Vitamin A Status in Preterm Infants Is Associated with Inflammation and Dexamethasone Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Nutritional Management
2.3. Data Collection and Nutritional Assessment
2.4. Data Analyses and Statistics
3. Results
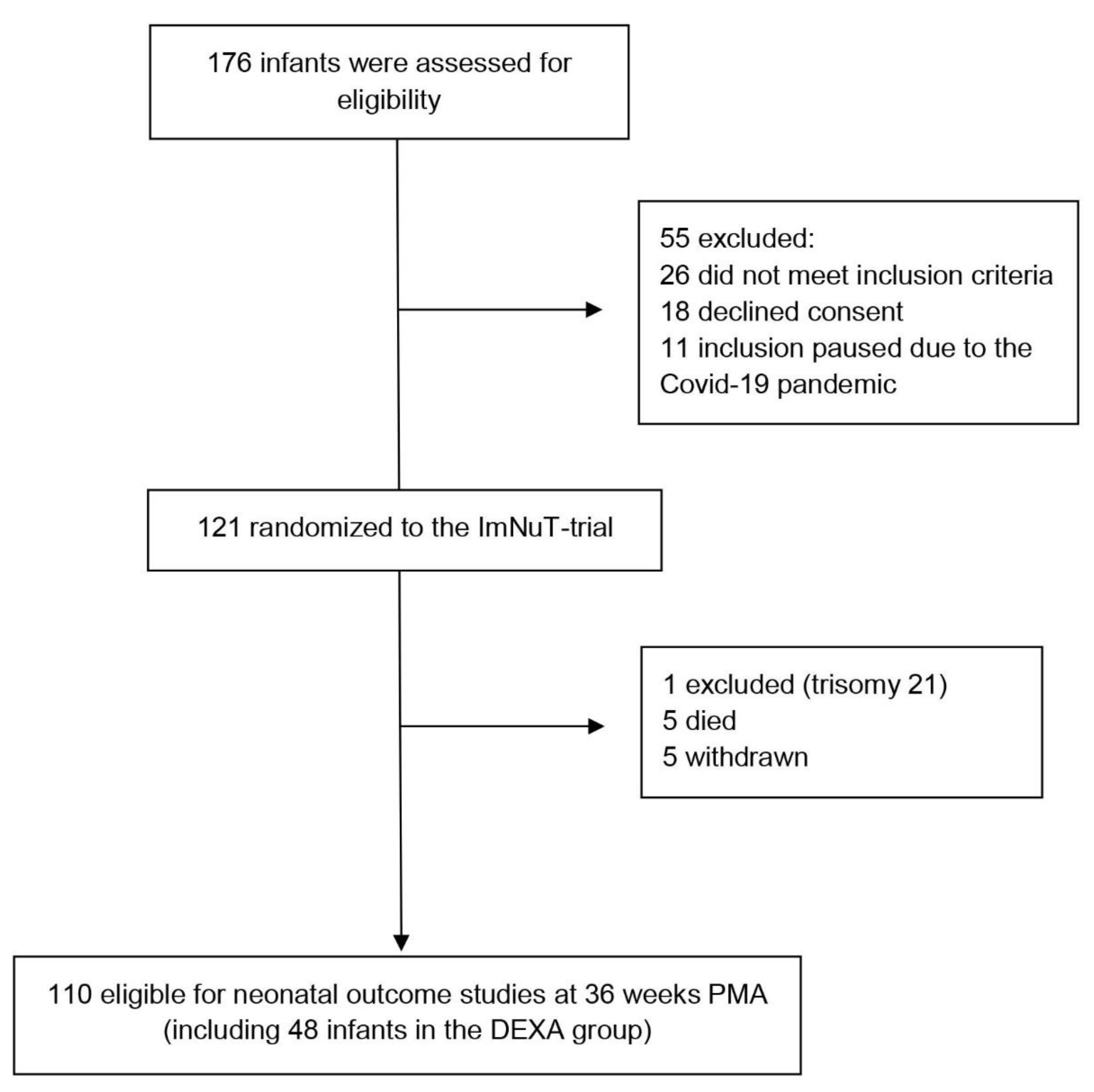
3.1. Study Population
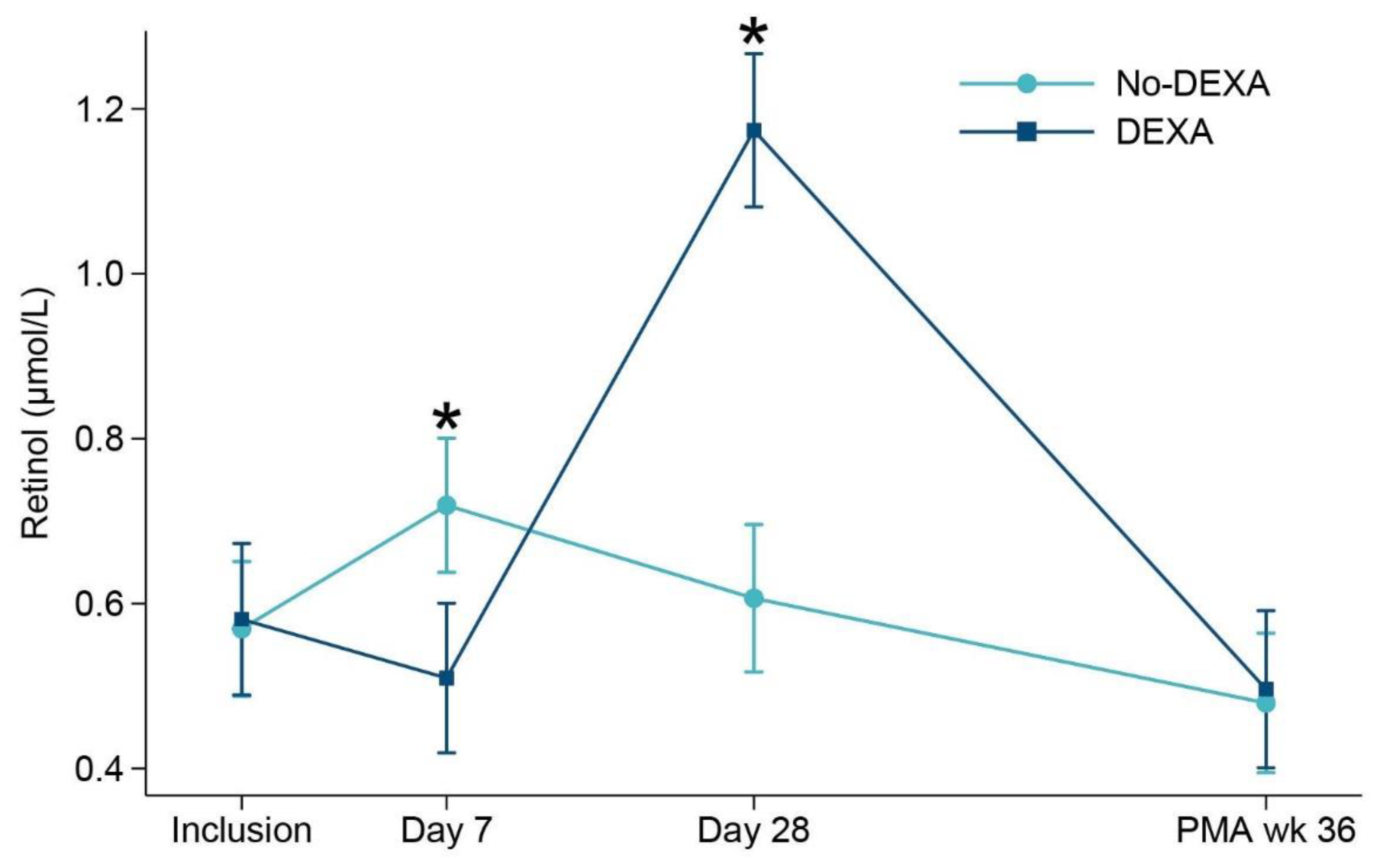
3.2. Vitamin A Concentrations
3.3. Vitamin A Deficiency
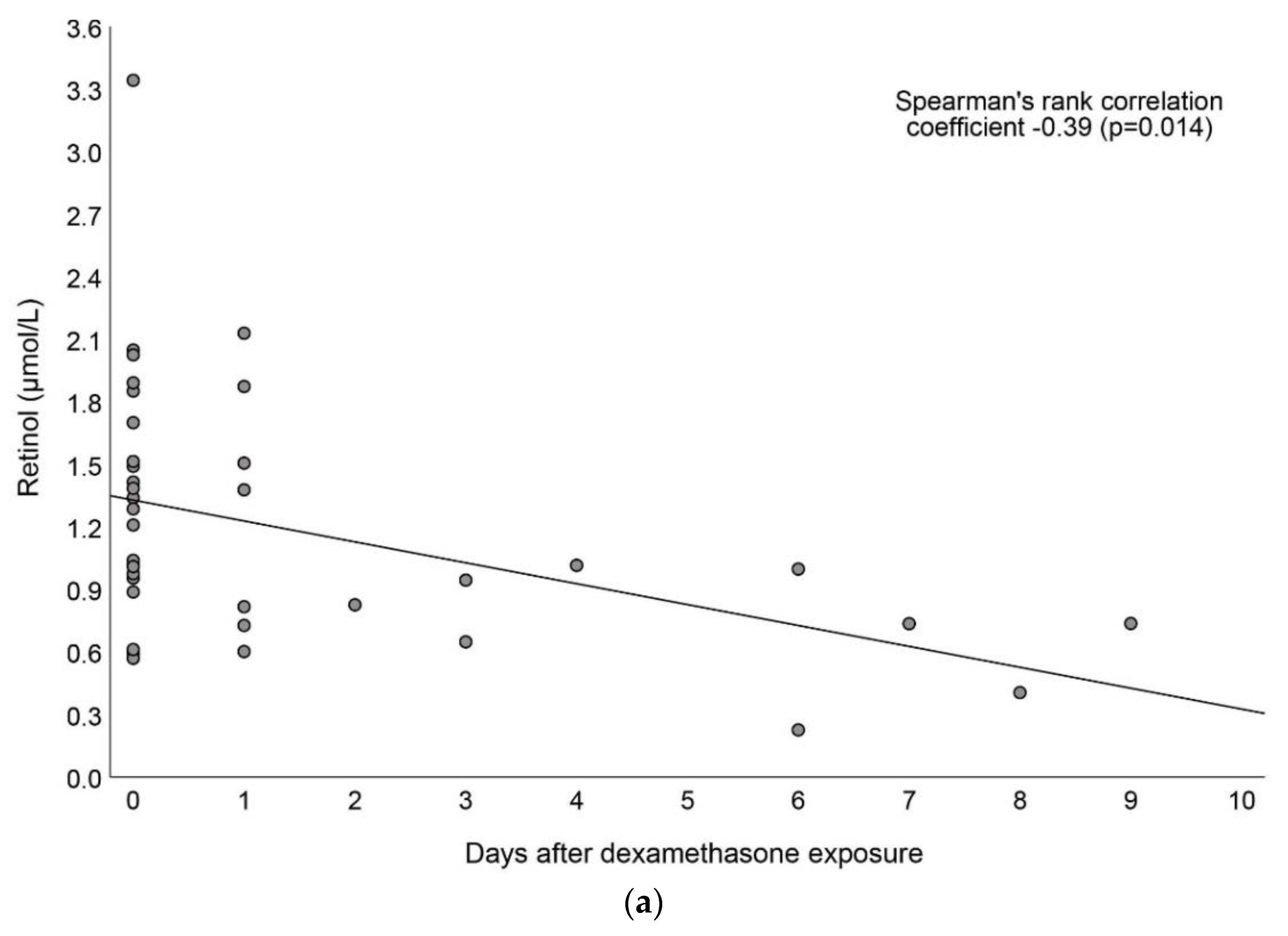
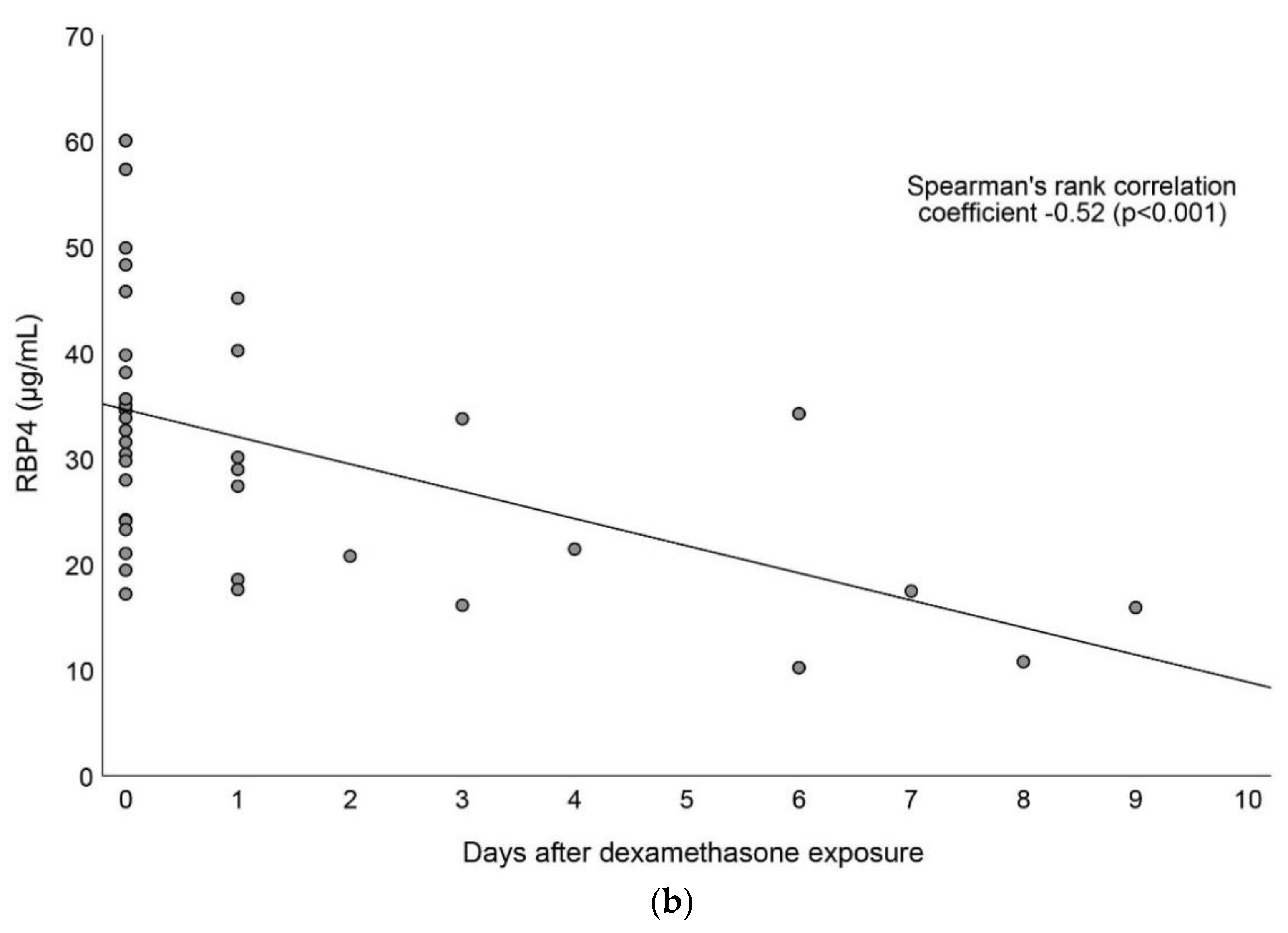
3.4. Associations between Vitamin A and Dexamethasone Exposure
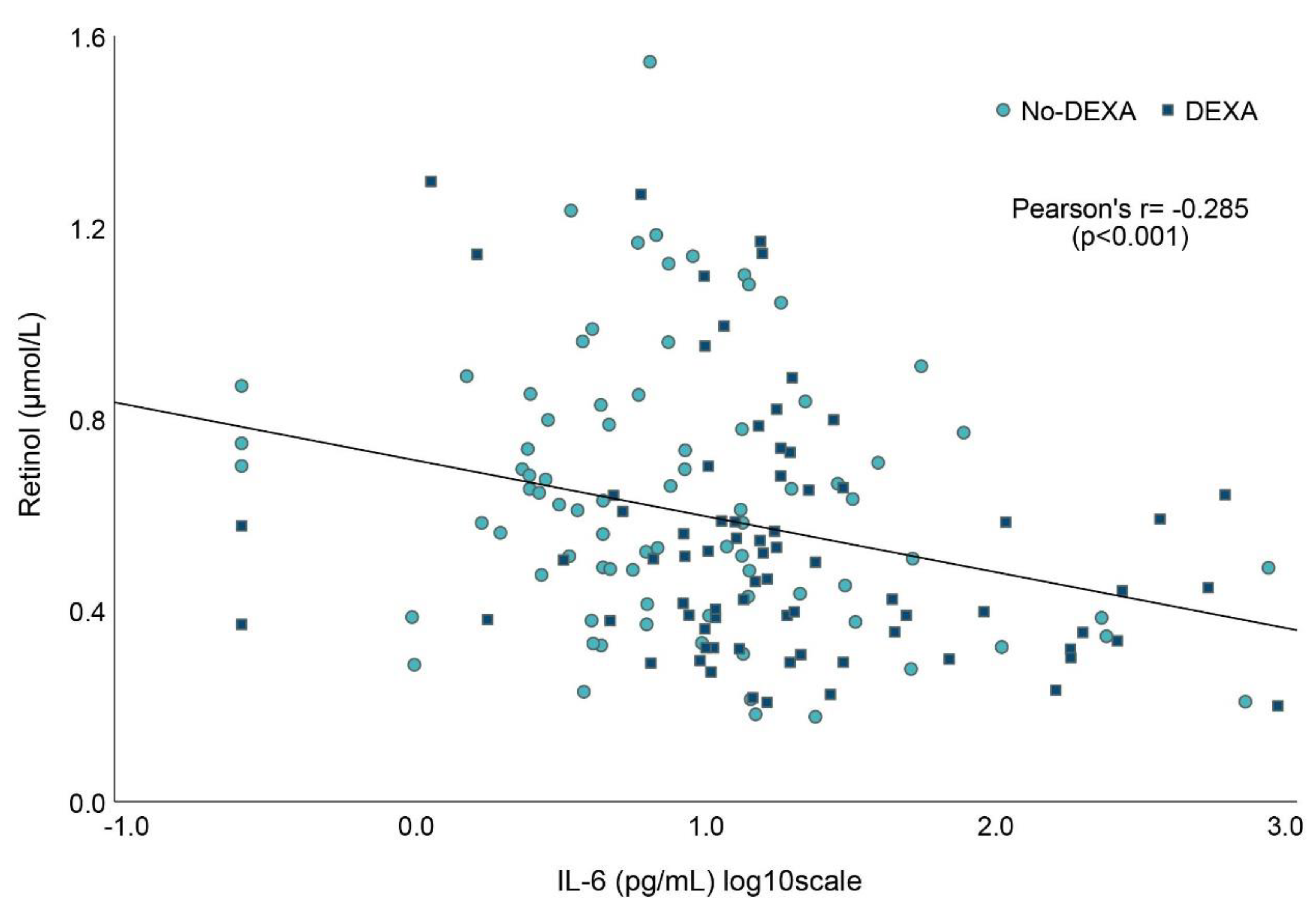
3.5. Associations between Vitamin A and Markers of Inflammation
3.6. Associations between Vitamin A Biochemistry and Intake
3.7. Multivariable Analyses between Vitamin A and Potential Predictors at Day 7
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGA | Appropriate for gestational age |
| BPD | Bronchopulmonary dysplasia |
| CRIB | Clinical Risk Index for Babies |
| CRP | C-reactive protein |
| DBS | Dried blood spot |
| GA | Gestational age |
| IL | Interleukin |
| PDA | Patent ductus arteriosus |
| PMA | Postmenstrual age |
| PN | Parenteral nutrition |
| RCT | Randomized controlled trial |
| RBP4 | Retinol-binding protein 4 |
| SGA | Small for gestational age |
Appendix A. Method Dried Blood Spot Analyses
References
- Higgins, R.D.; Jobe, A.H.; Koso-Thomas, M.; Bancalari, E.; Viscardi, R.M.; Hartert, T.V.; Ryan, R.M.; Kallapur, S.G.; Steinhorn, R.H.; Konduri, G.G.; et al. Bronchopulmonary Dysplasia: Executive Summary of a Workshop. J. Pediatr. 2018, 197, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Norman, M.; Hallberg, B.; Abrahamsson, T.; Bjorklund, L.J.; Domellof, M.; Farooqi, A.; Foyn Bruun, C.; Gadsboll, C.; Hellstrom-Westas, L.; Ingemansson, F.; et al. Association Between Year of Birth and 1-Year Survival Among Extremely Preterm Infants in Sweden During 2004–2007 and 2014–2016. Jama 2019, 321, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- Savani, R.C. Modulators of inflammation in Bronchopulmonary Dysplasia. Semin. Perinatol. 2018, 42, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, S.; Nobili, B.; Rossi, F.; Di Pinto, D.; Cucciolla, V.; Borriello, A.; Oliva, A.; Della Ragione, F. Vitamin A and infancy. Biochemical, functional, and clinical aspects. Vitam. Horm. 2003, 66, 457–591. [Google Scholar] [CrossRef] [PubMed]
- Gürbüz, M. Understanding the role of vitamin A and its precursors in the immune system. Nutr. Clin. Et Métabolisme 2022, 36, 89–98. [Google Scholar] [CrossRef]
- Blaner, W.S.; Shmarakov, I.O.; Traber, M.G. Vitamin A and Vitamin E: Will the Real Antioxidant Please Stand Up? Annu. Rev. Nutr. 2021, 41, 105–131. [Google Scholar] [CrossRef]
- Biesalski, H.K.; Nohr, D. Importance of vitamin-A for lung function and development. Mol. Asp. Med. 2003, 24, 431–440. [Google Scholar] [CrossRef]
- Massaro, D.; Massaro, G.D. Lung development, lung function, and retinoids. N. Engl. J. Med. 2010, 362, 1829–1831. [Google Scholar] [CrossRef]
- Shenai, J.P. Vitamin A supplementation in very low birth weight neonates: Rationale and evidence. Pediatrics 1999, 104, 1369–1374. [Google Scholar] [CrossRef]
- Agostoni, C.; Buonocore, G.; Carnielli, V.P.; De Curtis, M.; Darmaun, D.; Decsi, T.; Domellof, M.; Embleton, N.D.; Fusch, C.; Genzel-Boroviczeny, O.; et al. Enteral nutrient supply for preterm infants: Commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 85–91. [Google Scholar] [CrossRef]
- Darlow, B.A.; Graham, P.J.; Rojas-Reyes, M.X. Vitamin A supplementation to prevent mortality and short- and long-term morbidity in very low birth weight infants. Cochrane Database Syst. Rev. 2016, 8, Cd000501. [Google Scholar] [CrossRef] [PubMed]
- Mactier, H. Vitamin A for preterm infants; where are we now? Semin. Fetal Neonatal Med. 2013, 18, 166–171. [Google Scholar] [CrossRef]
- Inder, T.E.; Graham, P.J.; Winterbourn, C.C.; Austin, N.C.; Darlow, B.A. Plasma vitamin A levels in the very low birthweight infant--relationship to respiratory outcome. Early Hum. Dev. 1998, 52, 155–168. [Google Scholar] [CrossRef]
- Spears, K.; Cheney, C.; Zerzan, J. Low plasma retinol concentrations increase the risk of developing bronchopulmonary dysplasia and long-term respiratory disability in very-low-birth-weight infants. Am. J. Clin. Nutr. 2004, 80, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Rakshasbhuvankar, A.A.; Pillow, J.J.; Simmer, K.N.; Patole, S.K. Vitamin A supplementation in very-preterm or very-low-birth-weight infants to prevent morbidity and mortality: A systematic review and meta-analysis of randomized trials. Am. J. Clin. Nutr. 2021, 114, 2084–2096. [Google Scholar] [CrossRef]
- Shenai, J.P.; Chytil, F. Vitamin A storage in lungs during perinatal development in the rat. Biol Neonate 1990, 57, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Tanumihardjo, S.A.; Russell, R.M.; Stephensen, C.B.; Gannon, B.M.; Craft, N.E.; Haskell, M.J.; Lietz, G.; Schulze, K.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)-Vitamin A Review. J. Nutr. 2016, 146, 1816s–1848s. [Google Scholar] [CrossRef]
- Gerasimidis, K.; Bronsky, J.; Catchpole, A.; Embleton, N.; Fewtrell, M.; Hojsak, I.; Indrio, F.; Hulst, J.; Koglmeier, J.; de Koning, B.; et al. Assessment and Interpretation of Vitamin and Trace Element Status in Sick Children. A Position Paper from the ESPGHAN Committee in Nutrition. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 873–881. [Google Scholar] [CrossRef]
- Duncan, A.; Talwar, D.; McMillan, D.C.; Stefanowicz, F.; O’Reilly, D.S. Quantitative data on the magnitude of the systemic inflammatory response and its effect on micronutrient status based on plasma measurements. Am. J. Clin. Nutr. 2012, 95, 64–71. [Google Scholar] [CrossRef]
- Doyle, L.W.; Cheong, J.L.; Hay, S.; Manley, B.J.; Halliday, H.L. Late (≥7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst. Rev. 2021, 11, Cd001145. [Google Scholar] [CrossRef]
- Shenai, J.P.; Mellen, B.G.; Chytil, F. Vitamin A status and postnatal dexamethasone treatment in bronchopulmonary dysplasia. Pediatrics 2000, 106, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Georgieff, M.K.; Mammel, M.C.; Mills, M.M.; Gunter, E.W.; Johnson, D.E.; Thompson, T.R. Effect of postnatal steroid administration on serum vitamin A concentrations in newborn infants with respiratory compromise. J. Pediatr. 1989, 114, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Georgieff, M.K.; Radmer, W.J.; Sowell, A.L.; Yeager, P.R.; Blaner, W.S.; Gunter, E.W.; Johnson, D.E. The effect of glucocorticosteroids on serum, liver, and lung vitamin A and retinyl ester concentrations. J. Pediatr. Gastroenterol. Nutr. 1991, 13, 376–382. [Google Scholar] [CrossRef]
- Geevarghese, S.K.; Chytil, F. Depletion of retinyl esters in the lungs coincides with lung prenatal morphological maturation. Biochem. Biophys. Res. Commun. 1994, 200, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Wendel, K.; Pfeiffer, H.C.V.; Fugelseth, D.M.; Nestaas, E.; Domellöf, M.; Skålhegg, B.S.; Elgstøen, K.B.P.; Rootwelt, H.; Pettersen, R.D.; Pripp, A.H.; et al. Effects of nutrition therapy on growth, inflammation and metabolism in immature infants: A study protocol of a double-blind randomized controlled trial (ImNuT). BMC Pediatr. 2021, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Mihatsch, W.A.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; Darmaun, D.; Desci, T.; Domellof, M.; Embleton, N.; et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition. Clin. Nutr. 2018, 37, 2303–2305. [Google Scholar] [CrossRef]
- Rossholt, M.E.; Bratlie, M.; Wendel, K.; Aas, M.F.; Gunnarsdottir, G.; Fugelseth, D.; Stiris, T.; Domellöf, M.; Størdal, K.; Moltu, S.J. A standardized feeding protocol ensured recommended nutrient intakes and prevented growth faltering in preterm infants < 29 weeks gestation. Clin. Nutr. ESPEN 2022. [Google Scholar] [CrossRef]
- Greer, F. Vitamins A, E and K. In Nutrition of the Preterm Infant, 2nd ed.; Tsang, R.C., Ricardo, U., Koletzko, B.V., Zlotkin, S.H., Eds.; Digital Educational Publishing: Cincinnati, OH, USA, 2005; pp. 141–172. [Google Scholar]
- Doyle, L.W.; Davis, P.G.; Morley, C.J.; McPhee, A.; Carlin, J.B. Low-dose dexamethasone facilitates extubation among chronically ventilator-dependent infants: A multicenter, international, randomized, controlled trial. Pediatrics 2006, 117, 75–83. [Google Scholar] [CrossRef]
- Niklasson, A.; Albertsson-Wikland, K. Continuous growth reference from 24th week of gestation to 24 months by gender. BMC Pediatr. 2008, 8, 8. [Google Scholar] [CrossRef]
- Embleton, N.D.; Moltu, S.J.; Lapillonne, A.; van den Akker, C.H.P.; Carnielli, V.; Fusch, C.; Gerasimidis, K.; van Goudoever, J.B.; Haiden, N.; Iacobelli, S.; et al. Enteral Nutrition in Preterm Infants (2022): A Position Paper from the ESPGHAN Committee on Nutrition and invited experts. J. Pediatr. Gastroenterol. Nutr. 2022. [Google Scholar] [CrossRef]
- Shenai, J.P.; Chytil, F.; Jhaveri, A.; Stahlman, M.T. Plasma vitamin A and retinol-binding protein in premature and term neonates. J. Pediatr. 1981, 99, 302–305. [Google Scholar] [CrossRef]
- Chen, H.J.; Hsu, C.H.; Chiang, B.L. Serum retinol levels and neonatal outcomes in preterm infants. J. Formos. Med. Assoc 2017, 116, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Jobe, A.H. Mechanisms of Lung Injury and Bronchopulmonary Dysplasia. Am. J. Perinatol. 2016, 33, 1076–1078. [Google Scholar] [CrossRef] [PubMed]
- Bose, C.L.; Laughon, M.M.; Allred, E.N.; O’Shea, T.M.; Van Marter, L.J.; Ehrenkranz, R.A.; Fichorova, R.N.; Leviton, A. Systemic inflammation associated with mechanical ventilation among extremely preterm infants. Cytokine 2013, 61, 315–322. [Google Scholar] [CrossRef]
- Wardle, S.P.; Hughes, A.; Chen, S.; Shaw, N.J. Randomised controlled trial of oral vitamin A supplementation in preterm infants to prevent chronic lung disease. Arch. Dis. Childhood. Fetal Neonatal Ed. 2001, 84, F9–F13. [Google Scholar] [CrossRef] [PubMed]
- Gerasimidis, K.; Haiden, N. Vitamin Requirements for Preterm Infants. World Rev. Nutr. Diet. 2021, 122, 149–166. [Google Scholar] [CrossRef]
- Rakshasbhuvankar, A.A.; Simmer, K.; Patole, S.K.; Stoecklin, B.; Nathan, E.A.; Clarke, M.W.; Pillow, J.J. Enteral Vitamin A for Reducing Severity of Bronchopulmonary Dysplasia: A Randomized Trial. Pediatrics 2021, 147, e2020009985. [Google Scholar] [CrossRef]
- Basu, S.; Khanna, P.; Srivastava, R.; Kumar, A. Oral vitamin A supplementation in very low birth weight neonates: A randomized controlled trial. Eur. J. Pediatr. 2019, 178, 1255–1265. [Google Scholar] [CrossRef]
- Londhe, V.A.; Nolen, T.L.; Das, A.; Higgins, R.D.; Tyson, J.E.; Oh, W.; Devaskar, S.U. Vitamin A supplementation in extremely low-birth-weight infants: Subgroup analysis in small-for-gestational-age infants. Am. J. Perinatol. 2013, 30, 771–780. [Google Scholar] [CrossRef]
- Bronsky, J.; Campoy, C.; Braegger, C. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Vitamins. Clin. Nutr. 2018, 37, 2366–2378. [Google Scholar] [CrossRef]
| ImNuT Cohort (n = 120) | No DEXA (n = 71) | DEXA (n = 49) | |
|---|---|---|---|
| Maternal characteristics | |||
| Antenatal glucocorticoids any dose, n (%) | 120 (100) | 71 (100) | 49 (100) |
| Antenatal glucocorticoids 2 doses, n (%) | 83 (69) | 53 (75) | 30 (61) |
| Birth characteristics | |||
| Gestational age, weeks, median (range) | 26+6 (22+6–28+6) | 27+4 (23+5–28+6) | 24+5 (22+6–28+6) |
| Weight, g, median (IQR) | 798 (666, 1070) | 995 (720, 1140) | 712 (602, 804) |
| Weight, z score, median (IQR) | −0.88 (−1.7, −0.29) | −1.1 (−2.1, −0.32) | −0.74 (−1.3, −0.23) |
| Female, n (%) | 54 (45) | 34 (48) | 20 (41) |
| SGA, n (%) | 23 (19) | 17 (24) | 6 (12) |
| Apgar score at 5 min, median (IQR) | 8 (6, 8) | 8 (7, 9) | 7 (6, 8) |
| CRIB score, median (IQR) | 6 (2, 9) | 4 (1, 7) | 9 (7, 11) |
| Clinical outcomes | |||
| NEC, n (%) | 4 (3) | 3 (4) | 1 (2) |
| Mechanical ventilation, days, median (IQR) | 5 (0, 23) | 0 (0, 3) | 26 (17, 35) |
| BPD, n (%) a | 49 (41) | 13 (21) | 40 (83) |
| ROP, n (%) b | |||
| No ROP | 70 (64) | 45 (73) | 25 (52) |
| Stage ≥ 3 | 10 (9) | 2 (3) | 8 (17) |
| IVH, n (%) | |||
| No IVH | 95 (79) | 64 (90) | 31 (63) |
| Grade 3–4 | 11 (9) | 5 (7) | 6 (12) |
| PDA requiring treatment, n (%) c | 51 (45) | 15 (23) | 36 (74) |
| Any septicemia, n (%) | 61 (51) | 27 (38) | 34 (69) |
| Retinol (log10 µmol/L) Day 7 | |||
|---|---|---|---|
| Beta | Standard Error | p-Value | |
| MV at day 7 (yes = 1) | −0.19 | 0.047 | <0.001 |
| SGA (yes = 1) | −0.17 | 0.056 | 0.003 |
| log10 IL-6 (pg/mL) | −0.085 | 0.040 | 0.038 |
| log10 CRP (mg/L) | −0.085 | 0.050 | 0.094 |
| Adjusted R2 | 0.367 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossholt, M.E.; Wendel, K.; Bratlie, M.; Aas, M.F.; Gunnarsdottir, G.; Fugelseth, D.; Pripp, A.H.; Domellöf, M.; Størdal, K.; Stiris, T.; et al. Vitamin A Status in Preterm Infants Is Associated with Inflammation and Dexamethasone Exposure. Nutrients 2023, 15, 441. https://doi.org/10.3390/nu15020441
Rossholt ME, Wendel K, Bratlie M, Aas MF, Gunnarsdottir G, Fugelseth D, Pripp AH, Domellöf M, Størdal K, Stiris T, et al. Vitamin A Status in Preterm Infants Is Associated with Inflammation and Dexamethasone Exposure. Nutrients. 2023; 15(2):441. https://doi.org/10.3390/nu15020441
Chicago/Turabian StyleRossholt, Madelaine Eloranta, Kristina Wendel, Marianne Bratlie, Marlen Fossan Aas, Gunnthorunn Gunnarsdottir, Drude Fugelseth, Are Hugo Pripp, Magnus Domellöf, Ketil Størdal, Tom Stiris, and et al. 2023. "Vitamin A Status in Preterm Infants Is Associated with Inflammation and Dexamethasone Exposure" Nutrients 15, no. 2: 441. https://doi.org/10.3390/nu15020441
APA StyleRossholt, M. E., Wendel, K., Bratlie, M., Aas, M. F., Gunnarsdottir, G., Fugelseth, D., Pripp, A. H., Domellöf, M., Størdal, K., Stiris, T., & Moltu, S. J. (2023). Vitamin A Status in Preterm Infants Is Associated with Inflammation and Dexamethasone Exposure. Nutrients, 15(2), 441. https://doi.org/10.3390/nu15020441





