Hypoxia-Driven Changes in a Human Intestinal Organoid Model and the Protective Effects of Hydrolyzed Whey
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Tissues and Ethics
2.2. Small Intestinal Crypt Isolation
2.3. HIO Maintenance
2.4. HIO Culture Medium
2.5. HIO Monolayer Culture
2.6. Immunofluorescence Staining of HIO Monolayers
2.7. Measurement of Paracellular Barrier Function of 3D HIO
2.8. Human PBMC Isolation and Culture
2.9. Flow Cytometry Analysis of Human PBMC
2.10. RNA Isolation and Quantitative Real-Time PCR
2.11. Microbiology Experiments
2.12. Whey Protein Isolate and Whey Protein Hydrolysates
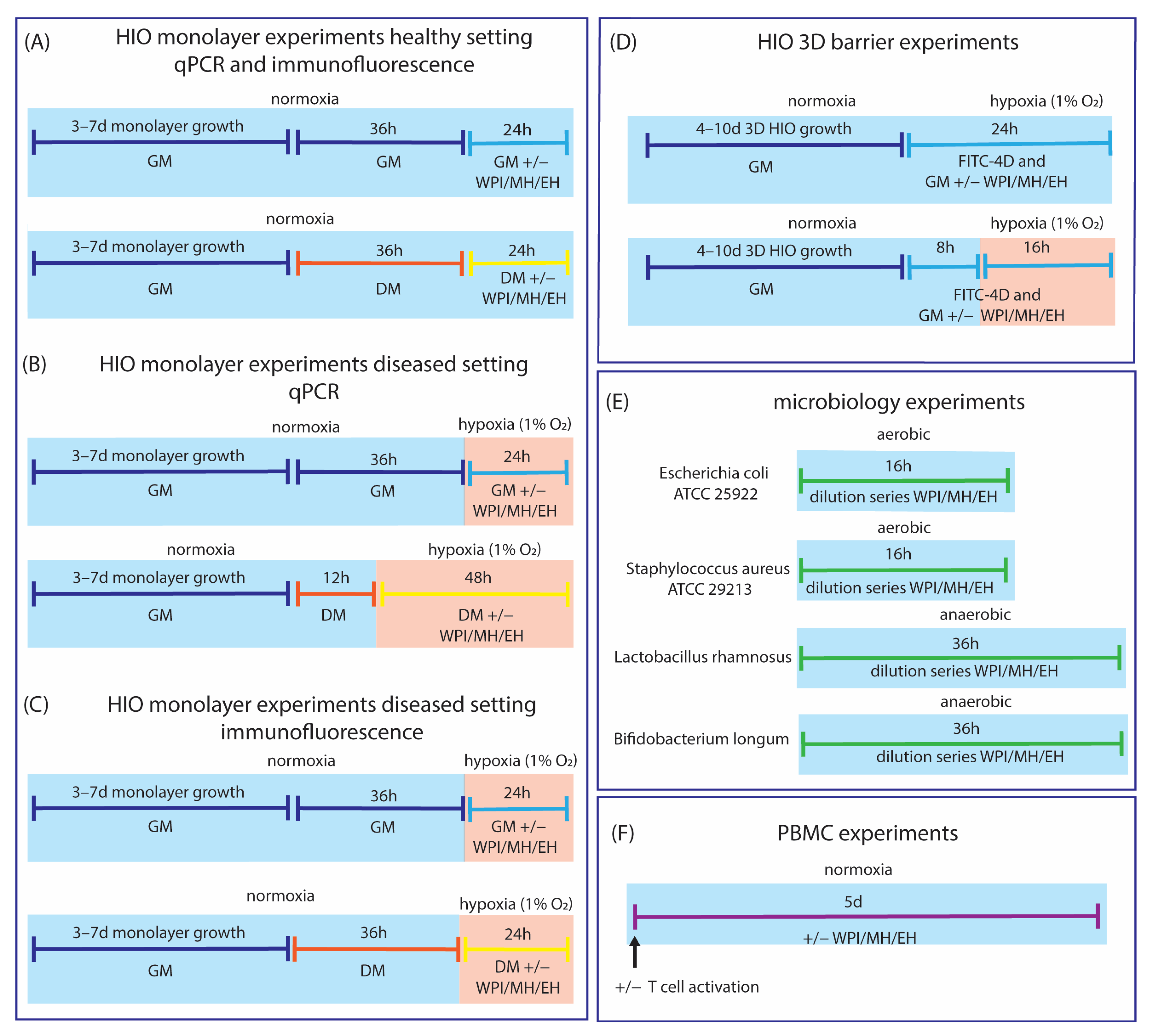
2.13. Experimental Set-Up
2.14. Statistical Analyses
3. Results
3.1. Model Development
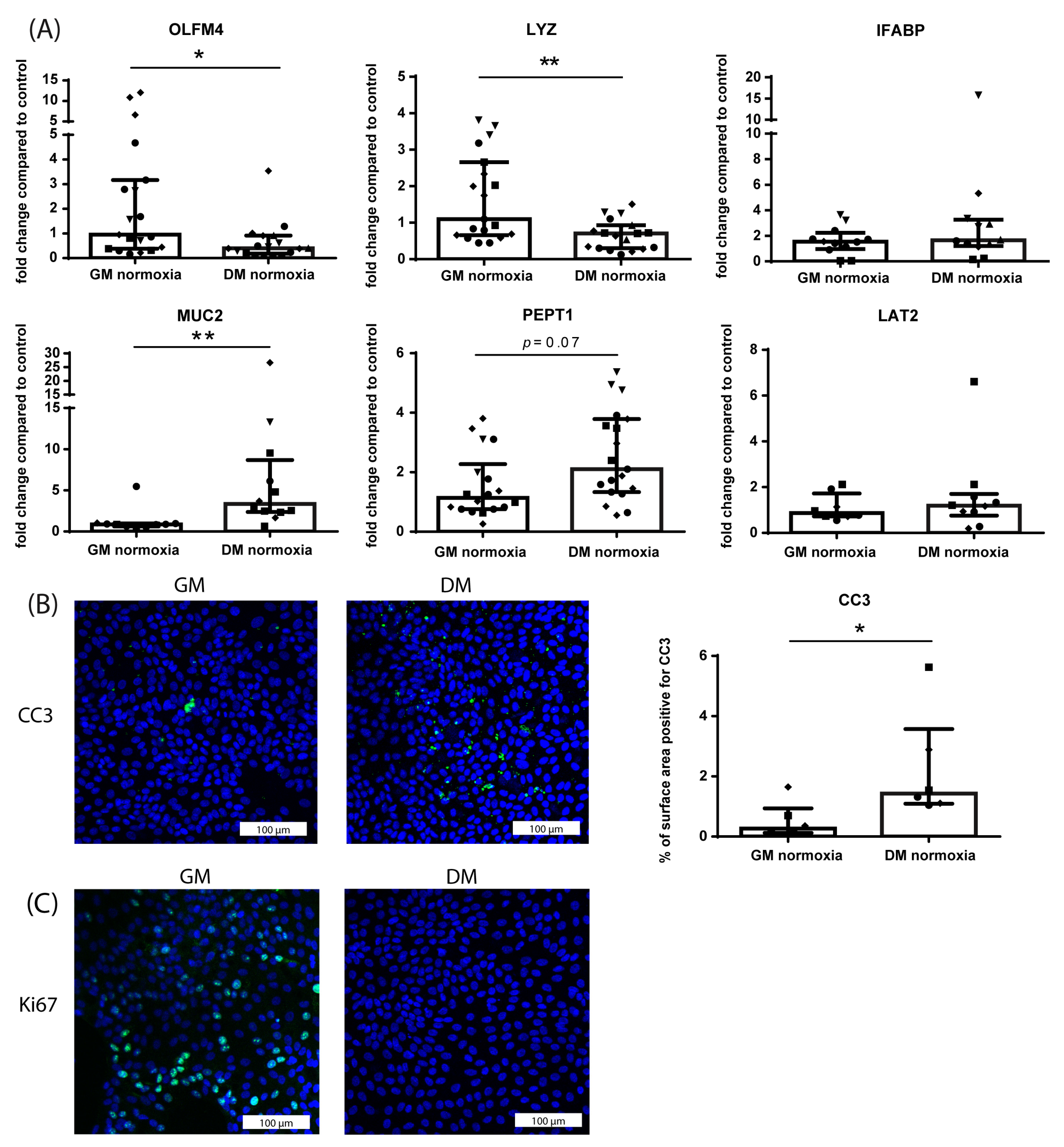
3.1.1. Differentiation from Crypt-Like to Villus-like HIO Monolayers
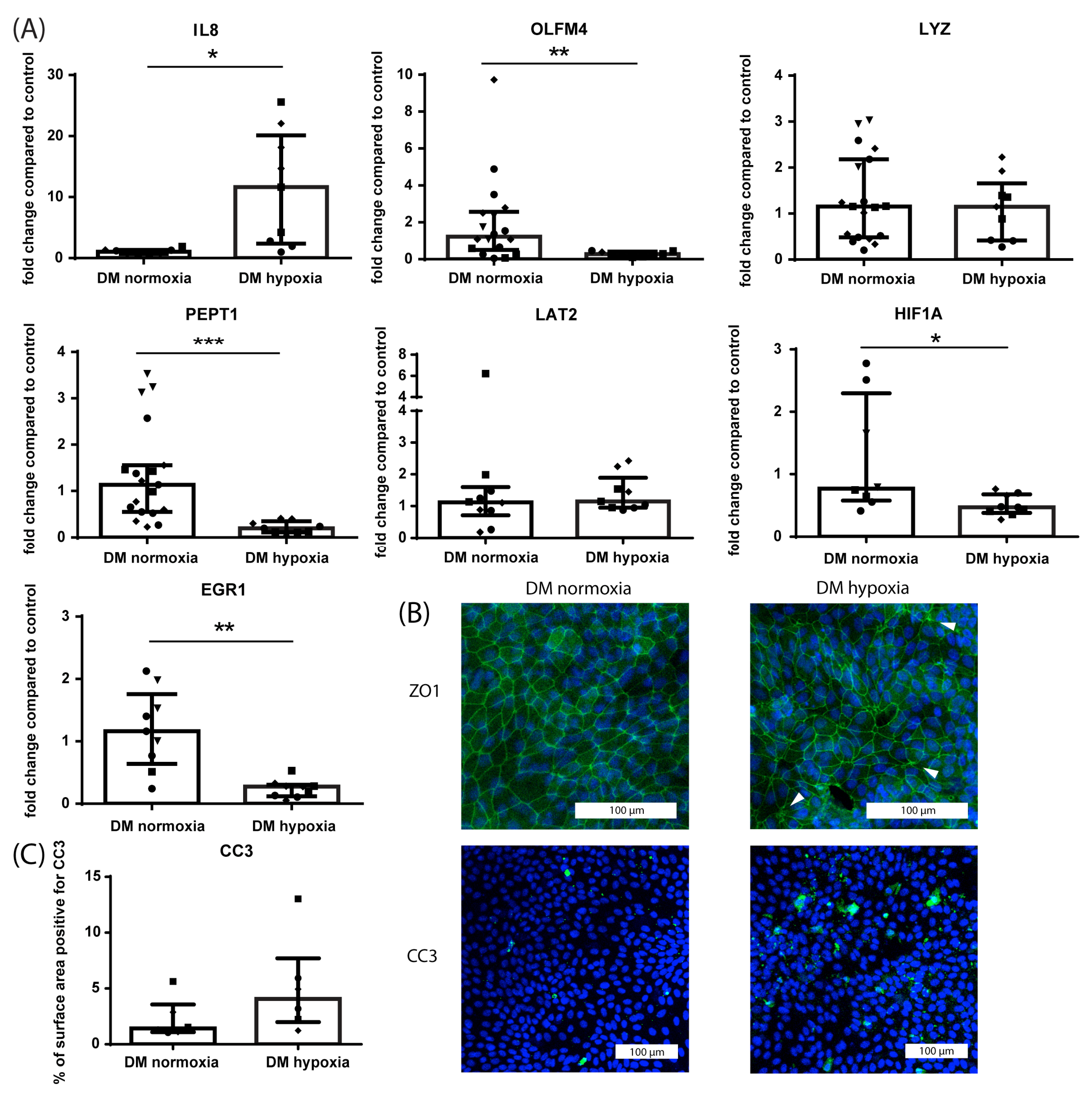
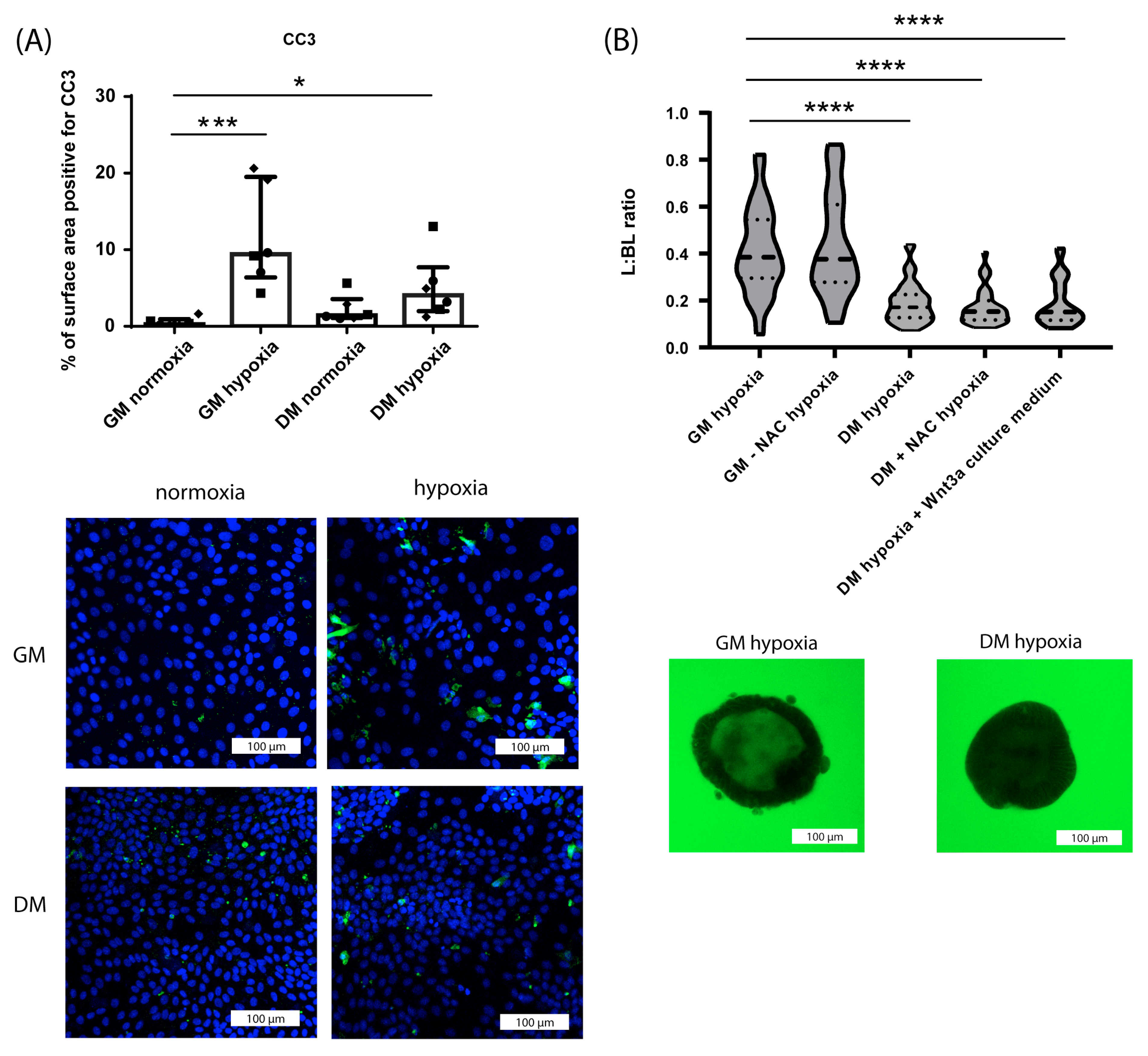
3.1.2. Effect of Hypoxia on Crypt-like HIO Monolayers and 3D HIO
3.1.3. Effect of Hypoxia on Villus-like HIO Monolayers
3.1.4. Differential Effect of Hypoxia on Crypt-like and Villus-like HIO Monolayers and 3D HIO
3.2. Comprehensive Screening of Whey Protein Fractions
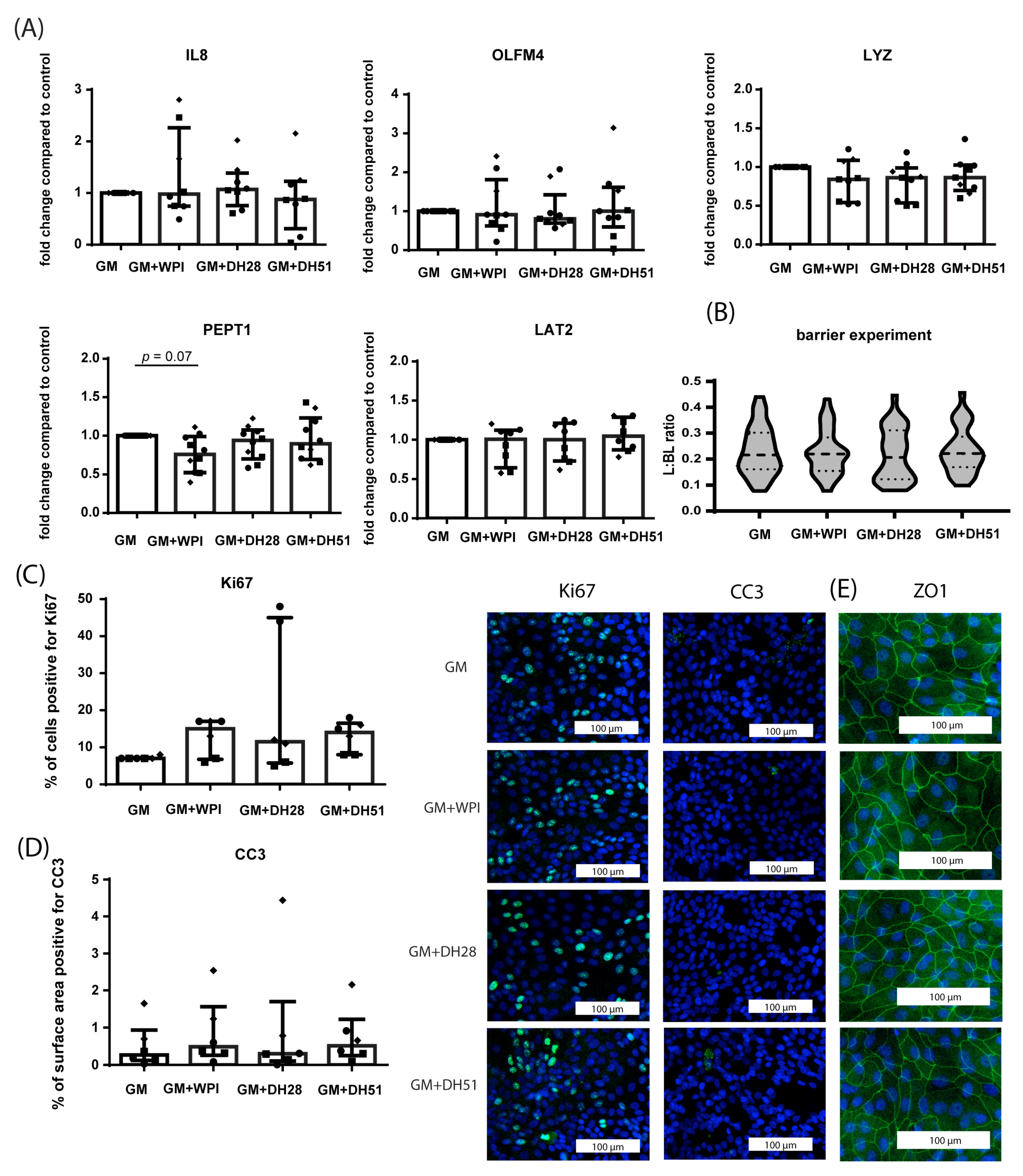
3.2.1. Effect of WPI, DH28 and DH51 on Crypt-like HIO (Monolayer- and 3D-Cultured) in a Healthy Setting (Normoxia)
3.2.2. Effect of WPI, DH28 and DH51 on Villus-like HIO (Monolayer- and 3D-Cultured) in a Healthy Setting (Normoxia)
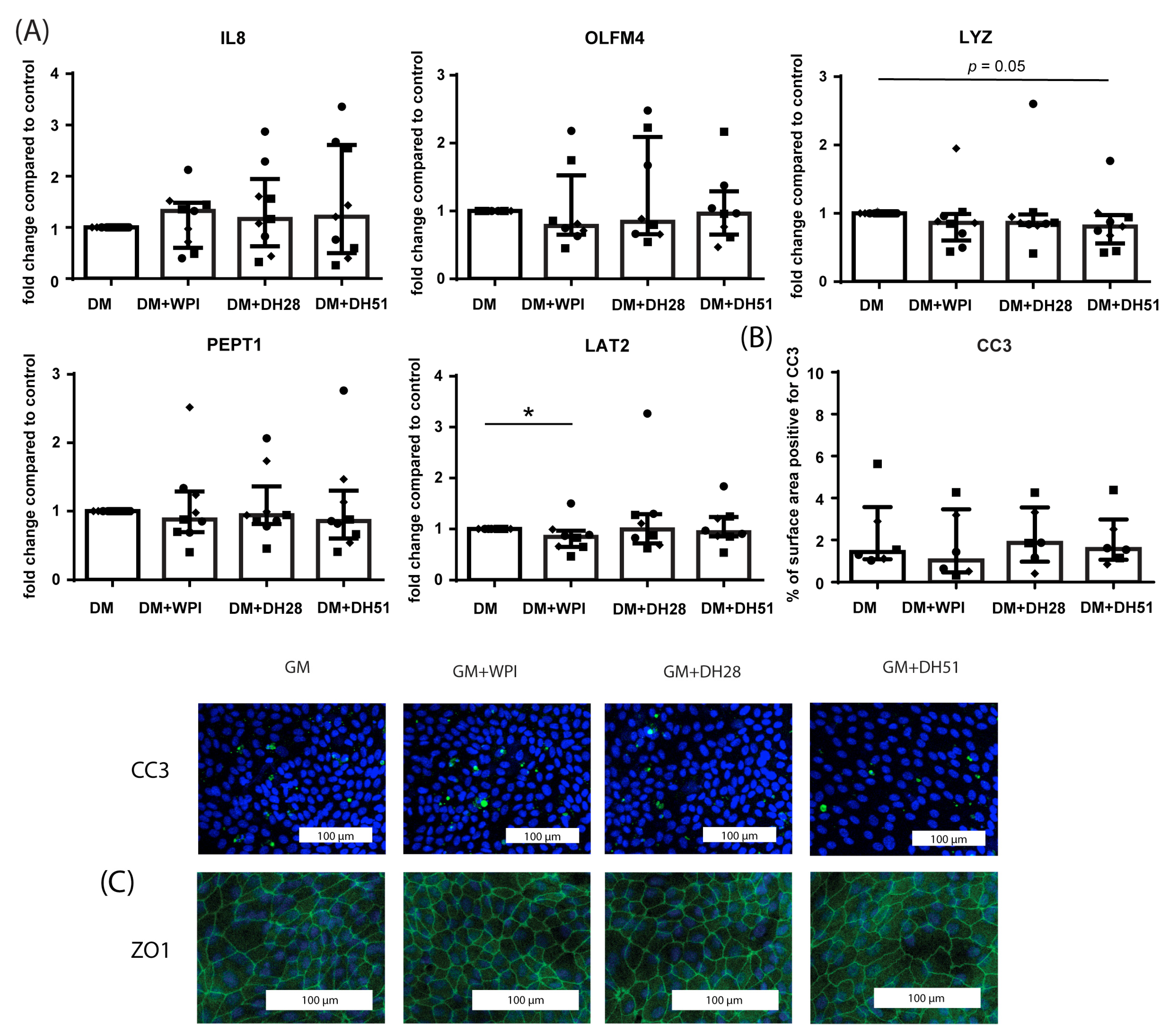
3.2.3. Effect of WPI, DH28 and DH51 on Crypt-like HIO (Monolayer- and 3D-Cultured) in a Diseased Setting (Hypoxia)
3.2.4. Effect of WPI, DH28 and DH51 on Villus-like HIO (Monolayer- and 3D-Cultured) in a Diseased Setting (Hypoxia)
3.2.5. Effect of WPI, DH28 and DH51 on T Cell Subsets and T Cell Proliferation
3.2.6. Effect of WPI, DH28 and DH51 on PBMC Cytokine Expression and Activation Makers
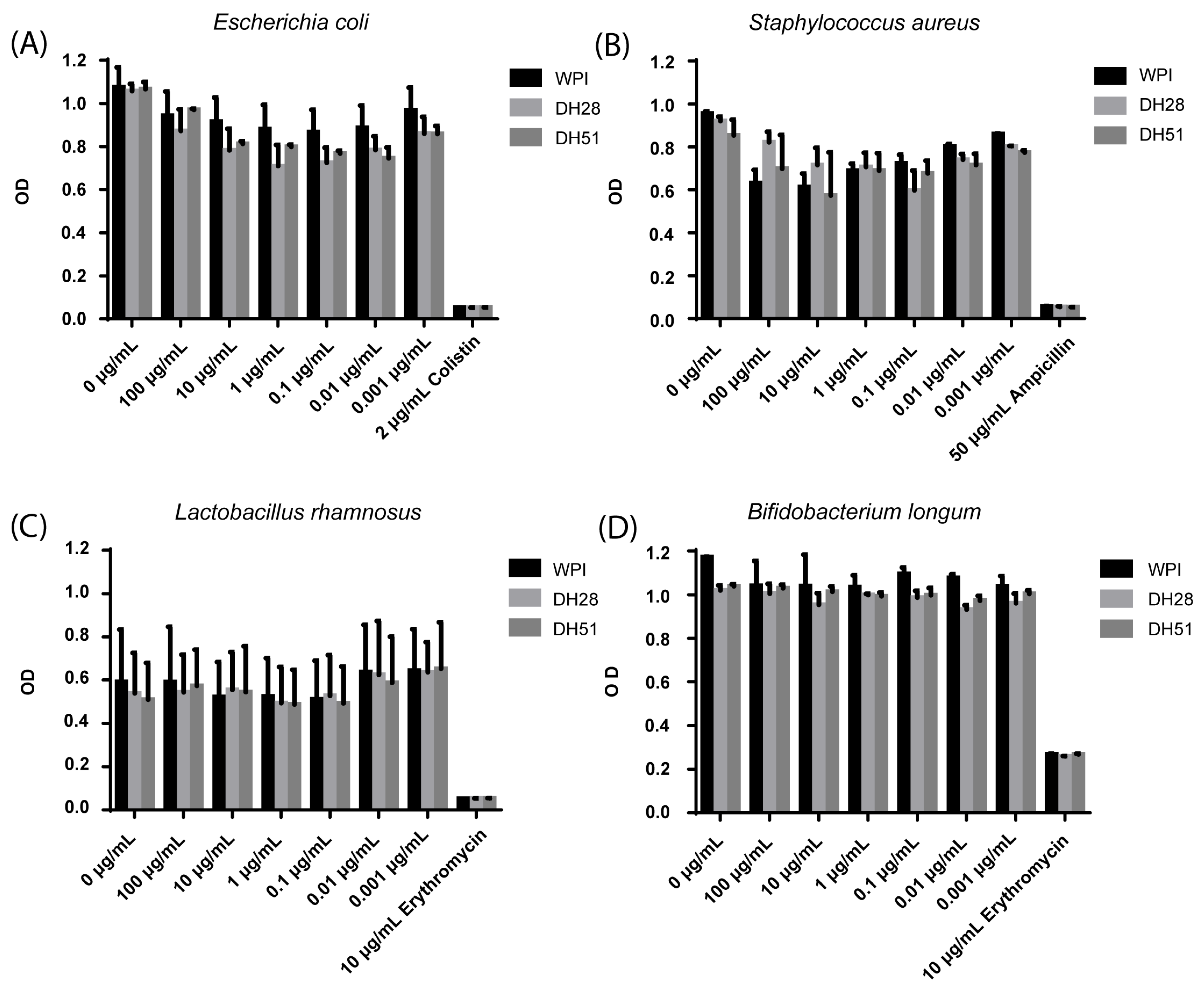
3.2.7. Effect of WPI, DH28 and DH51 on Four Microbial Strains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smithers, G.W. Whey and whey proteins—From ‘gutter-to-gold’. Int. Dairy J. 2008, 18, 695–704. [Google Scholar] [CrossRef]
- Madureira, A.R.; Pereira, C.I.; Gomes, A.M.P.; Pintado, M.E.; Xavier Malcata, F. Bovine whey proteins—Overview on their main biological properties. Food Res. Int. 2007, 40, 1197–1211. [Google Scholar] [CrossRef]
- Layman, D.K.; Lönnerdal, B.; Fernstrom, J.D. Applications for α-lactalbumin in human nutrition. Nutr. Rev. 2018, 76, 444–460. [Google Scholar] [CrossRef] [PubMed]
- Fenelon, M.A.; Hickey, R.M.; Buggy, A.; McCarthy, N.; Murphy, E.G. Whey Proteins in Infant Formula. In Whey Proteins; Deeth, H.C., Bansal, N., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 439–494. [Google Scholar]
- American Academy of Pediatrics; Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics 2000, 106 Pt 1, 346–349. [Google Scholar] [CrossRef]
- Boyle, R.J.; Ierodiakonou, D.; Khan, T.; Chivinge, J.; Robinson, Z.; Geoghegan, N.; Jarrold, K.; Afxentiou, T.; Reeves, T.; Cunha, S.; et al. Hydrolysed formula and risk of allergic or autoimmune disease: Systematic review and meta-analysis. BMJ 2016, 352, i974. [Google Scholar] [CrossRef]
- Ng, D.H.C.; Klassen, J.R.; Embleton, N.D.; McGuire, W. Protein hydrolysate versus standard formula for preterm infants. Cochrane Database Syst. Rev. 2019, 7, Cd012412. [Google Scholar] [PubMed]
- Pennings, B.; Boirie, Y.; Senden, J.M.; Gijsen, A.P.; Kuipers, H.; van Loon, L.J. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 2011, 93, 997–1005. [Google Scholar] [CrossRef]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef]
- Davies, R.W.; Carson, B.P.; Jakeman, P.M. The Effect of Whey Protein Supplementation on the Temporal Recovery of Muscle Function Following Resistance Training: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 221. [Google Scholar] [CrossRef]
- Liao, Y.; Peng, Z.; Chen, L.; Zhang, Y.; Cheng, Q.; Nüssler, A.K.; Bao, W.; Liu, L.; Yang, W. Prospective Views for Whey Protein and/or Resistance Training Against Age-Related Sarcopenia. Aging Dis. 2019, 10, 157–173. [Google Scholar] [CrossRef]
- Badely, M.; Sepandi, M.; Samadi, M.; Parastouei, K.; Taghdir, M. The effect of whey protein on the components of metabolic syndrome in overweight and obese individuals; a systematic review and meta-analysis. Diabetes Metab. Syndr. 2019, 13, 3121–3131. [Google Scholar] [CrossRef]
- Wirunsawanya, K.; Upala, S.; Jaruvongvanich, V.; Sanguankeo, A. Whey Protein Supplementation Improves Body Composition and Cardiovascular Risk Factors in Overweight and Obese Patients: A Systematic Review and Meta-Analysis. J. Am. Coll. Nutr. 2018, 37, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Sprong, R.C.; Schonewille, A.J.; van der Meer, R. Dietary cheese whey protein protects rats against mild dextran sulfate sodium-induced colitis: Role of mucin and microbiota. J. Dairy Sci. 2010, 93, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Chatterton, D.E.; Nguyen, D.N.; Bering, S.B.; Sangild, P.T. Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. Int. J. Biochem. Cell Biol. 2013, 45, 1730–1747. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, G. Health issues of Whey Proteins: 3. Gut Health Promotion. Curr. Top. Nutraceutical Res. 2007, 5, 29–34. [Google Scholar]
- Hering, N.A.; Andres, S.; Fromm, A.; van Tol, E.A.; Amasheh, M.; Mankertz, J.; Schulzke, J.D. Transforming growth factor-β, a whey protein component, strengthens the intestinal barrier by upregulating claudin-4 in HT-29/B6 cells. J. Nutr. 2011, 141, 783–789. [Google Scholar] [CrossRef]
- Xiao, K.; Jiao, L.; Cao, S.; Song, Z.; Hu, C.; Han, X. Whey protein concentrate enhances intestinal integrity and influences transforming growth factor-β1 and mitogen-activated protein kinase signalling pathways in piglets after lipopolysaccharide challenge. Br. J. Nutr. 2016, 115, 984–993. [Google Scholar] [CrossRef]
- Diederen, K.; Li, J.V.; Donachie, G.E.; de Meij, T.G.; de Waart, D.R.; Hakvoort, T.B.M.; Kindermann, A.; Wagner, J.; Auyeung, V.; Velde, A.A.T.; et al. Exclusive enteral nutrition mediates gut microbial and metabolic changes that are associated with remission in children with Crohn’s disease. Sci. Rep. 2020, 10, 18879. [Google Scholar] [CrossRef]
- Souza, A.L.; Fiorini Aguiar, S.L.; Gonçalves Miranda, M.C.; Lemos, L.; Freitas Guimaraes, M.A.; Reis, D.S.; Barros, P.A.V.; Veloso, E.S.; Carvalho, T.G.; Ribeiro, F.M.; et al. Consumption of Diet Containing Free Amino Acids Exacerbates Colitis in Mice. Front Immunol. 2017, 8, 1587. [Google Scholar] [CrossRef]
- Triantafillidis, J.K.; Tzouvala, M.; Triantafyllidi, E. Enteral Nutrition Supplemented with Transforming Growth Factor-β, Colostrum, Probiotics, and Other Nutritional Compounds in the Treatment of Patients with Inflammatory Bowel Disease. Nutrients 2020, 12, 1048. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, C.; Ming, Z.; Cao, J.; Yan, Y.; Zhao, P.; Pang, G.; Deng, Z.; Yao, Y.; Chen, Q. Molecular mechanisms by which casein glycomacropeptide maintains internal homeostasis in mice with experimental ulcerative colitis. PLoS ONE 2017, 12, e0181075. [Google Scholar] [CrossRef] [PubMed]
- Hvas, C.L.; Dige, A.; Bendix, M.; Wernlund, P.G.; Christensen, L.A.; Dahlerup, J.F.; Agnholt, J. Casein glycomacropeptide for active distal ulcerative colitis: A randomized pilot study. Eur. J. Clin. Investig. 2016, 46, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Uchida, M. Alpha-lactalbumin suppresses interleukin-6 release after intestinal ischemia/reperfusion via nitric oxide in rats. Inflammopharmacology 2007, 15, 43–47. [Google Scholar] [CrossRef] [PubMed]
- de Lange, I.H.; van Gorp, C.; Eeftinck Schattenkerk, L.D.; van Gemert, W.G.; Derikx, J.P.M.; Wolfs, T.G.A.M. Enteral Feeding Interventions in the Prevention of Necrotizing Enterocolitis: A Systematic Review of Experimental and Clinical Studies. Nutrients 2021, 13, 1726. [Google Scholar] [CrossRef] [PubMed]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.P.; Maubois, J.L.; Beaufrère, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef]
- Manninen, A.H. Protein hydrolysates in sports nutrition. Nutr. Metab. 2009, 6, 38. [Google Scholar] [CrossRef]
- Morifuji, M.; Ishizaka, M.; Baba, S.; Fukuda, K.; Matsumoto, H.; Koga, J.; Kanegae, M.; Higuchi, M. Comparison of different sources and degrees of hydrolysis of dietary protein: Effect on plasma amino acids, dipeptides, and insulin responses in human subjects. J. Agric. Food Chem. 2010, 58, 8788–8797. [Google Scholar] [CrossRef]
- Brandelli, A.; Daroit, D.J.; Corrêa, A.P.F. Whey as a source of peptides with remarkable biological activities. Food Res. Int. 2015, 73, 149–161. [Google Scholar] [CrossRef]
- Nielsen, S.D.; Beverly, R.L.; Qu, Y.; Dallas, D.C. Milk bioactive peptide database: A comprehensive database of milk protein-derived bioactive peptides and novel visualization. Food Chem. 2017, 232, 673–682. [Google Scholar] [CrossRef]
- Silvestre, M.P.C.; da Silva, M.C.; de Souza, M.W.S.; Silva, V.D.M.; de Aguiar, M.J.B.; Silva, M.R. Hydrolysis degree, peptide profile and phenylalanine removal from whey protein concentrate hydrolysates obtained by various proteases. Int. J. Food Sci. Technol. 2013, 48, 588–595. [Google Scholar] [CrossRef]
- Morais, H.A.; Silvestre, M.P.C.; Silva, V.D.M.; Silva, M.R.; Simoes e Silva, A.C.; Silveira, J.N. Correlation between the Degree of Hydrolysis and the Peptide Profile of Whey Protein Concentrate Hydrolysates: Effect of the Enzyme Type and Reaction Time. Am. J. Food Technol. 2013, 8, 1–16. [Google Scholar] [CrossRef]
- Iskandar, M.M.; Lands, L.C.; Sabally, K.; Azadi, B.; Meehan, B.; Mawji, N.; Skinner, C.D.; Kubow, S. High Hydrostatic Pressure Pretreatment of Whey Protein Isolates Improves Their Digestibility and Antioxidant Capacity. Foods 2015, 4, 184–207. [Google Scholar] [CrossRef] [PubMed]
- Poolman, J.T.; Anderson, A.S. Escherichia coli and Staphylococcus aureus: Leading bacterial pathogens of healthcare associated infections and bacteremia in older-age populations. Expert Rev. Vaccines 2018, 17, 607–618. [Google Scholar] [CrossRef]
- Coggins, S.A.; Wynn, J.L.; Weitkamp, J.-H. Infectious Causes of Necrotizing Enterocolitis. Clin. Perinatol. 2015, 42, 133–154. [Google Scholar] [CrossRef]
- Price, L.B.; Hungate, B.A.; Koch, B.J.; Davis, G.S.; Liu, C.M. Colonizing opportunistic pathogens (COPs): The beasts in all of us. PLoS Pathog. 2017, 13, e1006369. [Google Scholar] [CrossRef]
- Capurso, L. Thirty Years of Lactobacillus Rhamnosus GG: A Review. J. Clin. Gastroenterol. 2019, 53, S1–S41. [Google Scholar] [CrossRef]
- Arboleya, S.; Watkins, C.; Stanton, C.; Ross, R.P. Gut Bifidobacteria Populations in Human Health and Aging. Front Microbiol. 2016, 7, 1204. [Google Scholar] [CrossRef]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.; Van Es, J.H.; Van den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef]
- VanDussen, K.L.; Marinshaw, J.M.; Shaikh, N.; Miyoshi, H.; Moon, C.; Tarr, P.I.; Ciorba, M.A.; Stappenbeck, T.S. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut 2015, 64, 911–920. [Google Scholar] [CrossRef]
- Kozuka, K.; He, Y.; Koo-McCoy, S.; Kumaraswamy, P.; Nie, B.; Shaw, K.; Chan, P.; Leadbetter, M.; He, L.; Lewis, J.G.; et al. Development and Characterization of a Human and Mouse Intestinal Epithelial Cell Monolayer Platform. Stem Cell Rep. 2017, 9, 1976–1990. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Xu, P.; Becker, H.; Elizalde, M.; Masclee, A.; Jonkers, D. Intestinal organoid culture model is a valuable system to study epithelial barrier function in IBD. Gut 2018, 67, 1905–1906. [Google Scholar] [CrossRef]
- Wang, X.; Spandidos, A.; Wang, H.; Seed, B. PrimerBank: A PCR primer database for quantitative gene expression analysis, 2012 update. Nucleic Acid Res. 2012, 40, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, C.; Eaton, A.D.; Lanter, B.B.; Roper, J.; Hurley, B.P.; Delaney, B. Extended exposure duration of cultured intestinal epithelial cell monolayers in characterizing hazardous and non-hazardous proteins. Food Chem. Toxicol. 2018, 115, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, C.; Inagaki, M.; Nohara, M.; Fukuoka, M.; Yabe, T.; Kanamarua, Y. The effects of denatured major bovine whey proteins on the digestive tract, assessed by Caco-2 cell differentiation and on viability of suckling mice. J. Dairy Res. 2021, 88, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, M.; Kawai, S.; Ijier, X.; Fukuoka, M.; Yabe, T.; Iwamoto, S.; Kanamaru, Y. Effects of heat treatment on conformation and cell growth activity of alpha- lactalbumin and beta-lactoglobulin from market milk. Biomed. Res. 2017, 38, 53–59. [Google Scholar] [CrossRef]
- Arbizu, S.; Chew, B.; Mertens-Talcott, S.U.; Noratto, G. Commercial whey products promote intestinal barrier function with glycomacropeptide enhanced activity in downregulating bacterial endotoxin lipopolysaccharides (LPS)-induced inflammation in vitro. Food Funct. 2020, 11, 5842–5852. [Google Scholar] [CrossRef]
- Kiewiet, M.B.G.; Dekkers, R.; Gros, M.; van Neerven, R.J.J.; Groeneveld, A.; de Vos, P.; Faas, M.M. Toll-like receptor mediated activation is possibly involved in immunoregulating properties of cow’s milk hydrolysates. PLoS ONE 2017, 12, e0178191. [Google Scholar] [CrossRef]
- Rodríguez-Carrio, J.; Fernández, A.; Riera, F.A.; Suárez, A. Immunomodulatory activities of whey β-lactoglobulin tryptic-digested fractions. Int. Dairy J. 2014, 34, 65–73. [Google Scholar] [CrossRef]
- Chang, J.T. Pathophysiology of Inflammatory Bowel Diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef]
- Grootjans, J.; Lenaerts, K.; Buurman, W.A.; Dejong, C.H.C.; Derikx, J.P.M. Life and death at the mucosal-luminal interface: New perspectives on human intestinal ischemia-reperfusion. World J. Gastroenterol. 2016, 22, 2760–2770. [Google Scholar] [CrossRef]
- Neu, J.; Walker, W.A. Necrotizing enterocolitis. N. Engl. J. Med. 2011, 364, 255–264. [Google Scholar] [CrossRef]
- Cavadas, M.A.S.; Mesnieres, M.; Crifo, B.; Manresa, M.C.; Selfridge, A.C.; Scholz, C.C.; Cummins, E.; Cheong, A.; Taylor, C. REST mediates resolution of HIF-dependent gene expression in prolonged hypoxia. Sci. Rep. 2015, 5, 17851. [Google Scholar] [CrossRef] [PubMed]
- Bruning, U.; Cerone, L.; Neufeld, Z.; Fitzpatrick, S.F.; Cheong, A.; Scholz, C.C.; Cummins, E.; Cheong, A.; Taylor, C. MicroRNA-155 promotes resolution of hypoxia-inducible factor 1alpha activity during prolonged hypoxia. Mol. Cell. Biol. 2011, 31, 4087–4096. [Google Scholar] [CrossRef]
- Noel, G.; Baetz, N.W.; Staab, J.F.; Donowitz, M.; Kovbasnjuk, O.; Pasetti, M.F.; Zachos, N.C. A primary human macrophage-enteroid co-culture model to investigate mucosal gut physiology and host-pathogen interactions. Sci. Rep. 2017, 7, 45270. [Google Scholar] [CrossRef] [PubMed]
- Blander, J.M. On cell death in the intestinal epithelium and its impact on gut homeostasis. Curr. Opin. Gastroenterol. 2018, 34, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.W.; Stoll, B.J. Necrotising enterocolitis. Lancet 2006, 368, 1271–1283. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Stringari, C.; Edwards, R.A.; Pate, K.T.; Waterman, M.L.; Donovan, P.J.; Gratton, E. Metabolic trajectory of cellular differentiation in small intestine by Phasor Fluorescence Lifetime Microscopy of NADH. Sci. Rep. 2012, 2, 568. [Google Scholar] [CrossRef]
- Urbauer, E.; Rath, E.; Haller, D. Mitochondrial Metabolism in the Intestinal Stem Cell Niche-Sensing and Signaling in Health and Disease. Front. Cell Dev. Biol. 2020, 8, 602814. [Google Scholar] [CrossRef]
- Okkelman, I.A.; Neto, N.; Papkovsky, D.B.; Monaghan, M.G.; Dmitriev, R.I. A deeper understanding of intestinal organoid metabolism revealed by combining fluorescence lifetime imaging microscopy (FLIM) and extracellular flux analyses. Redox Biol. 2020, 30, 101420. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Kelly, C.J.; Colgan, S.P. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A Review in the Theme: Cellular Responses to Hypoxia. Am. J. Physiol. Cell Physiol. 2015, 309, C350–C360. [Google Scholar] [CrossRef] [PubMed]
- Kip, A.M.; Soons, Z.; Mohren, R.; Duivenvoorden, A.A.M.; Röth, A.A.J.; Cillero-Pastor, B.; Dejong, C.H.C.; Heeren, R.M.A.; Damink, S.W.M.O.; Lenaerts, K. Proteomics analysis of human intestinal organoids during hypoxia and reoxygenation as a model to study ischemia-reperfusion injury. Cell Death Dis. 2021, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Dengler, V.L.; Galbraith, M.; Espinosa, J.M. Transcriptional regulation by hypoxia inducible factors. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 1–15. [Google Scholar] [CrossRef]
- Muenchau, S.; Deutsch, R.; de Castro, I.J.; Hielscher, T.; Heber, N.; Niesler, B.; Lusic, M.; Stanifer, M.L.; Boulant, S. Hypoxic Environment Promotes Barrier Formation in Human Intestinal Epithelial Cells through Regulation of MicroRNA 320a Expression. Mol. Cell. Biol. 2019, 39, e00553-18. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. HIF-1: Mediator of physiological and pathophysiological responses to hypoxia. J. Appl. Physiol. 2000, 88, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.B.; Colorado, I.; Reino, D.; Palange, D.; Lu, Q.; Qin, X.; Abungu, B.; Watkins, A.; Caputo, F.J.; Xu, D.-Z.; et al. Hypoxia-inducible factor plays a gut-injurious role in intestinal ischemia reperfusion injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G853–G861. [Google Scholar] [CrossRef]
- Greer, S.N.; Metcalf, J.L.; Wang, Y.; Ohh, M. The updated biology of hypoxia-inducible factor. EMBO J. 2012, 31, 2448–2460. [Google Scholar] [CrossRef]
- Movafagh, S.; Crook, S.; Vo, K. Regulation of hypoxia-inducible factor-1a by reactive oxygen species: New developments in an old debate. J. Cell. Biochem. 2015, 116, 696–703. [Google Scholar] [CrossRef]
- Lim, J.H.; Lee, Y.M.; Chun, Y.S.; Chen, J.; Kim, J.E.; Park, J.W. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol. Cell 2010, 38, 864–878. [Google Scholar] [CrossRef]
- Liang, D.; Zhuo, Y.; Guo, Z.; He, L.; Wang, X.; He, Y.; Li, L.; Dai, H. SIRT1/PGC-1 pathway activation triggers autophagy/mitophagy and attenuates oxidative damage in intestinal epithelial cells. Biochimie 2020, 170, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Lu, C.; An, L.; Gao, Q.; Xie, W.; Miao, F.; Chen, X.; Pan, Y.; Wang, Q. SIRT1 Relieves Necrotizing Enterocolitis through Inactivation of Hypoxia-Inducible Factor (HIF)-1a. Cell Cycle 2020, 19, 2018–2027. [Google Scholar] [CrossRef]
- Hwang, J.S.; Han, S.G.; Lee, C.H.; Seo, H.G. Whey Protein Attenuates Angiotensin II-Primed Premature Senescence of Vascular Smooth Muscle Cells through Upregulation of SIRT1. Korean J. Food Sci. Anim. Resour. 2017, 37, 917–925. [Google Scholar]
- Liu, W.; Rodgers, G.P. Olfactomedin 4 expression and functions in innate immunity, inflammation, and cancer. Cancer Metastasis Rev. 2016, 35, 201–212. [Google Scholar] [CrossRef]
- Wang, X.Y.; Chen, S.H.; Zhang, Y.N.; Xu, C.F. Olfactomedin-4 in digestive diseases: A mini-review. World J. Gastroenterol. 2018, 24, 1881–1887. [Google Scholar] [CrossRef]
- Gersemann, M.; Becker, S.; Nuding, S.; Antoni, L.; Ott, G.; Fritz, P.; Oue, N.; Yasui, W.; Wehkamp, J.; Stange, E.F. Olfactomedin-4 is a glycoprotein secreted into mucus in active IBD. J. Crohns Colitis 2012, 6, 425–434. [Google Scholar] [CrossRef]
- Yu, L.; Yang, F.; Zhang, F.; Guo, D.; Li, L.; Wang, X.; Liang, T.; Wang, J.; Cai, Z.; Jin, H. CD69 enhances immunosuppressive function of regulatory T-cells and attenuates colitis by prompting IL-10 production. Cell Death Dis. 2018, 9, 905. [Google Scholar] [CrossRef]
- Cortés, J.R.; Sánchez-Díaz, R.; Bovolenta, E.R.; Barreiro, O.; Lasarte, S.; Matesanz-Marín, A.; Toribio, M.L.; Sánchez-Madrid, F.; Martín, P. Maintenance of immune tolerance by Foxp3+ regulatory T cells requires CD69 expression. J. Autoimmun. 2014, 55, 51–62. [Google Scholar] [CrossRef]
- Chen, J.H.; Huang, P.H.; Lee, C.C.; Chen, P.Y.; Chen, H.C. A bovine whey protein extract can induce the generation of regulatory T cells and shows potential to alleviate asthma symptoms in a murine asthma model. Br. J. Nutr. 2013, 109, 1813–1820. [Google Scholar] [CrossRef]
- Holvoet, S.; Perrot, M.; de Groot, N.; Prioult, G.; Mikogami, T.; Verhasselt, V.; Nutten, S. Oral Tolerance Induction to Newly Introduced Allergen is Favored by a Transforming Growth Factor-β-Enriched Formula. Nutrients 2019, 11, 2210. [Google Scholar] [CrossRef]
- Wan, Y.Y.; Flavell, R.A. TGF-beta and regulatory T cell in immunity and autoimmunity. J. Clin. Immunol. 2008, 28, 647–659. [Google Scholar] [CrossRef]
- Zhang, L.; Yi, H.; Xia, X.P.; Zhao, Y. Transforming growth factor-beta: An important role in CD4+CD25+ regulatory T cells and immune tolerance. Autoimmunity 2006, 39, 269–276. [Google Scholar] [CrossRef]
- Ando, T.; Hatsushika, K.; Wako, M.; Ohba, T.; Koyama, K.; Ohnuma, Y.; Katoh, R.; Ogawa, H.; Okumura, K.; Luo, J.; et al. Orally administered TGF-beta is biologically active in the intestinal mucosa and enhances oral tolerance. J. Allergy Clin. Immunol. 2007, 120, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Weitkamp, J.H.; Koyama, T.; Rock, M.T.; Correa, H.; Goettel, J.A.; Matta, P.; Oswald-Richter, K.; Rosen, M.J.; Engelhardt, B.G.; Moore, D.J.; et al. Necrotising enterocolitis is characterised by disrupted immune regulation and diminished mucosal regulatory (FOXP3)/effector (CD4, CD8) T cell ratios. Gut 2013, 62, 73–82. [Google Scholar] [CrossRef]
- Durrant, D.M.; Metzger, D.W. Emerging roles of T helper subsets in the pathogenesis of asthma. Immunol. Invest. 2010, 39, 526–549. [Google Scholar] [CrossRef]
- Chen, M.L.; Sundrud, M.S. Cytokine Networks and T-Cell Subsets in Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2016, 22, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Hirahara, K.; Nakayama, T. CD4+ T-cell subsets in inflammatory diseases: Beyond the Th1/Th2 paradigm. Int. Immunol. 2016, 28, 163–171. [Google Scholar] [CrossRef]
- Farmer, D.G.; Ke, B.; Shen, X.-D.; Kaldas, F.M.; Gao, F.; Watson, M.J.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Interleukin-13 protects mouse intestine from ischemia and reperfusion injury through regulation of innate and adaptive immunity. Transplantation 2011, 91, 737–743. [Google Scholar] [CrossRef]
- Imam, T.; Park, S.; Kaplan, M.H.; Olson, M.R. Effector T Helper Cell Subsets in Inflammatory Bowel Diseases. Front Immunol. 2018, 9, 1212. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Pan, X.; Gao, F.; Sangild, P.T.; Nguyen, D.N. Prenatal inflammation suppresses blood Th1 polarization and gene clusters related to cellular energy metabolism in preterm newborns. FASEB J. 2020, 34, 2896–2911. [Google Scholar] [CrossRef] [PubMed]
- Debock, I.; Flamand, V. Unbalanced Neonatal CD4(+) T-Cell Immunity. Front Immunol. 2014, 5, 393. [Google Scholar] [CrossRef] [PubMed]
- Magnan, A.O.; Mély, L.G.; Camilla, C.A.; Badier, M.M.; Montero-Julian, F.A.; Guillot, C.M.; Casano, B.B.; Prato, S.J.; Fert, V.; Bongrand, P.; et al. Assessment of the Th1/Th2 paradigm in whole blood in atopy and asthma. Increased IFN-gamma-producing CD8(+) T cells in asthma. Am. J. Respir. Crit. Care Med. 2000, 161, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Ozorio, L.; Mellinger-Silva, C.; Cabral, L.M.C.; Jardin, J.; Boudry, G.; Dupont, D. The Influence of Peptidases in Intestinal Brush Border Membranes on the Absorption of Oligopeptides from Whey Protein Hydrolysate: An Ex Vivo Study Using an Ussing Chamber. Foods 2020, 9, 1415. [Google Scholar] [CrossRef] [PubMed]
- Schulz, O.; Pabst, O. Antigen sampling in the small intestine. Trends Immunol. 2013, 34, 155–161. [Google Scholar] [CrossRef]





| Primer | Forward | Reverse |
|---|---|---|
| β-actin | 5′-ATTGCCGACAGGATGCAGAAG-3′ | 5′-TTGCTGATCCACATCTGCTGG-3′ |
| GAPDH | 5′-GGAAGCTCACTGGCATGGC-3′ | 5′-CCTGCTTCACCACCTTCTTG-3′ |
| YWHAZ | 5′-TGAACTCCCCTGAGAAAGCC-3′ | 5′-TCCGATGTCCACAATGTCAAGT-3′ |
| CD3e | 5′-TGCTGCTGGTTTACTACTGGA-3′ | 5′-GGATGGGCTCATAGTCTGGG-3′ |
| IL8 | 5′-GCCGGAATACCTGGACTATGC-3′ | 5′-TTCCTTGGGGTCCAGACAGA-3′ |
| OLFM4 | 5′-TGGACAGAGTGGAACGCTTG-3′ | 5′-TCAGAGCCACGATTTCTCGG-3′ |
| LYS | 5′-GATAACATCGCTGATGCTGTAGCT-3′ | 5′-CATGCCACCCATGCTCTAATG-3′ |
| IFABP | 5′-ACGGACAGACAATGGAAACGA-3′ | 5′-ACTGTGCGCCAAGAATAATGC-3′ |
| MUC2 | 5′-CTACTGGTGTGAGTCCAAGG-3′ | 5′-GGCACTTGGAGGAATAAACTG-3′ |
| PEPT1 | 5′-TGTCCACCGCCATCTACCATA-3′ | 5′-CCACGAGTCGGCGATAAGAG -3′ |
| LAT2 | 5′-AGGCTGGAACTTTCTGAATTACG-3′ | 5′-ACATAAGCGACATTGGCAAAGA-3′ |
| HIF1a | 5′-ATCCATGTGACCATGAGGAAATG-3′ | 5′-TCGGCTAGTTAGGGTACACTTC-3′ |
| IL4 | 5′-AGTGTCCTTCTCATGGTGGC-3′ | 5′-CACCGAGTTGACCGTAACAG-3′ |
| IL17 | 5′-CACTTTGCCTCCCAGATCAC-3′ | 5′-ACCAATCCCAAAAGGTCCTC-3′ |
| IFNϒ | 5′-TGGCTTTTCAGCTCTGCATC-3′ | 5′-CCGCTACATCTGAATGACCTG-3′ |
| TNFα | 5′-TCAATCGGCCCGACTATCTC-3′ | 5′-CAGGGCAATGATCCCAAAGT-3′ |
| IL10 | 5′-TCCCTGTGAAAACAAGAGCA-3′ | 5′-ATAGAGTCGCCACCCTGATG-3′ |
| Foxp3 | 5′- CACCTGGCTGGGAAAATGG-3′ | 5′-GGAGCCCTTGTCGGATGAT-3′ |
| Strain | Culture Medium | Antibiotics Used as Growth Inhibition Control |
|---|---|---|
| Escherichia coli ATCC 25922 | BHI | colistin (2 μg/mL) |
| Straphylococcus aureus ATCC 29213 | BHI | ampicillin (50 μg/mL) |
| Lactobacillus rhamnosis | BHI | erythromycin (10 μg/mL) |
| Bifidobacterium longum | MRS (+0.05% cystein) | erythromycin (10 μg/mL) |
| Product | WPI | DH28 | DH51 |
|---|---|---|---|
| Protein (%) | 90.0 | 86.5 | 85.1 |
| Lactose (%) | 0.05 | 0.10 | 0.09 |
| Fat (%) | 0.10 | 0.10 | 0.07 |
| Ash (%) | 4.0 | 3.3 | 4.9 |
| Mn (Da) | N/A | 593 | 333 |
| Mw (Da) | N/A | 914 | 581 |
| <375 Da (%) | 16.1 | 37.3 | |
| 375–750 Da (%) | 35.9 | 36.4 | |
| 750–1250 Da (%) | 24.6 | 19.4 | |
| 1250–2500 Da (%) | 21.4 | 6.6 | |
| >2500 Da (%) | 2.1 | 0.3 | |
| DH (%) | 27.7 | 50.9 | |
| FAA (%) | 0.0 | 0.5 | 29.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Lange, I.H.; van Gorp, C.; Massy, K.R.I.; Kessels, L.; Kloosterboer, N.; Bjørnshave, A.; Stampe Ostenfeld, M.; Damoiseaux, J.G.M.C.; Derikx, J.P.M.; van Gemert, W.G.; et al. Hypoxia-Driven Changes in a Human Intestinal Organoid Model and the Protective Effects of Hydrolyzed Whey. Nutrients 2023, 15, 393. https://doi.org/10.3390/nu15020393
de Lange IH, van Gorp C, Massy KRI, Kessels L, Kloosterboer N, Bjørnshave A, Stampe Ostenfeld M, Damoiseaux JGMC, Derikx JPM, van Gemert WG, et al. Hypoxia-Driven Changes in a Human Intestinal Organoid Model and the Protective Effects of Hydrolyzed Whey. Nutrients. 2023; 15(2):393. https://doi.org/10.3390/nu15020393
Chicago/Turabian Stylede Lange, Ilse H., Charlotte van Gorp, Kimberly R. I. Massy, Lilian Kessels, Nico Kloosterboer, Ann Bjørnshave, Marie Stampe Ostenfeld, Jan G. M. C. Damoiseaux, Joep P. M. Derikx, Wim G. van Gemert, and et al. 2023. "Hypoxia-Driven Changes in a Human Intestinal Organoid Model and the Protective Effects of Hydrolyzed Whey" Nutrients 15, no. 2: 393. https://doi.org/10.3390/nu15020393
APA Stylede Lange, I. H., van Gorp, C., Massy, K. R. I., Kessels, L., Kloosterboer, N., Bjørnshave, A., Stampe Ostenfeld, M., Damoiseaux, J. G. M. C., Derikx, J. P. M., van Gemert, W. G., & Wolfs, T. G. A. M. (2023). Hypoxia-Driven Changes in a Human Intestinal Organoid Model and the Protective Effects of Hydrolyzed Whey. Nutrients, 15(2), 393. https://doi.org/10.3390/nu15020393







