Evaluation of Nutritional Interventions in the Care Plan for Cancer Patients: The NOA Project
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
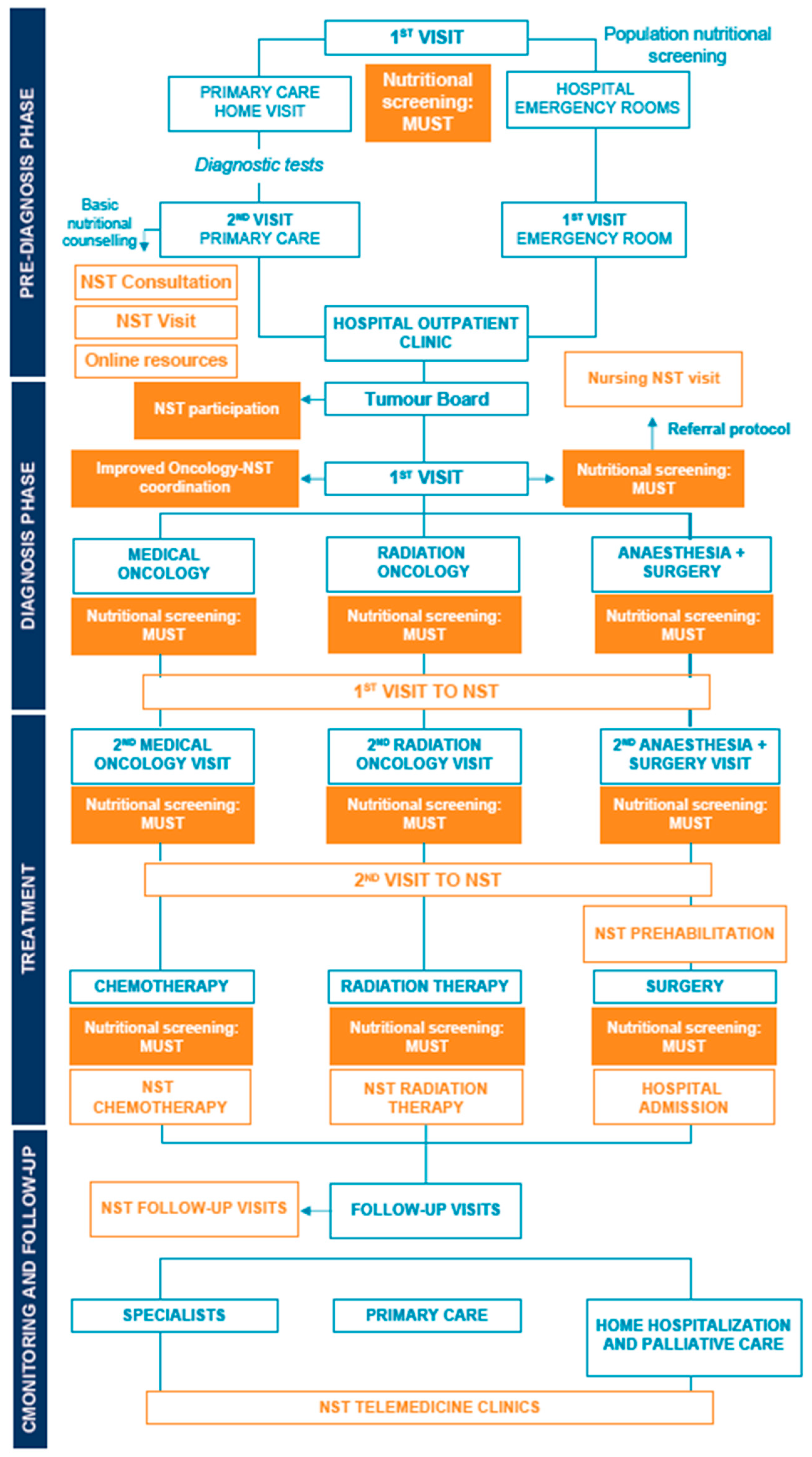
2.2. Methodology and Project Phases
2.3. Indicators of Success
2.3.1. In-Hospital Nutritional Screening
2.3.2. Nutrition Support Team Participation on Tumour Boards
2.3.3. Indicator Collection
3. Results
3.1. Improvement Ideas
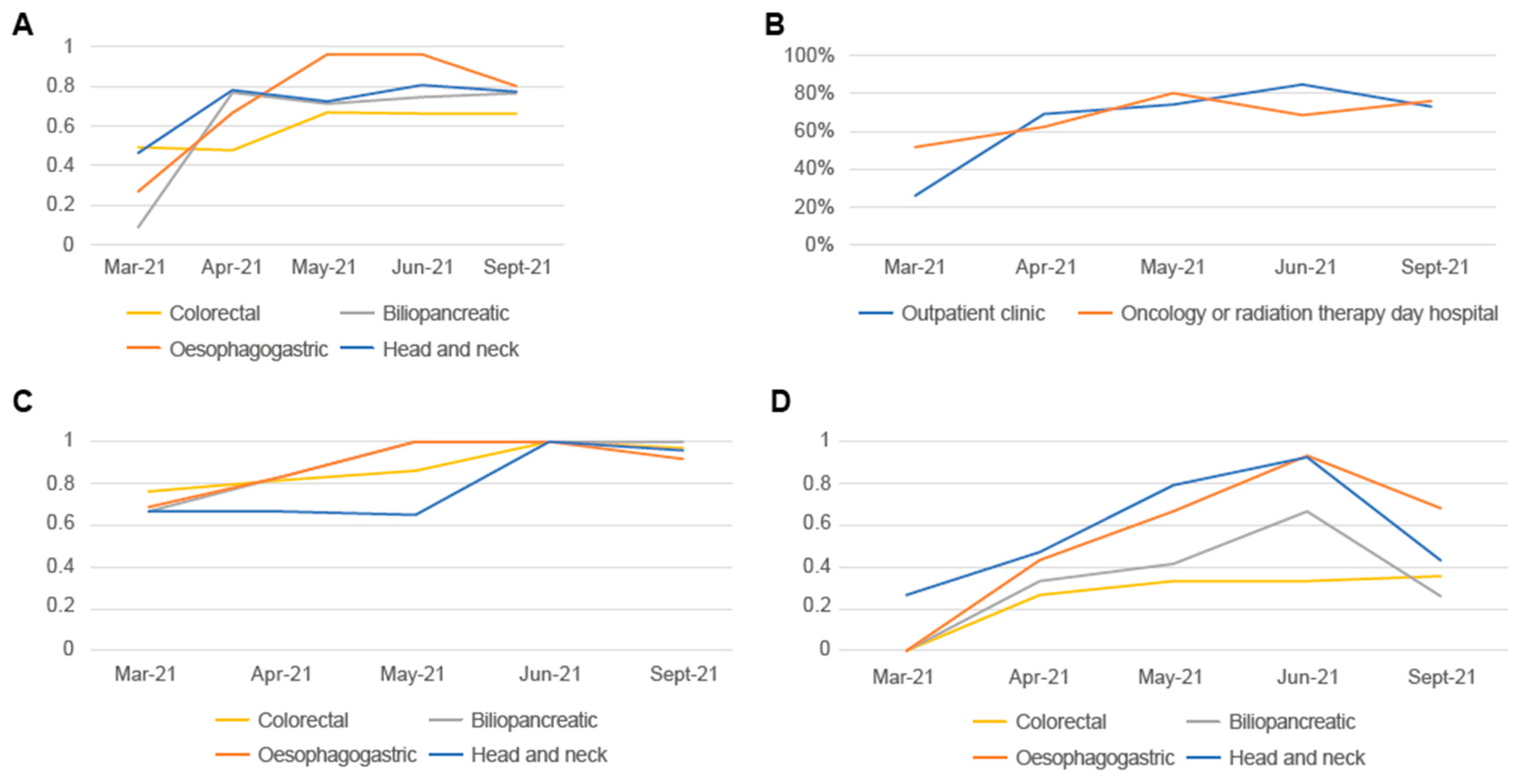
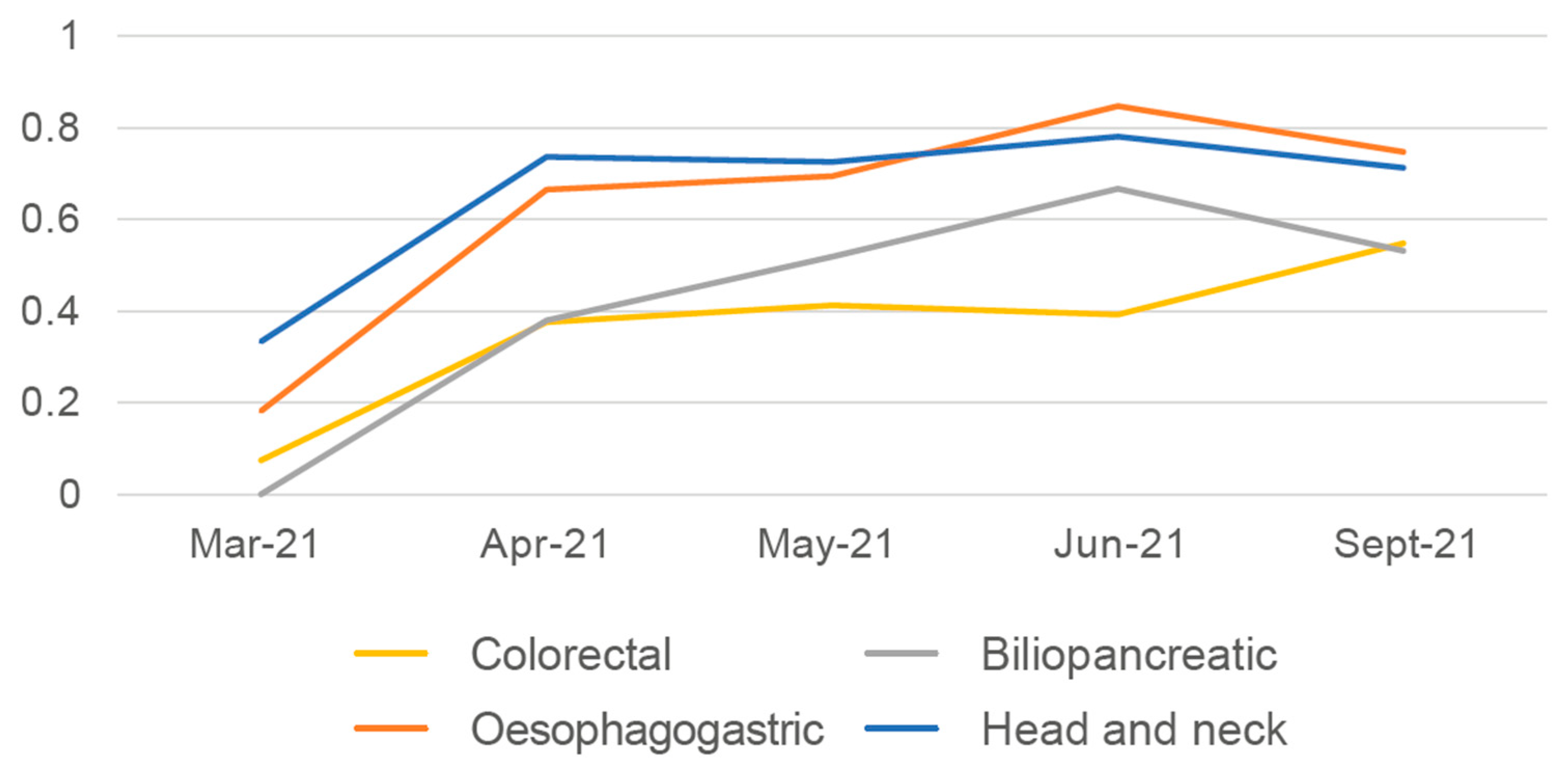
3.2. Results of Implementation in Clinical Practice of Proposals for Improvement with the Highest Impact/Feasibility Ratio
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Virizuela, J.A.; Camblor-Alvarez, M.; Luengo-Perez, L.M.; Grande, E.; Alvarez-Hernandez, J.; Sendros-Madrono, M.J.; Jimenez-Fonseca, P.; Cervera-Peris, M.; Ocon-Breton, M.J. Nutritional support and parenteral nutrition in cancer patients: An expert consensus report. Clin. Transl. Oncol. 2018, 20, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Yeom, E.; Yu, K. Understanding the molecular basis of anorexia and tissue wasting in cancer cachexia. Exp. Mol. Med. 2022, 54, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Argiles, J.M. Cancer-associated malnutrition. Eur. J. Oncol. Nurs. 2005, 9 (Suppl. 2), S39–S50. [Google Scholar] [CrossRef] [PubMed]
- Chow, R.; Bruera, E.; Chiu, L.; Chow, S.; Chiu, N.; Lam, H.; McDonald, R.; DeAngelis, C.; Vuong, S.; Ganesh, V.; et al. Enteral and parenteral nutrition in cancer patients: A systematic review and meta-analysis. Ann. Palliat. Med. 2016, 5, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Torralvo, F.J.; Gonzalez-Poveda, I.; Garcia-Olivares, M.; Porras, N.; Gonzalo-Marin, M.; Tapia, M.J.; Mera-Velasco, S.; Toval-Mata, J.A.; Ruiz-Lopez, M.; Carrasco-Campos, J.; et al. Poor Physical Performance Is Associated with Postoperative Complications and Mortality in Preoperative Patients with Colorectal Cancer. Nutrients 2022, 14, 1484. [Google Scholar] [CrossRef]
- Sanchez-Torralvo, F.J.; Contreras-Bolivar, V.; Ruiz-Vico, M.; Abuin-Fernandez, J.; Gonzalez-Almendros, I.; Barrios, M.; Olveira, G. Relationship between malnutrition and the presence of symptoms of anxiety and depression in hospitalized cancer patients. Support Care Cancer 2022, 30, 1607–1613. [Google Scholar] [CrossRef]
- Contreras-Bolivar, V.; Sanchez-Torralvo, F.J.; Ruiz-Vico, M.; Gonzalez-Almendros, I.; Barrios, M.; Padin, S.; Alba, E.; Olveira, G. GLIM Criteria Using Hand Grip Strength Adequately Predict Six-Month Mortality in Cancer Inpatients. Nutrients 2019, 11, 2043. [Google Scholar] [CrossRef]
- Ruiz-Garcia, I.; Contreras-Bolivar, V.; Sanchez-Torralvo, F.J.; Ulloa-Diaz, O.; Ruiz-Vico, M.; Abuin-Fernandez, J.; Barrios-Garcia, M.; Alba-Conejo, E.; Olveira, G. The economic cost of not coding disease-related malnutrition: A study in cancer inpatients. Clin. Nutr. 2022, 41, 186–191. [Google Scholar] [CrossRef]
- Planas, M.; Alvarez-Hernandez, J.; Leon-Sanz, M.; Celaya-Perez, S.; Araujo, K.; Garcia de Lorenzo, A. Prevalence of hospital malnutrition in cancer patients: A sub-analysis of the PREDyCES(R) study. Support Care Cancer 2016, 24, 429–435. [Google Scholar] [CrossRef]
- De Las Penas, R.; Majem, M.; Perez-Altozano, J.; Virizuela, J.A.; Cancer, E.; Diz, P.; Donnay, O.; Hurtado, A.; Jimenez-Fonseca, P.; Ocon, M.J. SEOM clinical guidelines on nutrition in cancer patients (2018). Clin. Transl. Oncol. 2019, 21, 87–93. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hutterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- European Commission. Europe’s Beating Cancer Plan. Available online: https://ec.europa.eu/health/system/files/2022-02/eu_cancer-plan_en_0.pdf (accessed on 10 November 2022).
- Vienna Declaration: Nutritional Care Is a Human Right. Available online: https://www.espen.org/files/Vienna-Declaration-2022.pdf (accessed on 10 November 2022).
- Ravasco, P. Nutrition in Cancer Patients. J. Clin. Med. 2019, 8, 1211. [Google Scholar] [CrossRef]
- Lluch Taltavull, J.I.; Mercadal Orfila, G.; Afonzo Gobbi, Y.S. Improvement of the nutritional status and quality of life of cancer patients through a protocol of evaluation and nutritional intervention. Nutr. Hosp. 2018, 35, 606–611. [Google Scholar] [CrossRef]
- Richards, J.; Arensberg, M.B.; Thomas, S.; Kerr, K.W.; Hegazi, R.; Bastasch, M. Impact of Early Incorporation of Nutrition Interventions as a Component of Cancer Therapy in Adults: A Review. Nutrients 2020, 12, 3403. [Google Scholar] [CrossRef]
- Reber, E.; Schonenberger, K.A.; Vasiloglou, M.F.; Stanga, Z. Nutritional Risk Screening in Cancer Patients: The First Step Toward Better Clinical Outcome. Front. Nutr. 2021, 8, 603936. [Google Scholar] [CrossRef]
- Elia, M.; Zellipour, L.; Stratton, R.J. To screen or not to screen for adult malnutrition? Clin. Nutr. 2005, 24, 867–884. [Google Scholar] [CrossRef]
- Duran-Poveda, M.; Jimenez-Fonseca, P.; Sirvent-Ochando, M.; Garcia-Luna, P.P.; Pereira-Cunill, J.L.; Lema-Marques, B.; Parejo-Arrondo, M.T.; Belda-Iniesta, C. Integral nutritional approach to the care of cancer patients: Results from a Delphi panel. Clin. Transl. Oncol. 2018, 20, 1202–1211. [Google Scholar] [CrossRef]
- Garcia Luna, P.P.; Calanas Continente, A.; Villarrubia Pozo, A.; Jimenez Lorente, C.P.; Vicente Baz, D.; Castanedo, O.I.; Salvador Bofill, J.; Rabat Restrepo, J.M.; Diaz Gomez, L.; Mediano Rambla, M.D.; et al. Analysis of nutritional interventions in the care process of oncological patients in Andalusia—The NOA project. Nutr. Hosp. 2021, 38, 758–764. [Google Scholar] [CrossRef]
- Plan Integral de Oncología de Andalucía. Available online: https://www.juntadeandalucia.es/export/drupaljda/salud_5af06533780a3_plan_oncologia_2007_12.pdf (accessed on 10 November 2022).
- Proceso de Soporte: Nutrición Clínica y Dietética. Available online: https://www.juntadeandalucia.es/export/drupaljda/salud_5af19571d66b8_proceso_soporte_nutricion.pdf (accessed on 10 November 2022).
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 207–217. [Google Scholar] [CrossRef]
- Stratton, R.J.; Hackston, A.; Longmore, D.; Dixon, R.; Price, S.; Stroud, M.; King, C.; Elia, M. Malnutrition in hospital outpatients and inpatients: Prevalence, concurrent validity and ease of use of the ‘malnutrition universal screening tool’ (‘MUST’) for adults. Br. J. Nutr. 2004, 92, 799–808. [Google Scholar] [CrossRef]
- Hettiarachchi, J.; Madubhashini, P.; Miller, M. Agreement between the Malnutrition Universal Screening Tool and the Patient-Generated Subjective Global Assessment for Cancer Outpatients Receiving Chemotherapy: A Cross-Sectional Study. Nutr. Cancer 2018, 70, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Bole, O.; Tome, C.; Monteiro-Grillo, I.; Camilo, M.; Ravasco, P. Validation of the Malnutrition Universal Screening Tool (MUST) in cancer. Br. J. Nutr. 2012, 108, 343–348. [Google Scholar] [CrossRef]
- Borges, N.P.; Silva, B.D.A.; Cohen, C.; Filho, P.E.P.; Medeiros, F.J. Comparison of the nutritional diagnosis obtained through different methods and indicators in patients with cancer. Nutr. Hosp. 2009, 24, 51–55. [Google Scholar]
- Leuenberger, M.; Kurmann, S.; Stanga, Z. Nutritional screening tools in daily clinical practice: The focus on cancer. Support Care Cancer 2010, 18 (Suppl. 2), S17–S27. [Google Scholar] [CrossRef] [PubMed]
- Stratton, R.J.; King, C.L.; Stroud, M.A.; Jackson, A.A.; Elia, M. ‘Malnutrition Universal Screening Tool’ predicts mortality and length of hospital stay in acutely ill elderly. Br. J. Nutr. 2006, 95, 325–330. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Meng, L.; Wei, J.; Ji, R.; Wang, B.; Xu, X.; Xin, Y.; Jiang, X. Effect of Early Nutrition Intervention on Advanced Nasopharyngeal Carcinoma Patients Receiving Chemoradiotherapy. J. Cancer 2019, 10, 3650–3656. [Google Scholar] [CrossRef]
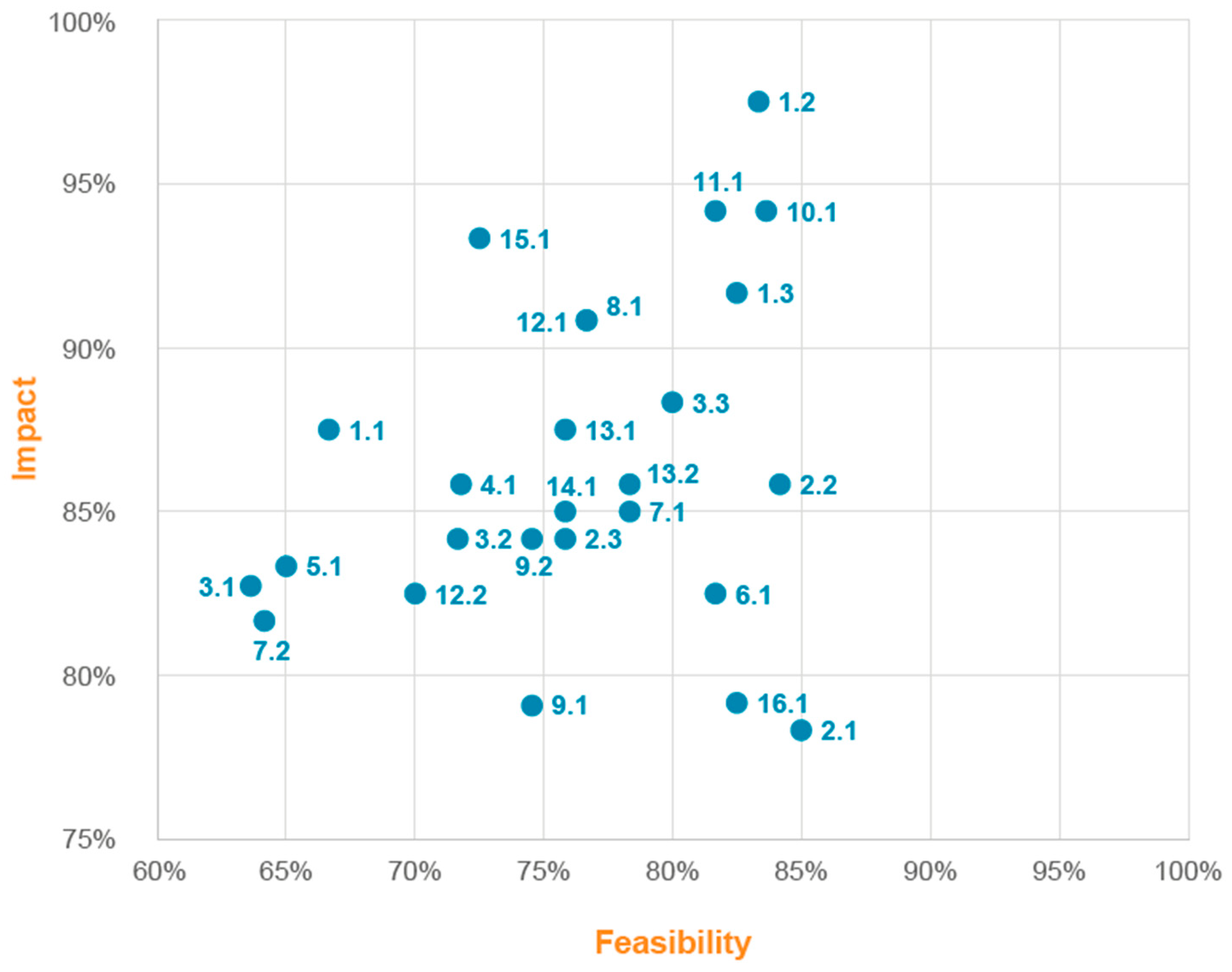
| Opportunity for Improvement | Description of the Improvement Idea | Obstacles | Phase | ||
|---|---|---|---|---|---|
| 1 | No nutritional screening by family doctor, specialists, or oncologists | 1.1 | Nutritional screening in primary care | Time and human resources | Pre-diagnosis, diagnosis |
| 1.2 | In-hospital nutritional screening | Time and human resources | Pre-diagnosis, diagnosis, treatment | ||
| 1.3 | Nutritional screening training | Time and human resources | Diagnosis, treatment, monitoring, and follow-up | ||
| 2 | Inadequate documentation, nutritional information, and existing resources and support services for patients and relatives | 2.1 | Patient information materials | Necessary materials, technology, time, and economic investment | Treatment, monitoring, and follow-up |
| 2.2 | Basic nutritional counselling | Time and human resources | Pre-diagnosis, diagnosis, treatment, monitoring, and follow-up | ||
| 2.3 | Advanced nutritional counselling | Time and human resources | Diagnosis, treatment, monitoring, and follow-up | ||
| 3 | Lack of nutritional training for patients and caregivers | 3.1 | Unreliable nutrition information resource alert system | Technology, time, and legal aspects | Pre-diagnosis, diagnosis, treatment, monitoring, and follow-up |
| 3.2 | Nutritional training for patients and relatives | Time and human resources | Diagnosis | ||
| 3.3 | Follow-up of patient nutritional status | Time and human resources | Treatment, monitoring, and follow-up | ||
| 4 | Inadequate involvement of family doctors in the nutrition of cancer patients | 4.1 | Improve training of primary care professionals on nutrition in cancer patients | Time and human resources | Pre-diagnosis |
| 5 | Inadequate cancer-specific psychological support for cancer patients and relatives, related to nutrition | 5.1 | Enhance cancer-specific psychological support in relation to nutritional status | Human resources | |
| 6 | Excessive paperwork for nutritional supplements | 6.1 | Simplify prescription procedures for nutritional supplements | Legal aspects | Diagnosis, treatment |
| 7 | Inadequate financial and social support for nutrition for patients and caregivers | 7.1 | Review the indications of HEN covered by the NHS | Legal aspects and human resources | Monitoring and follow-up |
| 7.2 | Referrals to social worker as food and nutrition support in cancer patients | Economic investment and human resources | |||
| 8 | Inadequate nutritional assessment and patient preparation prior to radiation therapy and chemotherapy | 8.1 | Improve nutritional assessment and preparation prior to cancer treatment | Time and human resources | Pre-diagnosis, diagnosis |
| 9 | Inadequate pre-habilitation before surgery (with major variability between centres) | 9.1 | Increase the surgical and anaesthesia teams’ understanding of nutrition | Time and human resources | Diagnosis, treatment |
| 9.2 | Incorporate coadjuvant nutritional measures before cancer surgery | Time, economic investment, and human resources | |||
| 10 | Non-participation of the NST on and lack of nutritional protocols for the tumour board. | 10.1 | NST participation on hospital tumour boards | Time and human resources | Diagnosis, treatment |
| 11 | Improve protocol for referral to NST | 11.1 | Improve coordination between the oncology department and the NST | Time and human resources | Diagnosis, treatment, monitoring, and follow-up |
| 12 | Inadequate access and communication between the NST and professionals, PCU and home hospitalisation | 12.1 | Improve coordination between PCU, home hospitalisation and NST | Time and human resources | Monitoring and follow-up |
| 12.2 | Implement telemedicine in all its versions | Economic investment and technological resources | Treatment, monitoring, and follow-up | ||
| 13 | Inadequate specific nutrition consultations during treatment (in-person or telephone) for patients | 13.1 | Raise awareness of nutrition | Time and human resources | Pre-diagnosis, diagnosis, treatment, monitoring and follow-up |
| 13.2 | Improve accessibility and care provision in the NST | Time, technology, and human resources | Pre-diagnosis | ||
| 14 | More accessible clinical history | 14.1 | Specific section on nutrition in the electronic clinical history of the DIRAYA | Technology | Pre-diagnosis, diagnosis, treatment, monitoring, and follow-up |
| 15 | Lack of exercise and rehabilitation programs | 15.1 | Prescription of exercise as coadjuvant to nutrition | Economic investment and human resources | Diagnosis, treatment, monitoring, and follow-up |
| 16 | Improved organoleptic characteristics of preparations and supplements | 16.1 | Different nutritional supplement forms | Economic investment and technological resources | Monitoring and follow-up |
| 17 | Parenteral nutrition. | 17.1 | Parenteral nutrition support | Not evaluated | |
| 18 | Enteral nutrition. | 18.1 | Nutritional support via the enteral route | Not evaluated | Diagnosis, treatment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Luna, P.P.; Rabat Restrepo, J.M.; Muñoz-Ayllón, M.; de la Calle Gil, M.; Remón, P.; Sánchez-Torralvo, F.J.; Pachón, J.; García-González, J.J.; García-Manrique, T.; Salvador-Bofill, J.; et al. Evaluation of Nutritional Interventions in the Care Plan for Cancer Patients: The NOA Project. Nutrients 2023, 15, 292. https://doi.org/10.3390/nu15020292
García-Luna PP, Rabat Restrepo JM, Muñoz-Ayllón M, de la Calle Gil M, Remón P, Sánchez-Torralvo FJ, Pachón J, García-González JJ, García-Manrique T, Salvador-Bofill J, et al. Evaluation of Nutritional Interventions in the Care Plan for Cancer Patients: The NOA Project. Nutrients. 2023; 15(2):292. https://doi.org/10.3390/nu15020292
Chicago/Turabian StyleGarcía-Luna, Pedro Pablo, Juana M. Rabat Restrepo, Marta Muñoz-Ayllón, Milagros de la Calle Gil, Pablo Remón, Francisco José Sánchez-Torralvo, Jerónimo Pachón, Juan J. García-González, Teresa García-Manrique, Javier Salvador-Bofill, and et al. 2023. "Evaluation of Nutritional Interventions in the Care Plan for Cancer Patients: The NOA Project" Nutrients 15, no. 2: 292. https://doi.org/10.3390/nu15020292
APA StyleGarcía-Luna, P. P., Rabat Restrepo, J. M., Muñoz-Ayllón, M., de la Calle Gil, M., Remón, P., Sánchez-Torralvo, F. J., Pachón, J., García-González, J. J., García-Manrique, T., Salvador-Bofill, J., Vicente, D., & Olveira, G. (2023). Evaluation of Nutritional Interventions in the Care Plan for Cancer Patients: The NOA Project. Nutrients, 15(2), 292. https://doi.org/10.3390/nu15020292







