High Consumption of Sugar-Sweetened Beverages Is Associated with Low Bone Mineral Density in Young People: The Brazilian Birth Cohort Consortium
Abstract
1. Introduction
2. Methods
2.1. Study Design and Sample
2.2. Food and Sugar-Sweetened Beverage Intake
2.3. Bone Mineral Density (BMD)
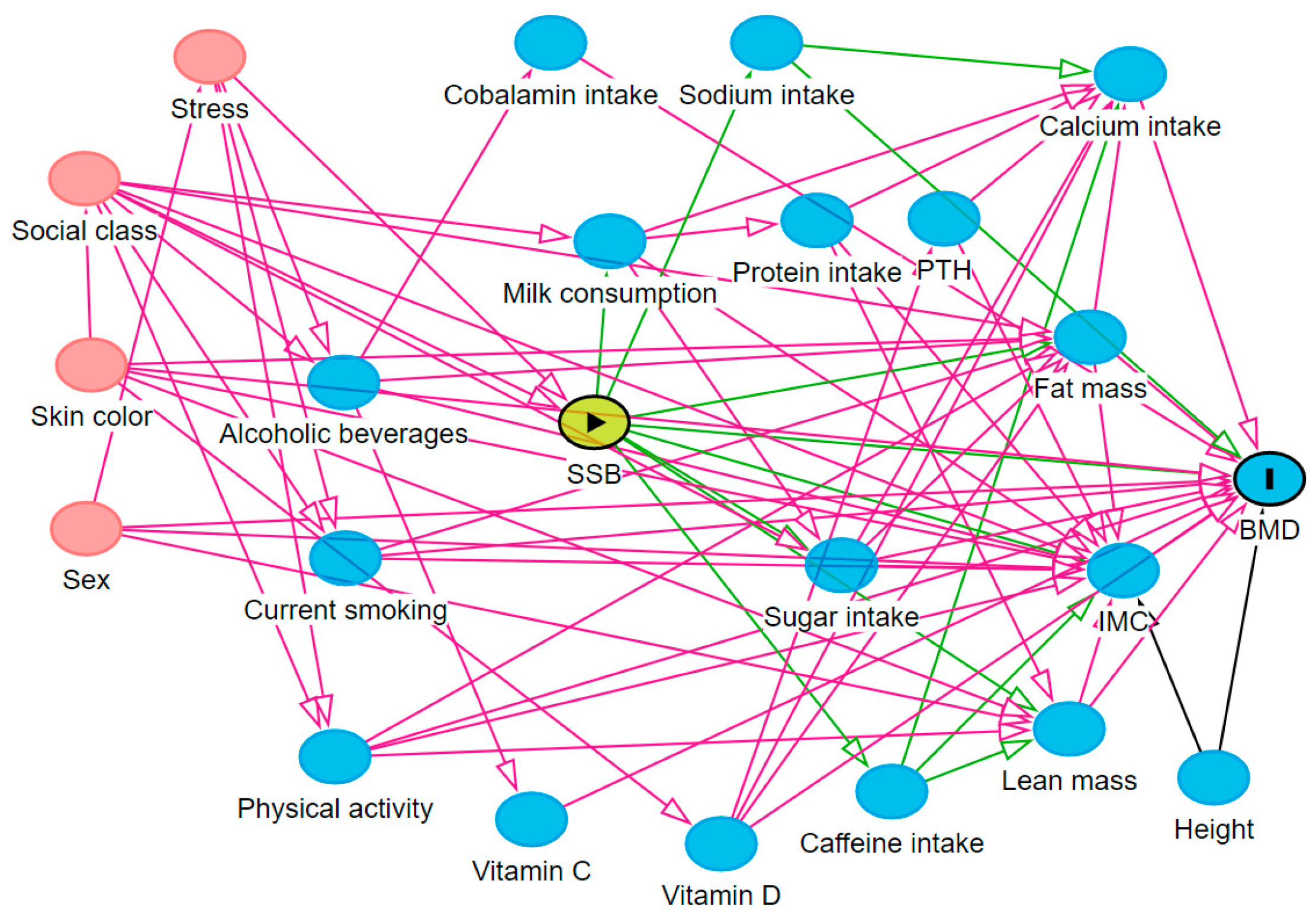
2.4. Theoretical Model
2.5. Covariates
2.6. Statistical Analysis
2.7. Ethical Criteria
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bachrach, L.K.; Gordon, C.M. Bone densitometry in children and adolescents. Pediatrics 2016, 138, e20162398. [Google Scholar] [CrossRef] [PubMed]
- Brandão, C.M.A.; Camargos, B.M.; Zerbini, C.A.; Plapler, P.G.; Mendonça, L.M.C.; Albergaria, B.-H.; Pinheiro, M.M.; Prado, M.d.; Eis, S.R. Posições oficiais 2008 da Sociedade Brasileira de Densitometria Clínica (SBDens). Arq. Bras. De Endocrinol. Metabol. 2009, 53, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Schlüssel, M.M.; Castro, J.A.; Kac, G.; Silva, A.A.; Cardoso, V.C.; Bettiol, H.B.M. Birth weight and bone mass in young adults from Brazil. Bone 2010, 46, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Karpouzos, A.; Diamantis, E.; Farmaki, P.; Savvanis, S.; Troupis, T. Nutritional Aspects of Bone Health and Fracture Healing. J. Osteoporos. 2017, 2017, 4218472. [Google Scholar] [CrossRef] [PubMed]
- Libuda, L.; Alexy, U.; Remer, T.; Stehle, P.; Schoenau, E.; Kersting, M. Association between long-term consumption of soft drinks and variables of bone modeling and remodeling in a sample of healthy German children and adolescents. Am. J. Clin. Nutr. 2008, 88, 1670–1677. [Google Scholar] [CrossRef]
- Rosinger, A.; Herrick, K.; Gahche, J.J.; Park, S. Sugar-sweetened Beverage Consumption Among U.S. Adults, 2011–2014. NCHS Data Brief Natl. Cent. Health Stat. 2017, 271. [Google Scholar]
- Whiting, S.J.; Healey, A.; Psiuk, S.; Mirwald, R.; Kowalski, K.; Bailey, D.A. Relationship between carbonated and other low nutrient dense beverages and bone mineral content of adolescents. Nutr. Res. 2001, 21, 1107–1115. [Google Scholar] [CrossRef]
- Braun, A. Relations among Bone Health Measures and Beverage Intakes during The Bone Building Years. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2011. [Google Scholar]
- Scapin, T.; Fernandes, A.C.; Proença, R.P.C. Added sugars: Definitions, classifications, metabolism and health implications. Rev. De Nutr. 2017, 30, 663–677. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Mehta, V.; Zaman, S.B.; O’Keefe, J.H. Not Salt But Sugar as Aetiological in Osteoporosis: A Review. Mo. Med. 2018, 115, 247–252. [Google Scholar]
- Claro, R.M.; Santos, M.A.S.; Oliveira, T.P.; Pereira, C.A.; Szwarcwald, C.L.; Malta, D.C. Consumo de alimentos não saudáveis relacionados a doenças crônicas não transmissíveis no Brasil: Pesquisa Nacional de Saúde, 2013. Epidemiol. E Serviços De Saúde 2015, 24, 257–265. [Google Scholar] [CrossRef]
- Levy, R.B.; Sichierii, R. Alimentos mais consumidos no Brasil: Inquérito nacional de alimentação 2008–2009. Rev. De Saude Publica 2013, 47, 190s–199s. [Google Scholar]
- Brazil Ministry of Health, Department of Primary Care. Surveillance of Risk and Protective Factors for Chronic Diseases by Telephone Survey; Brazil Ministry of Health, Department of Primary Care: Brasilia, Brazil, 2015. [Google Scholar]
- Confortin, S.C.; Ribeiro, M.R.C.; Barros, A.J.D.; Menezes, A.M.B.; Horta, B.L.; Victora, C.G.; Barros, F.C.; Helen, G.; Bettiol, H.; dos Santos, I.S.; et al. RPS Brazilian Birth Cohorts Consortium (Ribeirão Preto, Pelotas and São Luís): History, Objectives and Methods. Cad. De Saúde Pública 2021, 37. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, H.; Wehrmeister, F.C.; Assunção, M.C.F.; Tovo-Rodrigues, L.; Oliveira, I.O.; Murray, J.; Anselmi, L.; Barros, F.C.; Victora, C.G.; Menezes, A.M.B. Cohort profile update: The 1993 Pelotas (Brazil) Birth Cohort follow-up at 22 years. Int. J. Epidemiol. 2018, 47, 1389–1390e. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, V.C.; Simões, V.M.F.; Barbieri, M.A.; Silva, A.A.M.; Bettiol, H.; Alves, M.T.S.S.B.; Goldani, M.Z. Profile of three Brazilian birth cohort studies in Ribeirão Preto, SP and São Luís, MA. Braz. J. Med. Biol. Res. 2007, 40, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Schneider, B.C.; Motta, J.V.D.S.; Muniz, L.C.; Bielemann, R.M.; Madruga, S.W.; Orlandi, S.P.; Gigante, D.P.; Assunção, M.C.F. Desenho de um questionário de frequência alimentar digital autoaplicado para avaliar o consumo alimentar de adolescentes e adultos jovens: Coortes de nascimentos de Pelotas, Rio Grande do Sul. Rev. Bras. De Epidemiol. 2016, 19, 419–432. [Google Scholar] [CrossRef]
- Vaz, J.S.; Buffarini, R.; Schneider, B.C.; Bielemann, R.M.; Gonçalves, H.A.M. Relative validity of a computer-based semi-quantitative FFQ for use in the Pelotas (Brazil) Birth Cohort Studies. Public Health Nutr. 2021, 24, 34–42. [Google Scholar] [CrossRef]
- Bogea, E.G.; França, A.K.T.C.; Bragança, M.L.B.M.; Vaz, J.S.; Assunção, M.C.; Barbieri, M.A.; Bettiol, H.; Silva, A.A.M. Relative validity of a food frequency questionnaire for adolescents from a capital in the Northeastern region of Brazil. Braz. J. Med. Biol. Res. 2021, 54, 1–9. [Google Scholar] [CrossRef]
- Sousa, R.D.S.; Bragança, M.L.B.M.; Oliveira, B.R.; Coelho, C.C.N.D.S.; Silva, A.A.M.D. Association between the Degree of Processing of consumed foods and sleep quality in adolescents. Nutrients 2020, 12, 462. [Google Scholar] [CrossRef]
- Kroke, A.; Klipstein-Grobusch, K.; Voss, S.; Möseneder, J.; Thielecke, F.; Noack, R.; Boeing, H. Validation of a self-administered food-frequency questionnaire administered in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: Comparison of energy, protein, and macronutrient intakes estimated with the doubly labeled water, urinary nitrogen, and repeated 24-h dietary recall methods. Am. J. Clin. Nutr. 1999, 70, 439–947. [Google Scholar]
- Benzecry, E.; Pinheiro, A.B.; Lacerda, E.M.; Gomes, M.C.C.V. Tabela de Avaliação de Consumo Alimentar em Medidas Caseiras, 5th ed; Editora Atheneu: São Paulo, Brazil, 2005; p. 240. [Google Scholar]
- Núcleo de Estudos e Pesquisas em Alimentação (NEPA/UNICAMP). Tabela Brasileira de Composição de Alimentos, 4th ed.; NEPA-UNICAMP: Campinas, Brazil, 2011. [Google Scholar]
- IBGE Instituto Brasileiro de Geografia e Estatística. Tabela de Composição Nutricional dos Alimentos Consumidos No Brasil; IBGE: Rio de Janeiro, Brazil, 2011. [Google Scholar]
- USDA US Department of Agriculture. Nutrient Database for Standard Reference; US Department of Agriculture: Washington, DC, USA, 2011.
- Textor, J.; Hardt, J.K.S. DAGitty: A graphical tool for analyzing causal diagrams. Epidemiology 2011, 22, 745. [Google Scholar] [CrossRef] [PubMed]
- Cortes, T.R.; Faerstein, E.; Struchiner, C.J. Use of causal diagrams in epidemiology: Application to a situation with confounding. Cad. De Saude Publica 2016, 32, e00103115. [Google Scholar]
- Robins, J.M.; Hernán, M.Á.; Brumback, B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000, 11, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Werneck, G.L. Diagramas causais: A epidemiologia Brasileira de volta para o futuro. Cad. De Saude Publica 2016, 32, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Brasil. Critério de Classificação Econômica Brasil—CCEB; Associação Brasileira de Empresas de Pesquisa: São Paulo, Brazil, 2015. [Google Scholar]
- Sallis, J.F.; Strikmiller, P.K.; Harsha, D.W.; Feldman, H.A.; Ehlinger, S.; Stone, E.J.; Williston, J.W.S. Validation of interviewer-and self-administered physical activity checklists for fifth grade students. Med. Sci. Sport Exerc. 1996, 28, 840–851. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-Country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Fairchild, A.J.; McDaniel, H.L. Statistical Commentary Best (but oft-forgotten) practices: Mediation analysis 1,2. Am. J. Clin. Nutr. 2017, 105, 1259–1271. [Google Scholar]
- van Leeuwen, J.; Koes, B.W.; Paulis, W.D.; van Middelkoop, M. Differences in bone mineral density between normal-weight children and children with overweight and obesity: A systematic review and meta-analysis. Obes. Rev. 2017, 18, 526–546. [Google Scholar] [CrossRef]
- Kline, R. Principles and Practice of Structural Equation Modeling, 4th ed.; Guilford Publications: New York, NY, USA, 2016. [Google Scholar]
- Xue, S.; Kemal, O.; Lu, M.; Lix, L.M.; Leslie, W.D.; Yang, S. Age at attainment of peak bone mineral density and its associated factors: The National Health and Nutrition Examination Survey 2005–2014. Bone 2020, 131, 115163. [Google Scholar] [CrossRef]
- Akinlawon, O.; Noel, S.; Flanagan, K.; Zhang, X.T.K.T. The Association Between Sugar Sweetened Beverages and Bone Health Among Older Puerto Rican Adults. Curr. Dev. Nutr. 2020, 4, 1373. [Google Scholar] [CrossRef]
- Bukhar, H.M.; Nada, I.S.; Header, E.A. Effect of Obesity and Dietary Factors on Bone Mineral Density Levels Among Female Students in Umm Al-Qura University. Egypt J. Hosp. Med. 2012, 49, 678–691. [Google Scholar] [CrossRef]
- Tsanzi, E.; Light, H.R.; Tou, J.C. The effect of feeding different sugar-sweetened beverages to growing female Sprague-Dawley rats on bone mass and strength. Bone 2008, 42, 960–968. [Google Scholar] [CrossRef]
- Yoon, V.; Adams-Huet, B.; Sakhaee, K.M.N. Hyperinsulinemia and urinary calcium excretion in calcium stone formers with idiopathic hypercalciuria. J. Clin. Endocrinol. Metab. 2013, 98, 2589–2594. [Google Scholar] [CrossRef] [PubMed]
- Terada, M.; Inaba, M.; Yano, Y.; Hasuma, T.; Nishizawa, Y.; Morii, H.; Otani, S. Growth-inhibitory effect of a high glucose concentration on osteoblast-like cells. Bone 1998, 22, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Lana, A.; Rodríguez-Artalejo, F.; Lopez-Garcia, E. Consumption of sugar-sweetened beverages is positively related to insulin resistance and higher plasma leptin concentrations in men and nonoverweight women. J. Nutr. 2014, 144, 1099–1105. [Google Scholar] [CrossRef]
- Barzel, U.S. The skeleton as an ion exchange system: Implications for the role of acid-base imbalance in the genesis of osteoporosis. J. Bone Miner. Res. 1995, 10, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Caliceti, C.; Calábria, D.; Roda, A.; Cicero, A. Frutose Intake, Soro Úrico Acid, and Cardiometabolic Disorders: A Critical Review. Nutrients 2017, 9, 395. [Google Scholar] [CrossRef]
- Willet, W.C. Nutritional Epidemiology; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
| Variable | n | % |
|---|---|---|
| Age in years | ||
| 18 | 1640 | 24.8 |
| 19 | 753 | 11.4 |
| 21 | 134 | 2.0 |
| 22 | 3241 | 48.9 |
| 23 | 852 | 12.9 |
| Sex | ||
| Male | 3060 | 46.2 |
| Female | 3560 | 53.8 |
| Socioeconomic class * | ||
| A | 507 | 7.7 |
| B | 2521 | 38.1 |
| C | 2737 | 41.3 |
| D/E | 384 | 5.8 |
| Unknown | 471 | 7.1 |
| Current smoking | ||
| No | 5901 | 89.1 |
| Yes | 719 | 10.9 |
| Current alcohol consumption | ||
| No | 2356 | 35.6 |
| Yes | 4060 | 61.3 |
| Unknown | 204 | 3.1 |
| Physical activity | ||
| Insufficiently active | 2424 | 36.6 |
| Active | 4196 | 63.4 |
| Total | 6620 | 100.0 |
| Variable | n | Minimum | Maximum | Mean | SD |
|---|---|---|---|---|---|
| Total body BMD (g/cm2) | 6620 | 0.7 | 1.7 | 1.2 | 0.1 |
| Lumbar spine BMD (g/cm2) | 6620 | 0.6 | 1.8 | 1.2 | 0.1 |
| Daily SSB consumption frequency | 6620 | 0 | 16.7 | 1.6 | 1.7 |
| 1st tertile | 2253 | 0 | 0.6 | 0.3 | 0.3 |
| 2nd tertile | 2179 | 0.7 | 2.0 | 1.2 | 0.6 |
| 3rd tertile | 2188 | 2.1 | 16.7 | 3.4 | 2.0 |
| Daily SSB consumption (mL/day) | 6620 | 0 | 3862.5 | 281.5 | 374.1 |
| 1st tertile | 2212 | 0 | 83.2 | 39.9 | 34.7 |
| 2nd tertile | 2205 | 83.4 | 275.5 | 176.6 | 82.1 |
| 3rd tertile | 2203 | 275.6 | 3862.5 | 629.0 | 471.5 |
| Daily energy contribution of SSB (%) | 6620 | 0 | 25.7 | 5.9 | 5.7 |
| 1st tertile | 2207 | 0 | 2.4 | 1.5 | 1.5 |
| 2nd tertile | 2207 | 2.5 | 6.8 | 4.8 | 2.6 |
| 3rd tertile | 2206 | 6.9 | 25.7 | 11.4 | 6.2 |
| Daily Consumption Frequency | Total Body Bone Mineral Density | Lumbar Spine Bone Mineral Density | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tertile | n | Number of | βa | 95% CI | p | Standardized | βa | 95% CI | p | Standardized |
| Times | βb | βb | ||||||||
| Unadjusted analysis | ||||||||||
| 1st | 2253 | 0 to 0.6 | Ref. | - | - | - | Ref. | - | - | - |
| 2nd | 2179 | 0.7 to 2.0 | 0.005 | −0.002; 0.011 | 0.159 | 0.02 | 0 | −0.007; 0.008 | 0.913 | 0.001 |
| 3rd | 2188 | 2.1 to 16.7 | −0.003 | −0.009; 0.004 | 0.406 | −0.120 | −0.009 | −0.017; −0.001 | 0.032 | −0.030 |
| Adjusted analysis c | ||||||||||
| 1st | 2253 | 0 to 0.6 | Ref. | - | - | - | Ref. | - | - | - |
| 2nd | 2179 | 0.7 to 2.0 | −0.001 | −0.007; 0.005 | 0.689 | −0.005 | −0.001 | −0.009; 0.007 | 0.781 | 0.004 |
| 3rd | 2188 | 2.1 to 16.7 | −0.009 | −0.014; −0.003 | 0.005 | −0.036 | −0.012 | −0.021; −0.004 | 0.003 | −0.043 |
| Adjusted analysis d | ||||||||||
| 1st | 2253 | 0 to 0.6 | Ref. | - | - | - | Ref. | - | - | - |
| 2nd | 2179 | 0.7 to 2.0 | −0.001 | −0.005; 0.005 | 0.971 | −0.001 | −0.000 | −0.008; 0.008 | 0.975 | −0.001 |
| 3rd | 2188 | 2.1 to 16.7 | −0.004 | −0.009; 0.001 | 0.131 | −0.017 | −0.008 | −0.016; −0.001 | 0.036 | −0.030 |
| Daily Consumption (mL/day) | Total Body Bone Mineral Density | Lumbar Spine Bone Mineral Density | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tertile | n | mL/day | βa | 95% CI | p | Standardized β b | βa | 95% CI | p | Standardized β b |
| Unadjusted analysis | ||||||||||
| 1st | 2212 | 0 to 83.2 | Ref. | - | - | - | Ref. | - | - | - |
| 2nd | 2205 | 83.4 to 275.5 | 0.006 | −0.001; 0.013 | 0.073 | 0.025 | −0.002 | −0.010; 0.006 | 0.647 | −0.006 |
| 3rd | 2203 | 275.6 to 3862.5 | 0.008 | −0.002; 0.015 | 0.072 | 0.035 | 0.001 | −0.007; 0.009 | 0.726 | 0.005 |
| Adjusted analysis c | ||||||||||
| 1st | 2212 | 0 to 83.2 | Ref. | - | - | - | Ref. | - | - | - |
| 2nd | 2205 | 83.4 to 275.5 | −0.001 | −0.007; 0.005 | 0.793 | −0.003 | −0.003 | −0.011; 0.005 | 0.477 | −0.010 |
| 3rd | 2203 | 275.6 to 3862.5 | −0.004 | −0.011; 0.002 | 0.159 | −0.018 | −0.004 | −0.012; 0.004 | 0.362 | −0.014 |
| Adjusted analysis d | ||||||||||
| 1st | 2212 | 0 to 83.2 | Ref. | - | - | - | Ref. | - | - | - |
| 2nd | 2205 | 83.4 to 275.5 | −0.001 | −0.006; 0.004 | 0.778 | −0.003 | −0.003 | −0.011; 0.005 | 0.474 | −0.010 |
| 3rd | 2203 | 275.6 to 3862.5 | −0.003 | −0.008; 0.002 | 0.286 | −0.012 | −0.003 | −0.011; 0.005 | 0.488 | −0.010 |
| Daily Energy Contribution (%) | Total Body Bone Mineral Density | Lumbar Spine Bone Mineral Density | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tertile | n | % | βa | 95% CI | p Value | Standardized | βa | 95% CI | p | Standardized |
| βb | βb | |||||||||
| Unadjusted analysis | ||||||||||
| 1st | 2207 | 0 to 2.4 | Ref. | - | - | - | Ref. | - | - | - |
| 2nd | 2207 | 2.5 to 6.8 | 0.006 | −0.000; 0.013 | 0.052 | 0.028 | 0.001 | −0.007; 0.008 | 0.877 | 0.002 |
| 3rd | 2206 | 6.9 to 25.7 | 0.004 | −0.002; 0.011 | 0.202 | 0.018 | −0.001 | −0.009; 0.007 | 0.84 | −0.003 |
| Adjusted analysis c | ||||||||||
| 1st | 2207 | 0 to 2.4 | Ref. | - | - | - | Ref. | - | - | - |
| 2nd | 2207 | 2.5 to 6.8 | 0 | −0.006; 0.006 | 0.929 | −0.001 | 0 | −0.008; 0.008 | 0.937 | 0.001 |
| 3rd | 2206 | 6.9 to 25.7 | 0.002 | −0.018; 0.004 | 0.46 | −0.010 | −0.002 | −0.010; 0.006 | 0.66 | −0.006 |
| Adjusted analysis d | ||||||||||
| 1st | 2207 | 0 to 2.4 | Ref. | - | - | - | Ref. | - | - | - |
| 2nd | 2207 | 2.5 to 6.8 | 0 | −0.005; 0.006 | 0.835 | 0.002 | 0.001 | −0.007; 0.009 | 0.804 | 0.003 |
| 3rd | 2206 | 6.9 to 25.7 | −0.002 | −0.007; 0.003 | 0.415 | −0.009 | −0.002 | −0.010; 0.006 | 0.645 | −0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bragança, M.L.B.M.; Bogea, E.G.; de Almeida Fonseca Viola, P.C.; dos Santos Vaz, J.; Confortin, S.C.; Menezes, A.M.B.; Gonçalves, H.; Bettiol, H.; Barbieri, M.A.; Cardoso, V.C.; et al. High Consumption of Sugar-Sweetened Beverages Is Associated with Low Bone Mineral Density in Young People: The Brazilian Birth Cohort Consortium. Nutrients 2023, 15, 324. https://doi.org/10.3390/nu15020324
Bragança MLBM, Bogea EG, de Almeida Fonseca Viola PC, dos Santos Vaz J, Confortin SC, Menezes AMB, Gonçalves H, Bettiol H, Barbieri MA, Cardoso VC, et al. High Consumption of Sugar-Sweetened Beverages Is Associated with Low Bone Mineral Density in Young People: The Brazilian Birth Cohort Consortium. Nutrients. 2023; 15(2):324. https://doi.org/10.3390/nu15020324
Chicago/Turabian StyleBragança, Maylla Luanna Barbosa Martins, Eduarda Gomes Bogea, Poliana Cristina de Almeida Fonseca Viola, Juliana dos Santos Vaz, Susana Cararo Confortin, Ana Maria Baptista Menezes, Helen Gonçalves, Heloisa Bettiol, Marco Antonio Barbieri, Viviane Cunha Cardoso, and et al. 2023. "High Consumption of Sugar-Sweetened Beverages Is Associated with Low Bone Mineral Density in Young People: The Brazilian Birth Cohort Consortium" Nutrients 15, no. 2: 324. https://doi.org/10.3390/nu15020324
APA StyleBragança, M. L. B. M., Bogea, E. G., de Almeida Fonseca Viola, P. C., dos Santos Vaz, J., Confortin, S. C., Menezes, A. M. B., Gonçalves, H., Bettiol, H., Barbieri, M. A., Cardoso, V. C., & da Silva, A. A. M. (2023). High Consumption of Sugar-Sweetened Beverages Is Associated with Low Bone Mineral Density in Young People: The Brazilian Birth Cohort Consortium. Nutrients, 15(2), 324. https://doi.org/10.3390/nu15020324







