Herbal Honey Preparations of Curcuma Xanthorriza and Black Cumin Protect against Carcinogenesis through Antioxidant and Immunomodulatory Activities in Sprague Dawley (SD) Rats Induced with Dimethylbenz(a)anthracene
Abstract
1. Introduction
2. Materials and Methods
2.1. Instruments and Materials
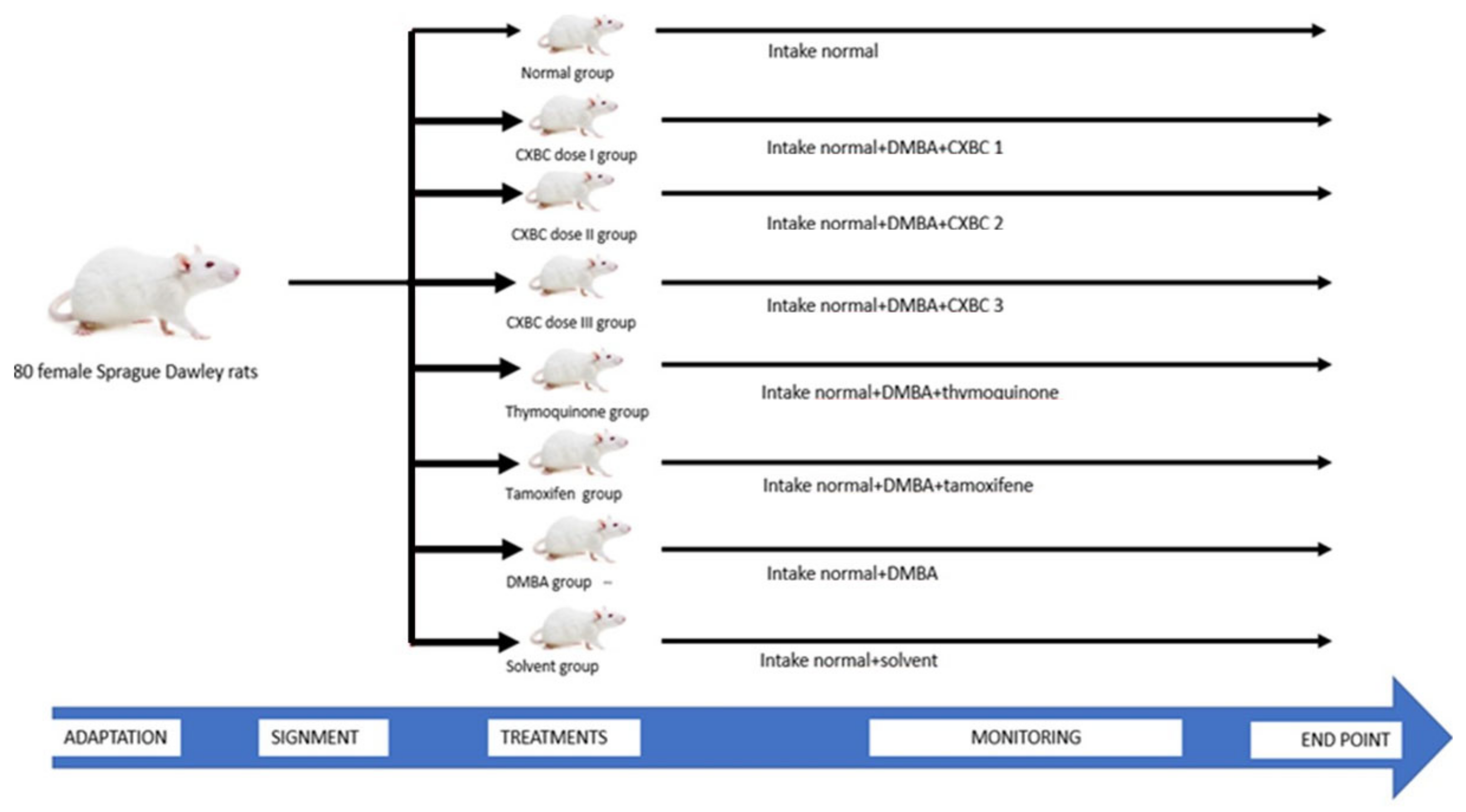
2.1.1. Research Protocol, Test Materials, Positive Control, and Carcinogen
2.1.2. Experimental Cells and Animals
2.1.3. Instruments and Materials
2.2. Preparation of CXBC Herbal Honey, Phytochemical Analysis, and Active Substance Content Testing
2.3. Reactive Radical Binding Activity Test of CXBCH Preparations
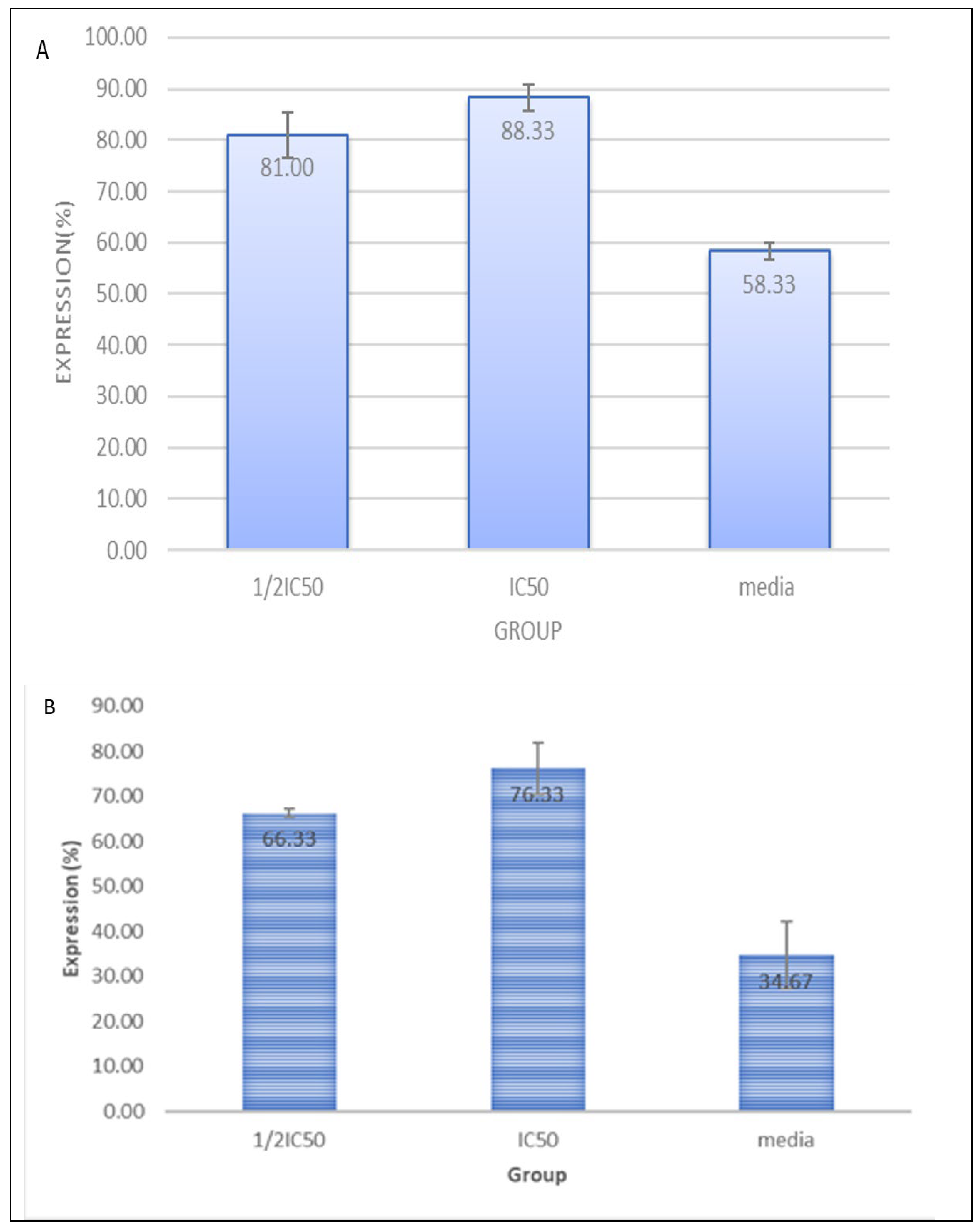
2.4. Viability and Chemopreventive Mechanism Test of CXBCH
2.5. Chemopreventive Testing in Animal Models of Cancer
2.5.1. DMBA Induction and CXBCH Administration in Experimental Animals
2.5.2. Examination Procedure of the Chemopreventive Effect of CXBCH Preparations
Observation of Clinical Manifestations and Nodule Formation
Examination of Nodule Incidence and Multiplication
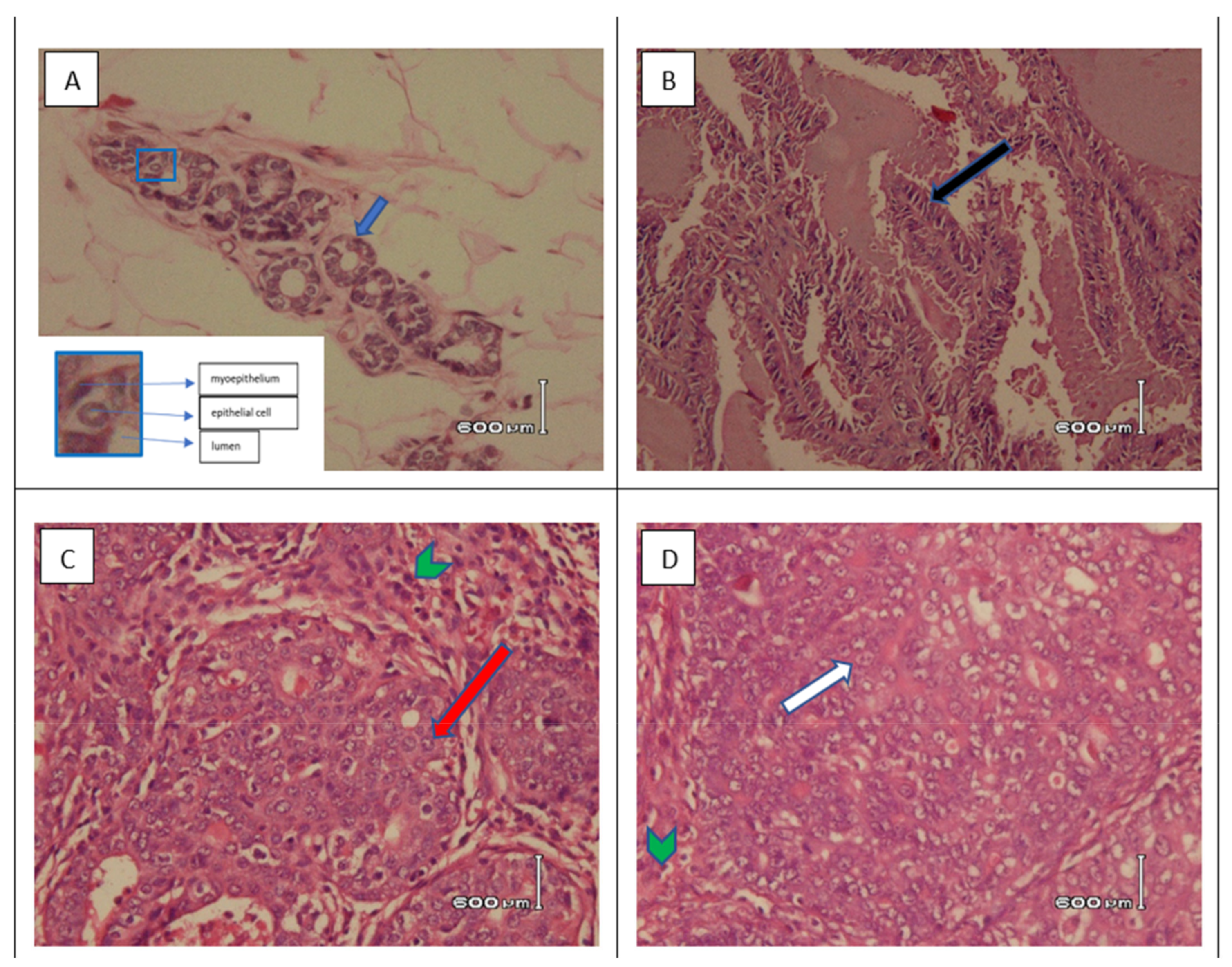
Histopathological Examination
2.6. CXBC Antioxidant Activity Examination Procedure
2.6.1. Examination of Serum NO Levels
2.6.2. Liver and Spleen GST Enzyme Activity
2.7. Monitoring the Immune Response
Number and Types of CD4, CD8 and CD4CD25 Lymphocytes by Flowcytometer
2.8. Data Analysis
3. Results
3.1. Active Substance Content of CXBCH Preparations
3.1.1. Bioactive Compound Profile on CXBC with GCMS and LC-HRMS
3.1.2. Profile of Bioactive Compounds in CXBCH Preparations Observed Using Liquid Chromatography High Resolution Mass Spectrometry (LC-HRMS)
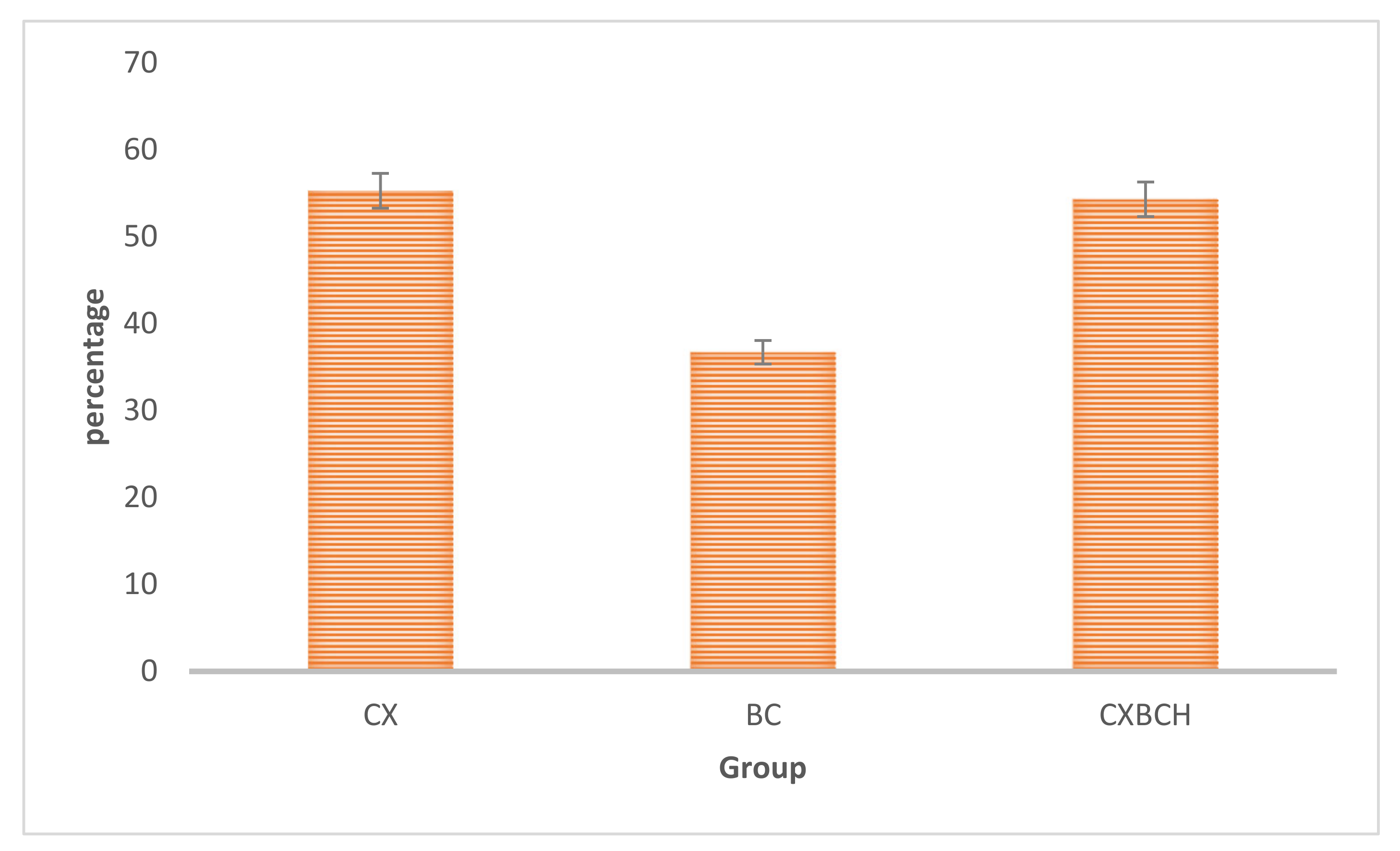
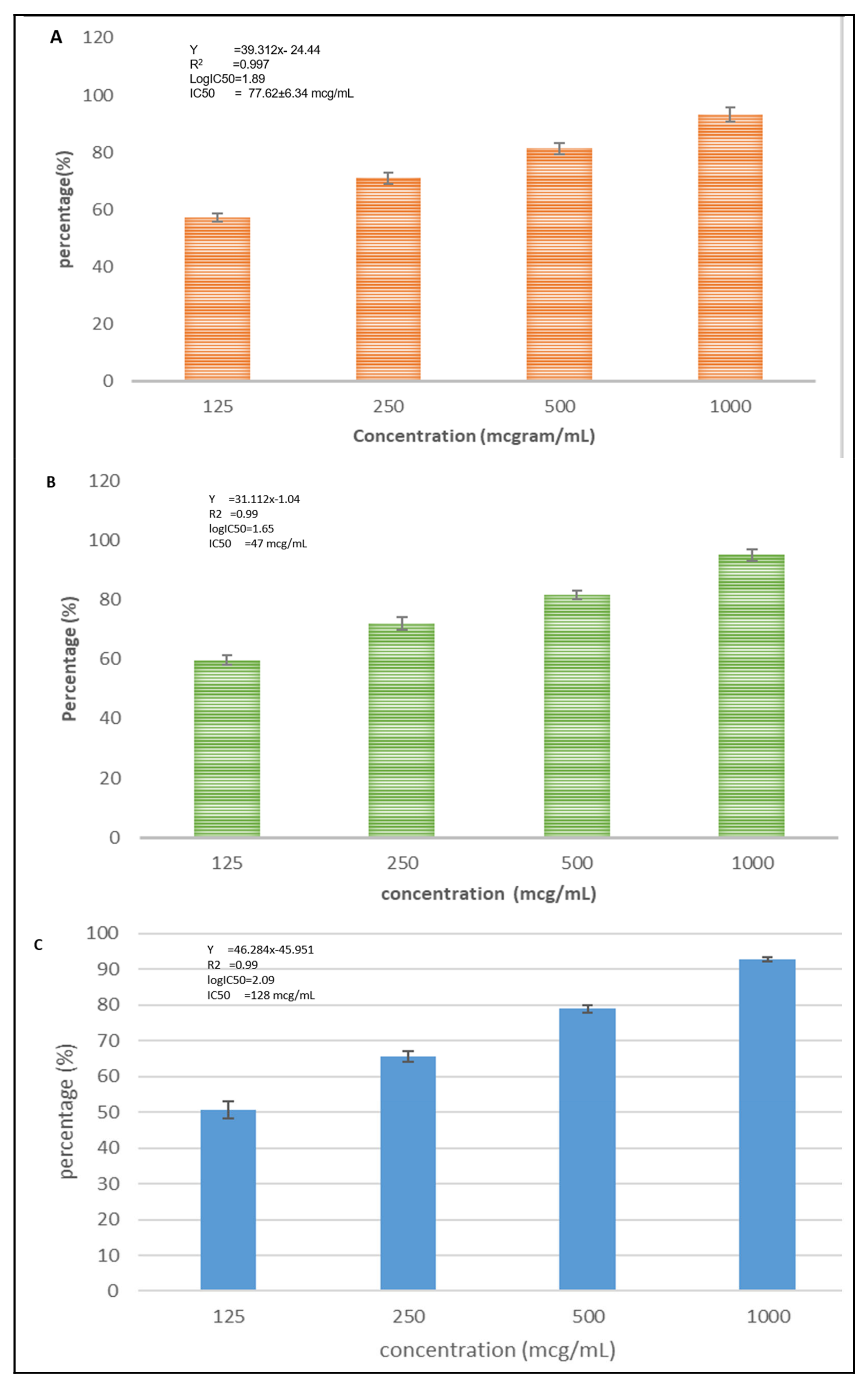
3.2. Cytotoxic and Antioxidant Activity of CXBC Preparations
3.2.1. Radical Scavenging Activities CXBCH Preparation
3.2.2. Cytotoxicity Activity of CXBCH Preparation
3.3. CXBCH Chemopreventive Activity in SD Rats
3.3.1. Clinical Conditions of Test Animals
3.3.2. Nodule Formation
3.3.3. Histopathological Examination of Tumor Tissue in SD Rats Induced with DMBA
3.4. CXBCH by Increasing GST Activity and Decreasing Serum NO Levels
3.5. CXBC Preparations Increase the Number of CD4, CD8 and CD4CD25
3.6. CXBCH Increases Absolute CD8 and CD8CD25 Counts
4. Discussion
4.1. CXBCH Preparation Ingredients and Activities
4.2. CXBCH Chemopreventive Activity
4.3. CXBC Antioxidant Activity through Increased GST Expression and Decreases NO Levels
4.4. CXBCH as Immunomodulator
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, L.L.; Lee, J.S.C.; Waldman, S.D.; Casper, R.F.; Grynpas, M.D. Polycyclic aromatic hydrocarbons present in cigarette smoke cause bone loss in an ovariectomized rat model. Bone 2002, 30, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Kerdelhué, B.; Forest, C.; Coumoul, X. Dimethyl-Benz(a)anthracene: A mammary carcinogen and a neuroendocrine disruptor. Biochim. Open 2016, 3, 49–55. [Google Scholar] [CrossRef]
- De Oliveira, K.D.; Avanzo, G.U.; Tedardi, M.V.; Rangel, M.M.M.; Avanzo, J.L.; Fukumasu, H.; Rao, K.V.K.; Sinhorini, I.L.; Dagli, M.L.Z. Chemical carcinogenesis by DMBA (7,12-dimethylbenzanthracene)in female BALB/c mice: New facts. Braz. J. Vet. Res. Anim. Sci. 2015, 52, 125–133. [Google Scholar] [CrossRef]
- Gopalakrishnan, T.; Ganapathy, S.; Veeran, V.; Namasivayam, N. Preventive effect of D-carvone during DMBA induced mouse skin tumorigenesis by modulating xenobiotic metabolism and induction of apoptotic events. Biomed. Pharmacother. 2019, 111, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Reitsma, M.B.; Kendrick, P.J.; Ababneh, E.; Abbafati, C.; Abbasi-Kangevari, M.; Abdoli, A.; Abedi, A.; Abhilash, E.S.; Abila, D.B.; Aboyans, V.; et al. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet 2021, 397, 2337–2360. [Google Scholar] [CrossRef]
- Crosby, A.; Dunn, J.L.; Aditjondro, E. Rachfiansyah Tobacco Control Is a Wicked Problem: Situating Design Responses in Yogyakarta and Banjarmasin. She Ji 2019, 5, 261–284. [Google Scholar] [CrossRef]
- Ahsan, A. Bunga Rampai Fakta Tembakau dan Permasalahannya di Indonesia 2014; Tobacco Control Support Center—IAKMI, IAKMI Secretariat: Jakarta, Indonesia, 2014. [Google Scholar]
- Hua, M.; Talbot, P. Potential health effects of electronic cigarettes: A systematic review of case reports. Prev. Med. Rep. 2016, 4, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Forster, M.; McAughey, J.; Prasad, K.; Mavropoulou, E.; Proctor, C. Assessment of tobacco heating product THP1.0. Part 4: Characterisation of indoor air quality and odour. Regul. Toxicol. Pharmacol. 2018, 93, 34–51. [Google Scholar] [CrossRef]
- Control, D.; Cdc, P. GATS (Global Adult Tobacco Survey) Comparison Fact Sheet, Indonesia 2011 and 2021. 2021. Available online: https://cdn.who.int/media/docs/default-source/ncds/ncd-surveillance/data-reporting/indonesia/indonesia-national-2021-factsheet.pdf?sfvrsn=53eac4fd_1&download=true (accessed on 13 November 2022).
- Almeida-da-Silva, C.L.C.; Matshik Dakafay, H.; O’Brien, K.; Montierth, D.; Xiao, N.; Ojcius, D.M. Effects of electronic cigarette aerosol exposure on oral and systemic health. Biomed. J. 2020, 44, 252–259. [Google Scholar] [CrossRef]
- Xi, B.; Liang, Y.; Liu, Y.; Yan, Y.; Zhao, M.; Ma, C.; Bovet, P. Tobacco use and second-hand smoke exposure in young adolescents aged 12–15 years: Data from 68 low-income and middle-income countries. Lancet Glob. Health 2016, 4, e795–e805. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Yang, L.; Zhou, Q.; Xing, W.; Toriba, A. Characteristics of Polycyclic Aromatic Hydrocarbons (PAHs) and Common Air Pollutants at Wajima, a Remote Background Site in Japan. Int. J. Environ. Res. Public Health 2020, 17, 957. [Google Scholar] [CrossRef]
- Meckley, D.R.; Hayes, J.R.; Van Kampen, K.R.; Ayres, P.H.; Mosberg, A.T.; Swauger, J.E. Comparative study of smoke condensates from 1R4F cigarettes that burn tobacco versus ECLIPSE cigarettes that primarily heat tobacco in the SENCAR mouse dermal tumor promotion assay. Food Chem. Toxicol. 2004, 42, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Septiono, W.; Kuipers, M.A.G.; Ng, N.; Kunst, A.E. Changes in adolescent smoking with implementation of local smoke-free policies in Indonesia: Quasi-experimental repeat cross-sectional analysis of national surveys of 2007 and 2013. Drug Alcohol Depend. 2020, 209, 107954. [Google Scholar] [CrossRef] [PubMed]
- Miyata, M.; Furukawa, M.; Takahashi, K.; Gonzalez, F.J.; Yamazoe, Y. Mechanism of 7,12-dimethylbenz[a]anthracene-induced immunotoxicity: Role of metabolic activation at the target organ. Jpn. J. Pharmacol. 2001, 86, 302–309. [Google Scholar] [CrossRef]
- Galván, N.; Page, T.J.; Czuprynski, C.J.; Jefcoate, C.R. Benzo(a)pyrene and 7,12-dimethylbenz(a)anthrecene differentially affect bone marrow cells of the lymphoid and myeloid lineages. Toxicol. Appl. Pharmacol. 2006, 213, 105–116. [Google Scholar] [CrossRef]
- Pugalendhi, P.; Manoharan, S.; Panjamurthy, K.; Balakrishnan, S.; Nirmal, M.R. Antigenotoxic effect of genistein against 7,12-dimethylbenz[a]anthracene induced genotoxicity in bone marrow cells of female Wistar rats. Pharmacol. Rep. 2009, 61, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Chełchowska, M.; Ambroszkiewicz, J.; Gajewska, J.; Mazur, J.; Lewandowski, L.; Reśko-Zachara, M.; Maciejewski, T.M. Influence of active exposure to Tobacco smoke on nitric oxide status of pregnant women. Int. J. Environ. Res. Public Health 2018, 15, 2719. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.; Costa, C. Oxidative Stress, Inflammation and Angiogenesis in the Metabolic Syndrome; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009; ISBN 9781402097003. [Google Scholar] [CrossRef]
- Abdel-Wahhab, M.A.; Aljawish, A.; El-Nekeety, A.A.; Abdel-Aiezm, S.H.; Abdel-Kader, H.A.M.; Rihn, B.H.; Joubert, O. Chitosan nanoparticles and quercetin modulate gene expression and prevent the genotoxicity of aflatoxin B1 in rat liver. Toxicol. Rep. 2015, 2, 737–747. [Google Scholar] [CrossRef]
- Gao, J.; Mitchell, L.A.; Lauer, F.T.; Burchiel, S.W. p53 and ATM/ATR regulate 7,12-dimethylbenz[a]anthracene-induced immunosuppression. Mol. Pharmacol. 2008, 73, 137–146. [Google Scholar] [CrossRef]
- Gao, J.; Lauer, F.T.; Mitchell, L.A.; Burchiel, S.W. Microsomal expoxide hydrolase Is required for 7,12-dimethylbenz[a]anthracene (DMBA)—Induced immunotoxicity in mice. Toxicol. Sci. 2007, 98, 137–144. [Google Scholar] [CrossRef]
- Sun, X.; Bernhardt, S.M.; Glynn, D.J.; Hodson, L.J.; Woolford, L.; Evdokiou, A.; Yan, C.; Du, H.; Robertson, S.A.; Ingman, W.V. Attenuated TGFB signalling in macrophages decreases susceptibility to DMBA-induced mammary cancer in mice. Breast Cancer Res. 2021, 23, 39. [Google Scholar] [CrossRef]
- Fujii, S.I.; Shimizu, K.; Shimizu, T.; Lotze, M.T. Interleukin-10 promotes the maintenance of antitumor CD8+ T-cell effector function in situ. Blood 2001, 98, 2143–2151. [Google Scholar] [CrossRef]
- Mattes, J.; Hulett, M.; Xie, W.; Hogan, S.; Rothenberg, M.E.; Foster, P.; Parish, C. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: An eotaxin and STAT6-dependent process. J. Exp. Med. 2003, 197, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Tarigan, S.P.; Soeroso, N.N.; Tumanggor, C.A.K.; Gani, S.; Pradana, A. Clinical profile of male patients with non-small cell lung cancer in Adam Malik General Hospital, Medan, Indonesia. Open Access Maced. J. Med. Sci. 2019, 7, 2612–2614. [Google Scholar] [CrossRef] [PubMed]
- Anisimov, V.N.; Popovich, I.G.; Zabezhinski, M.A.; Anisimov, S.V.; Vesnushkin, G.M.; Vinogradova, I.A. Melatonin as antioxidant, geroprotector and anticarcinogen. Biochim. Biophys. Acta Bioenerg. 2006, 1757, 573–589. [Google Scholar] [CrossRef]
- Kleiner, H.E. Oral administration of naturally occurring coumarins leads to altered phase I and II enzyme activities and reduced DNA adduct formation by polycyclic aromatic hydrocarbons in various tissues of SENCAR mice. Carcinogenesis 2001, 22, 73–82. [Google Scholar] [CrossRef]
- Shimada, T.; Fujii-Kuriyama, Y. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and 1B1. Cancer Sci. 2004, 95, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Hall, P.; Smith, M.; Kirk, M.; Prasain, J.K.; Barnes, S.; Grubbs, C. Chemoprevention by Grape Seed Extract and Genistein in Carcinogen-induced Mammary Cancer in Rats Is Diet Dependent. J. Nutr. 2004, 134, 3445S–3452S. [Google Scholar] [CrossRef]
- Nugraha, R.V.; Ridwansyah, H.; Ghozali, M.; Khairani, A.F.; Atik, N. Traditional Herbal Medicine Candidates as Complementary Treatments for COVID-19: A Review of Their Mechanisms, Pros and Cons. Evid. Based Complement. Altern. Med. 2020, 2020, 2560645. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Mishra, R.K.; Vyawahare, A.; Kumar, A.; Rehman, M.U.; Qamar, W.; Khan, A.Q.; Khan, R. Thymoquinone (2-Isoprpyl-5-methyl-1, 4-benzoquinone) as a chemopreventive/anticancer agent: Chemistry and biological effects. Saudi Pharm. J. 2019, 27, 1113–1126. [Google Scholar] [CrossRef]
- Hidayati, T.; Akrom; Indrayanti; Sagiran. Chemopreventive effect of black cumin seed oil (BCSO) by increasing p53 expression in dimethylbenzanthracene (DMBA)-induced Sprague Dawley rats. Res. J. Chem. Environ. 2019, 23, 24–32. [Google Scholar]
- Mansour, M.A.; Nagi, M.N.; El-Khatib, A.S.; Al-Bekairi, A.M. Effects of thymoquinone on antioxidant enzyme activities, lipid peroxidation and dt-diaphorase in different tissues of mice: A possible mechanism of action. Cell Biochem. Funct. 2002, 20, 143–151. [Google Scholar] [CrossRef]
- Wani, M.R.; Shadab, G.G.H.A. Low doses of thymoquinone protect isolated human blood cells from TiO2 nanoparticles induced oxidative stress, hemolysis, cytotoxicity, DNA damage and collapse of mitochondrial activity. Phytomed. Plus 2021, 1, 100056. [Google Scholar] [CrossRef]
- Fu, Y.S.; Chen, T.H.; Weng, L.; Huang, L.; Lai, D.; Weng, C.F. Pharmacological properties and underlying mechanisms of curcumin and prospects in medicinal potential. Biomed. Pharmacother. 2021, 141, 111888. [Google Scholar] [CrossRef]
- Zhao, G.; Qi, L.; Wang, Y.; Li, X.; Li, Q.; Tang, X.; Wang, X.; Wu, C. Antagonizing effects of curcumin against mercury-induced autophagic death and trace elements disorder by regulating PI3K/AKT and Nrf2 pathway in the spleen. Ecotoxicol. Environ. Saf. 2021, 222, 112529. [Google Scholar] [CrossRef]
- Oon, S.F.; Nallappan, M.; Tee, T.T.; Shohaimi, S.; Kassim, N.K.; Sa’ariwijaya, M.S.F.; Cheah, Y.H. Xanthorrhizol: A review of its pharmacological activities and anticancer properties. Cancer Cell Int. 2015, 15, 100. [Google Scholar] [CrossRef]
- Salleh, N.; Ismail, S.; Ab Halim, M.R. Effects of Curcuma xanthorrhiza extracts and their constituents on phase II drug-metabolizing enzymes activity. Pharmacogn. Res. 2016, 8, 309–315. [Google Scholar] [CrossRef]
- Singgih Wahono, C.; Diah Setyorini, C.; Kalim, H.; Nurdiana, N.; Handono, K. Effect of Curcuma xanthorrhiza Supplementation on Systemic Lupus Erythematosus Patients with Hypovitamin D Which Were Given Vitamin D 3 towards Disease Activity (SLEDAI), IL-6, and TGF- β 1 Serum. Int. J. Rheumatol. 2017, 2017, 7687053. [Google Scholar] [CrossRef]
- Silva, T.M.S.; Santos, F.P.; Evangelista-Rodrigues, A.; da Silva, E.M.S.; da Silva, G.S.; de Novais, J.S.; de Assis Ribeiro dos Santos, F.; Camara, C.A. Phenolic compounds, melissopalynological, physicochemical analysis and antioxidant activity of jandaíra (Melipona subnitida) honey. J. Food Compos. Anal. 2013, 29, 10–18. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Silva, B.; Bergamo, G.; Brugnerotto, P.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. An overview of physicochemical characteristics and health-promoting properties of honeydew honey. Food Res. Int. 2019, 119, 44–66. [Google Scholar] [CrossRef]
- Khan, S.U.; Anjum, S.I.; Ansari, M.J.; Khan, M.H.U.; Kamal, S.; Rahman, K.; Shoaib, M.; Man, S.; Khan, A.J.; Khan, S.U.; et al. Antimicrobial potentials of medicinal plant’s extract and their derived silver nanoparticles: A focus on honey bee pathogen. Saudi J. Biol. Sci. 2019, 26, 1815–1834. [Google Scholar] [CrossRef] [PubMed]
- Hidayati, T.; Indrayanti; Sagiran. DMBA induction increases H-ras gene expression and decreases CD8 count in sprague dawley rats. In Proceedings of the 2019 6th International Conference on Biomedical and Bioinformatics Engineering, Shanghai, China, 13–15 November 2019; pp. 111–117. [Google Scholar] [CrossRef]
- Ibrahim, A.B.; Zaki, H.F.; Ibrahim, W.W.; Omran, M.M.; Shouman, S.A. Evaluation of tamoxifen and simvastatin as the combination therapy for the treatment of hormonal dependent breast cancer cells. Toxicol. Rep. 2019, 6, 1114–1126. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, T.G.; Carriço, T.; Fernandes, E.; Abessa, D.M.S.; Tavares, A.; Bebianno, M.J. Impacts of in vivo and in vitro exposures to tamoxifen: Comparative effects on human cells and marine organisms. Environ. Int. 2019, 129, 256–272. [Google Scholar] [CrossRef] [PubMed]
- Yamanoshita, O.; Ichihara, S.; Hama, H.; Ichihara, G.; Chiba, M.; Kamijima, M.; Takeda, I.; Nakajima, T. Chemopreventive effect of selenium-enriched Japanese radish sprout against breast cancer induced by 7,12-dimethylbenz[a]anthracene in rats. Tohoku J. Exp. Med. 2007, 212, 191–198. [Google Scholar] [CrossRef]
- Panda, V.; Deshmukh, A.; Singh, S.; Shah, T.; Hingorani, L. An Ayurvedic formulation of Emblica officinalis and Curcuma longa alleviates insulin resistance in diabetic rats: Involvement of curcuminoids and polyphenolics. J. Ayurveda Integr. Med. 2021, 12, 506–513. [Google Scholar] [CrossRef]
- Soumaya, K.J.; Zied, G.; Nouha, N.; Mounira, K.; Kamel, G.; Genviève, F.D.M.; Leila, G.C. Evaluation of in vitro antioxidant and apoptotic activities of Cyperus rotundus. Asian Pac. J. Trop. Med. 2014, 7, 105–112. [Google Scholar] [CrossRef]
- Akanni, O.O.; Owumi, S.E.; Adaramoye, O.A. In vitro studies to assess the antioxidative, radical scavenging and arginase inhibitory potentials of extracts from Artocarpus altilis, Ficus exasperate and Kigelia africana. Asian Pac. J. Trop. Biomed. 2014, 4, S492–S499. [Google Scholar] [CrossRef]
- Khazdair, M.R.; Gholamnezhad, Z.; Rezaee, R.; Boskabady, M.H. A qualitative and quantitative comparison of Crocus sativus and Nigella sativa immunomodulatory effects. Biomed. Pharmacother. 2021, 140, 111774. [Google Scholar] [CrossRef]
- Noori, S.; Kiasat, A.R.; Kolahi, M.; Mirzajani, R.; Seyyed Nejad, S.M. Determination of secondary metabolites including curcumin in Rheum ribes L. and surveying of its antioxidant and anticancer activity. J. Saudi Chem. Soc. 2022, 26, 101479. [Google Scholar] [CrossRef]
- Aryal, B.; Adhikari, B.; Aryal, N.; Bhattarai, B.R.; Khadayat, K.; Parajuli, N. LC-HRMS Profiling and Antidiabetic, Antioxidant, and Antibacterial Activities of Acacia catechu (L.f.) Willd. Biomed. Res. Int. 2021, 2021, 7588711. [Google Scholar] [CrossRef]
- Dalhoumi, W.; Guesmi, F.; Bouzidi, A.; Akermi, S.; Hfaiedh, N.; Saidi, I. Therapeutic strategies of Moringa oleifera Lam. (Moringaceae) for stomach and forestomach ulceration induced by HCl/EtOH in rat model. Saudi J. Biol. Sci. 2022, 29, 103284. [Google Scholar] [CrossRef] [PubMed]
- Majdalawieh AF & Fayyad MW Recent advances on the anti-cancer properties of Nigella sativa, a widely used food additive. J. Ayurveda Integr. Med. 2016, 7, 173–180.
- Roy, A.M.; Baliga, M.S.; Katiyar, S.K. Epigallocatechin-3-gallate induces apoptosis in estrogen receptor-negative human breast carcinoma cells via modulation in protein expresssion of p53 and Bax and caspase-3 activation. Mol. Cancer Ther. 2005, 4, 81–90. [Google Scholar] [CrossRef]
- Hamza, A.A.; Khasawneh, M.A.; Elwy, H.M.; Hassanin, S.O.; Elhabal, S.F.; Fawzi, N.M. Salvadora persica attenuates DMBA-induced mammary cancer through downregulation oxidative stress, estrogen receptor expression and proliferation and augmenting apoptosis. Biomed. Pharmacother. 2022, 147, 112666. [Google Scholar] [CrossRef] [PubMed]
- Aydin, M.S.; Caliskan, A.; Kocarslan, A.; Kocarslan, S.; Yildiz, A.; Günay, S.; Savik, E.; Hazar, A.; Yalcin, F. Intraperitoneal curcumin decreased lung, renal and heart injury in abdominal aorta ischemia/reperfusion model in rat. Int. J. Surg. 2014, 12, 601–605. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Ni, Z.; Oveisi, F.; Liang, K.; Pandian, R. Enhanced nitric oxide inactivation and protein nitration by reactive oxygen species in renal insufficiency. Hypertension 2002, 39, 135–141. [Google Scholar] [CrossRef]
- Gholamnezhad, Z.; Boskabady, M.H.; Hosseini, M. The effect of chronic supplementation of Nigella sativa on splenocytes response in rats following treadmill exercise. Drug Chem. Toxicol. 2019, 29, 487–492. [Google Scholar] [CrossRef]
- Monton, C.; Settharaksa, S.; Luprasong, C.; Songsak, T. An optimization approach of dynamic maceration of Centella asiatica to obtain the highest content of four centelloids by response surface methodology. Rev. Bras. Farmacogn. 2019, 29, 254–261. [Google Scholar] [CrossRef]
- Aljohar, H.I.; Maher, H.M.; Albaqami, J.; Al-Mehaizie, M.; Orfali, R.; Orfali, R.; Alrubia, S. Physical and chemical screening of honey samples available in the Saudi market: An important aspect in the authentication process and quality assessment. Saudi Pharm. J. 2018, 26, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Anjum, S.I.; Rahman, K.; Ansari, M.J.; Khan, W.U.; Kamal, S.; Khattak, B.; Muhammad, A.; Khan, H.U. Honey: Single food stuff comprises many drugs. Saudi J. Biol. Sci. 2018, 25, 320–325. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Lamas, L.B.; Flórez, S.M.; Toyos, P.A.; et al. Phenolic compounds in honey and their associated health benefits: A review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [PubMed]
- Hatipoglu, D.; Keskin, E. The effect of curcumin on some cytokines, antioxidants and liver function tests in rats induced by Aflatoxin B1. Heliyon 2022, 8, e09890. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Khan, M.R.; Mishra, S.K. An updated literature-based review: Phytochemistry, pharmacology and therapeutic promises of Nigella sativa L. Orient. Pharm. Exp. Med. 2019, 19, 115–129. [Google Scholar] [CrossRef]
- Akrom, A.; Darmawan, E.; Yuhelvia, L. Black Cumin Seed Oilas Hepatoprotector in Decreasing SGPT and SGOT Activity and Increasing p53 Gene Expression in Sprague Dawley Rats Induced by Alloxan. Int. J. Public Health Sci. 2015, 4, 159. [Google Scholar] [CrossRef]
- Burits, M.; Bucar, F. Antioxidant activity of Nigella sativa essential oil. Phyther. Res. 2000, 14, 323–328. [Google Scholar] [CrossRef]
- Çelik, F.; Göçmez, C.; Karaman, H.; Kamaşak, K.; Kaplan, I.; Akil, E.; Tufek, A.; Guzel, A.; Uzar, E. Therapeutic Effects of Thymoquinone in a Model of Neuropathic Pain. Curr. Ther. Res. Clin. Exp. 2014, 76, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Farkhondeh, T.; Samarghandian, S.; Borji, A. An overview on cardioprotective and anti-diabetic effects of thymoquinone. Asian Pac. J. Trop. Med. 2017, 10, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Shaterzadeh-Yazdi, H.; Noorbakhsh, M.-F.; Samarghandian, S.; Farkhondeh, T. An Overview on Renoprotective Effects of Thymoquinone. Kidney Dis. 2018, 4, 74–82. [Google Scholar] [CrossRef]
- Umar, S.; Shah, M.A.A.; Munir, M.T.; Yaqoob, M.; Fiaz, M.; Anjum, S.; Kaboudi, K.; Bouzouaia, M.; Younus, M.; Nisa, Q.; et al. Synergistic effects of thymoquinone and curcumin on immune response and anti-viral activity against avian influenza virus (H9N2) in turkeys. Poult. Sci. 2016, 95, 1513–1520. [Google Scholar] [CrossRef]
- Ali, M.Y.; Akter, Z.; Mei, Z.; Zheng, M.; Tania, M.; Khan, M.A. Thymoquinone in autoimmune diseases: Therapeutic potential and molecular mechanisms. Biomed. Pharmacother. 2021, 134, 111157. [Google Scholar] [CrossRef]
- Begum, S.; Mannan, A. A Review on Nigella sativa: A Marvel Herb. J. Drug Deliv. Ther. 2020, 10, 213–219. [Google Scholar] [CrossRef]
- Rajkamal, G.; Suresh, K.; Sugunadevi, G.; Vijayaanand, M.A.; Rajalingam, K. Evaluation of chemopreventive effects of Thymoquinone on cell surface glycoconjugates and cytokeratin expression during DMBA induced hamster buccal pouch carcinogenesis. BMB Rep. 2010, 43, 664–669. [Google Scholar] [CrossRef]
- Singletary, K.; MacDonald, C.; Iovinelli, M.; Fisher, C.; Wallig, M. Effect of the β-diketones diferuloylmethane (curcumin) and dibenzoylmethane on rat mammary DNA adducts and tumors induced by 7,12-dimethylbenz[a]anthracene. Carcinogenesis 1998, 19, 1039–1043. [Google Scholar] [CrossRef]
- Yeh, H.C. and G.; Cellular The flavonoid galangin is an inhibitor of CYP1A1 activity and an agonist/antagonist of the aryl hydrocarbon receptor. Br. J. Cancer 1999, 79, 1340–1346. [Google Scholar]
- Jensen, B.A.; Leeman, R.J.; Schlezinger, J.J.; Sherr, D.H. Aryl hydrocarbon receptor (AhR) agonists suppress interleukin-6 expression by bone marrow stromal cells: An immunotoxicology study. Environ. Health Glob. Access Sci. Source 2003, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lutterodt, H.; Luther, M.; Slavin, M.; Yin, J.J.; Parry, J.; Gao, J.M.; Yu, L.L. Fatty acid profile, thymoquinone content, oxidative stability, and antioxidant properties of cold-pressed black cumin seed oils. LWT—Food Sci. Technol. 2010, 43, 1409–1413. [Google Scholar] [CrossRef]
- Abbas, F.; Eladl, M.A.; El-Sherbiny, M.; Abozied, N.; Nabil, A.; Mahmoud, S.M.; Mokhtar, H.I.; Zaitone, S.A.; Ibrahim, D. Celastrol and thymoquinone alleviate aluminum chloride-induced neurotoxicity: Behavioral psychomotor performance, neurotransmitter level, oxidative-inflammatory markers, and BDNF expression in rat brain. Biomed. Pharmacother. 2022, 151, 113072. [Google Scholar] [CrossRef]
- El Aziz, M.A.; Hassan, H.A.; Mohammed, M.H.; Meki, A. The biochemical and morphological alterations following administration of melatonin, retinoic acid and Nigella sativa in mammary carcinoma:an animal model. Int. J. Exp. Pathol 2005, 86, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Yuk-kwan, C.; Shui-sang, H.; Li-min, L.I.N. The mRNA expression of inducible nitric oxide synthase in DMBA-induced hamster buccal-pouch carcinomas: An In Situ RT-PCR study. Int. J. Exp. Pathol. 2002, 88, 133–137. [Google Scholar] [CrossRef]
- Alghamdi, F.; Al-Seeni, M.N.; Ghoneim, M.A. Potential synergistic antioxidant effect of thymoquinone and vitamin E on cisplatin-induced acute nephropathy in rats. Clin. Nutr. Exp. 2020, 32, 29–37. [Google Scholar] [CrossRef]
- Fusi, J.; Bianchi, S.; Daniele, S.; Pellegrini, S.; Martini, C.; Galetta, F.; Giovannini, L.; Franzoni, F. An In Vitro comparative study of the antioxidant activity and SIRT1 modulation of natural compounds. Biomed. Pharmacother. 2018, 101, 805–819. [Google Scholar] [CrossRef]
- Ramaswami, G.; Chai, H.; Yao, Q.; Lin, P.H.; Lumsden, A.B.; Chen, C. Curcumin blocks homocysteine-induced endothelial dysfunction in porcine coronary arteries. J. Vasc. Surg. 2004, 40, 1216–1222. [Google Scholar] [CrossRef]
- Tabassum, S.; Rosli, N.; Ichwan, S.J.A.; Mishra, P. Thymoquinone and its pharmacological perspective: A review. Pharmacol. Res. Mod. Chin. Med. 2021, 1, 100020. [Google Scholar] [CrossRef]
- Kassab, R.B.; El-Hennamy, R.E. The role of thymoquinone as a potent antioxidant in ameliorating the neurotoxic effect of sodium arsenate in female rat. Egypt. J. Basic Appl. Sci. 2017, 4, 160–167. [Google Scholar] [CrossRef]
- Yaman, I.; Balikci, E. Protective effects of nigella sativa against gentamicin-induced nephrotoxicity in rats. Exp. Toxicol. Pathol. 2010, 62, 183–190. [Google Scholar] [CrossRef]
- Petroni, G.; Galluzzi, L. Impact of treatment schedule on the efficacy of cytostatic and immunostimulatory agents. Oncoimmunology 2021, 10, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.S.; Chen, Y.Q.; Lin, S.H.; Xie, K.; Wang, C.J.; Yang, Y.Z.; Xu, F. Curcumin regulates the differentiation of naïve CD4+T cells and activates IL-10 immune modulation against acute lung injury in mice. Biomed. Pharmacother. 2020, 125, 109946. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.S.; Begum, N.; Mutha, S.; Bakshi, V. Beneficial effect of Curcumin in Letrozole induced polycystic ovary syndrome. Asian Pac. J. Reprod. 2016, 5, 116–122. [Google Scholar] [CrossRef]
- Salem, M.L. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int. Immunopharmacol. 2005, 5, 1749–1770. [Google Scholar] [CrossRef] [PubMed]
- Thimmulappa, R.K.; Mudnakudu-Nagaraju, K.K.; Shivamallu, C.; Subramaniam, K.J.T.; Radhakrishnan, A.; Bhojraj, S.; Kuppusamy, G. Antiviral and immunomodulatory activity of curcumin: A case for prophylactic therapy for COVID-19. Heliyon 2021, 7, e06350. [Google Scholar] [CrossRef]
- Shaterzadeh-Yazdi, H.; Noorbakhsh, M.-F.; Hayati, F.; Samarghandian, S.; Farkhondeh, T. Immunomodulatory and Anti-inflammatory Effects of Thymoquinone. Cardiovasc. Hematol. Disord. Targets 2018, 18, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Finlay, T.M.; Abdulkhalek, S.; Gilmour, A.; Guzzo, C.; Jayanth, P.; Amith, S.R.; Gee, K.; Beyaert, R.; Szewczuk, M.R. Thymoquinone-induced Neu4 sialidase activates NFκB in macrophage cells and pro-inflammatory cytokines In Vivo. Glycoconj. J. 2010, 27, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Aljohani, Z.M.; Alharbi, A.A.; Alsaedi, M.; Alahmadi, A.F.; Alahmadi, O.A.; Alharbi, A.A.; Abo-Haded, H.M. Evaluation of the potential beneficial effects of thymoquinone against nicotine induced toxicity. Int. J. Pharm. Clin. Res. 2015, 7, 395–398. [Google Scholar]
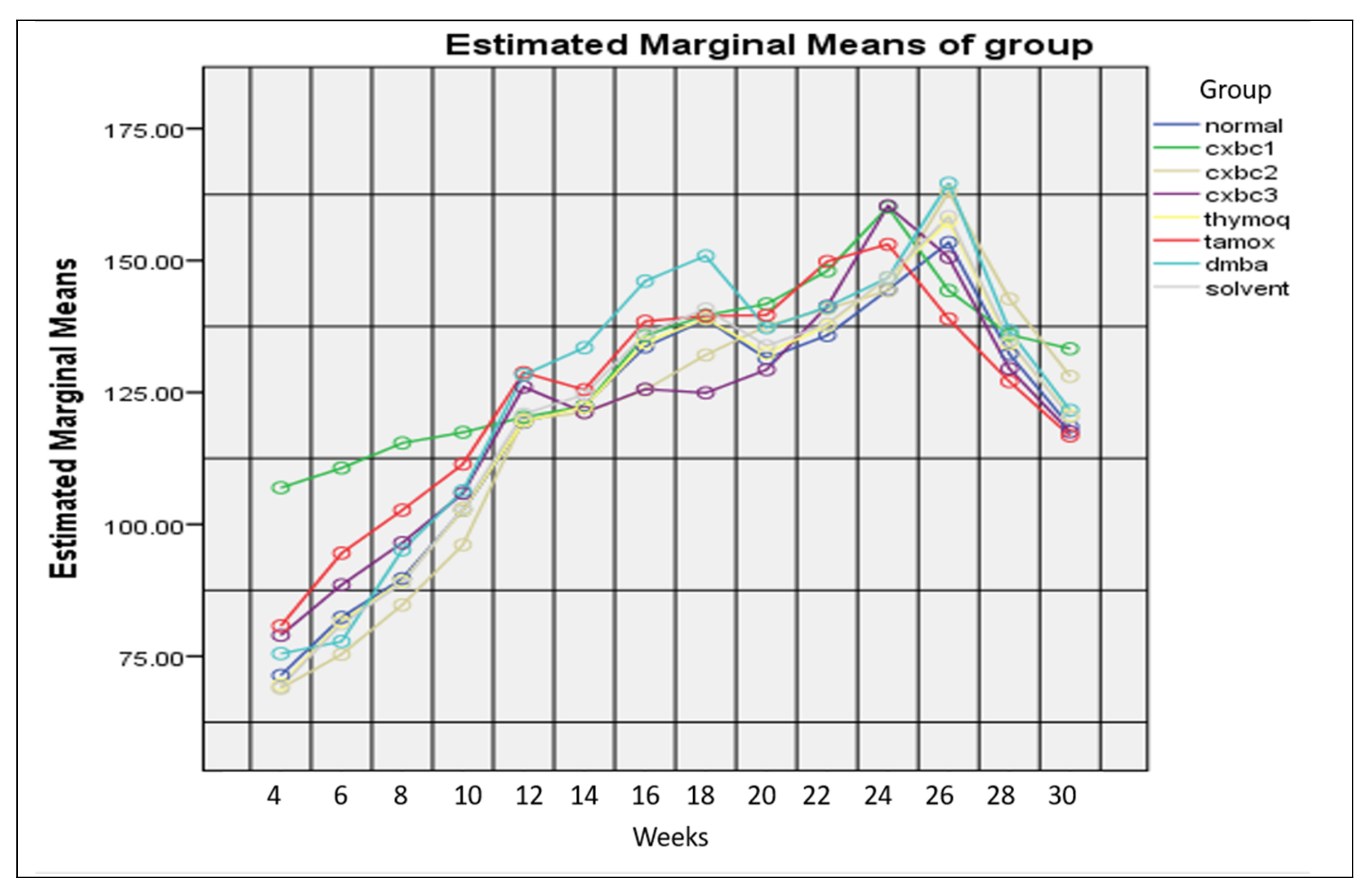
| Test Groups | n | Dead Beginning | Livability of Test Animals (%) Week | Percentage of Total Deaths (%) | ||
|---|---|---|---|---|---|---|
| 16 | 20 | 30 | ||||
| Normal | 10 | 1 | 90.00 | 90.00 | 90.00 | 10.00 |
| CXBCH 1 | 10 | 1 | 90.00 | 90.00 | 90.00 | 10.00 |
| CXBCH 2 | 10 | 2 | 80.00 | 80.00 | 80.00 | 20.00 |
| CXBCH 3 | 10 | 1 | 90.00 | 90.00 | 80.00 | 20.00 |
| Thymoquinone | 10 | 1 | 90.00 | 90.00 | 90.00 | 10.00 |
| Tamoxifen | 10 | 2 | 80.0 | 80.00 | 70.00 | 30.00 |
| DMBA | 10 | 3 | 70.00 | 40.00 | 30.00 | 100.00 |
| Solvent | 10 | 1 | 90.00 | 90.00 | 90.00 | 10.00 |
| Group | Leukocyte Count (×103/µL) (Mean ± sd) | Erythrocyte Count (×106/µL) (Mean ± sd) | Platelet Count (×103/µL) (Mean ± sd) | Hb Level (Mean ± sd) | MCV (Mean ± sd) | MCH (Mean ± sd) |
|---|---|---|---|---|---|---|
| Normal (10) | 6.33 ± 1.37 * | 8.32 ± 0.40b,c,* | 981.33 ± 95.37 * | 14.67 ± 0.52 * | 58.33 ± 1.37 * | 20.00 ± 0.89 * |
| CXBCH 1 (10) | 9.86 ± 0.90 a* | 7.87 ± 0.27 * | 668.71 ± 50.91 a,* | 14.74 ± 0.24 * | 56.86 ± 0.89 a,* | 18.71 ± 0.49 a,* |
| CXBCH 2 (10) | 6.29 ± 0.49 * | 7.35 ± 0.28 a* | 724.71 ± 181.25 * | 14.44 ± 0.80 * | 59.29 ± 0.49 * | 19.29 ± 0.49 * |
| CXBCH 3 (10) | 7.00 ± 0.93 * | 7.65 ± 0.14 a* | 802.00 ± 106.23 * | 14.59 ± 0.85 * | 59.38 ± 0.52 * | 19.38 ± 0.52 * |
| Thymoquinone (10) | 6.86 ± 0.90 * | 6.53 ± 0.94 a* | 734.29 ± 151.14 * | 13.34 ± 0.74 * | 57.34 ± 2.30 * | 21.17 ± 1.21 * |
| Tamoxifen (10) | 7.00 ± 2.68 * | 6.59 ± 0.21 a* | 889.17 ± 387.24 * | 13.83 ± 2.99 * | 57.33 ± 1.63 * | 19.67 ± 0.52 * |
| DMBA (10) | 2.80 ± 1.10 a,b,c | 4.18 ± 0.94 a,b,c | 255.00 ± 70.31 a,b,c | 6.60 ± 2.07 a,b,c | 54.40 ± 1.52 a,b,c | 16.33 ± 0.52 a,b,c |
| Solvent (10) | 6.67 ± 1.03 * | 9.14 ± 0.42 * | 908.00 ± 120.10 * | 15.67 ± 0.52 * | 56.67 ± 1.37 a,* | 19.33 ± 0.52 * |
| Test Group | Serum Urea Level (Mean ± sd) (mg/dL) | Serum Creatinine Level (Mean ± sd) (mg/dL) | The Average Level of SGPT (Mean ± sd) (U/L) | Average Serum SGOT Level (Mean ± sd) (U/L) |
|---|---|---|---|---|
| Normal (10) | 26.33 ± 1.37 c,* | 0.40 ± 0.00 b,c,* | 44.00 ± 0.89 c,* | 130.33 ± 1.37 c,* |
| CXBCH 1 (10) | 27.14 ± 4.81 * | 0.26 ± 0.05 a,b,c,* | 52.00 ± 4.86 a,b,* | 107.19 ± 21.78 a,c,* |
| CXBCH 2 (10) | 30.29 ± 2.06 a,* | 0.30 ± 0.00 a,b,c,* | 58.00 ± 11.65 a,* | 88.00 ± 4.89 a,b,c,* |
| CXBCH 3 (10) | 33.63 ± 4.17 a,* | 0.30 ± 0.00 a,b,c,* | 55.38 ± 9.04 a,* | 83.50 ± 3.70 a,b,c,* |
| Thymoquinone (10) | 31.11 ± 7.26 * | 0.34 ± 0.05 a,* | 44.31 ± 12.81 * | 124.14 ± 5.46 a,c,* |
| Tamoxifen (10) | 37.00 ± 6.39 a | 0.35 ± 0.08 a,* | 95.33 ± 74.29 a,* | 206.17 ± 2.43 a,b,* |
| DMBA(10) | 40.80 ± 0.84 a,b,c | 0.54 ± 0.05a a,b,c | 156.80 ± 50.58 a,b,c | 830.40 ± 92.66 a,b,c |
| Solvent (10) | 35.00 ± 4.73 * | 0.33 ± 0.05 a,* | 60.33 ± 7.23 * | 92.00 ± 8.80 * |
| Test Group (n) | Incidence of Tumor Formation (%) | Number of Nodules Formed | Tumor Multiplicity (Nodule/Rat) | Total Weight of Nodules (g) |
|---|---|---|---|---|
| Normal (10) | 0 | 0 | 0.0 ± 0.0 | 0 |
| CXBCH 1 (10) | 50% | 8 | 0.50 ± 0.50 | 2.51 |
| CXBCH 2 (10) | 50% | 8 | 0.80 ± 0.92 | 3.25 |
| CXBCH 3 (10) | 50% | 6 | 0.73 ± 0.79 | 4.17 |
| Thymoquinone (10) | 30% | 3 | 0.30 ± 0.48 | 1.20 |
| Tamoxifen (10) | 30% | 5 | 0.46 ± 0.93 | 1.40 |
| DMBA (10) | 100% | 14 | 1.40 ± 1.1 | 10.53 |
| Solvent (10) | 0 | 0 | 0.0 ± 0.0 | 0 |
| Test Groups | Type of Histopathological Picture (%) | % Inhibition of ACM | ||
|---|---|---|---|---|
| TAP | PP | ACM (Invasive) | ||
| Normal (10) | 100.00 | 0 | 0 | - |
| CXBCH 1 (10) | 50.00 | 30.00 | 20.00 | 80 |
| CXBCH 2 (10) | 60.00 | 10.00 | 30.00 | 70 |
| CXBCH 3 (10) | 55.00 | 40.00 | 10.00 | 90 |
| Thymoquinone (10) | 70.00 | 30.00 | 0 | 100 |
| Tamoxifen (10) | 72.70 | 0 | 27.30 | 73 |
| DMBA (10) | 0 | 0 | 100.00 | 0 |
| Solvent (10) | 100 | 0 | 0 | 0 |
| Test Group | Average Serum NO Level (µM) (Mean ± sd) |
|---|---|
| Normal (10) | 0.20 ± 0.07 b,c,* |
| CXBCH 1 (10) | 0.21 ± 0.05 a,b,c,* |
| CXBCH 2 (10) | 0.24 ± 0.05 a,b,c,* |
| CXBCH 3 (10) | 0.18 ± 0.12 a,b,c,* |
| Thymoquinone (10) | 0.29 ± 0.09 a,c,* |
| Tamoxifen (10) | 0.23 ± 0.01 a,b,* |
| DMBA (10) | 0.38 ± 0.09 a,b,c |
| Solvent (10) | 0.18 ± 0.02 a,b,c,* |
| Group | GST Activity in Liver and Spleen Tissue (Mean ± sd) (ug/min/mL) | |
|---|---|---|
| Spleen | Liver | |
| Normal (10) | 8.70 ± 0.89 * | 82.91 ± 7.93 * |
| CXBCH 1 (10) | 17.39 ± 2.17 a,* | 106.98 ± 5.45 a,* |
| CXBCH 2 (10) | 18.87 ± 1.30 a,* | 112.21 ± 8.87 a,* |
| CXBCH 3 (10) | 20.44 ± 0.98 a,* | 113.83 ± 10.08 a,* |
| Thymoquinone (10) | 18.74 ± 2.38 a,* | 91.14 ± 7.18 a,* |
| Tamoxifen (10) | 17.62 ± 2.61 a,* | 83.29 ± 11.14 * |
| DMBA (10) | 6.86 ± 0.91 a | 65.54 ± 3.31 a |
| Solvent (10) | 8.41 ± 0.76 * | 83.50 ± 7.31 * |
| Test Group | Absolute Amount CD4 (Mean ± SD) | Absolute CD4CD25 Count (Mean ± SD) | Percentage of CD4CD25 to CD4 (Mean ± SD) |
|---|---|---|---|
| Normal (10) | 1575.67 ± 131.70 * | 70.50 ± 11.76 | 4.41 ± 0.01 b,c,* |
| CXBC 1 (10) | 1619.57 ± 519.86 * | 80.86 ± 17.78 * | 4.19 ± 0.01 * |
| CXBC 2 (10) | 1868.57 ± 382.55 * | 113.32 ± 20.58 a,* | 6.14 ± 0.01 a,b,c,* |
| CXBC 3 (10) | 1668.75 ± 398.01 * | 92.50 ± 20.53 * | 5.66 ± 0.01 a,* |
| Thymoquinone (10) | 1799.83 ± 429.90 * | 97.50 ± 21.69 * | 5.62 ± 0.02 a,* |
| Tamoxifen (10) | 1940.00 ± 203.76 a* | 84.50 ± 13.46 * | 4.34 ± 0.00 a,b,* |
| DMBA (10) | 484.17 ± 33.98 a,b,c | 45.17 ± 9.07 a,b,c | 11.50 ± 0.02 a,b,c |
| Solvent (10) | 1490.33 ± 508.98 * | 109.33 ± 64.06 * | 7.35 ± 0.04 a,b,c,* |
| Test Group | Absolute CD8 Count (Mean ± SD) | The Absolute Number of CD8CD25 (Mean ± SD) | Percentage of CD8CD25 to CD8 (Mean ± SD) |
|---|---|---|---|
| Normal (10) | 580.00 ± 66.63 b,c,* | 52.50 ± 9.39 * | 9.00 ± 1.30 * |
| CXBCH 1 (10) | 840.43 ± 48.64 a,c,* | 77.00 ± 2.15 a,* | 10.70 ± 3.67 * |
| CXBCH 2 (10) | 860.00 ± 33.95 a,b,c,* | 85.28 ± 2.6 a,b,* | 10.84 ± 3.00 * |
| CXBCH 3 (10) | 512.50 ± 47.42 b,c,* | 53.00 ± 3.86 * | 13.18 ± 3.72 * |
| Thymoquinone (10) | 813.33 ± 18.17 a,c,* | 67.16 ± 16.77 * | 8.70 ± 3.09 * |
| Tamoxifen (10) | 915.00 ± 17.13 a,b,* | 68.16 ± 20.62 * | 7.52 ± 2.32 * |
| DMBA (10) | 137.00 ± 18.48 a,b,c | 32.66 ± 6.43 a,b,c | 23.75 ± 2.5 a,b,c |
| Solvent (10) | 668.33 ± 39.56 a,b,c,* | 59.33 ± 16.94 b,* | 10.74 ± 5.6 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidayati, T.; Indrayanti, I.; Darmawan, E.; Akrom, A. Herbal Honey Preparations of Curcuma Xanthorriza and Black Cumin Protect against Carcinogenesis through Antioxidant and Immunomodulatory Activities in Sprague Dawley (SD) Rats Induced with Dimethylbenz(a)anthracene. Nutrients 2023, 15, 371. https://doi.org/10.3390/nu15020371
Hidayati T, Indrayanti I, Darmawan E, Akrom A. Herbal Honey Preparations of Curcuma Xanthorriza and Black Cumin Protect against Carcinogenesis through Antioxidant and Immunomodulatory Activities in Sprague Dawley (SD) Rats Induced with Dimethylbenz(a)anthracene. Nutrients. 2023; 15(2):371. https://doi.org/10.3390/nu15020371
Chicago/Turabian StyleHidayati, Titiek, Indrayanti Indrayanti, Endang Darmawan, and Akrom Akrom. 2023. "Herbal Honey Preparations of Curcuma Xanthorriza and Black Cumin Protect against Carcinogenesis through Antioxidant and Immunomodulatory Activities in Sprague Dawley (SD) Rats Induced with Dimethylbenz(a)anthracene" Nutrients 15, no. 2: 371. https://doi.org/10.3390/nu15020371
APA StyleHidayati, T., Indrayanti, I., Darmawan, E., & Akrom, A. (2023). Herbal Honey Preparations of Curcuma Xanthorriza and Black Cumin Protect against Carcinogenesis through Antioxidant and Immunomodulatory Activities in Sprague Dawley (SD) Rats Induced with Dimethylbenz(a)anthracene. Nutrients, 15(2), 371. https://doi.org/10.3390/nu15020371







