Interaction between SIDT2 and ABCA1 Variants with Nutrients on HDL-c Levels in Mexican Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Dietary Assessment
2.3. Genomic DNA Extraction and Genotyping
2.4. HDL-c Levels
2.5. Covariates
2.6. Statistical Power of the Study
2.7. Statistical Analysis
3. Results
3.1. Population Characteristics and Genotype Frequencies
3.2. Association Analyses between the rs1784042, rs17120425, and rs9282541 Polymorphisms with HDL-c Levels
3.3. Conditional Analysis
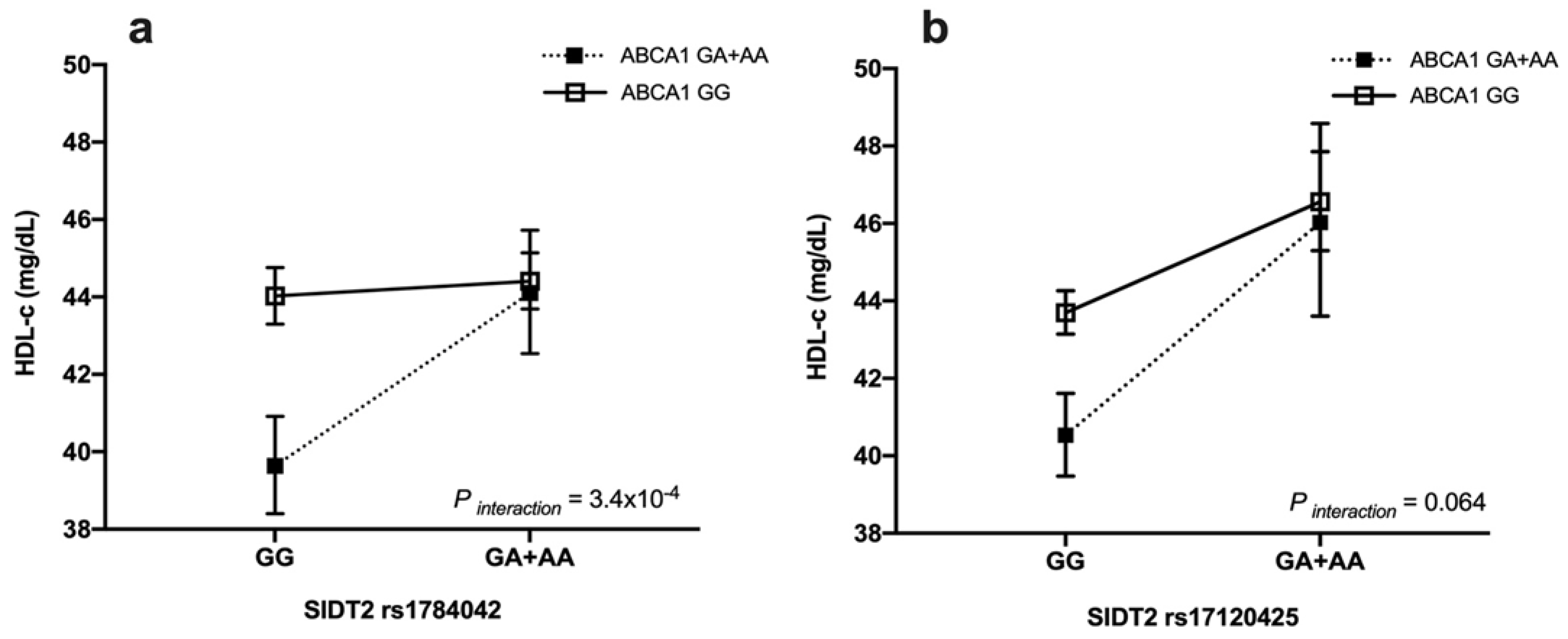
3.4. Gene–Gene Interaction with HDL-c Levels
3.5. Association Analyses between Nutrients with HDL-c Levels
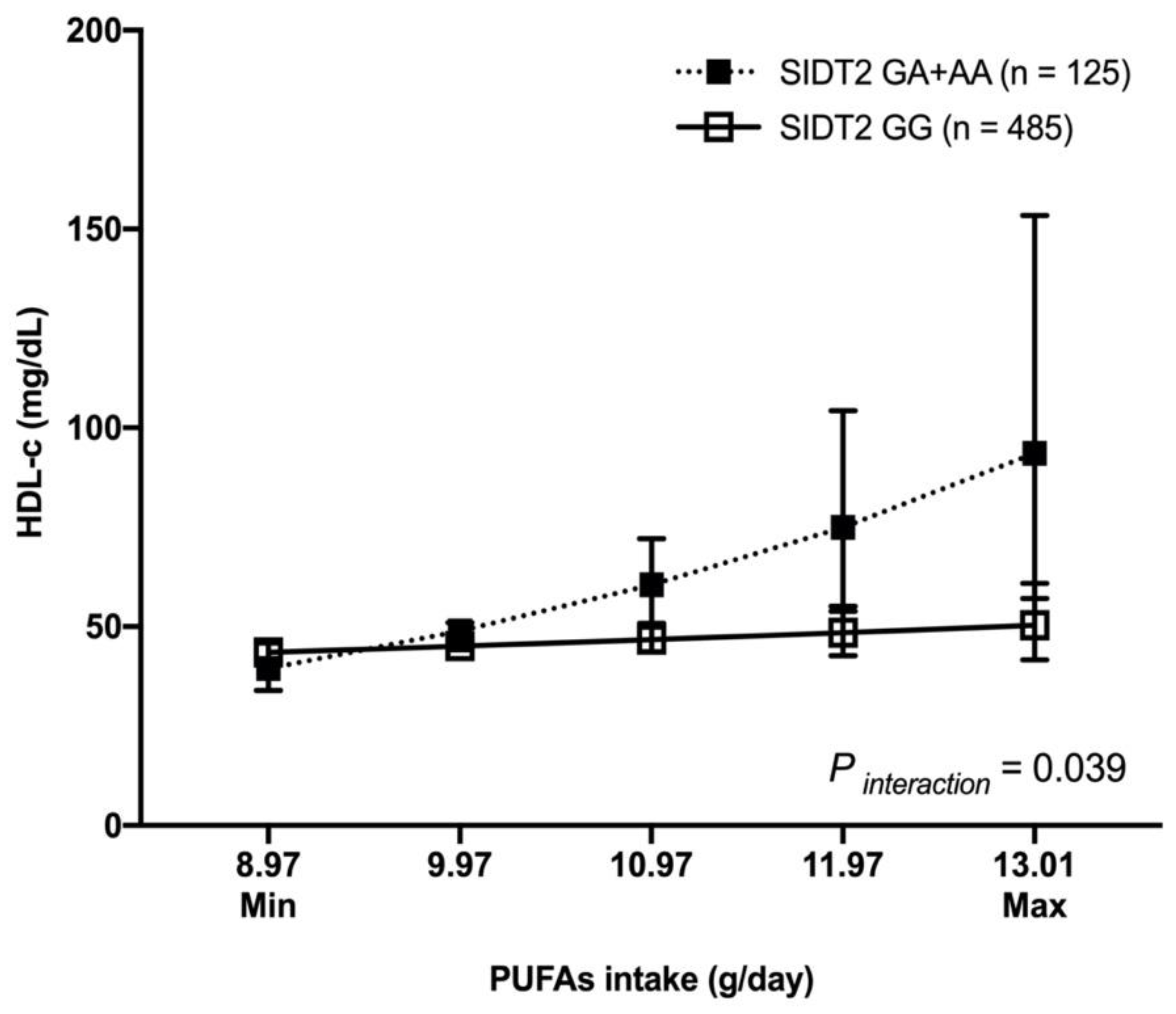
3.6. Gene–Diet Interaction with HDL-c Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kopin, L.; Lowenstein, C. Dyslipidemia. Ann. Intern. Med. 2017, 167, ITC81–ITC95. [Google Scholar] [CrossRef]
- Riwanto, M.; Landmesser, U. High Density Lipoproteins and Endothelial Functions: Mechanistic Insights and Alterations in Cardiovascular Disease. J. Lipid Res. 2013, 54, 3227–3243. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Alcaraz, C.; Aguilar-Salinas, C.A.; Mendoza-Herrera, K.; Pedroza-Tobías, A.; Villalpando, S.; Shamah-Levy, T.; Rivera-Dommarco, J.; Hernández-Ávila, M.; Barquera, S. Dyslipidemia Prevalence, Awareness, Treatment and Control in Mexico: Results of the Ensanut 2012. Salud Publica Mex. 2020, 62, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Munguía-Miranda, C.; Sánchez-Barrera, R.G.; Hernández-Saavedra, D.; Cruz-López, M. Dyslipidemia Prevalence and Its Relationship with Insulin Resistance in a Population of Apparently Healthy Subjects. Salud Publica Mex. 2008, 50, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Salinas, C.A.; Canizales-Quinteros, S.; Rojas-Martínez, R.; Mehta, R.; Rodriguez-Guillén, R.; Ordoñez-Sanchez, M.L.; Riba, L.; Tusié-Luna, M.T. The Non-Synonymous Arg230Cys Variant (R230C) of the ATP-Binding Cassette Transporter A1 Is Associated with Low HDL Cholesterol Concentrations in Mexican Adults: A Population Based Nation Wide Study. Atherosclerosis 2011, 216, 146–150. [Google Scholar] [CrossRef]
- Wang, X.; Paigen, B. Genetics of Variation in HDL Cholesterol in Humans and Mice. Circ. Res. 2005, 96, 27–42. [Google Scholar] [CrossRef]
- Weissglas-Volkov, D.; Pajukanta, P. Genetic Causes of High and Low Serum HDL-Cholesterol. J. Lipid Res. 2010, 51, 2032–2057. [Google Scholar] [CrossRef]
- Singh, I.M.; Shishehbor, M.H.; Ansell, B.J. High-Density Lipoprotein as a Therapeutic Target: A Systematic Review. JAMA 2007, 298, 786–798. [Google Scholar] [CrossRef]
- Willer, C.J.; Mohlke, K.L. Finding Genes and Variants for Lipid Levels after Genome-Wide Association Analysis. Curr. Opin. Lipidol. 2012, 23, 98–103. [Google Scholar] [CrossRef]
- Andaleon, A.; Mogil, L.S.; Wheeler, H.E. Gene-Based Association Study for Lipid Traits in Diverse Cohorts Implicates BACE1 and SIDT2 Regulation in Triglyceride Levels. PeerJ 2018, 6, e4314. [Google Scholar] [CrossRef]
- Teslovich, T.M.; Musunuru, K.; Smith, A.V.; Edmondson, A.C.; Stylianou, I.M.; Koseki, M.; Pirruccello, J.P.; Ripatti, S.; Chasman, D.I.; Willer, C.J.; et al. Biological, Clinical and Population Relevance of 95 Loci for Blood Lipids. Nature 2010, 466, 707–713. [Google Scholar] [CrossRef]
- Suarez-Sanchez, F.; Vazquez-Moreno, M.; Herrera-Lopez, E.; Gomez-Zamudio, J.H.; Peralta-Romero, J.J.; Castelan-Martinez, O.D.; Cruz, M.; Parra, E.J.; Valladares-Salgado, A. Association of Rs2000999 in the Haptoglobin Gene with Total Cholesterol, HDL-C, and LDL-C Levels in Mexican Type 2 Diabetes Patients. Medicine 2019, 98, e17298. [Google Scholar] [CrossRef]
- León-Reyes, G.; Rivera-Paredez, B.; López, J.C.F.; Ramírez-Salazar, E.G.E.G.; Aquino-Gálvez, A.; Gallegos-Carrillo, K.; Denova-Gutiérrez, E.; Salmerón, J.; Velázquez-Cruz, R. The Variant Rs1784042 of the SIDT2 Gene Is Associated with Metabolic Syndrome through Low HDL-c Levels in a Mexican Population. Genes 2020, 11, 1192. [Google Scholar] [CrossRef]
- León-Mimila, P.; Villamil-Ramírez, H.; MacÍas-Kauffer, L.R.; Jacobo-Albavera, L.; López-Contreras, B.E.; Posadas-Sánchez, R.; Posadas-Romero, C.; Romero-Hidalgo, S.; Morán-Ramos, S.; Domínguez-Pérez, M.; et al. Genome-Wide Association Study Identifies a Functional SIDT2 Variant Associated With HDL-C (High-Density Lipoprotein Cholesterol) Levels and Premature Coronary Artery Disease. Arter. Thromb. Vasc. Biol. 2021, 41, 2494–2508. [Google Scholar] [CrossRef]
- Guevara-Cruz, M.; Torres, N.; Tovar, A.R.; Tejero, M.E.; Castellanos-Jankiewicz, A.; del Bosque-Plata, L. A Genetic Variant of the CAPN10 Gene in Mexican Subjects with Dyslipidemia Is Associated with Increased HDL-Cholesterol Concentrations after the Consumption of a Soy Protein and Soluble Fiber Dietary Portfolio. Nutr. Hosp. 2014, 30, 671–677. [Google Scholar] [CrossRef]
- Guerra-García, M.T.; Moreno-Macías, H.; Ochoa-Guzmán, A.; Ordoñez-Sánchez, M.L.; Rodríguez-Guillen, R.; Vázquez-Cárdenas, P.; Ortíz-Ortega, V.M.; Peimbert-Torres, M.; Aguilar-Salinas, C.A.; Tusié-Luna, M.T. The -514C>T Polymorphism in the LIPC Gene Modifies Type 2 Diabetes Risk through Modulation of HDL-Cholesterol Levels in Mexicans. J. Endocrinol. Investig. 2021, 44, 557–565. [Google Scholar] [CrossRef]
- González-Mercado, A.; Magaña-Torres, M.T.; Sánchez-López, J.Y.; Ríos-Silva, M.; Ibarra-Cortés, B.; Trujillo, X.; Huerta, M. The Relationship of Single Nucleotide Polymorphisms in the TRPV1 Gene with Lipid Profile, Glucose, and Blood Pressure in Mexican Population. Genet. Test Mol. Biomark. 2020, 24, 420–424. [Google Scholar] [CrossRef]
- Hanson, R.L.; Leti, F.; Tsinajinnie, D.; Kobes, S.; Puppala, S.; Curran, J.E.; Almasy, L.; Lehman, D.M.; Blangero, J.; Duggirala, R.; et al. The Arg59Trp Variant in ANGPTL8 (Betatrophin) Is Associated with Total and HDL-Cholesterol in American Indians and Mexican Americans and Differentially Affects Cleavage of ANGPTL3. Mol. Genet Metab. 2016, 118, 128–137. [Google Scholar] [CrossRef]
- Cahua-Pablo, J.Á.; Gómez-Zamudio, J.H.; Reséndiz-Abarca, C.A.; Tello-Flores, V.A.; Eulogio-Metodio, Y.; Ramírez-Vargas, M.A.; Cruz, M.; del Carmen Alarcón-Romero, L.; Matia-García, I.; Marino-Ortega, L.A.; et al. Genetic Variants in SLC22A1 Are Related to Serum Lipid Levels in Mexican Women. Lipids 2022, 57, 105–114. [Google Scholar] [CrossRef]
- Gao, J.; Yu, C.; Xiong, Q.; Zhang, Y.; Wang, L. Lysosomal Integral Membrane Protein Sidt2 Plays a Vital Role in Insulin Secretion. Int. J. Clin. Exp. Pathol. 2015, 8, 15622–15631. [Google Scholar]
- Gombojav, B.; Lee, S.J.; Kho, M.; Song, Y.M.; Lee, K.; Sung, J. Multiple Susceptibility Loci at Chromosome 11q23.3 Are Associated with Plasma Triglyceride in East Asians. J. Lipid Res. 2016, 57, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Lee, Y.; Won, S.; Lee, J. Multiple Genotype-Phenotype Association Study Reveals Intronic Variant Pair on SIDT2 Associated with Metabolic Syndrome in a Korean Population. Hum. Genom. 2018, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhang, S.; Guo, Z.; Xing, D.; Chen, W. The Crosstalk of ABCA1 and ANXA1: A Potential Mechanism for Protection against Atherosclerosis. Mol. Med. 2020, 26, 84. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Alonzo, V.; Flores-Dorantes, T.; Kruit, J.K.; Villarreal-Molina, T.; Arellano-Campos, O.; Hünemeier, T.; Moreno-Estrada, A.; Ortiz-López, M.G.; Villamil-Ramírez, H.; León-Mimila, P.; et al. A Functional ABCA1 Gene Variant Is Associated with Low HDL-Cholesterol Levels and Shows Evidence of Positive Selection in Native Americans. Hum. Mol. Genet. 2010, 19, 2877–2885. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Molina, T.; Posadas-Romero, C.; Romero-Hidalgo, S.; Antúnez-Argüelles, E.; Bautista-Grande, A.; Vargas-Alarcón, G.; Kimura-Hayama, E.; Canizales-Quinteros, S.; Juárez-Rojas, J.G.; Posadas-Sánchez, R.; et al. The ABCA1 Gene R230C Variant Is Associated with Decreased Risk of Premature Coronary Artery Disease: The Genetics of Atherosclerotic Disease (GEA) Study. PLoS ONE 2012, 7, e49285. [Google Scholar] [CrossRef]
- Flores-Dorantes, T.; Arellano-Campos, O.; Posadas-Sánchez, R.; Villarreal-Molina, T.; Medina-Urrutia, A.; Romero-Hidalgo, S.; Yescas-Gómez, P.; Pérez-Méndez, O.; Jorge-Galarza, E.; Tusié-Luna, T.; et al. Association of R230C ABCA1 Gene Variant with Low HDL-C Levels and Abnormal HDL Subclass Distribution in Mexican School-Aged Children. Clin. Chim. Acta 2010, 411, 1214–1217. [Google Scholar] [CrossRef]
- Ochoa-Guzmán, A.; Moreno-Macías, H.; Guillén-Quintero, D.; Chávez-Talavera, O.; Ordoñez-Sánchez, M.L.; Segura-Kato, Y.; Ortíz, V.; Díaz-Díaz, E.; Muñoz-Hernández, L.; García, A.; et al. R230C but Not-565C/T Variant of the ABCA1 Gene Is Associated with Type 2 Diabetes in Mexicans through an Effect on Lowering HDL-Cholesterol Levels. J. Endocrinol. Investig. 2020, 43, 1061–1071. [Google Scholar] [CrossRef]
- Zhou, Y.; Mägi, R.; Milani, L.; Lauschke, V.M. Global Genetic Diversity of Human Apolipoproteins and Effects on Cardiovascular Disease Risk. J. Lipid Res. 2018, 59, 1987–2000. [Google Scholar] [CrossRef]
- de Luis, D.A.; Izaola, O.; Primo, D.; Aller, R. Influence of Rs670 Variant of APOA1 Gene on Serum HDL Response to an Enriched-Polyunsaturated vs. an Enriched-Monounsaturated Fat Hypocaloric Diet. Nutr. Hosp. 2019, 36, 1288–1295. [Google Scholar] [CrossRef]
- Ordovas, J.M. The Quest for Cardiovascular Health in the Genomic Era: Nutrigenetics and Plasma Lipoproteins. Proc. Nutr. Soc. 2004, 63, 145–152. [Google Scholar] [CrossRef]
- Costello, K.R.; Schones, D.E. Chromatin Modifications in Metabolic Disease: Potential Mediators of Long-Term Disease Risk. Wiley Interdiscip Rev. Syst. Biol. Med. 2018, 10, e1416. [Google Scholar] [CrossRef]
- Ma, Y.; Ordovas, J.M. The Integration of Epigenetics and Genetics in Nutrition Research for CVD Risk Factors. Proc. Nutr. Soc. 2017, 76, 333–346. [Google Scholar] [CrossRef]
- Denova-Gutiérrez, E.; Flores, Y.N.; Gallegos-Carrillo, K.; Ramírez-Palacios, P.; Rivera-Paredez, B.; Muñoz-Aguirre, P.; Velázquez-Cruz, R.; Torres-Ibarra, L.; Meneses-León, J.; Méndez-Hernández, P.; et al. Health Workers Cohort Study: Methods and Study Design. Salud Publica Mex. 2016, 58, 708–716. [Google Scholar] [CrossRef]
- Hernández-Avila, M.; Romieu, I.; Parra, S.; Hernández-Avila, J.; Madrigal, H.; Willett, W. Validity and Reproducibility of a Food Frequency Questionnaire to Assess Dietary Intake of Women Living in Mexico City. Salud Publica Mex. 1998, 40, 133–140. [Google Scholar] [CrossRef]
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/12485966/ (accessed on 28 March 2022).
- Deckers, J.G.M.; Schellevis, F.G.; Fleming, D.M. WHO Diagnostic Criteria as a Validation Tool for the Diagnosis of Diabetes Mellitus: A Study in Five European Countries. Eur. J. Gen. Pract. 2006, 12, 108–113. [Google Scholar] [CrossRef]
- Gauderman, W.J. Sample Size Requirements for Association Studies of Gene-Gene Interaction. Am. J. Epidemiol. 2002, 155, 478–484. [Google Scholar] [CrossRef]
- Aguilar-Salinas, C.A.; Canizales-Quinteros, S.; Rojas-Martfnez, R.; Mehta, R.; Ma, T.V.M.; Arellano-Campos, O.; Riba, L.; Gómez-Pérez, F.J.; Tusié-Luna, M.T. Hypoalphalipoproteinemia in Populations of Native American Ancestry: An Opportunity to Assess the Interaction of Genes and the Environment. Curr Opin Lipidol. 2009, 20, 92–97. [Google Scholar] [CrossRef]
- Rangel-Baltazar, E.; Cuevas-Nasu, L.; Shamah-Levy, T.; Rodríguez-Ramírez, S.; Méndez-Gómez-Humarn, I.; Rivera, J.A. Association of MARC1, ADCY5, and BCO1 Variants with the Lipid Profile, Suggests an Additive Effect for Hypertriglyceridemia in Mexican Adult Men. Nutrients 2019, 11, 11815. [Google Scholar]
- Siri-Tarino, P.W. Effects of Diet on High-Density Lipoprotein Cholesterol. Curr. Atheroscler. Rep. 2011, 13, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Boes, E.; Coassin, S.; Kollerits, B.; Heid, I.M.; Kronenberg, F. Genetic-Epidemiological Evidence on Genes Associated with HDL Cholesterol Levels: A Systematic in-Depth Review. Exp. Gerontol. 2009, 44, 136–160. [Google Scholar] [CrossRef]
- Flores-Viveros, K.L.; Aguilar-Galarza, B.A.; Ordóñez-Sánchez, M.L.; Anaya-Loyola, M.A.; Moreno-Celis, U.; Vázquez-Cárdenas, P.; García-Gasca, T. Contribution of Genetic, Biochemical and Environmental Factors on Insulin Resistance and Obesity in Mexican Young Adults. Obes. Res. Clin. Pract. 2019, 13, 533–540. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, L.; Ling, L. Changes of Lysosomal Membrane Permeabilization and Lipid Metabolism in Sidt2 Deficient Mice. Exp. Ther. Med. 2018, 16, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Jialin, G.; Xuefan, G.; Huiwen, Z. SID1 Transmembrane Family, Member 2 (Sidt2): A Novel Lysosomal Membrane Protein. Biochem. Biophys. Res. Commun. 2010, 402, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.Y.; Xiong, C.Q.; Wang, L.Z.; Gao, J.L. Effect of Sidt2 Gene on Cell Insulin Resistance and Its Molecular Mechanism. J. Diabetes Res. 2020, 2020, 4217607. [Google Scholar] [CrossRef]
- Ramasamy, I. Recent Advances in Physiological Lipoprotein Metabolism. Clin. Chem. Lab. Med. 2014, 52, 1695–1727. [Google Scholar] [CrossRef]
- Cavelier, C.; Lorenzi, I.; Rohrer, L.; von Eckardstein, A. Lipid Efflux by the ATP-Binding Cassette Transporters ABCA1 and ABCG1. Biochim. Biophys. Acta 2006, 1761, 655–666. [Google Scholar] [CrossRef]
- Xu, B.; Gillard, B.K.; Gotto, A.M.J.; Rosales, C.; Pownall, H.J. ABCA1-derived nascent high-density lipoprotein–apolipoprotein AI and lipids metabolically segregate. Arter. Thromb. Vasc. Biol. 2017, 37, 2260–2270. [Google Scholar] [CrossRef]
- Zhang, Y.; Klein, K.; Sugathan, A.; Nassery, N.; Dombkowski, A.; Zanger, U.M.; Waxman, D.J. Transcriptional Profiling of Human Liver Identifies Sex-Biased Genes Associated with Polygenic Dyslipidemia and Coronary Artery Disease. PLoS ONE 2011, 6, e23506. [Google Scholar] [CrossRef]
- Almeida, S.; Hutz, M.H. Estrogen Receptor 1 Gene Polymorphisms in Premenopausal Women: Interaction between Genotype and Smoking on Lipid Levels. Braz. J. Med. Biol. Res. 2008, 41, 872–876. [Google Scholar] [CrossRef]
- Mauerer, R.; Ebert, S.; Langmann, T. High Glucose, Unsaturated and Saturated Fatty Acids Differentially Regulate Expression of ATP-Binding Cassette Transporters ABCA1 and ABCG1 in Human Macrophages. Exp. Mol. Med. 2009, 41, 126–132. [Google Scholar] [CrossRef]
- Villard, E.F.; Ei Khoury, P.; Frisdal, E.; Bruckert, E.; Clement, K.; Bonnefont-Rousselot, D.; Bittar, R.; le Goff, W.; Guerin, M. Genetic Determination of Plasma Cholesterol Efflux Capacity Is Gender-Specific and Independent of HDL-Cholesterol Levels. Arter. Thromb. Vasc. Biol. 2013, 33, 822–828. [Google Scholar] [CrossRef]
- Chiba-Falek, O.; Nichols, M.; Suchindran, S.; Guyton, J.; Ginsburg, G.S.; Barrett-Connor, E.; McCarthy, J.J. Impact of Gene Variants on Sex-Specific Regulation of Human Scavenger Receptor Class B Type 1 (SR-BI) Expression in Liver and Association with Lipid Levels in a Population-Based Study. BMC Med. Genet. 2010, 11, 9. [Google Scholar] [CrossRef]
- Delgado-Lista, J.; Perez-Martinez, P.; Perez-Jimenez, F.; Garcia-Rios, A.; Fuentes, F.; Marin, C.; Gómez-Luna, P.; Camargo, A.; Parnell, L.D.; Maria Ordovas, J.; et al. ABCA1 Gene Variants Regulate Postprandial Lipid Metabolism in Healthy Men. Arter. Thromb. Vasc. Biol. 2010, 30, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Lista, J.; Perez-Jimenez, F.; Tanaka, T.; Perez-Martinez, P.; Jimenez-Gomez, Y.; Marin, C.; Ruano, J.; Parnell, L.; Ordovas, J.M.; Lopez-Miranda, J. An Apolipoprotein A-II Polymorphism (-265T/C, Rs5082) Regulates Postprandial Response to a Saturated Fat Overload in Healthy Men. J. Nutr. 2007, 137, 2024–2028. [Google Scholar] [CrossRef]
- Gardner, C.D.; Tribble, D.L.; Young, D.R.; Ahn, D.; Fortmann, S.P. Population Frequency Distributions of HDL, HDL(2), and HDL(3) Cholesterol and Apolipoproteins A-I and B in Healthy Men and Women and Associations with Age, Gender, Hormonal Status, and Sex Hormone Use: The Stanford Five City Project. Prev. Med. 2000, 31, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, B.N.; Kang, S.; Mukherjee, B. A Latent Variable Approach to Study Gene-Environment Interactions in the Presence of Multiple Correlated Exposures. Biometrics 2012, 68, 466–476. [Google Scholar] [CrossRef]
- Frikke-Schmidt, R.; Nordestgaard, B.G.; Stene, M.C.A.; Sethi, A.A.; Remaley, A.T.; Schnohr, P.; Grande, P.; Tybjærg-Hansen, A. Association of Loss-of-Function Mutations in the ABCA1 Gene with High-Density Lipoprotein Cholesterol Levels and Risk of Ischemic Heart Disease. JAMA 2008, 299, 2524–2532. [Google Scholar] [CrossRef]
- Chen, M.-M.; Huang, X.; Xu, C.; Song, X.-H.; Liu, Y.-M.; Yao, D.; Lu, H.; Wang, G.; Zhang, G.-L.; Chen, Z.; et al. High Remnant Cholesterol Level Potentiates the Development of Hypertension. Front. Endocrinol. 2022, 13, 830347. [Google Scholar] [CrossRef]
- Méndez-Acevedo, K.M.; Valdes, V.J.; Asanov, A.; Vaca, L. A Novel Family of Mammalian Transmembrane Proteins Involved in Cholesterol Transport. Sci. Rep. 2017, 7, 7450. [Google Scholar] [CrossRef]
- Szpiech, Z.A.; Hernandez, R.D. Selscan: An Efficient Multithreaded Program to Perform EHH-Based Scans for Positive Selection. Mol. Biol. Evol. 2014, 31, 2824–2827. [Google Scholar] [CrossRef]
- Chen, X.; Gu, X.; Zhang, H. Sidt2 Regulates Hepatocellular Lipid Metabolism through Autophagy. J. Lipid Res. 2018, 59, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Mukhamedova, N.; Hoang, A.; Cui, H.L.; Carmichael, I.; Fu, Y.; Bukrinsky, M.; Sviridov, D. Small GTPase ARF6 Regulates Endocytic Pathway Leading to Degradation of ATP-Binding Cassette Transporter A1. Arter. Thromb. Vasc. Biol. 2016, 36, 2292–2303. [Google Scholar] [CrossRef]
- du Plessis, J.P.; Melse-Boonstra, A.; Zandberg, L.; Nienaber-Rousseau, C. Gene Interactions Observed with the HDL-c Blood Lipid, Intakes of Protein, Sugar and Biotin in Relation to Circulating Homocysteine Concentrations in a Group of Black South Africans. Mol. Genet. Metab. Rep. 2019, 22, 100556. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Du, M.-H.; Wen, H.; Wang, W.-Q.; Tang, J.; Shen, L.-R. Effects of N-6 PUFA-Rich Soybean Oil, MUFA-Rich Olive Oil and Camellia Seed Oil on Weight and Cardiometabolic Profiles among Chinese Women: A 3-Month Double-Blind Randomized Controlled-Feeding Trial. Food Funct. 2022, 13, 4375–4383. [Google Scholar] [CrossRef]
- Pérez-Beltrán, Y.E.; Rivera-Iñiguez, I.; Gonzalez-Becerra, K.; Pérez-Naitoh, N.; Tovar, J.; Sáyago-Ayerdi, S.G.; Mendivil, E.J. Personalized Dietary Recommendations Based on Lipid-Related Genetic Variants: A Systematic Review. Front. Nutr. 2022, 9, 830283. [Google Scholar] [CrossRef]
- Guevara-Cruz, M.; Tovar, A.R.; Larrieta, E.; Canizales-Quinteros, S.; Torres, N. Increase in HDL-C Concentration by a Dietary Portfolio with Soy Protein and Soluble Fiber Is Associated with the Presence of the ABCA1R230C Variant in Hyperlipidemic Mexican Subjects. Mol. Genet. Metab. 2010, 101, 268–272. [Google Scholar] [CrossRef]
- Ayyappa, K.A.; Shatwan, I.; Bodhini, D.; Bramwell, L.R.; Ramya, K.; Sudha, V.; Anjana, R.M.; Lovegrove, J.A.; Mohan, V.; Radha, V.; et al. High Fat Diet Modifies the Association of Lipoprotein Lipase Gene Polymorphism with High Density Lipoprotein Cholesterol in an Asian Indian Population. Nutr. Metab. 2017, 14, 8. [Google Scholar] [CrossRef]
- AlSaleh, A.; O’Dell, S.D.; Frost, G.S.; Griffin, B.A.; Lovegrove, J.A.; Jebb, S.A.; Sanders, T.A.B. Interaction of PPARG Pro12Ala with Dietary Fat Influences Plasma Lipids in Subjects at Cardiometabolic Risk. J. Lipid Res. 2011, 52, 2298–2303. [Google Scholar] [CrossRef]
- Berryman, C.E.; Lieberman, H.R.; Fulgoni, V.L.; Pasiakos, S.M. Greater Protein Intake at Breakfast or as Snacks and Less at Dinner Is Associated with Cardiometabolic Health in Adults. Clin. Nutr. 2021, 40, 4301–4308. [Google Scholar] [CrossRef]
- Surampudi, P.; Enkhmaa, B.; Anuurad, E.; Berglund, L. Lipid Lowering with Soluble Dietary Fiber. Curr. Atheroscler. Rep. 2016, 18, 75. [Google Scholar] [CrossRef]
- Woodard, G.A.; Brooks, M.M.; Barinas-Mitchell, E.; MacKey, R.H.; Matthews, K.A.; Sutton-Tyrrell, K. Lipids, Menopause, and Early Atherosclerosis in Study of Women’s Health Across the Nation Heart Women. Menopause 2011, 18, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.; Kastelein, J.; Nunn, A.; Hobbs, R.; Shepherd, J.; Ballantyne, C.; Brown, V.; Bruckert, E.; Carmena, R.; Davidson, M.; et al. High Density Lipoproteins (HDLs) and Atherosclerosis; the Unanswered Questions. Atherosclerosis 2003, 168, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Brites, F.D.; Bonavita, C.D.; de Geitere, C.; Cloës, M.; Delfly, B.; Yael, M.J.; Fruchart, J.C.; Wikinski, R.W.; Castro, G.R. Alterations in the Main Steps of Reverse Cholesterol Transport in Male Patients with Primary Hypertriglyceridemia and Low HDL-Cholesterol Levels. Atherosclerosis 2000, 152, 181–192. [Google Scholar] [CrossRef]
- Kontush, A.; Chantepie, S.; Chapman, M.J. Small, Dense HDL Particles Exert Potent Protection of Atherogenic LDL against Oxidative Stress. Arter. Thromb. Vasc. Biol. 2003, 23, 1881–1888. [Google Scholar] [CrossRef]
- Hansel, B.; Giral, P.; Nobecourt, E.; Chantepie, S.; Bruckert, E.; Chapman, M.J.; Kontush, A. Metabolic Syndrome Is Associated with Elevated Oxidative Stress and Dysfunctional Dense High-Density Lipoprotein Particles Displaying Impaired Antioxidative Activity. J. Clin. Endocrinol. Metab. 2004, 89, 4963–4971. [Google Scholar] [CrossRef]
- Romero-Hidalgo, S.; Villarreal-Molina, T.; González-Barrios, J.A.; Canizales-Quinteros, S.; Rodríguez-Arellano, M.E.; Yañez-Velazco, L.B.; Bernal-Alcantara, D.A.; Villa, A.R.; Antuna-Puente, B.; Acuña-Alonzo, V.; et al. Carbohydrate Intake Modulates the Effect of the ABCA1-R230C Variant on HDL Cholesterol Concentrations in Premenopausal Women. J. Nutr. 2012, 142, 278–283. [Google Scholar] [CrossRef]
- Jacobo-Albavera, L.; Posadas-Romero, C.; Vargas-Alarcón, G.; Romero-Hidalgo, S.; Posadas-Sánchez, R.; González-Salazar, M.D.C.; Carnevale, A.; Canizales-Quinteros, S.; Medina-Urrutia, A.; Antúnez-Argüelles, E.; et al. Dietary Fat and Carbohydrate Modulate the Effect of the ATP-Binding Cassette A1 (ABCA1) R230C Variant on Metabolic Risk Parameters in Premenopausal Women from the Genetics of Atherosclerotic Disease (GEA) Study. Nutr. Metab. 2015, 12, 45. [Google Scholar] [CrossRef]
- Rothman, K. No Adjustments Are Needed for Multiple Comparisons-PubMed. Epidemiology 1990, 1, 43–46. [Google Scholar] [CrossRef]
| Characteristics | Men | Women ** | p * |
|---|---|---|---|
| n = 601 | n = 1381 | ||
| Age, years a | 46(36–56) | 54(43–63) | <0.001 |
| Body mass index a, Kg/m2 | 26.5(24.1–29.0) | 26.8(24.0–30.1) | 0.161 |
| LDL-c a, mg/dL | 115.5(96.7–143.6) | 121(99.1–146.6) | 0.006 |
| HDL-c a, mg/dL | 39(34–46) | 46(39–54) | <0.001 |
| Low HDL-c (%) | 51.8 | 63.9 | <0.001 |
| Triglycerides a, mg/dL | 169(118–247) | 151(109–201) | <0.001 |
| Total cholesterol a, mg/dL | 192(168–221) | 199(172–227) | 0.0002 |
| Dietary intake | |||
| Energy a, kcal/day | 1957(1458–2556) | 1687(1255–2227) | <0.001 |
| Carbohydrates a, g/day | 312(237–411) | 278(204–372) | <0.001 |
| Protein a, g/day | 59.5(44.2–78.3) | 52.2(38.1–71.0) | <0.001 |
| Total fat a, g/day | 43.2(30.7–61.9) | 37.5(27.0–52.7) | <0.001 |
| Monounsaturated fat a, g/day | 17.7(12.9–25.0) | 15.6(11.2–22.3) | <0.001 |
| Polyunsaturated fat a, g/day | 8.6(6.4–12.1) | 7.7(5.5–10.7) | <0.001 |
| Saturated fat a, g/day | 15.2(10.2–22.0) | 12.8(8.8–18.9) | <0.001 |
| SIDT2-rs1784042, n (%) | |||
| GG | 298 (50.6) | 690 (50.5) | 0.974 |
| GA + AA | 291 (49.4) | 676 (49.5) | 0.974 |
| SIDT2-rs17120425, n (%) | |||
| GG | 487 (93.0) | 1112 (81.4) | 0.414 |
| GA + AA | 100 (17.0) | 254 (18.6) | 0.414 |
| ABCA1-rs9282541, n (%) | |||
| GG | 486 (81.3) | 1106 (80.3) | 0.623 |
| GA + AA | 112 (18.7) | 271 (19.7) | 0.623 |
| Characteristics | SIDT2-rs1784042 | SIDT2-rs17120425 | ABCA1-rs9282541 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| GG | GA + AA | p * | GG | GA + AA | p * | GG | GA + AA | p * | |
| n = 988 (50.6%) | n = 967 (49.4%) | n = 1599 (81.9%) | n = 354 (18.1%) | n = 1592 (80.6%) | n = 383 (19.4%) | ||||
| Age, years a | 51 (40–61) | 52 (40–62) | 0.347 | 52 (40–62) | 52 (41–60) | 0.493 | 52 (40–62) | 52 (42–62) | 0.296 |
| Women n (%) | 690 (69.8) | 676 (69.9) | 0.967 | 1112 (69.5) | 254 (71.7) | 0.490 | 1106 (69.5) | 271 (70.8) | 0.676 |
| Body mass index a, Kg/m2 | 26.9 (24.2–30.0) | 26.5 (23.9–29.4) | 0.070 | 26.7 (24.1–29.7) | 26.7 (23.8–30.0) | 0.751 | 26.7 (23.9–29.7) | 26.9 (24.3–30.1) | 0.266 |
| LDL-c a, mg/dL | 117.8 (97.9–142) | 122 (100–148) | 0.004 | 119.9 (98–144.7) | 121 (100–148) | 0.349 | 119.5 (98–145) | 121 (99.7–146.7) | 0.580 |
| HDL-c a, mg/dL | 43.1 (36.5–50.6) | 44.8 (38–52.8) | 0.002 | 43 (36.8–50.8) | 47 (40–55) | <0.001 | 44.1 (38–52) | 41.4 (35.2–49.3) | <0.001 |
| HDL-c categories, n (%) | |||||||||
| ≥40 men and ≥50 women | 359 (36.3) | 421 (43.5) | 0.041 | 590 (36.9) | 186 (52.5) | <0.001 | 655 (41.1) | 128 (33.4) | 0.102 |
| <40 men and <50 women | 629 (63.7) | 545 (56.5) | 0.011 | 1009 (63.1) | 168 (47.5) | <0.001 | 937 (58.9) | 255 (66.6) | 0.025 |
| Triglycerides a, mg/dL | 159 (116–218) | 152 (109–204) | 0.018 | 158 (112–210) | 147 (109–200) | 0.182 | 156 (111.5–210.5) | 154 (113–201) | 0.597 |
| Total cholesterola, mg/dL | 195 (170–225) | 198 (171–226) | 0.223 | 195 (170–225) | 199 (178–228) | 0.025 | 197 (171–225) | 194 (166–228) | 0.390 |
| Gene SNP | Model | Normal HDL-c n(%) | Low HDL-c n(%) | Adjusted Model 1OR (95% CI) | p | Adjusted Model 2OR (95% CI) | p |
|---|---|---|---|---|---|---|---|
| SIDT2 rs1784042 | Dominant GG | 359 (46.0) | 629 (53.5) | Ref | Ref | ||
| GA + AA | 421 (54.0) | 546 (46.5) | 0.74 (0.61–0.90) | 0.002 | 0.75 (0.62–0.91) | 0.004 | |
| Additive | 780 (39.9) | 1175 (60.1) | 0.77 (0.66–0.89) | 0.001 | 0.77 (0.66–0.90) | 0.001 | |
| SIDT2 rs17120425 | Dominant GG | 590 (76.0) | 1009 (85.7) | Ref | Ref | ||
| GA + AA | 186 (24.0) | 168 (14.3) | 0.48 (0.38–0.62) | 7.4 × 10−9 | 0.48 (0.37–0.62) | 9.6 × 10−9 | |
| Additive | 776 (39.7) | 1177 (60.3) | 0.53 (0.42–0.66) | 4.1 × 10−8 | 0.52 (0.42–0.66) | 5.4 × 10−8 | |
| ABCA1 rs9282541 | Dominant GG | 655 (83.7) | 937 (78.6) | Ref | Ref | ||
| GA + AA | 128 (16.3) | 255 (21.4) | 1.38 (1.08–1.76) | 0.011 | 1.41 (1.09–1.81) | 0.008 | |
| Additive | 783 (39.7) | 1192 (60.3) | 1.32 (1.05–1.66) | 0.016 | 1.34 (1.06–1.69) | 0.013 |
| Women (n = 1381) | Men (n = 601) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene SNP | Model | Normal HDL-c n(%) | Low HDL-c n(%) | Model 1 OR (95%CI) | p | Model 2 OR (95%CI) | p | Normal HDL-c n(%) | Low HDL-cn(%) | Model 1 OR (95%CI) | p | Model 2 OR (95%CI) | p |
| SIDT2 rs1784042 | Dominant GG | 220(44.5) | 470(53.9) | Ref | Ref | 139(48.6) | 159(52.5) | Ref | Ref | ||||
| GA + AA | 274(55.5) | 402(46.1) | 0.70 (0.55–0.88) | 0.003 | 0.71 (0.56–0.90) | 0.005 | 147(51.4) | 144(47.5) | 0.83 (0.59–1.16) | 0.278 | 0.84 (0.60–1.17) | 0.312 | |
| Additive | 494(36.2) | 872(63.8) | 0.76 (0.63–0.90) | 0.002 | 0.76 (0.63–0.92) | 0.004 | 286(48.6) | 303(51.4) | 0.81 (0.61–1.06) | 0.119 | 0.81 (0.61–1.06) | 0.128 | |
| SIDT2 rs17120425 | Dominant GG | 365(74.2) | 747(85.5) | Ref | Ref | 225(79.2) | 262(86.5) | Ref | Ref | ||||
| GA + AA | 127(25.8) | 127(14.5) | 0.48 (0.36–0.64) | 5.3 × 10−7 | 0.43 (0.32–0.59) | 6.0 × 10−8 | 59(20.8) | 41(13.5) | 0.56 (0.36–0.88) | 0.012 | 0.59 (0.37–0.93) | 0.022 | |
| Additive | 492(36.0) | 874(64.0) | 0.53 (0.40–0.69) | 2.5 × 10−6 | 0.48 (0.37–0.64) | 4.3 × 10−7 | 284(48.4) | 303(51.6) | 0.59 (0.39–0.88) | 0.012 | 0.61 (0.40–0.93) | 0.023 | |
| ABCA1 rs9282541 | Dominant GG | 413(83.3) | 693(78.7) | Ref | Ref | 242(84.3) | 244(78.5) | Ref | Ref | ||||
| GA + AA | 83(16.7) | 188(21.3) | 1.37 (1.01–1.85) | 0.042 | 1.41 (1.03–1.93) | 0.029 | 45(15.7) | 67(21.5) | 1.42 (0.93–2.19) | 0.103 | 1.44 (0.93–2.26) | 0.103 | |
| Additive | 496(36.0) | 881(64.0) | 1.30 (0.99–1.72) | 0.053 | 1.34 (1.01–1.77) | 0.040 | 287(48.0) | 311(52.0) | 1.37 (0.92–2.06) | 0.123 | 1.39 (0.92–2.12) | 0.116 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
León-Reyes, G.; Argoty-Pantoja, A.D.; Rivera-Paredez, B.; Hidalgo-Bravo, A.; Flores, Y.N.; Salmerón, J.; Velázquez-Cruz, R. Interaction between SIDT2 and ABCA1 Variants with Nutrients on HDL-c Levels in Mexican Adults. Nutrients 2023, 15, 370. https://doi.org/10.3390/nu15020370
León-Reyes G, Argoty-Pantoja AD, Rivera-Paredez B, Hidalgo-Bravo A, Flores YN, Salmerón J, Velázquez-Cruz R. Interaction between SIDT2 and ABCA1 Variants with Nutrients on HDL-c Levels in Mexican Adults. Nutrients. 2023; 15(2):370. https://doi.org/10.3390/nu15020370
Chicago/Turabian StyleLeón-Reyes, Guadalupe, Anna D. Argoty-Pantoja, Berenice Rivera-Paredez, Alberto Hidalgo-Bravo, Yvonne N. Flores, Jorge Salmerón, and Rafael Velázquez-Cruz. 2023. "Interaction between SIDT2 and ABCA1 Variants with Nutrients on HDL-c Levels in Mexican Adults" Nutrients 15, no. 2: 370. https://doi.org/10.3390/nu15020370
APA StyleLeón-Reyes, G., Argoty-Pantoja, A. D., Rivera-Paredez, B., Hidalgo-Bravo, A., Flores, Y. N., Salmerón, J., & Velázquez-Cruz, R. (2023). Interaction between SIDT2 and ABCA1 Variants with Nutrients on HDL-c Levels in Mexican Adults. Nutrients, 15(2), 370. https://doi.org/10.3390/nu15020370






