Oral Bioavailability and Metabolism of Hydroxytyrosol from Food Supplements
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Investigational Products (IPs)
2.3. Participants and Study Design
2.4. Sampling
2.5. Sample Processing
2.6. Analysis of HT and Its Metabolites
2.7. Data Analysis
3. Results
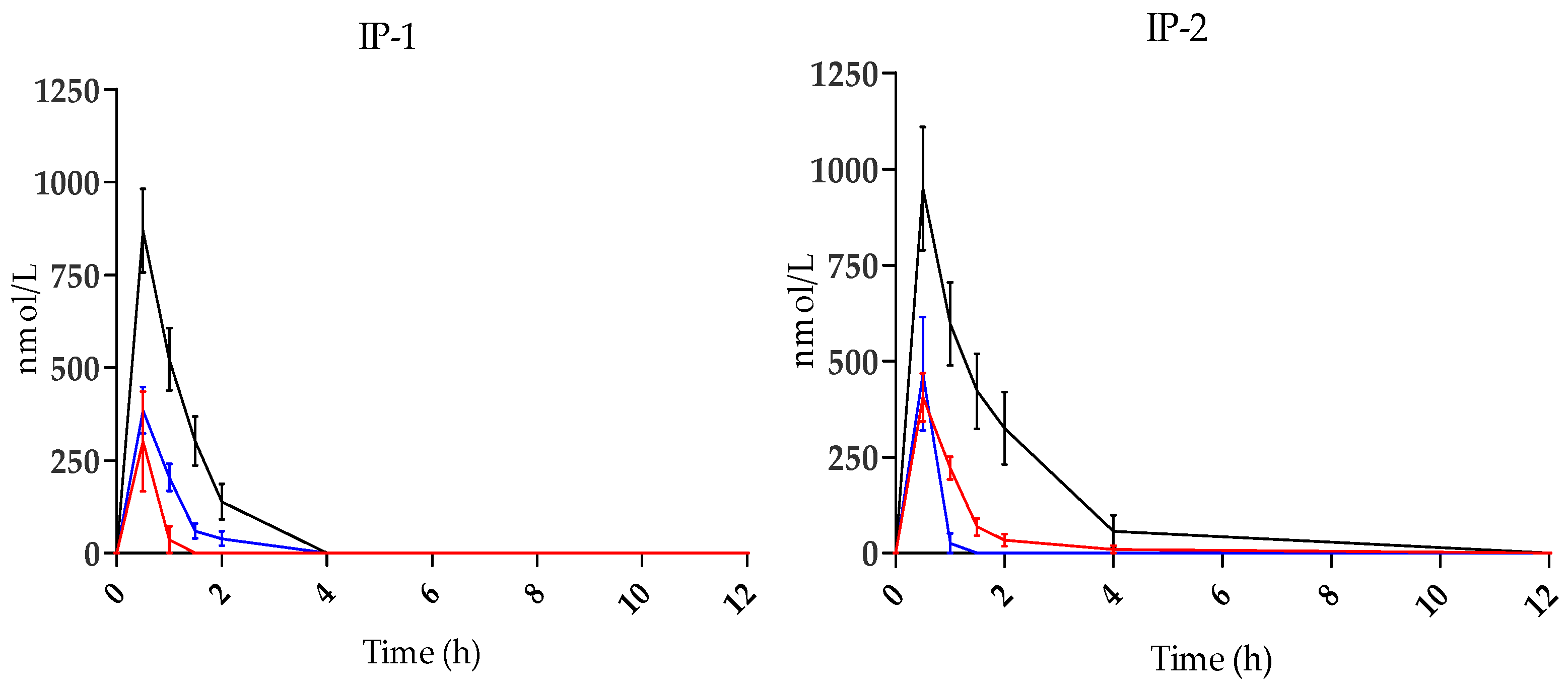
3.1. HT Metabolites in Plasma
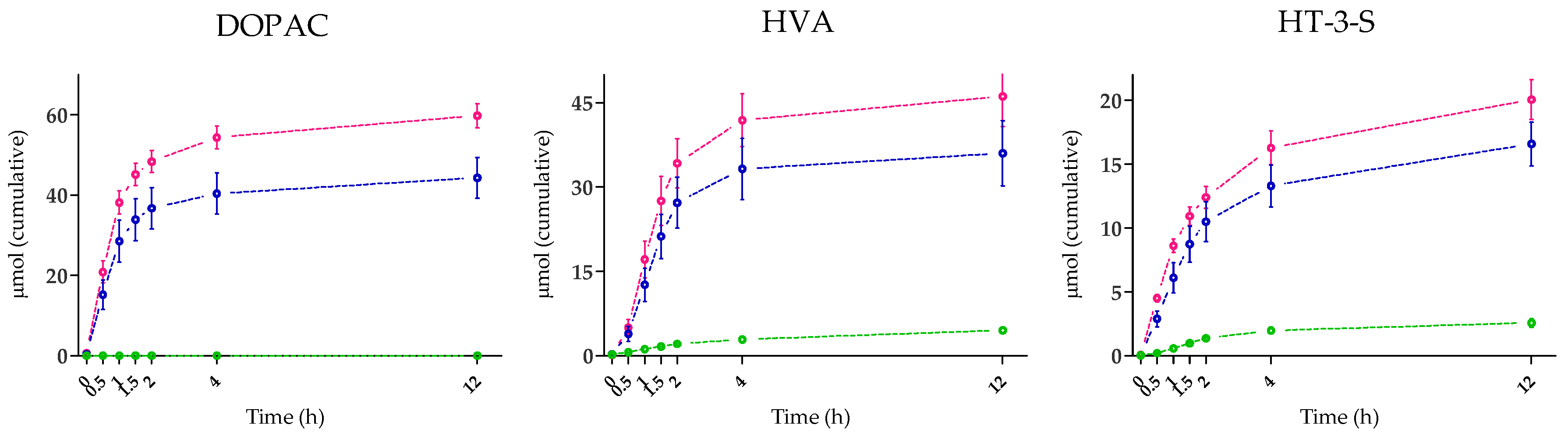
3.2. Urinary Excretion of HT Metabolites
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rothwell, J.A.; Pérez-Jiménez, J.; Neveu, V.; Medina-Ramon, A.; M’Hiri, N.; Garcia Lobato, P.; Manach, C.; Knox, K.; Eisner, R.; Wishart, D.; et al. Phenol-Explorer 3.0: A Major Update of the Phenol-Explorer Database to Incorporate Data on the Effects of Food Processing on Polyphenol Content. Database 2013. https://doi.org/10.1093/database/bat070. Available online: http://www.phenol-explorer.eu (accessed on 3 February 2022).
- Amiot, M.J.; Fleuriet, A.; Macheix, J.J. Importance and evolution of phenolic compounds in olive during growth and maturation. J. Agric. Food Chem. 1986, 34, 823–826. [Google Scholar] [CrossRef]
- Ryan, D.; Robards, K.; Lavee, S. Changes in phenolic content of olive during maturation. Int. J. FoodSci. Technol. 1999, 34, 265–274. [Google Scholar] [CrossRef]
- Geropoulos, N.K.; Kaliora, A.C. Effect of Fruit Maturity on Olive Oil Phenolic Composition and Antioxidant Capacity. In Olive and Olive Oil Bioactive Constituents; AOCS Press: Urbana, Il, USA, 2015; pp. 123–145. [Google Scholar] [CrossRef]
- Blekas, G.; Vassilakis, C.; Harizanis, C.; Tsimidou, M.; Boskou, D.G. Biophenolsin table olives. J. Agric. Food Chem. 2002, 50, 3688–3692. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, C.D.; Cramer, H.; Michalsen, A.; Kessler, C.; Steckhan, N.; Choi, K.; Dobos, G. Effects of high phenolic olive oil on cardiovascular risk factors: A systematic review and meta-analysis. Phytomedicine 2015, 22, 631–640. [Google Scholar] [CrossRef]
- Covas, M.I.; de la Torre, R.; Fitó, M. Virgin olive oil: A key food for cardiovascular risk protection. Br. J. Nutr. 2015, 113, S19–S28. [Google Scholar] [CrossRef]
- Tejada, S.; Pinya, S.; Del Mar Bibiloni, M.; Tur, J.A.; Pons, A.; Sureda, A. Cardioprotective Effects of the Polyphenol Hydroxytyrosol from Olive Oil. Curr. Drug Targets 2017, 18, 1477–1486. [Google Scholar] [CrossRef]
- Karković-Marković, A.; Torić, J.; Barbarić, M.; Jakobušić-Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- European Commission. Regulation EC No. 432/2012 Establishing a List of Permitted Health Claims Made on Foods, Other Than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health. Off. J. Eur. Union 2012, L136, 1. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32012R0432 (accessed on 3 February 2022).
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), “anti-inflammatory properties”(ID 1882), “contributes to the upper respiratory tract health” (ID 3468), “can help to maintain a normal function of gastrointestinal tract”(3779), and “contributes to body defences. EFSA J. 2011, 9, 2033. [Google Scholar]
- González-Santiago, M.; Fonollá, J.; Lopez-Huertas, E. Human absorption of a supplement containing purified hydroxytyrosol, a natural antioxidant from olive oil, and evidence for its transient association with low-density lipoproteins. Pharmacol. Res. 2010, 61, 364–370. [Google Scholar] [CrossRef]
- Covas, M.I.; Nyyssönen, K.; Poulsen, H.E.; Kaikkonen, J.; Zunft, H.J.; Kiesewetter, H.; Gaddi, A.; de la Torre, R.; Mursu, J.; Bäumler, H.; et al. EUROLIVE Study Group. The effect of polyphenols in olive oil on heart disease risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 333–341. [Google Scholar] [CrossRef]
- Fitó, M.; Guxens, M.; Corella, D.; Sáez, G.; Estruch, R.; de la Torre, R.; Francés, F.; Cabezas, C.; López-Sabater, M.D.C.; Marrugat, J.; et al. Effect of a traditional Mediterranean diet on lipoprotein oxidation: A randomized controlled trial. Arch Intern. Med. 2007, 167, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- de la Torre-Carbot, K.; Chávez-Servín, J.L.; Jaúregui, O.; Castellote, A.I.; Lamuela-Raventós, R.M.; Nurmi, T.; Poulsen, H.E.; Gaddi, A.V.; Kaikkonen, J.; Zunft, H.F.; et al. Elevated circulating LDL phenol levels in men who consumed virgin rather than refined olive oil are associated with less oxidation of plasma LDL. J. Nutr. 2010, 140, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Martínez-López, S.; BaezaArévalo, G.; Amigo-Benavent, M.; Sarriá, B.; Bravo-Clemente, L. Hydroxytyrosol in functional hydroxytyrosol-enriched biscuits is highly bioavailable and decreases oxidised low density lipoprotein levels in humans. Food Chem. 2016, 205, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Caruso, D.; Visioli, F.; Patelli, R.; Galli, C.; Galli, G. Urinary excretion of olive oil phenols and their metabolites in humans. Metabolism 2001, 50, 1426–1428. [Google Scholar] [CrossRef] [PubMed]
- Miro-Casas, E.; Covas, M.I.; Farre, M.; Fito, M.; Ortuño, J.; Weinbrenner, T.; Roset, P.; de la Torre, R. Hydroxytyrosol disposition in humans. Clin. Chem. 2003, 49, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Bonanome, A.; Pagnan, A.; Caruso, D.; Toia, A.; Xamin, A.; Fedeli, E.; Berra, B.; Zamburlini, A.; Ursini, F.; Galli, G. Evidence of postprandial absorption of olive oil phenols in humans. Nutr. Metab. Cardiovasc. Dis. 2000, 10, 111–120. [Google Scholar]
- Miró-Casas, E.; Covas, M.I.; Fitó, M.; Farré-Albadalejo, M.; Marrugat, J.; de la Torre, R. Tyrosol and hydroxytyrosol are absorbed from moderate and sustained doses of virgin olive oil in humans. Eur. J. Clin. Nutr. 2003, 57, 186–190. [Google Scholar] [CrossRef]
- Tuck, K.L.; Hayball, P.J. Major phenolic compounds in olive oil: Metabolism and health effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef]
- Suárez, M.; Valls, R.M.; Romero, M.P.; Macià, A.; Fernández, S.; Giralt, M.; Solà, R.; Motilva, M.J. Bioavailability of phenols from a phenol-enriched olive oil. Br. J. Nutr. 2011, 106, 1691–1701. [Google Scholar] [CrossRef]
- Vissers, M.N.; Zock, P.L.; Roodenburg, A.J.; Leenen, R.; Katan, M.B. Olive oil phenols are absorbed in humans. J. Nutr. 2002, 132, 409–417. [Google Scholar] [CrossRef]
- Kountouri, A.M.; Mylona, A.; Kaliora, A.C.; Andrikopoulos, N.K. Bioavailability of the phenolic compounds of the fruits (drupes) of Olea europaea (olives): Impact on plasma antioxidant status in humans. Phytomedicine 2007, 14, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Galli, C.; Bornet, F.; Mattei, A.; Patelli, R.; Galli, G.; Caruso, D. Olive oil phenolics are dose-dependently absorbed in humans. FEBS Lett. 2000, 468, 159–160. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, R. Bioavailability of olive oil phenolic compounds in humans. Inflammopharmacology 2008, 16, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Rubió, L.; Valls, R.M.; Macià, A.; Pedret, A.; Giralt, M.; Romero, M.P.; de la Torre, R.; Covas, M.I.; Solà, R.; Motilva, M.J. Impact of olive oil phenolic concentration on human plasmatic phenolic metabolites. Food Chem. 2012, 135, 2922–2929. [Google Scholar] [CrossRef]
- Khymenets, O.; Farre, M.; Pujadas, M.; Ortiz, E.; Joglar, J.; Covas, M.I.; de la Torre, R. Direct analysis of glucuronidated metabolites of main olive oil phenols in human urine after dietary consumption of virgin olive oil. Food Chem. 2011, 126, 306–314. [Google Scholar] [CrossRef]
- Rodríguez-Morató, J.; Boronat, A.; Kotronoulas, A.; Pujadas, M.; Pastor, A.; Olesti, E.; Pérez-Mañá, C.; Khymenets, O.; Fitó, M.; Farré, M.; et al. Metabolic disposition and biological significance of simple phenols of dietary origin: Hydroxytyrosol and tyrosol. Drug Metab. Rev. 2016, 48, 218–236. [Google Scholar] [CrossRef]
- D’Angelo, S.; Manna, C.; Migliardi, V.; Mazzoni, O.; Morrica, P.; Capasso, G.; Pontoni, G.; Galletti, P.; Zappia, V. Pharmacokinetics and metabolism of hydroxytyrosol, a natural antioxidant from olive oil. Drug Metab. Dispos. 2001, 29, 1492–1498. [Google Scholar]
- Kotronoulas, A.; Pizarro, N.; Serra, A.; Robledo, P.; Joglar, J.; Rubió, L.; Hernaéz, A.; Tormos, C.; Motilva, M.J.; Fitó, M.; et al. Dose-dependent metabolic disposition of hydroxytyrosol and formation of mercapturates in rats. Pharmacol. Res. 2013, 77, 47–56. [Google Scholar] [CrossRef]
- López de las Hazas, M.C.; Rubió, L.; Kotronoulas, A.; de la Torre, R.; Solà, R.; Motilva, M.J. Dose effect on the uptake and accumulation of hydroxytyrosol and its metabolites in target tissues in rats. Mol. Nutr. Food Res. 2015, 59, 1395–1399. [Google Scholar] [CrossRef]
- López de las Hazas, M.C.; Piñol, C.; Macià, A.; Romero, M.P.; Pedret, A.; Solà, R.; Rubió, L.; Motilva, M.J. Differential absorption and metabolism of hydroxytyrosol and its precursors oleuropein and secoiridoids. J. Funct. Foods 2016, 22, 52–63. [Google Scholar] [CrossRef]
- Obied, H.K.; Allen, M.S.; Bedgood, D.R.; Prenzler, P.D.; Robards, K.; Stockmann, R. Bioactivity and analysis of biophenols recovered from olive mill waste. J. Agric. Food Chem. 2005, 53, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Galli, C.; Grande, S.; Colonnelli, K.; Patelli, C.; Galli, G.; Caruso, D. Hydroxytyrosol excretion differs between rats and humans and depends on the vehicle of administration. J. Nutr. 2003, 133, 2612–2615. [Google Scholar] [CrossRef] [PubMed]
- Alemán-Jiménez, C.; Domínguez-Perles, R.; Medina, S.; Prgomet, I.; López-González, I.; Simonelli-Muñoz, A.; Campillo-Cano, M.; Auñón, D.; Ferreres, F.; Gil-Izquierdo, Á. Pharmacokinetics and bioavailability of hydroxytyrosol are dependent on the food matrix in humans. Eur. J. Nutr. 2021, 60, 905–915. [Google Scholar] [CrossRef]
- Bender, C.; Candi, I.; Rogel, E. Bioefficacy of hydroxytyrosol-rich food supplements on preventing lipid peroxidation in healthy men. bioRxiv 2022, 508834. [Google Scholar] [CrossRef]
- Bender, C.; Straßmann, S.; Heidrich, P. Cellular Antioxidant Effects and Bioavailability of Food Supplements Rich in Hydroxytyrosol. Appl. Sci. 2021, 11, 4763. [Google Scholar] [CrossRef]
- Feliciano, R.P.; Mecha, E.; Bronze, M.R.; Rodriguez-Mateos, A. Development and validation of a high-throughput micro solid-phase extraction method coupled with ultra-high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry for rapid identification and quantification of phenolic metabolites in human plasma and urine. J. Chromatogr. A 2016, 1464, 21–31. [Google Scholar] [CrossRef]
- Suárez, M.; Romero, M.P.; Macià, A.; Valls, R.M.; Fernández, S.; Solà, R.; Motilva, M.J. Improved method for identifying and quantifying olive oil phenolic compounds and their metabolites in human plasma by microelution solid-phase extraction plate and liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 4097–4106. [Google Scholar] [CrossRef]
- Marrugat, J.; Covas, M.I.; Fitó, M.; Schröder, H.; Miró-Casas, E.; Gimeno, E.; López-Sabater, M.C.; de la Torre, R.; Farré, M. Effects of differing phenolic content in dietary olive oils on lipids and LDL oxidation—A randomized controlled trial. Eur. J. Nutr. 2004, 43, 140–147. [Google Scholar] [CrossRef]
- Weinbrenner, T.; Fitó, M.; de la Torre, R.; Saez, G.T.; Rijken, P.; Tormos, C.; Coolen, S.; Albaladejo, M.F.; Abanades, S.; Schroder, H.; et al. Olive oils high in phenolic compounds modulate oxidative/antioxidative status in men. J. Nutr. 2004, 134, 2314–2321. [Google Scholar] [CrossRef]
- Covas, M.I.; de la Torre, K.; Farré-Albaladejo, M.; Kaikkonen, J.; Fitó, M.; López-Sabater, C.; Pujadas-Bastardes, M.A.; Joglar, J.; Weinbrenner, T.; Lamuela-Raventós, R.M.; et al. Postprandial LDL phenolic content and LDL oxidation are modulated by olive oil phenolic compounds in humans. Free RadicBiol. Med. 2006, 40, 608–616. [Google Scholar] [CrossRef] [PubMed]
- de Bock, M.; Thorstensen, E.B.; Derraik, J.G.; Henderson, H.V.; Hofman, P.L.; Cutfield, W.S. Human absorption and metabolism of oleuropein and hydroxytyrosol ingested as olive (Olea europaea L.) leaf extract. Mol. Nutr. Food Res. 2013, 57, 2079–2085. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef] [PubMed]
- Khymenets, O.; Crespo, M.; Dangles, O.; Njara, R.; Andres-Lacueva, C.; Visioli, F. Human hydroxytyrosol’s absorption and excretion from a nutraceutical. J. Funct. Foods 2016, 23, 278–282. [Google Scholar] [CrossRef]
| Nutrional Value * (g/100 mL) | IP-1 | IP-2 |
|---|---|---|
| Energy (kJ) | 75.00 | 555.00 |
| Fat | 0.00 | 0.05 |
| of which saturates | 0.00 | 0.00 |
| Carbohydrate | 4.17 | 32.03 |
| of which sugars | 1.05 | 27.52 |
| Protein | 0.20 | 0.34 |
| Salt | 0.028 | 0.019 |
| Potassium | 0.804 | 0.642 |
| HT and Derivatives (mg/L) | ||
| HT | 1223 | 2459 |
| Ole | 1.65 | 2.99 |
| HVA | n.d | n.d |
| DOPAC | n.d | n.d |
| Total polyphenols * | 10,980 | 10,100 |
| Hydroxytyrosol (Metabolites) | [M − H]− (m/z) | Most Abundant Fragment (m/z) (Used for Quantification) |
|---|---|---|
| HT | 153 | 123 |
| Ole | 539 | 275 |
| DOPAC | 167 | 123 |
| HVA | 181 | 137 |
| HT-S | 233 | 153 |
| HT-G | 329 | 153 |
| Plasma | Urine | |
|---|---|---|
| HT | 0.02 ± 0.01 | 0.01 ± 0.00 |
| Ole | 0.01 ± 0.00 | 0.01 ± 0.00 |
| HT-3-S | 0.05 ± 0.01 | 0.05 ± 0.00 |
| HVA | 0.08 ± 0.02 | 0.14 ± 0.08 |
| DOPAC | 0.25 ± 0.04 | 0.09 ± 0.03 |
| HT-3-G | 0.64 ± 0.22 | 0.05 ± 0.00 |
| HT-3-S | HVA | DOPAC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| IP | Cmax | tmax | AUC | Cmax | tmax | AUC | Cmax | tmax | AUC |
| IP-1 | 384.89 n.s ± 62.63 | 30 | 372 n.s | 868.87 n.s ± 111.86 | 30 | 1019 n.s | 301.07 n.s ± 134.43 | 30 | 169 n.s |
| IP-2 | 406.28 n.s ± 62.68 | 30 | 443 n.s | 948.76 n.s ± 160.19 | 30 | 1672 n.s | 466.96 n.s ± 147.68 | 30 | 247 n.s |
| IP | n | HVA | DOPAC | HT-3-S | HT-4-S | HT-3-G | HT-4-G |
|---|---|---|---|---|---|---|---|
| IP-1 | 12 | 36.01 * ± 20.08 | 44.31 * ± 17.52 | 16.58 * ± 6.0 | 5.32 n.s ± 16.23 | 0.46 * ± 0.35 | 0.15 n.s ± 0.10 |
| IP-2 | 13 | 46.16 * ± 5.37 | 59.74 * ± 3.00 | 20.05 * ± 1.55 | 0.16 n.s ± 0.05 | 0.87 * ± 0.13 | 0.41 * ± 0.10 |
| EVOO | 12 | 4.84 ± 3.93 | 4.53 ± 1.76 | 2.57 ± 1.17 | 0.05 ± 0.19 | 0.06 ± 0.09 | 0.01 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bender, C.; Strassmann, S.; Golz, C. Oral Bioavailability and Metabolism of Hydroxytyrosol from Food Supplements. Nutrients 2023, 15, 325. https://doi.org/10.3390/nu15020325
Bender C, Strassmann S, Golz C. Oral Bioavailability and Metabolism of Hydroxytyrosol from Food Supplements. Nutrients. 2023; 15(2):325. https://doi.org/10.3390/nu15020325
Chicago/Turabian StyleBender, Cecilia, Sarah Strassmann, and Christian Golz. 2023. "Oral Bioavailability and Metabolism of Hydroxytyrosol from Food Supplements" Nutrients 15, no. 2: 325. https://doi.org/10.3390/nu15020325
APA StyleBender, C., Strassmann, S., & Golz, C. (2023). Oral Bioavailability and Metabolism of Hydroxytyrosol from Food Supplements. Nutrients, 15(2), 325. https://doi.org/10.3390/nu15020325






