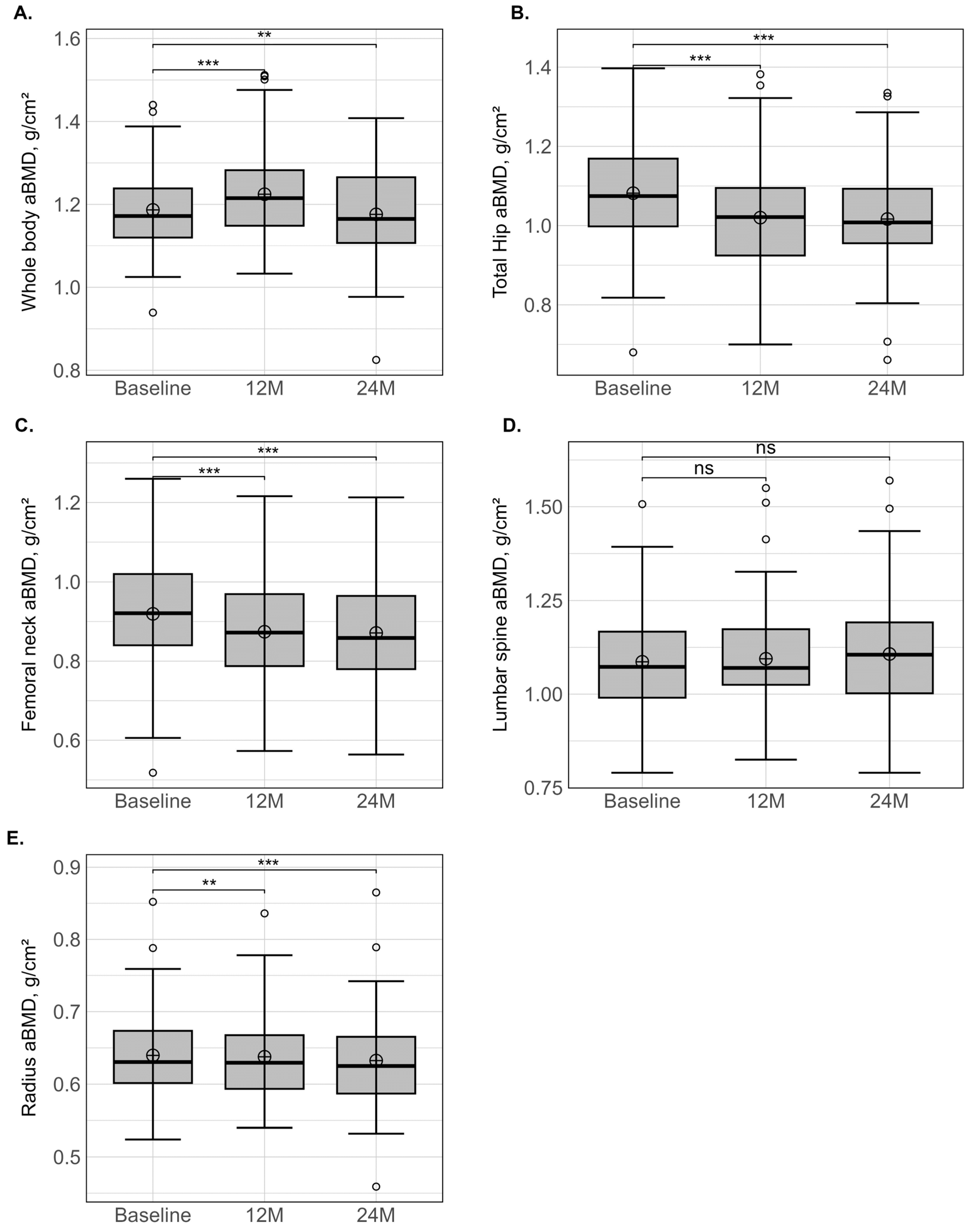
| Whole body | |||||
| aBMD (g/cm2) | 1.187 ± 0.098 | 1.225 ± 0.110 | 1.176 ± 0.113 | 2.4 ± 2.9 *** | −2.4 ± 5.4 ** |
| T-score (SD) | 0.71 ± 1.02 | 1.14 ± 1.02 | 0.54 ± 1.24 | ||
| Z-score (SD) | 0.63 ± 0.87 | 0.99 ± 0.92 | 0.55 ± 1.05 | ||
| Total hip | |||||
| aBMD (g/cm2) | 1.083 ± 0.133 | 1.020 ± 0.137 | 1.017 ± 0.133 | −6.6 ± 4.2 *** | −7.9 ± 4.6 *** |
| T-score (SD) | 0.84 ± 0.97 | 0.43 ± 0.93 | 0.36 ± 0.90 | ||
| Z-score (SD) | 1.09 ± 0.97 | 0.66 ± 0.96 | 0.69 ± 0.91 | ||
| Osteoporosis n (%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Osteopenia n (%) | 2 (2.4%) | 5 (6.6%) | 5 (8.3%) | ||
| Femoral neck | |||||
| aBMD (g/cm2) | 0.920 ± 0.150 | 0.874 ± 0.141 | 0.871 ± 0.140 | −5.4 ± 7.0 *** | −5.8 ± 7.6 *** |
| Lumbar spine | |||||
| aBMD (g/cm2) | 1.087 ± 0.139 | 1.095 ± 0.142 | 1.107 ± 0.159 | −0.1 ± 4.1 | −0.4 ± 5.0 |
| T-score (SD) | 0.58 ± 1.31 | 0.63 ± 1.30 | 0.71 ± 1.44 | ||
| Z-score (SD) | 0.86 ± 1.27 | 0.92 ± 1.31 | 1.11 ± 1.4 | ||
| Osteoporosis n (%) | 1 (1.2%) | 0 (0%) | 1 (1.7%) | ||
| Osteopenia n (%) | 9 (10.8%) | 8 (10.5%) | 8 (13.3%) | ||
| Radius | |||||
| aBMD (g/cm2) | 0.641 ± 0.061 | 0.638 ± 0.061 | 0.632 ± 0.069 | −0.8 ± 1.6 ** | −2.1 ± 2.4 *** |
| T-score (SD) | 1.71 ± 1.36 | 1.67 ± 1.25 | 1.43 ± 1.41 | ||
| Z-score (SD) | 2.11 ± 1.45 | 2.08 ± 1.40 | 1.95 ± 1.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maïmoun, L.; Aouinti, S.; Puech, M.; Lefebvre, P.; Deloze, M.; de Santa Barbara, P.; Cristol, J.-P.; Brabant, S.; Gautier, T.; Nedelcu, M.; et al. Effect of Nutritional Deprivation after Sleeve Gastrectomy on Bone Mass, Periostin, Sclerostin and Semaphorin 4D: A Two-Year Longitudinal Study. Nutrients 2023, 15, 4310. https://doi.org/10.3390/nu15204310
Maïmoun L, Aouinti S, Puech M, Lefebvre P, Deloze M, de Santa Barbara P, Cristol J-P, Brabant S, Gautier T, Nedelcu M, et al. Effect of Nutritional Deprivation after Sleeve Gastrectomy on Bone Mass, Periostin, Sclerostin and Semaphorin 4D: A Two-Year Longitudinal Study. Nutrients. 2023; 15(20):4310. https://doi.org/10.3390/nu15204310
Chicago/Turabian StyleMaïmoun, Laurent, Safa Aouinti, Marion Puech, Patrick Lefebvre, Mélanie Deloze, Pascal de Santa Barbara, Jean-Paul Cristol, Séverine Brabant, Thomas Gautier, Marius Nedelcu, and et al. 2023. "Effect of Nutritional Deprivation after Sleeve Gastrectomy on Bone Mass, Periostin, Sclerostin and Semaphorin 4D: A Two-Year Longitudinal Study" Nutrients 15, no. 20: 4310. https://doi.org/10.3390/nu15204310
APA StyleMaïmoun, L., Aouinti, S., Puech, M., Lefebvre, P., Deloze, M., de Santa Barbara, P., Cristol, J.-P., Brabant, S., Gautier, T., Nedelcu, M., Renard, E., Picot, M.-C., Mariano-Goulart, D., & Nocca, D. (2023). Effect of Nutritional Deprivation after Sleeve Gastrectomy on Bone Mass, Periostin, Sclerostin and Semaphorin 4D: A Two-Year Longitudinal Study. Nutrients, 15(20), 4310. https://doi.org/10.3390/nu15204310







