Integrating Baseline Nutritional and Inflammatory Parameters with Post-Treatment EBV DNA Level to Predict Outcomes of Patients with De Novo Metastatic Nasopharyngeal Carcinoma Receiving Chemotherapy Combination PD-1 Inhibitor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Extraction and Study Population
2.2. Data Collection and Classification
2.3. Treatments
2.4. Endpoints and Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics and Treatment Outcomes
3.2. Factors Associated with Disease Progression
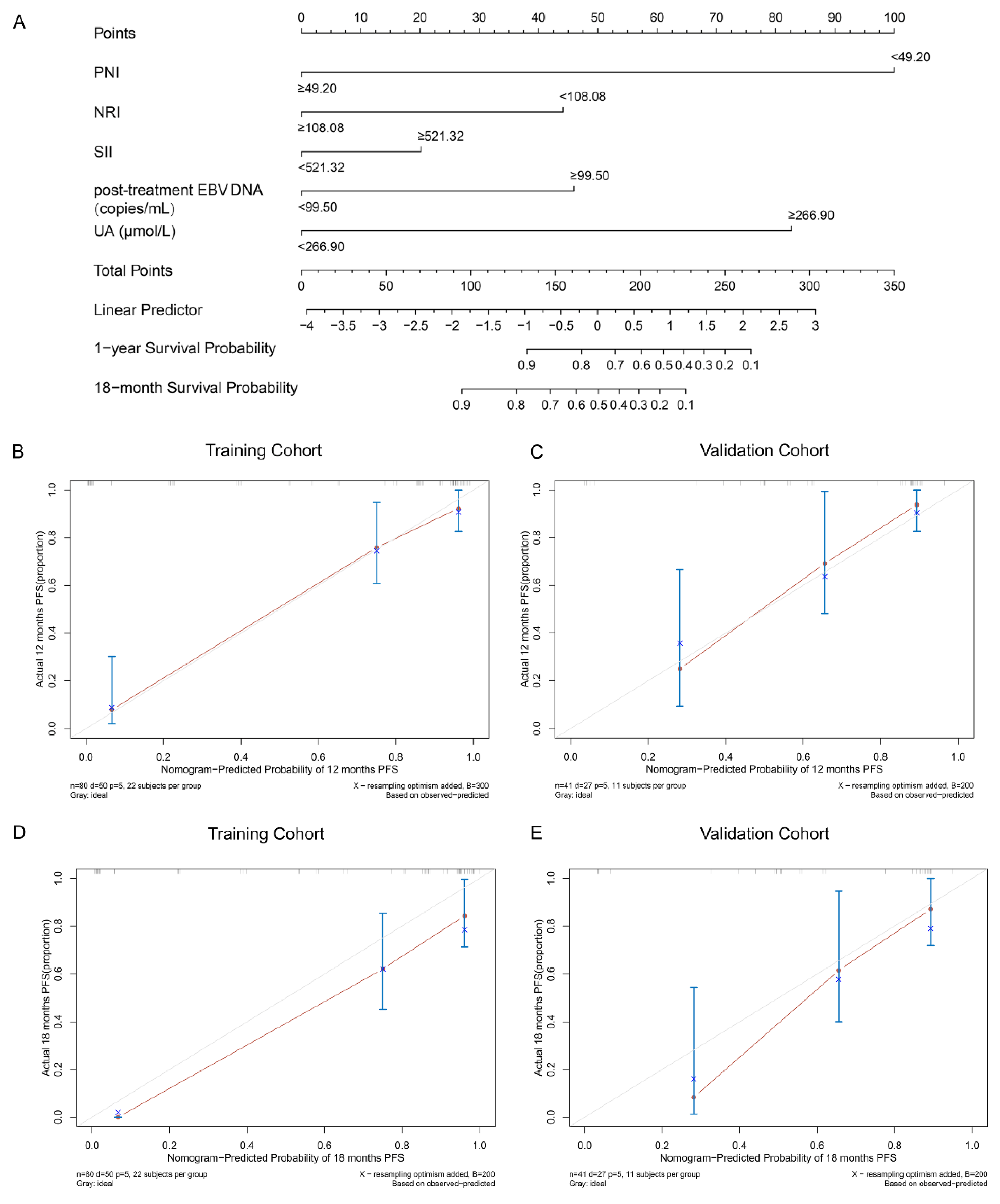
3.3. Development of a Nomogram Model for PFS
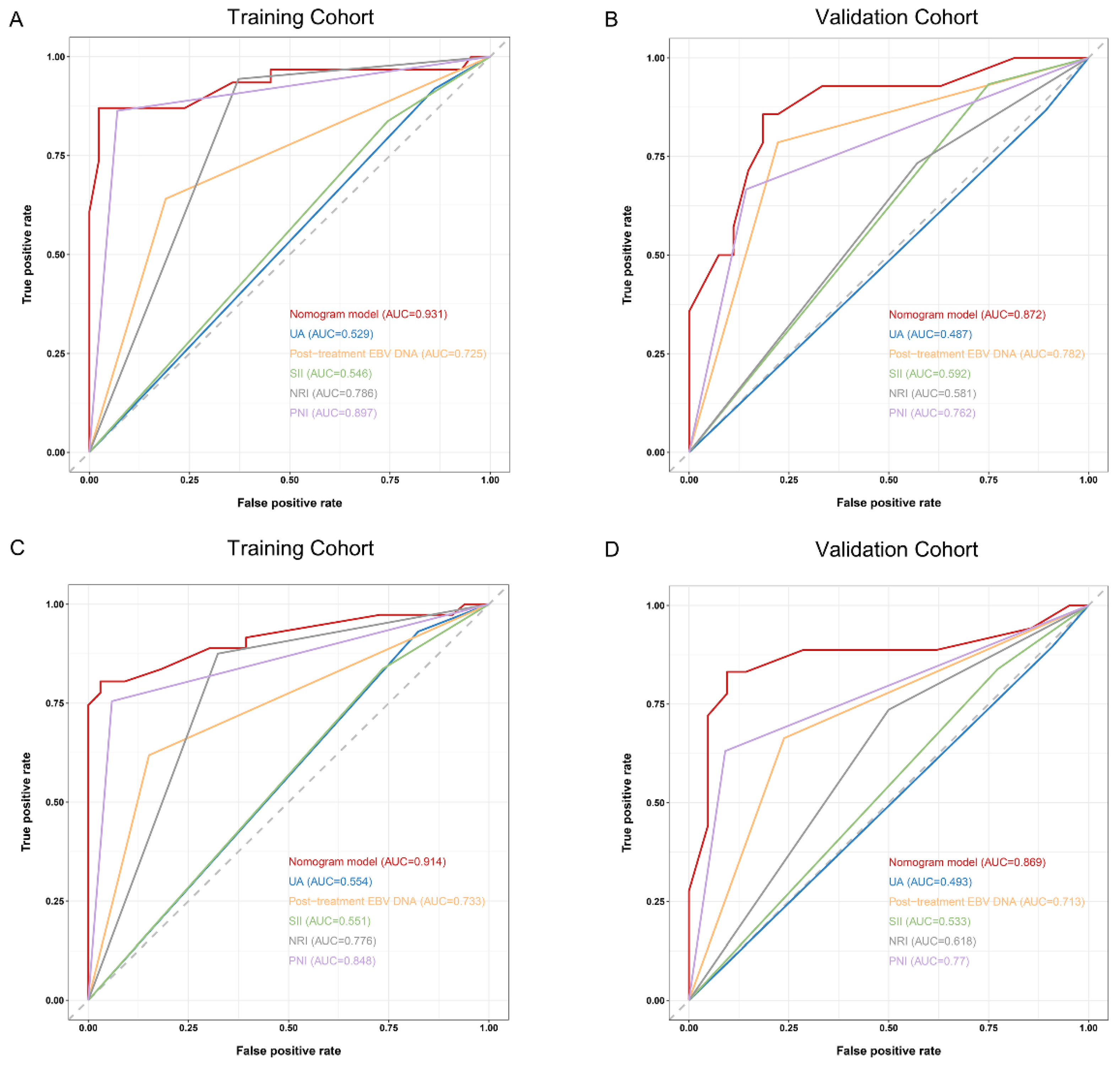
3.4. Comparison and Validation of the Predictive Accuracy of Nomogram and Other Traditional Factors
3.5. Comparison and Validation of the Predictive Accuracy of Nomogram and Other Constituent Factors
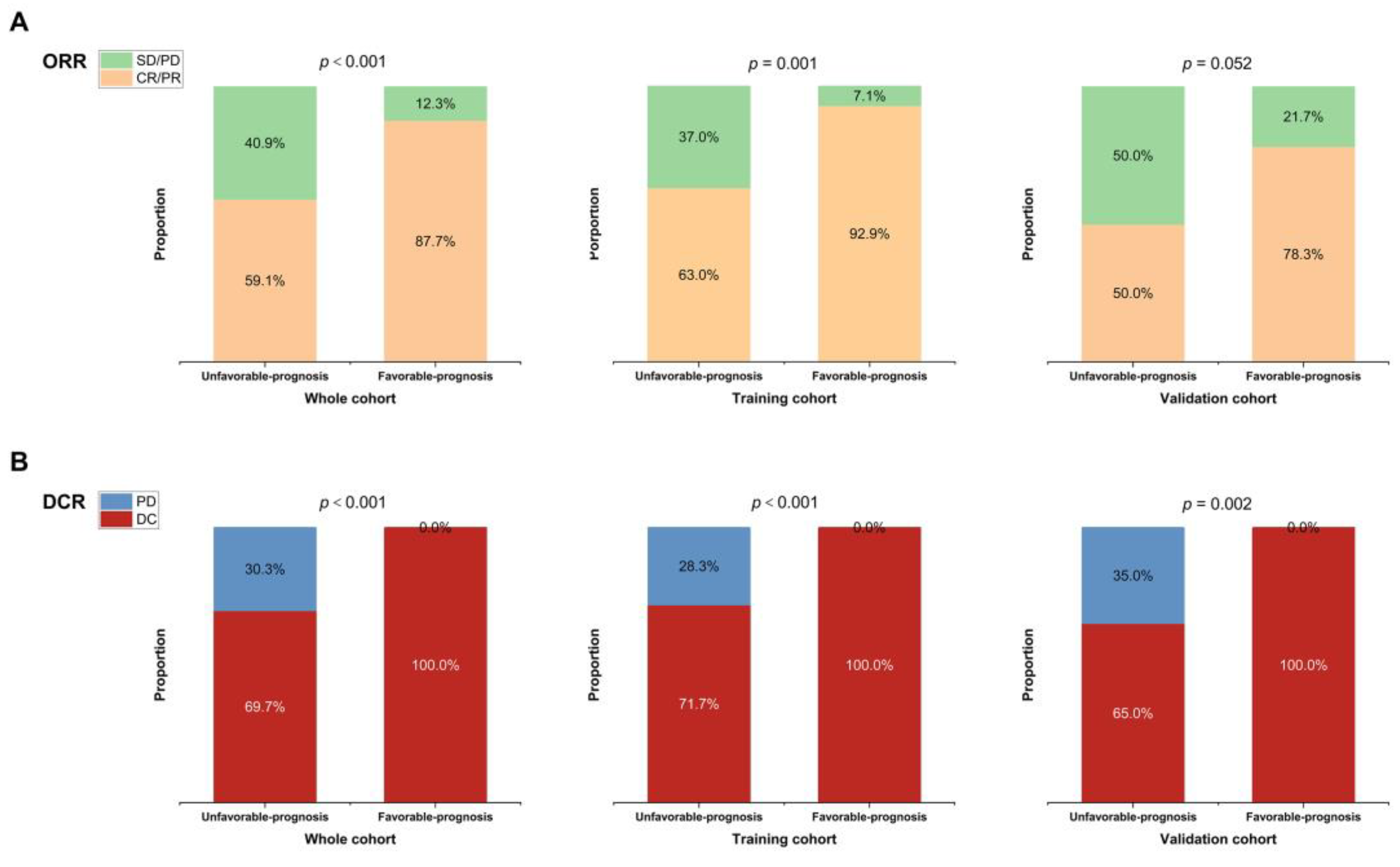
3.6. Separating Patients into Different Risk Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Li, S.; Zhang, M.; Qiao, Q. Pattern and Prognosis of Distant Metastases in Nasopharyngeal Carcinoma: A Large-Population Retrospective Analysis. Cancer Med. 2020, 9, 6147–6158. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qu, S.; Li, J.; Hu, C.; Xu, M.; Li, W.; Zhou, T.; Shen, L.; Wu, H.; Lang, J.; et al. Camrelizumab versus Placebo in Combination with Gemcitabine and Cisplatin as First-Line Treatment for Recurrent or Metastatic Nasopharyngeal Carcinoma (CAPTAIN-1st): A Multicentre, Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol. 2021, 22, 1162–1174. [Google Scholar] [CrossRef] [PubMed]
- Alwarawrah, Y.; Kiernan, K.; MacIver, N.J. Changes in Nutritional Status Impact Immune Cell Metabolism and Function. Front. Immunol. 2018, 9, 1055. [Google Scholar] [CrossRef]
- Tu, X.; Ren, J.; Zhao, Y. Prognostic Value of Prognostic Nutritional Index in Nasopharyngeal Carcinoma: A Meta-Analysis Containing 4511 Patients. Oral. Oncol. 2020, 110, 104991. [Google Scholar] [CrossRef]
- Miao, J.; Xiao, W.; Wang, L.; Han, F.; Wu, H.; Deng, X.; Guo, X.; Zhao, C. The Value of the Prognostic Nutritional Index (PNI) in Predicting Outcomes and Guiding the Treatment Strategy of Nasopharyngeal Carcinoma (NPC) Patients Receiving Intensity-Modulated Radiotherapy (IMRT) with or without Chemotherapy. J. Cancer Res. Clin. Oncol. 2017, 143, 1263–1273. [Google Scholar] [CrossRef]
- Lu, H.; Guo, S.; Liu, L.; Chen, Q.; Liang, Y.; Liu, S.; Sun, X.; Tang, Q.; Li, X.; Guo, L.; et al. Prognostic Significance of a Combined and Controlled Nutritional Status Score and EBV-DNA in Patients with Advanced Nasopharyngeal Carcinoma: A Long-Term Follow-up Study. Cancer Biol. Med. 2021, 19, 551–564. [Google Scholar] [CrossRef]
- Jin, S.; Li, R.; Chen, M.-Y.; Yu, C.; Tang, L.-Q.; Liu, Y.-M.; Li, J.-P.; Liu, Y.-N.; Luo, Y.-L.; Zhao, Y.; et al. Single-Cell Transcriptomic Analysis Defines the Interplay between Tumor Cells, Viral Infection, and the Microenvironment in Nasopharyngeal Carcinoma. Cell Res. 2020, 30, 950–965. [Google Scholar] [CrossRef]
- Hu, B.; Yang, X.-R.; Xu, Y.; Sun, Y.-F.; Sun, C.; Guo, W.; Zhang, X.; Wang, W.-M.; Qiu, S.-J.; Zhou, J.; et al. Systemic Immune-Inflammation Index Predicts Prognosis of Patients after Curative Resection for Hepatocellular Carcinoma. Clin. Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef]
- Liu, D.; Czigany, Z.; Heij, L.R.; Bouwense, S.A.W.; van Dam, R.; Lang, S.A.; Ulmer, T.F.; Neumann, U.P.; Bednarsch, J. The Value of Platelet-to-Lymphocyte Ratio as a Prognostic Marker in Cholangiocarcinoma: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 438. [Google Scholar] [CrossRef]
- Valero, C.; Lee, M.; Hoen, D.; Weiss, K.; Kelly, D.W.; Adusumilli, P.S.; Paik, P.K.; Plitas, G.; Ladanyi, M.; Postow, M.A.; et al. Pretreatment Neutrophil-to-Lymphocyte Ratio and Mutational Burden as Biomarkers of Tumor Response to Immune Checkpoint Inhibitors. Nat. Commun. 2021, 12, 729. [Google Scholar] [CrossRef]
- Chan, K.C.A.; Woo, J.K.S.; King, A.; Zee, B.C.Y.; Lam, W.K.J.; Chan, S.L.; Chu, S.W.I.; Mak, C.; Tse, I.O.L.; Leung, S.Y.M.; et al. Analysis of Plasma Epstein-Barr Virus DNA to Screen for Nasopharyngeal Cancer. N. Engl. J. Med. 2017, 377, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.M.; Chan, A.T.; Chan, L.Y.; Leung, S.F.; Lam, C.W.; Huang, D.P.; Johnson, P.J. Molecular Prognostication of Nasopharyngeal Carcinoma by Quantitative Analysis of Circulating Epstein-Barr Virus DNA. Cancer Res. 2000, 60, 6878–6881. [Google Scholar] [PubMed]
- Lo, Y.M.; Chan, L.Y.; Chan, A.T.; Leung, S.F.; Lo, K.W.; Zhang, J.; Lee, J.C.; Hjelm, N.M.; Johnson, P.J.; Huang, D.P. Quantitative and Temporal Correlation between Circulating Cell-Free Epstein-Barr Virus DNA and Tumor Recurrence in Nasopharyngeal Carcinoma. Cancer Res. 1999, 59, 5452–5455. [Google Scholar] [PubMed]
- Xu, J.-Y.; Wei, X.-L.; Ren, C.; Zhang, Y.; Hu, Y.-F.; Li, J.-Y.; Chen, J.-L.; Wang, Y.-Q.; Han, F.; Wang, F.-H. Association of Plasma Epstein-Barr Virus DNA with Outcomes for Patients with Recurrent or Metastatic Nasopharyngeal Carcinoma Receiving Anti-Programmed Cell Death 1 Immunotherapy. JAMA Netw. Open 2022, 5, e220587. [Google Scholar] [CrossRef]
- Tang, L.-Q.; Li, C.-F.; Li, J.; Chen, W.-H.; Chen, Q.-Y.; Yuan, L.-X.; Lai, X.-P.; He, Y.; Xu, Y.-X.-X.; Hu, D.-P.; et al. Establishment and Validation of Prognostic Nomograms for Endemic Nasopharyngeal Carcinoma. J. Natl. Cancer Inst. 2016, 108, djv291. [Google Scholar] [CrossRef]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.-P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A New Index for Evaluating at-Risk Elderly Medical Patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef]
- Onodera, T.; Goseki, N.; Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984, 85, 1001–1005. [Google Scholar]
- Ding, P.; Yang, P.; Sun, C.; Tian, Y.; Guo, H.; Liu, Y.; Li, Y.; Zhao, Q. Predictive Effect of Systemic Immune-Inflammation Index Combined with Prognostic Nutrition Index Score on Efficacy and Prognosis of Neoadjuvant Intraperitoneal and Systemic Paclitaxel Combined with Apatinib Conversion Therapy in Gastric Cancer Patients with Positive Peritoneal Lavage Cytology: A Prospective Study. Front. Oncol. 2021, 11, 791912. [Google Scholar] [CrossRef]
- Schietroma, M.; Romano, L.; Schiavi, D.; Pessia, B.; Mattei, A.; Fiasca, F.; Carlei, F.; Giuliani, A. Systemic Inflammation Response Index (SIRI) as Predictor of Anastomotic Leakage after Total Gastrectomy for Gastric Cancer. Surg. Oncol. 2022, 43, 101791. [Google Scholar] [CrossRef]
- Sun, Y.; Li, W.F.; Chen, N.Y.; Zhang, N.; Hu, G.Q.; Xie, F.Y.; Sun, Y.; Chen, X.Z.; Li, J.G.; Zhu, X.D.; et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: A phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016, 17, 1509–1520. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, T.; Chen, X.; Li, J.; Pan, J.; He, X.; Lin, L.; Shi, Y.-R.; Feng, W.; Xiong, J.; et al. Efficacy, Safety, and Biomarker Analysis of Camrelizumab in Previously Treated Recurrent or Metastatic Nasopharyngeal Carcinoma (CAPTAIN Study). J. Immunother. Cancer 2021, 9, e003790. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-H.; Wei, X.-L.; Feng, J.; Li, Q.; Xu, N.; Hu, X.-C.; Liao, W.; Jiang, Y.; Lin, X.-Y.; Zhang, Q.-Y.; et al. Efficacy, Safety, and Correlative Biomarkers of Toripalimab in Previously Treated Recurrent or Metastatic Nasopharyngeal Carcinoma: A Phase II Clinical Trial (POLARIS-02). J. Clin. Oncol. 2021, 39, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wu, L.; Jian, H.; Chen, Y.; Wang, Q.; Fang, J.; Wang, Z.; Hu, Y.; Sun, M.; Han, L.; et al. Sintilimab plus Bevacizumab Biosimilar IBI305 and Chemotherapy for Patients with EGFR-Mutated Non-Squamous Non-Small-Cell Lung Cancer Who Progressed on EGFR Tyrosine-Kinase Inhibitor Therapy (ORIENT-31): First Interim Results from a Randomised, Double-Blind, Multicentre, Phase 3 Trial. Lancet Oncol. 2022, 23, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Pan, J.; Wang, H.; Zhao, Y.; Qu, S.; Chen, N.; Chen, X.; Sun, Y.; He, X.; Hu, C.; et al. Tislelizumab plus Chemotherapy as First-Line Treatment for Recurrent or Metastatic Nasopharyngeal Cancer: A Multicenter Phase 3 Trial (RATIONALE-309). Cancer Cell 2023, 41, 1061–1072.e4. [Google Scholar] [CrossRef]
- Ma, B.B.Y.; Lim, W.-T.; Goh, B.-C.; Hui, E.P.; Lo, K.-W.; Pettinger, A.; Foster, N.R.; Riess, J.W.; Agulnik, M.; Chang, A.Y.C.; et al. Antitumor Activity of Nivolumab in Recurrent and Metastatic Nasopharyngeal Carcinoma: An International, Multicenter Study of the Mayo Clinic Phase 2 Consortium (NCI-9742). J. Clin. Oncol. 2018, 36, 1412–1418. [Google Scholar] [CrossRef]
- Ritchie, G.; Gasper, H.; Man, J.; Lord, S.; Marschner, I.; Friedlander, M.; Lee, C.K. Defining the Most Appropriate Primary End Point in Phase 2 Trials of Immune Checkpoint Inhibitors for Advanced Solid Cancers: A Systematic Review and Meta-Analysis. JAMA Oncol. 2018, 4, 522–528. [Google Scholar] [CrossRef]
- Wong, K.C.W.; Hui, E.P.; Lo, K.-W.; Lam, W.K.J.; Johnson, D.; Li, L.; Tao, Q.; Chan, K.C.A.; To, K.-F.; King, A.D.; et al. Nasopharyngeal Carcinoma: An Evolving Paradigm. Nat. Rev. Clin. Oncol. 2021, 18, 679–695. [Google Scholar] [CrossRef]
- Huang, Z.-L.; Liu, S.; Wang, G.-N.; Zheng, S.-H.; Ding, S.-R.; Tao, Y.-L.; Chen, C.; Liu, S.-R.; Yang, X.; Chang, H.; et al. The Prognostic Significance of PD-L1 and PD-1 Expression in Patients with Nasopharyngeal Carcinoma: A Systematic Review and Meta-Analysis. Cancer Cell Int. 2019, 19, 141. [Google Scholar] [CrossRef]
- Caudell, J.J.; Gillison, M.L.; Maghami, E.; Spencer, S.; Pfister, D.G.; Adkins, D.; Birkeland, A.C.; Brizel, D.M.; Busse, P.M.; Cmelak, A.J.; et al. NCCN Guidelines® Insights: Head and Neck Cancers, Version 1.2022. J. Natl. Compr. Cancer Netw. 2022, 20, 224–234. [Google Scholar] [CrossRef]
- Prawira, A.; Oosting, S.F.; Chen, T.W.; Delos Santos, K.A.; Saluja, R.; Wang, L.; Siu, L.L.; Chan, K.K.W.; Hansen, A.R. Systemic Therapies for Recurrent or Metastatic Nasopharyngeal Carcinoma: A Systematic Review. Br. J. Cancer 2017, 117, 1743–1752. [Google Scholar] [CrossRef]
- Barao, K.; Abe Vicente Cavagnari, M.; Silva Fucuta, P.; Manoukian Forones, N. Association Between Nutrition Status and Survival in Elderly Patients with Colorectal Cancer. Nutr. Clin. Pract. 2017, 32, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Shen, L.-J.; Guo, X.; Guo, X.-M.; Qian, C.-N.; Wu, P.-H. Critical Weight Loss Predicts Poor Prognosis in Nasopharyngeal Carcinoma. BMC Cancer 2016, 16, 169. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J. BMI-Related Errors in the Measurement of Obesity. Int. J. Obes. 2008, 32 (Suppl 3), S56–S59. [Google Scholar] [CrossRef]
- Tang, Q.-N.; Qiu, H.-Z.; Sun, X.-Q.; Guo, S.-S.; Liu, L.-T.; Wen, Y.-F.; Liu, S.-L.; Xie, H.-J.; Liang, Y.-J.; Sun, X.-S.; et al. Geriatric Nutritional Risk Index as an Independent Prognostic Factor in Locally Advanced Nasopharyngeal Carcinoma Treated Using Radical Concurrent Chemoradiotherapy: A Retrospective Cohort Study. Ann. Transl. Med. 2021, 9, 532. [Google Scholar] [CrossRef] [PubMed]
- Huemer, F.; Lang, D.; Westphal, T.; Gampenrieder, S.P.; Hutarew, G.; Weiss, L.; Hackl, H.; Lamprecht, B.; Rinnerthaler, G.; Greil, R. Baseline Absolute Lymphocyte Count and ECOG Performance Score Are Associated with Survival in Advanced Non-Small Cell Lung Cancer Undergoing PD-1/PD-L1 Blockade. J. Clin. Med. 2019, 8, 1014. [Google Scholar] [CrossRef]
- Ikeya, T.; Shibutani, M.; Maeda, K.; Sugano, K.; Nagahara, H.; Ohtani, H.; Hirakawa, K. Maintenance of the nutritional prognostic index predicts survival in patients with unresectable meta static colorectal cancer. J. Cancer Res. Clin. Oncol. 2015, 141, 307–313. [Google Scholar] [CrossRef]
- Schetter, A.J.; Heegaard, N.H.H.; Harris, C.C. Inflammation and Cancer: Interweaving microRNA, Free Radical, Cytokine and P53 Pathways. Carcinogenesis 2010, 31, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Kusumanto, Y.H.; Dam, W.A.; Hospers, G.A.P.; Meijer, C.; Mulder, N.H. Platelets and Granulocytes, in Particular the Neutrophils, Form Important Compartments for Circulating Vascular Endothelial Growth Factor. Angiogenesis 2003, 6, 283–287. [Google Scholar] [CrossRef]
- Marra, P.; Mathew, S.; Grigoriadis, A.; Wu, Y.; Kyle-Cezar, F.; Watkins, J.; Rashid, M.; De Rinaldis, E.; Hessey, S.; Gazinska, P.; et al. IL15RA Drives Antagonistic Mechanisms of Cancer Development and Immune Control in Lymphocyte-Enriched Triple-Negative Breast Cancers. Cancer Res. 2014, 74, 4908–4921. [Google Scholar] [CrossRef]
- Labelle, M.; Begum, S.; Hynes, R.O. Direct Signaling between Platelets and Cancer Cells Induces an Epithelial-Mesenchymal-like Transition and Promotes Metastasis. Cancer Cell 2011, 20, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Hui, E.P.; Li, W.F.; Ma, B.B.; Lam, W.K.J.; Chan, K.C.A.; Mo, F.; Ai, Q.Y.H.; King, A.D.; Wong, C.H.; Guo, R.; et al. Integrating Postradiotherapy Plasma Epstein-Barr Virus DNA and TNM Stage for Risk Stratification of Nasopharyngeal Carcinoma to Adjuvant Therapy. Ann. Oncol. 2020, 31, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Du, X.-J.; Chen, L.; Li, W.-F.; Tang, L.-L.; Mao, Y.-P.; Guo, R.; Sun, Y.; Lin, A.-H.; Ma, J. Use of Pretreatment Serum Uric Acid Level to Predict Metastasis in Locally Advanced Nasopharyngeal Carcinoma. Head. Neck 2017, 39, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Fini, M.A.; Elias, A.; Johnson, R.J.; Wright, R.M. Contribution of Uric Acid to Cancer Risk, Recurrence, and Mortality. Clin. Transl. Med. 2012, 1, 16. [Google Scholar] [CrossRef]
- Dhankhar, R.; Dahiya, K.; Sharma, T.K.; Ghalaut, V.S.; Atri, R.; Kaushal, V. Diagnostic Significance of Adenosine Deaminase, Uric Acid and C-Reactive Protein Levels in Patients of Head and Neck Carcinoma. Clin. Lab. 2011, 57, 795–798. [Google Scholar]



| Characteristic | Training Cohort (n = 88) No. (%) | Validation Cohort (n = 43) No. (%) | p Value |
|---|---|---|---|
| Sex | 0.842 | ||
| Female | 15 (17%) | 6 (14%) | |
| Male | 73 (83%) | 37 (86%) | |
| Smoking | 0.362 | ||
| No | 57 (64.8%) | 32 (74.4%) | |
| Yes | 31 (35.2%) | 11 (25.6%) | |
| Drinking | 0.480 | ||
| No | 72 (81.8%) | 38 (88.4%) | |
| Yes | 16 (18.2%) | 5 (11.6%) | |
| Family history | 0.394 | ||
| No | 85 (96.6%) | 40 (93%) | |
| Yes | 3 (3.4%) | 3 (7%) | |
| Age | 45.85 ± 11.14 | 47.63 ± 11.50 | 0.404 |
| Height (cm) | 165.85 ± 6.17 | 164.17 ± 8.09 | 0.233 |
| Weight (kg) | 63.30 ± 10.50 | 61.49 ± 11.58 | 0.174 |
| a Tumor stage | 0.487 | ||
| T1 | 3 (3.4%) | 0 (0%) | |
| T2 | 4 (4.5%) | 2 (4.7%) | |
| T3 | 45 (51.1%) | 19 (44.2%) | |
| T4 | 36 (40.9%) | 22 (51.2%) | |
| a Node stage | 0.488 | ||
| N1 | 8 (9.1%) | 6 (14.0%) | |
| N2 | 28 (31.8%) | 10 (23.3%) | |
| N3 | 52 (59.1%) | 27 (62.8%) | |
| Pretreatment EBV DNA, copies/mL | 0.527 | ||
| <4760 | 42 (47.7%) | 18 (41.9%) | |
| ≥4760 | 46 (52.3%) | 25 (58.1%) | |
| Post-treatment EBV DNA, copies/mL | 0.821 | ||
| <99.50 | 50 (62.5%) | 24 (58.5%) | |
| ≥99.50 | 30 (37.5%) | 17 (41.5%) | |
| Liver metastasis | 0.472 | ||
| No | 50 (56.8%) | 28 (65.1%) | |
| Yes | 38 (43.2%) | 15 (34.9%) | |
| Bone metastasis | 0.952 | ||
| No | 25 (28.4%) | 12 (27.9%) | |
| Yes | 63 (71.6%) | 31 (72.1%) | |
| Lung metastasis | 0.799 | ||
| No | 60 (68.2%) | 31 (72.1%) | |
| Yes | 28 (31.8%) | 12 (27.9%) | |
| Distance LN metastasis | 0.939 | ||
| No | 64 (72.7%) | 31 (72.1%) | |
| Yes | 24 (27.3%) | 12 (27.9%) | |
| No of metastatic sites | 0.333 | ||
| 1 | 42 (47.7%) | 26 (60.5%) | |
| 2–3 | 41 (46.6%) | 16 (37.2%) | |
| ≥4 | 5 (5.7%) | 1 (2.3%) | |
| Chemotherapy combination PD-1 inhibitor lines | 0.363 | ||
| 1 | 66 (75.0%) | 29 (67.4%) | |
| ≥2 | 22 (25.0%) | 14 (32.6%) | |
| Response | 0.233 | ||
| CR | 2 (2.3%) | 0 (0%) | |
| PR | 66 (75.0%) | 28 (65.1%) | |
| SD | 7 (8.0%) | 8 (18.6%) | |
| PD | 13 (14.8%) | 7 (16.3%) | |
| Anti-PD-1 agent | 0.651 | ||
| Camrelizumab | 27 (30.7%) | 14 (32.6%) | |
| Toripalimab | 44 (50.0%) | 18 (41.9%) | |
| Sintilimab | 7 (8.0%) | 7 (16.3%) | |
| Tislelizumab | 8 (9.1%) | 3 (7.0%) | |
| Nivolumab | 2 (2.3%) | 1 (2.3%) | |
| Chemotherapy regimens | 0.470 | ||
| GP | 60 (68.2%) | 23 (53.5%) | |
| PF | 3 (3.4%) | 3 (7.0%) | |
| TP | 10 (11.4%) | 6 (14.0%) | |
| Capecitabine | 4 (4.6%) | 4 (9.3%) | |
| TPF | 1 (1.1%) | 2 (4.7%) | |
| Others | 10 (11.4%) | 5 (11.6%) | |
| BMI (kg/m2) | 0.816 | ||
| <19.19 | 11 (12.5%) | 6 (14%) | |
| ≥19.19 | 77 (87.5%) | 37 (86%) | |
| NRI | 0.876 | ||
| <108.08 | 58 (65.9%) | 27 (62.8%) | |
| ≥108.08 | 30 (34.1%) | 16 (37.2%) | |
| PNI | 0.223 | ||
| <49.20 | 40 (45.5%) | 14 (32.6%) | |
| ≥49.20 | 48 (54.5%) | 29 (67.4%) | |
| SII | 0.754 | ||
| <521.32 | 20 (22.7%) | 8 (18.6%) | |
| ≥521.32 | 68 (77.3%) | 35 (81.4%) | |
| SIRI | 0.447 | ||
| <2.42 | 74 (84.1%) | 39 (90.7%) | |
| ≥2.42 | 14 (15.9%) | 4 (9.3%) | |
| GPS | |||
| 0 | 58 (65.9%) | 34 (79.1%) | |
| 1–2 | 30 (34.1%) | 9 (20.9%) | |
| CONUT score | |||
| 0–1 | 50 (56.8%) | 25 (58.1%) | |
| 2–6 | 38 (43.2%) | 18 (41.9%) | |
| NLR | 0.608 | ||
| <3.24 | 58 (65.9%) | 31 (72.1%) | |
| ≥3.24 | 30 (34.1%) | 12 (27.9%) | |
| LAR | 0.359 | ||
| <3.74 | 15 (17%) | 11 (25.6%) | |
| ≥3.74 | 73 (83%) | 32 (74.4%) | |
| LMR | 0.443 | ||
| <2.87 | 21 (23.9%) | 7 (16.3%) | |
| ≥2.87 | 67 (76.1%) | 36 (83.7%) | |
| PLR | 0.999 | ||
| <123.0 | 22 (25%) | 10 (23.3%) | |
| ≥123.0 | 66 (75%) | 33 (76.7%) | |
| WBC (109/L) | 0.652 | ||
| <9.18 | 72 (81.8%) | 33 (76.7%) | |
| ≥9.18 | 16 (18.2%) | 10 (23.3%) | |
| Neutrophil (109/L) | 0.390 | ||
| <7.26 | 78 (88.6%) | 35 (81.4%) | |
| ≥7.26 | 10 (11.4%) | 8 (18.6%) | |
| Lymphocyte (109/L) | 0.727 | ||
| <2.03 | 53 (60.2%) | 28 (65.1%) | |
| ≥2.03 | 35 (39.8%) | 15 (34.9%) | |
| Monocyte (109/L) | 0.987 | ||
| <0.55 | 61 (69.3%) | 29 (67.4%) | |
| ≥0.55 | 27 (30.7%) | 14 (32.6%) | |
| RBC (1012/L) | 0.987 | ||
| <4.49 | 18 (20.5%) | 8 (18.6%) | |
| ≥4.49 | 70 (79.5%) | 35 (81.4%) | |
| PLT (109/L) | 0.340 | ||
| <374.0 | 75 (85.2%) | 33 (76.7%) | |
| ≥374.0 | 13 (14.8%) | 10 (23.3%) | |
| HGB (g/L) | 0.421 | ||
| <145.0 | 47 (53.4%) | 19 (44.2%) | |
| ≥145.0 | 41 (46.6%) | 24 (55.8%) | |
| ALP (U/L) | 0.074 | ||
| <94.10 | 73 (83%) | 29 (67.4%) | |
| ≥94.10 | 15 (17%) | 14 (32.6%) | |
| GGT (U/L) | 0.356 | ||
| <32.80 | 54 (61.4%) | 22 (51.2%) | |
| ≥32.80 | 34 (38.6%) | 21 (48.8%) | |
| LDH (U/L) | 0.231 | ||
| <163.30 | 10 (11.4%) | 9 (20.9%) | |
| ≥163.30 | 78 (88.6%) | 34 (79.1%) | |
| UA (μmol/L) | 0.825 | ||
| <266.90 | 13 (14.8%) | 5 (11.6%) | |
| ≥266.90 | 75 (85.2%) | 38 (88.4%) | |
| GLU (mmol/L) | 0.921 | ||
| <4.97 | 34 (38.6%) | 17 (39.5%) | |
| ≥4.97 | 54 (61.4%) | 26 (60.5%) | |
| CRP (mg/L) | 0.772 | ||
| <27.0 | 77 (87.5%) | 39 (90.7%) | |
| ≥27.0 | 11 (12.5%) | 4 (9.3%) | |
| CK (U/L) | 0.822 | ||
| <47.0 | 13 (14.8%) | 7 (16.3%) | |
| ≥47.0 | 75 (85.2%) | 36 (83.7%) | |
| SAA (mg/L) | 0.444 | ||
| <19.30 | 56 (63.6%) | 31 (72.1%) | |
| ≥19.30 | 32 (36.4%) | 12 (27.9%) |
| Variables | Univariate HR (95%CI) | p Value | Multivariate HR (95%CI) | p Value |
|---|---|---|---|---|
| Sex | 0.555 | |||
| Female | Reference | |||
| Male | 1.254 (0.592, 2.654) | |||
| Smoking | 0.942 | |||
| No | Reference | |||
| Yes | 1.020 (0.593, 1.755) | |||
| Drinking | 0.247 | |||
| No | Reference | |||
| Yes | 1.451 (0.773, 2.722) | |||
| Family history | 0.665 | |||
| No | Reference | |||
| Yes | 1.369 (0.330, 5.675) | |||
| Age | 1.011 (0.987, 1.036) | 0.369 | ||
| a Tumor stage | 0.620 | |||
| T1 | Reference | |||
| T2 | 1.501 (0.136, 16.601) | 0.740 | ||
| T3 | 2.704 (0.368, 19.878) | 0.328 | ||
| T4 | 2.888 (0.389, 21.413) | 0.300 | ||
| a Node stage | 0.543 | |||
| N1 | Reference | |||
| N2 | 1.808 (0.618, 5.296) | 0.280 | ||
| N3 | 1.747 (0.616, 4.954) | 0.294 | ||
| Pretreatment EBV DNA, copies/mL | 0.128 | |||
| <4760 | Reference | |||
| ≥4760 | 1.516 (0.887, 2.592) | |||
| Post-treatment EBV DNA, copies/mL | <0.001 | 0.003 | ||
| <99.50 | Reference | Reference | ||
| ≥99.50 | 0.387 (0.253, 0.592) | 3.109 (1.502, 6.437) | ||
| Liver metastasis | 0.420 | |||
| No | Reference | |||
| Yes | 1.243 (0.733, 2.106) | |||
| Bone metastasis | 0.181 | |||
| No | Reference | |||
| Yes | 1.534 (0.820, 2.871) | |||
| Lung metastasis | 0.342 | |||
| No | Reference | |||
| Yes | 0.750 (0.415, 1.356) | |||
| Distance LN metastasis | 0.146 | |||
| No | Reference | |||
| Yes | 1.535 (0.861, 2.736) | |||
| No of metastatic sites | 0.175 | |||
| 1 | Reference | |||
| 2–3 | 2.200 (0.871, 5.559) | 0.096 | ||
| ≥4 | 2.884 (0.833, 9.987) | 0.095 | ||
| Chemotherapy combination PD-1 inhibitor lines | 0.395 | |||
| 1 | Reference | |||
| ≥2 | 1.339 (0.683, 2.626) | |||
| Response | <0.001 | 0.791 | ||
| CR | Reference | Reference | ||
| PR | 2.325 (0.318, 17.004) | 0.406 | 2.159 (0.242, 19.237) | 0.490 |
| SD | 2.489 (0.253, 24.469) | 0.434 | 1.651 (0.125, 21.738) | 0.703 |
| PD | 17.029 (2.108, 137.588) | 0.008 | 1.558 (0.139, 17.476) | 0.719 |
| Anti-PD-1 agent | 0.923 | |||
| Camrelizumab | Reference | |||
| Toripalimab | 0.958 (0.530, 1.733) | 0.888 | ||
| Sintilimab | 0.704 (2.108, 137.588) | 0.574 | ||
| Tislelizumab | 17.029 (2.108, 137.588) | 0.682 | ||
| Nivolumab | 17.029 (2.108, 137.588) | 0.573 | ||
| Chemotherapy regimens | 0.005 | 0.197 | ||
| GP | Reference | Reference | ||
| PF | 0.290 (0.040, 2.134) | 0.224 | 0.100 (0.012, 0.805) | 0.030 |
| TP | 0.742 (0.265, 2.081) | 0.571 | 0.526 (0.172, 1.603) | 0.258 |
| Capecitabine | 0.816 (0.195, 3.411) | 0.780 | 0.954 (0.168, 5.434) | 0.958 |
| TPF | 13.237 (1.608, 108.983) | 0.016 | 0 (0, -) | 0.983 |
| Others | 3.121 (1.467, 6.637) | 0.003 | 0.427 (0.147, 1.242) | 0.118 |
| BMI (kg/m2) | 0.064 | 0.291 | ||
| <19.19 | Reference | Reference | ||
| ≥19.19 | 0.507 (0.248, 1.040) | 1.849 (0.590, 5.791) | ||
| NRI | <0.001 | <0.001 | ||
| <108.08 | Reference | Reference | ||
| ≥108.08 | 0.201 (0.104, 0.389) | 0.176 (0.066, 0.464) | ||
| PNI | <0.001 | <0.001 | ||
| <49.20 | Reference | Reference | ||
| ≥49.20 | 0.092 (0.047, 0.180) | 0.096 (0.030, 0.309) | ||
| SII | 0.054 | 0.177 | ||
| <521.32 | Reference | Reference | ||
| ≥521.32 | 2.088 (0.987, 4.418) | 1.990 (0.732, 5.413) | ||
| SIRI | 0.583 | |||
| <2.42 | Reference | |||
| ≥2.42 | 0.810 (0.383, 1.717) | |||
| GPS | 0.875 | |||
| 0 | Reference | |||
| 1–2 | 0.958 (0.563, 1.631) | |||
| CONUT score | 0.222 | |||
| 0–1 | Reference | |||
| 2–6 | 1.130 (0.928, 1.376) | |||
| NLR | 0.130 | |||
| <3.24 | Reference | |||
| ≥3.24 | 1.824 (1.058, 3.143) | |||
| LAR | 0.289 | |||
| <3.74 | Reference | |||
| ≥3.74 | 1.536 (0.695, 3.395) | |||
| LMR | 0.276 | |||
| <2.87 | Reference | |||
| ≥2.87 | 1.444 (0.746, 2.794) | |||
| PLR | 0.088 | 0.754 | ||
| <123.0 | Reference | Reference | ||
| ≥123.0 | 1.815 (0.915, 3.601) | 1.154 (0.472, 2.821) | ||
| WBC (109/L) | 0.093 | 0.199 | ||
| <9.18 | Reference | Reference | ||
| ≥9.18 | 0.506 (0.228, 1.120) | 0.439 (0.125, 1.542) | ||
| Neutrophil (109/L) | 0.559 | |||
| <7.26 | Reference | |||
| ≥7.26 | 0.759 (0.301, 1.913) | |||
| Lymphocyte (109/L) | 0.168 | |||
| <2.03 | Reference | |||
| ≥2.03 | 0.682 (0.396, 1.175) | |||
| Monocyte (109/L) | 0.297 | |||
| <0.55 | Reference | |||
| ≥0.55 | 0.734 (0.411, 1.312) | |||
| RBC (1012/L) | 0.143 | |||
| <4.49 | Reference | |||
| ≥4.49 | 0.604 (0.308, 1.186) | |||
| PLT (109/L) | 0.386 | |||
| <374.0 | Reference | |||
| ≥374.0 | 0.703 (0.317, 1.558) | |||
| HGB (g/L) | 0.639 | |||
| <145.0 | Reference | |||
| ≥145.0 | 0.882 (0.521, 1.492) | |||
| ALP (U/L) | 0.282 | |||
| <94.10 | Reference | |||
| ≥94.10 | 0.662 (0.312, 1.404) | |||
| GGT (U/L) | 0.911 | |||
| <32.80 | Reference | |||
| ≥32.80 | 0.970 (0.568, 1.655) | |||
| LDH (U/L) | 0.530 | |||
| <163.30 | Reference | |||
| ≥163.30 | 1.344 (0.534, 3.382) | |||
| UA (μmol/L) | 0.077 | 0.008 | ||
| <266.90 | Reference | Reference | ||
| ≥266.90 | 2.513 (0.906, 6.969) | 5.026 (1.532, 16.497) | ||
| GLU (mmol/L) | 0.007 | 0.393 | ||
| <4.97 | Reference | Reference | ||
| ≥4.97 | 0.479 (0.279, 0.821) | 0.763 (0.411, 1.419) | ||
| CRP (mg/L) | 0.587 | |||
| <27.0 | Reference | |||
| ≥27.0 | 0.791 (0.339, 1.846) | |||
| CK (U/L) | 0.373 | |||
| <47.0 | Reference | |||
| ≥47.0 | 1.435 (0.649, 3.177) | |||
| SAA (mg/L) | 0.380 | |||
| <19.30 | Reference | |||
| ≥19.30 | 0.780 (0.448, 1.358) |
| Variable | PFS (Month) | OS (Month) | ||
|---|---|---|---|---|
| Median (95% CI) | p | Median (95% CI) | p | |
| Favorable- prognosis | 35.10 (27.36, 42.84) | 0.001 | - (-,-) | <0.001 |
| Unfavorable- prognosis | 7.23 (6.50, 7.97) | 33.73 (36.73, 40.73) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Yang, Q.; Jiang, Q.; Gu, L.-W.; Lin, H.-X.; Guo, L. Integrating Baseline Nutritional and Inflammatory Parameters with Post-Treatment EBV DNA Level to Predict Outcomes of Patients with De Novo Metastatic Nasopharyngeal Carcinoma Receiving Chemotherapy Combination PD-1 Inhibitor. Nutrients 2023, 15, 4262. https://doi.org/10.3390/nu15194262
Guo J, Yang Q, Jiang Q, Gu L-W, Lin H-X, Guo L. Integrating Baseline Nutritional and Inflammatory Parameters with Post-Treatment EBV DNA Level to Predict Outcomes of Patients with De Novo Metastatic Nasopharyngeal Carcinoma Receiving Chemotherapy Combination PD-1 Inhibitor. Nutrients. 2023; 15(19):4262. https://doi.org/10.3390/nu15194262
Chicago/Turabian StyleGuo, Jia, Qi Yang, Qi Jiang, Li-Wen Gu, Huan-Xin Lin, and Ling Guo. 2023. "Integrating Baseline Nutritional and Inflammatory Parameters with Post-Treatment EBV DNA Level to Predict Outcomes of Patients with De Novo Metastatic Nasopharyngeal Carcinoma Receiving Chemotherapy Combination PD-1 Inhibitor" Nutrients 15, no. 19: 4262. https://doi.org/10.3390/nu15194262
APA StyleGuo, J., Yang, Q., Jiang, Q., Gu, L.-W., Lin, H.-X., & Guo, L. (2023). Integrating Baseline Nutritional and Inflammatory Parameters with Post-Treatment EBV DNA Level to Predict Outcomes of Patients with De Novo Metastatic Nasopharyngeal Carcinoma Receiving Chemotherapy Combination PD-1 Inhibitor. Nutrients, 15(19), 4262. https://doi.org/10.3390/nu15194262





