Supplementation with Whey Protein, but Not Pea Protein, Reduces Muscle Damage Following Long-Distance Walking in Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
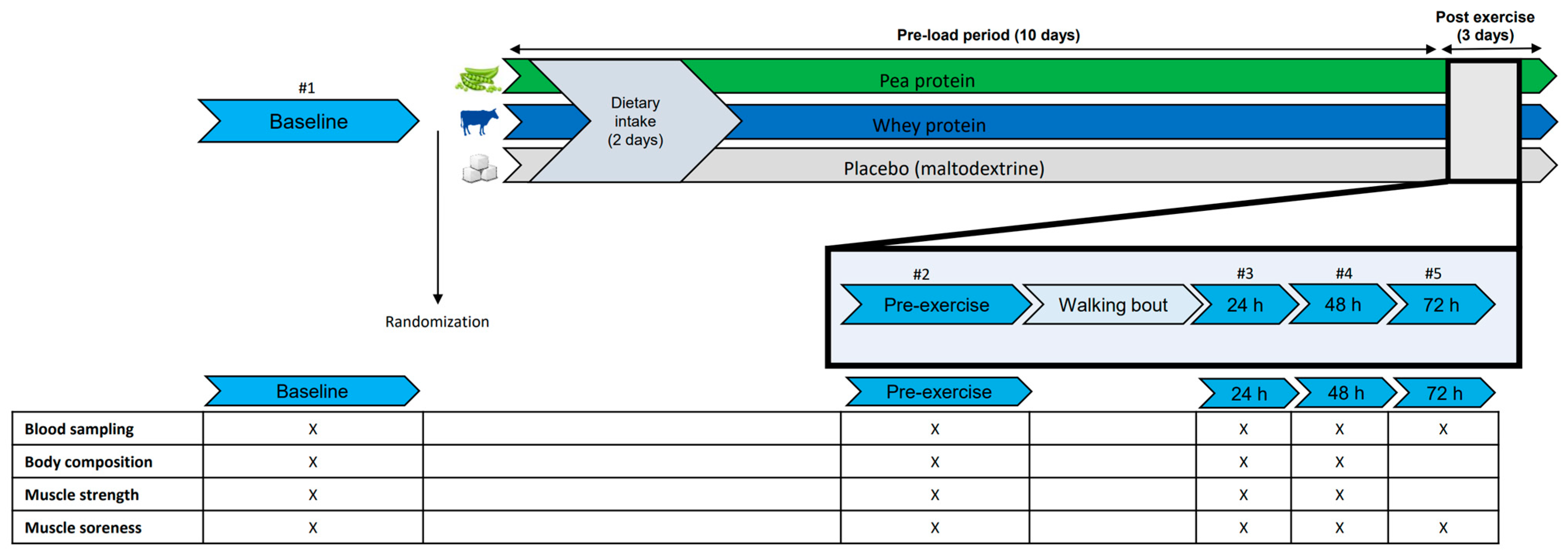
2.2. Study Design
2.3. Supplementation Protocol
2.4. Measurements
2.4.1. Blood Samples
2.4.2. Habitual Walking Characteristics
2.4.3. Exercise Intervention
2.4.4. Muscle Soreness
2.4.5. Dietary Intake
2.4.6. Muscle Strength
2.4.7. Anthropometrics and Muscle Mass
3. Statistical Analysis
4. Results
4.1. Participants
4.2. Habitual Dietary Intake and Walking Activity
4.3. Exercise Bout Characteristic
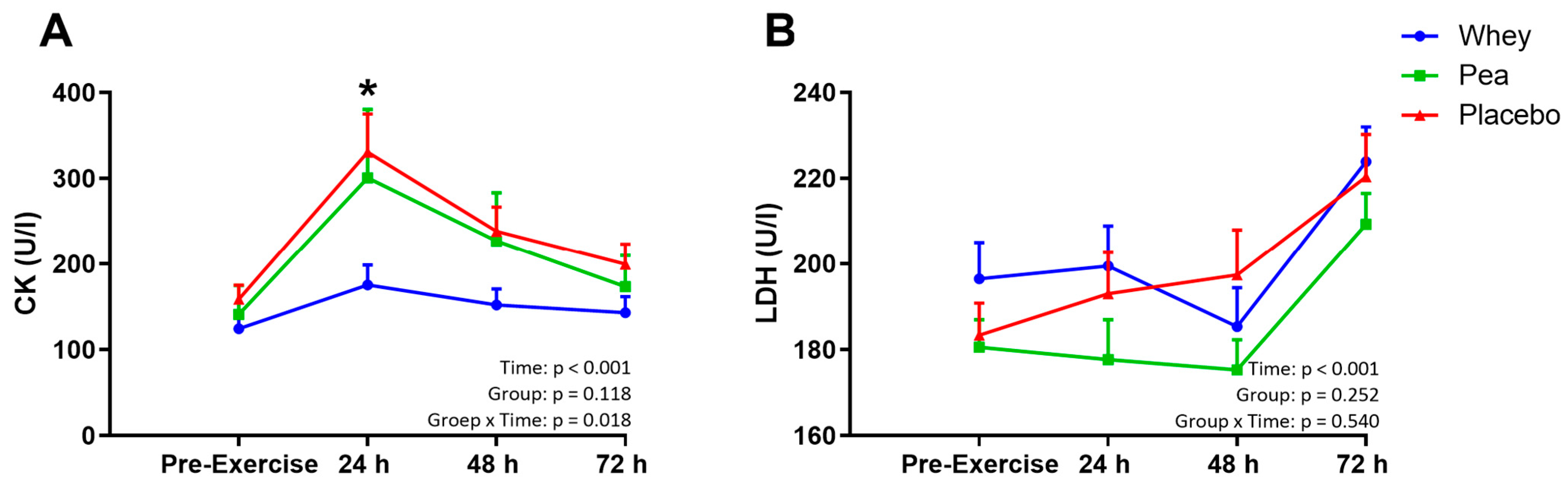
4.4. Muscle Damage
4.5. Muscle Parameters
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CK | Creatine Kinase |
| COPD | Chronic Obstructive Pulmonary Disease |
| EIMD | Exercise Induced Muscle Damage |
| LDH | Lactate Dehydrogenase |
| MPS | Muscle Protein Synthesis |
| NRS | Numeric (pain) Rating Scale |
| RM | Repetition Maximum |
References
- Markus, I.; Constantini, K.; Hoffman, J.R.; Bartolomei, S.; Gepner, Y. Exercise-Induced muscle damage: Mechanism, assessment and nutritional factors to accelerate recovery. Eur. J. Appl. Physiol. 2021, 121, 969–992. [Google Scholar] [CrossRef] [PubMed]
- Ten Haaf, D.S.M.; Bongers, C.; Hulshof, H.G.; Eijsvogels, T.M.H.; Hopman, M.T.E. The Impact of Protein Supplementation on Exercise-Induced Muscle Damage, Soreness and Fatigue Following Prolonged Walking Exercise in Vital Older Adults: A Randomized Double-Blind Placebo-Controlled Trial. Nutrients 2020, 12, 1806. [Google Scholar] [CrossRef] [PubMed]
- Clifford, T.; Hayes, E.J.; Scragg, J.H.; Taylor, G.; Smith, K.; Bowden Davies, K.A.; Stevenson, E.J. The Effects of a High-Protein Diet on Markers of Muscle Damage Following Exercise in Active Older Adults: A Randomized, Controlled Trial. Int. J. Sport Nutr. Exerc. Metab. 2020, 30, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Nieman, D.C.; Zwetsloot, K.A.; Simonson, A.J.; Hoyle, A.T.; Wang, X.; Nelson, H.K.; Lefranc-Millot, C.; Guerin-Deremaux, L. Effects of Whey and Pea Protein Supplementation on Post-Eccentric Exercise Muscle Damage: A Randomized Trial. Nutrients 2020, 12, 2382. [Google Scholar] [CrossRef]
- Clifford, T. Nutritional and Pharmacological Interventions to Expedite Recovery Following Muscle-Damaging Exercise in Older Adults: A Narrative Review of the Literature. J. Aging Phys. Act. 2019, 27, 914–928. [Google Scholar] [CrossRef] [PubMed]
- Ten Haaf, D.S.M.; Nuijten, M.A.H.; Maessen, M.F.H.; Horstman, A.M.H.; Eijsvogels, T.M.H.; Hopman, M.T.E. Effects of protein supplementation on lean body mass, muscle strength, and physical performance in nonfrail community-dwelling older adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018, 108, 1043–1059. [Google Scholar] [CrossRef]
- Davies, R.W.; Carson, B.P.; Jakeman, P.M. The Effect of Whey Protein Supplementation on the Temporal Recovery of Muscle Function Following Resistance Training: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 221. [Google Scholar] [CrossRef]
- Ten Haaf, D.S.M.; De Regt, M.F.; Visser, M.; Witteman, B.J.M.; de Vries, J.H.M.; Eijsvogels, T.M.H.; Hopman, M.T.E. Insufficient Protein Intakes is Highly Prevalent among Physically Active Elderly. J. Nutr. Health Aging 2018, 22, 1112–1114. [Google Scholar] [CrossRef]
- Aschemann-Witzel, J.; Gantriis, R.F.; Fraga, P.; Perez-Cueto, F.J.A. Plant-based food and protein trend from a business perspective: Markets, consumers, and the challenges and opportunities in the future. Crit. Rev. Food Sci. Nutr. 2021, 61, 3119–3128. [Google Scholar] [CrossRef]
- Babault, N.; Paizis, C.; Deley, G.; Guerin-Deremaux, L.; Saniez, M.H.; Lefranc-Millot, C.; Allaert, F.A. Pea proteins oral supplementation promotes muscle thickness gains during resistance training: A double-blind, randomized, Placebo-controlled clinical trial vs. Whey protein. J. Int. Soc. Sports Nutr. 2015, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, S.H.; Horstman, A.M.; Franssen, R.; Crombag, J.J.; Langer, H.; Bierau, J.; Respondek, F.; van Loon, L.J. Ingestion of Wheat Protein Increases In Vivo Muscle Protein Synthesis Rates in Healthy Older Men in a Randomized Trial. J. Nutr. 2016, 146, 1651–1659. [Google Scholar] [CrossRef]
- Oikawa, S.Y.; Bahniwal, R.; Holloway, T.M.; Lim, C.; McLeod, J.C.; McGlory, C.; Baker, S.K.; Phillips, S.M. Potato Protein Isolate Stimulates Muscle Protein Synthesis at Rest and with Resistance Exercise in Young Women. Nutrients 2020, 12, 1235. [Google Scholar] [CrossRef] [PubMed]
- Maessen, M.F.; Verbeek, A.L.; Bakker, E.A.; Thompson, P.D.; Hopman, M.T.; Eijsvogels, T.M. Lifelong Exercise Patterns and Cardiovascular Health. Mayo Clin. Proc. 2016, 91, 745–754. [Google Scholar] [CrossRef]
- Xia, Z.; Cholewa, J.M.; Dardevet, D.; Huang, T.; Zhao, Y.; Shang, H.; Yang, Y.; Ding, X.; Zhang, C.; Wang, H.; et al. Effects of oat protein supplementation on skeletal muscle damage, inflammation and performance recovery following downhill running in untrained collegiate men. Food Funct. 2018, 9, 4720–4729. [Google Scholar] [CrossRef]
- Rohling, M.; McCarthy, D.; Berg, A. Continuous Protein Supplementation Reduces Acute Exercise-Induced Stress Markers in Athletes Performing Marathon. Nutrients 2021, 13, 2929. [Google Scholar] [CrossRef] [PubMed]
- Ten Haaf, D.S.M.; Flipsen, M.A.; Horstman, A.M.H.; Timmerman, H.; Steegers, M.A.H.; de Groot, L.; Eijsvogels, T.M.H.; Hopman, M.T.E. The Effect of Protein Supplementation versus Carbohydrate Supplementation on Muscle Damage Markers and Soreness Following a 15-km Road Race: A Double-Blind Randomized Controlled Trial. Nutrients 2021, 13, 858. [Google Scholar] [CrossRef]
- Hjermstad, M.J.; Fayers, P.M.; Haugen, D.F.; Caraceni, A.; Hanks, G.W.; Loge, J.H.; Fainsinger, R.; Aass, N.; Kaasa, S.; European Palliative Care Research, C. Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: A systematic literature review. J. Pain Symptom Manag. 2011, 41, 1073–1093. [Google Scholar] [CrossRef]
- Boonstra, A.M.; Stewart, R.E.; Koke, A.J.; Oosterwijk, R.F.; Swaan, J.L.; Schreurs, K.M.; Schiphorst Preuper, H.R. Cut-Off Points for Mild, Moderate, and Severe Pain on the Numeric Rating Scale for Pain in Patients with Chronic Musculoskeletal Pain: Variability and Influence of Sex and Catastrophizing. Front. Psychol 2016, 7, 1466. [Google Scholar] [CrossRef] [PubMed]
- Ocke, M.; Dinnissen, C.; Stafleu, A.; de Vries, J.; van Rossum, C. Relative Validity of MijnEetmeter: A Food Diary App for Self-Monitoring of Dietary Intake. Nutrients 2021, 13, 1135. [Google Scholar] [CrossRef] [PubMed]
- Ottestad, I.; Lovstad, A.T.; Gjevestad, G.O.; Hamarsland, H.; Saltyte Benth, J.; Andersen, L.F.; Bye, A.; Biong, A.S.; Retterstol, K.; Iversen, P.O.; et al. Intake of a Protein-Enriched Milk and Effects on Muscle Mass and Strength. A 12-Week Randomized Placebo Controlled Trial among Community-Dwelling Older Adults. J. Nutr. Health Aging 2017, 21, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J.; Blue, M.N.M.; Hirsch, K.R.; Saylor, H.E.; Gould, L.M.; Nelson, A.G.; Smith-Ryan, A.E. Validation of InBody 770 bioelectrical impedance analysis compared to a four-compartment model criterion in young adults. Clin. Physiol. Funct. Imaging 2021, 41, 317–325. [Google Scholar] [CrossRef]
- Shenoy, S.; Dhawan, M.; Singh Sandhu, J. Four Weeks of Supplementation With Isolated Soy Protein Attenuates Exercise-Induced Muscle Damage and Enhances Muscle Recovery in Well Trained Athletes: A Randomized Trial. Asian J. Sports Med. 2016, 7, e33528. [Google Scholar] [CrossRef]
- Baum, J.I.; Kim, I.Y.; Wolfe, R.R. Protein Consumption and the Elderly: What Is the Optimal Level of Intake? Nutrients 2016, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, S.H.M.; Trommelen, J.; Kouw, I.W.K.; Holwerda, A.M.; Pennings, B.; Groen, B.B.L.; Wall, B.T.; Churchward-Venne, T.A.; Horstman, A.M.H.; Koopman, R.; et al. Protein Type, Protein Dose, and Age Modulate Dietary Protein Digestion and Phenylalanine Absorption Kinetics and Plasma Phenylalanine Availability in Humans. J. Nutr. 2020, 150, 2041–2050. [Google Scholar] [CrossRef]
- Deutz, N.E.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznaric, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; van Loon, L.J. Aging, exercise, and muscle protein metabolism. J. Appl. Physiol. 2009, 106, 2040–2048. [Google Scholar] [CrossRef] [PubMed]
- Wall, B.T.; Gorissen, S.H.; Pennings, B.; Koopman, R.; Groen, B.B.; Verdijk, L.B.; van Loon, L.J. Aging Is Accompanied by a Blunted Muscle Protein Synthetic Response to Protein Ingestion. PLoS ONE 2015, 10, e0140903. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J. Does exercise-induced muscle damage play a role in skeletal muscle hypertrophy? J. Strength Cond. Res. 2012, 26, 1441–1453. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, P.; Maffulli, N.; Limongelli, F.M. Creatine kinase monitoring in sport medicine. Br. Med. Bull. 2007, 81–82, 209–230. [Google Scholar] [CrossRef]
- Cooke, M.B.; Rybalka, E.; Stathis, C.G.; Cribb, P.J.; Hayes, A. Whey protein isolate attenuates strength decline after eccentrically-induced muscle damage in healthy individuals. J. Int. Soc. Sports Nutr. 2010, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Buckley, J.D.; Thomson, R.L.; Coates, A.M.; Howe, P.R.; DeNichilo, M.O.; Rowney, M.K. Supplementation with a whey protein hydrolysate enhances recovery of muscle force-generating capacity following eccentric exercise. J. Sci. Med. Sport 2010, 13, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Jagim, A.; Hagele, A.; Jager, R. Plant Proteins and Exercise: What Role Can Plant Proteins Have in Promoting Adaptations to Exercise? Nutrients 2021, 13, 1962. [Google Scholar] [CrossRef]
- van Vliet, S.; Burd, N.A.; van Loon, L.J. The Skeletal Muscle Anabolic Response to Plant- versus Animal-Based Protein Consumption. J. Nutr. 2015, 145, 1981–1991. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, F.; Li, Y.; Tang, Y.; Kong, X.; Feng, Z.; Anthony, T.G.; Watford, M.; Hou, Y.; Wu, G.; et al. The role of leucine and its metabolites in protein and energy metabolism. Amino Acids 2016, 48, 41–51. [Google Scholar] [CrossRef]
- Jager, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef]
- Corgneau, M.; Gaiani, C.; Petit, J.; Nikolova, Y.; Banon, S.; Ritié-Pertusa, L.; Le, D.T.L.; Scher, J. Nutritional quality evaluation of commercial protein supplements. Int. J. Food Sci. Technol. 2019, 54, 2586–2594. [Google Scholar] [CrossRef]
- Liu, J.; Klebach, M.; Visser, M.; Hofman, Z. Amino Acid Availability of a Dairy and Vegetable Protein Blend Compared to Single Casein, Whey, Soy, and Pea Proteins: A Double-Blind, Cross-Over Trial. Nutrients 2019, 11, 2613. [Google Scholar] [CrossRef]
- Minahan, C.L.; Poke, D.P.; Morrison, J.; Bellinger, P.M. Muscle Damage and Metabolic Responses to Repeated-Sprint Running With and Without Deceleration. J. Strength Cond. Res. 2020, 34, 3423–3430. [Google Scholar] [CrossRef]
- Callegari, G.A.; Novaes, J.S.; Neto, G.R.; Dias, I.; Garrido, N.D.; Dani, C. Creatine Kinase and Lactate Dehydrogenase Responses after Different Resistance and Aerobic Exercise Protocols. J. Hum. Kinet. 2017, 58, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Chapman, D.W.; Newton, M.J.; Zainuddin, Z.; Sacco, P.; Nosaka, K. Work and peak torque during eccentric exercise do not predict changes in markers of muscle damage. Br. J. Sports Med. 2008, 42, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Ispirlidis, I.; Fatouros, I.G.; Jamurtas, A.Z.; Nikolaidis, M.G.; Michailidis, I.; Douroudos, I.; Margonis, K.; Chatzinikolaou, A.; Kalistratos, E.; Katrabasas, I.; et al. Time-course of changes in inflammatory and performance responses following a soccer game. Clin. J. Sport Med. 2008, 18, 423–431. [Google Scholar] [CrossRef]
- Rodrigues, P.; Wassmansdorf, R.; Salgueirosa, F.M.; Hernandez, S.G.; Nascimento, V.B.; Daros, L.B.; Lee, W.; Ousicki, R. Time-course of changes in indirect markers of muscle damage responses following a 130-km cycling race. Rev. Bras. CIneantropom Hum. 2016, 18, 322–331. [Google Scholar] [CrossRef]
- Mehmet, H.; Yang, A.W.H.; Robinson, S.R. Measurement of hand grip strength in the elderly: A scoping review with recommendations. J. Bodyw. Mov. Ther. 2020, 24, 235–243. [Google Scholar] [CrossRef]
- Joy, J.M.; Lowery, R.P.; Wilson, J.M.; Purpura, M.; De Souza, E.O.; Wilson, S.M.; Kalman, D.S.; Dudeck, J.E.; Jager, R. The effects of 8 weeks of whey or rice protein supplementation on body composition and exercise performance. Nutr. J. 2013, 12, 86. [Google Scholar] [CrossRef]
- Kim, J.E.; O’Connor, L.E.; Sands, L.P.; Slebodnik, M.B.; Campbell, W.W. Effects of dietary protein intake on body composition changes after weight loss in older adults: A systematic review and meta-analysis. Nutr. Rev. 2016, 74, 210–224. [Google Scholar] [CrossRef]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Saito, Y.; Sanbongi, C.; Murata, K.; Urashima, T. Effects of low-dose milk protein supplementation following low-to-moderate intensity exercise training on muscle mass in healthy older adults: A randomized placebo-controlled trial. Eur. J. Nutr. 2021, 60, 917–928. [Google Scholar] [CrossRef]
- Tieland, M.; Dirks, M.L.; van der Zwaluw, N.; Verdijk, L.B.; van de Rest, O.; de Groot, L.C.; van Loon, L.J. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2012, 13, 713–719. [Google Scholar] [CrossRef]

| Nutrient | Whey Protein | Pea Protein | Placebo (Maltodextrin with Lemon Flavor) |
|---|---|---|---|
| Energy (kcal/100 g) | 390 | 382 | 385 |
| Fat (g/100 g) | 8 | 9 | 0 |
| Carbohydrate (g/100 g) | 6 | 3.8 | 94.8 |
| Of which lactose (g/100 g) | 6 | 0 | 0 |
| Protein (g/100) | 81 | 82 | 0 |
| Amino Acid content (g/100 g) | |||
| Alanine | 4.1 | 3.6 | 0 |
| Arginine | 2.4 | 7.2 | 0 |
| Aspartanic Acid | 9.2 | 9.2 | 0 |
| Cystine | 1.9 | 0.8 | 0 |
| Glutamic Acid | 12.3 | 13.2 | 0 |
| Glycine | 1.7 | 3.2 | 0 |
| Histidine | 1.5 | 2.0 | 0 |
| Isoleucine ** | 4.4 | 4.0 | 0 |
| Leucine ** | 9.2 | 7.2 | 0 |
| Lysine | 7.3 | 6.4 | 0 |
| Methionine | 2.1 | 0.8 | 0 |
| Phenylalanine | 2.8 | 4.8 | 0 |
| Proline | 5.1 | 3.6 | 0 |
| Serine | 3.9 | 4.4 | 0 |
| Threonine | 4.6 | 3.2 | 0 |
| Tryptofaan | 1.2 | 0.8 | 0 |
| Tyrosine | 2.4 | 3.2 | 0 |
| Valine ** | 4.5 | 4.4 | 0 |
| Total Group n = 45 | Whey Protein n = 15 | Pea Protein n = 15 | Placebo n = 15 | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 70 ± 6 | 72 ± 5 | 69 ± 6 | 69 ± 6 |
| Male n (%) | 36 (80) | 12 (80) | 12 (80) | 12 (80) |
| Anthropometrics | ||||
| Body weight (kg) | 75.4 ± 13.9 | 73.9 ± 10.1 | 75.8 ± 16.3 | 76.4 ± 15.4 |
| Height (m) | 1.76 ± 0.09 | 1.76 ± 0.07 | 1.75 ± 0.10 | 1.76 ± 0.10 |
| BMI (kg/m2) | 24.2 ± 2.8 | 23.7 ± 2.2 | 24.6 ± 3.3 | 24.4 ± 2.9 |
| Waist-hip ratio | 0.97 ± 0.06 | 0.98 ± 0.07 | 0.97 ± 0.05 | 0.97 ± 0.07 |
| Skeletal muscle mass (%) | 42.5 ± 3.3 | 42.6 ± 3.9 | 41.5 ± 3.2 | 43.4 ± 3.3 |
| Dietary intake | ||||
| Energy intake (kcal) | 2078 ± 528 | 2153 ± 529 | 1960 ± 496 | 2123 ± 570 |
| Protein intake (g/kg/d) | 1.13 ± 0.33 | 1.16 ± 0.39 | 1.09 ± 0.31 | 1.15 ± 0.31 |
| Protein intake (en%) | 22.0 ± 4.7 | 21.8 ± 5.3 | 22.1 ± 3.9 | 22.0 ± 5.1 |
| Number of participants with a protein intake below 1.0 g/kg/d (n (%)) | 18 (40) | 5 (33) | 7 (47) | 6 (40) |
| Walking activity | ||||
| Cumulative walking distance last year (km) | 645 [1074] | 800 [1784] | 280 [520] | 820 [988] |
| Walking bout distance (km) | 24.3 ± 4.9 | 23.8 ± 5.4 | 23.0 ± 4.4 | 26.1 ± 4.8 |
| Walking bout duration bout (h) | 5.2 ± 1.1 | 5.2 ± 1.3 | 4.8 ± 1.0 | 5.4 ± 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spoelder, M.; Koopmans, L.; Hartman, Y.A.W.; Bongers, C.C.W.G.; Schoofs, M.C.A.; Eijsvogels, T.M.H.; Hopman, M.T.E. Supplementation with Whey Protein, but Not Pea Protein, Reduces Muscle Damage Following Long-Distance Walking in Older Adults. Nutrients 2023, 15, 342. https://doi.org/10.3390/nu15020342
Spoelder M, Koopmans L, Hartman YAW, Bongers CCWG, Schoofs MCA, Eijsvogels TMH, Hopman MTE. Supplementation with Whey Protein, but Not Pea Protein, Reduces Muscle Damage Following Long-Distance Walking in Older Adults. Nutrients. 2023; 15(2):342. https://doi.org/10.3390/nu15020342
Chicago/Turabian StyleSpoelder, Marcia, Lotte Koopmans, Yvonne A. W. Hartman, Coen C. W. G. Bongers, Merle C. A. Schoofs, Thijs M. H. Eijsvogels, and Maria T. E. Hopman. 2023. "Supplementation with Whey Protein, but Not Pea Protein, Reduces Muscle Damage Following Long-Distance Walking in Older Adults" Nutrients 15, no. 2: 342. https://doi.org/10.3390/nu15020342
APA StyleSpoelder, M., Koopmans, L., Hartman, Y. A. W., Bongers, C. C. W. G., Schoofs, M. C. A., Eijsvogels, T. M. H., & Hopman, M. T. E. (2023). Supplementation with Whey Protein, but Not Pea Protein, Reduces Muscle Damage Following Long-Distance Walking in Older Adults. Nutrients, 15(2), 342. https://doi.org/10.3390/nu15020342






