A Higher Skeletal Muscle Mass and Lower Adiposity Phenotype Is Associated with Better Cardiometabolic Control in Adults with Hip and Knee Osteoarthritis: Results from the Chilean National Health Survey 2016–2017
Abstract
:1. Introduction
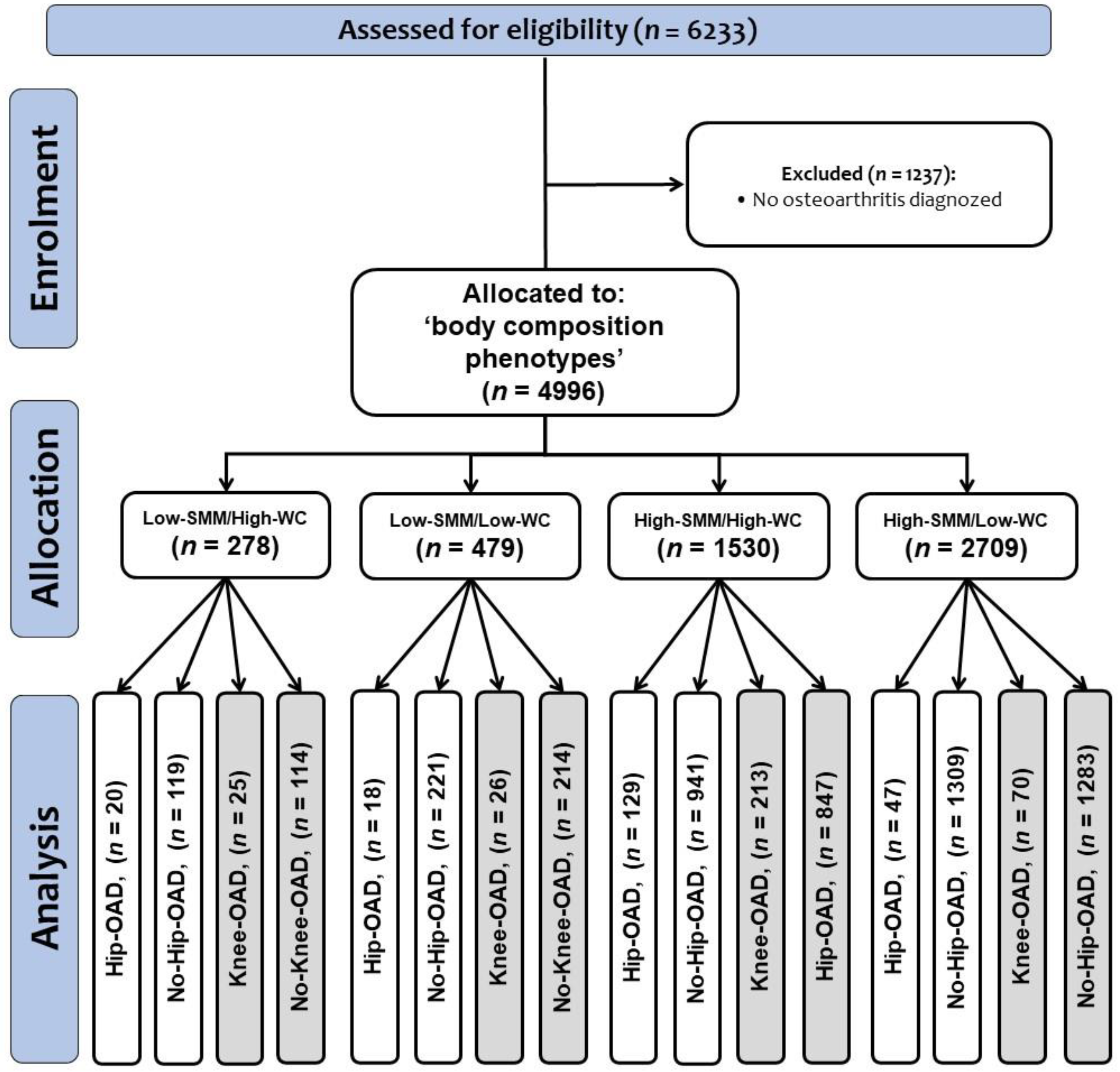
2. Materials and Methods
2.1. Participants
2.2. Phenotypes by Hip and Knee Osteoarthritis Diagnosed
2.3. Diabetes and Arterial Hypertension Markers (Main Outcomes)
2.4. Secondary Cardiometabolic Risk Factors (Secondary Outcomes)
2.5. Other Cardiovascular Risk Estimation
2.6. Statistical Analyses
3. Results
3.1. Baseline Characteristics
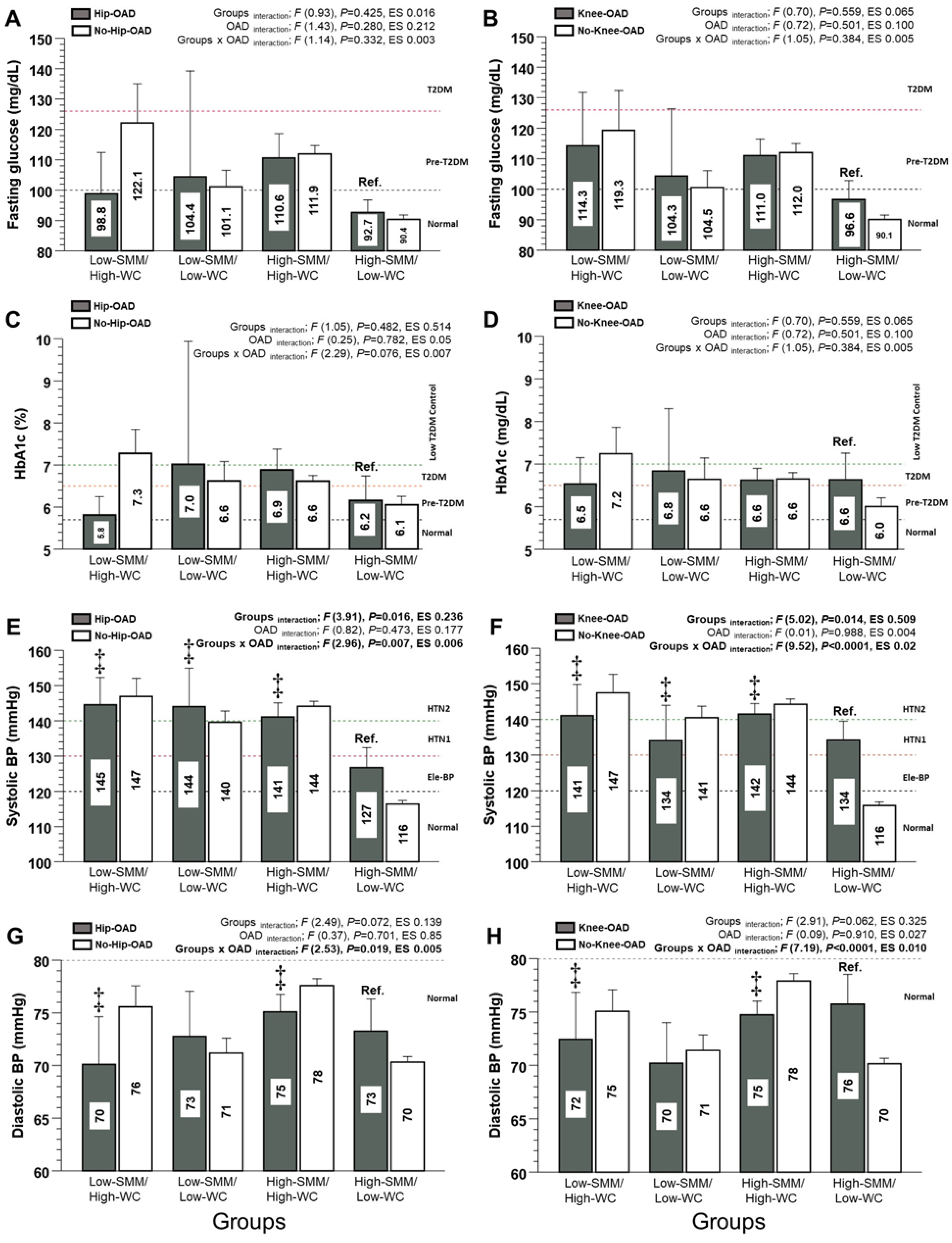
3.2. Diabetes and Arterial Hypertension Markers (Main Outcomes)
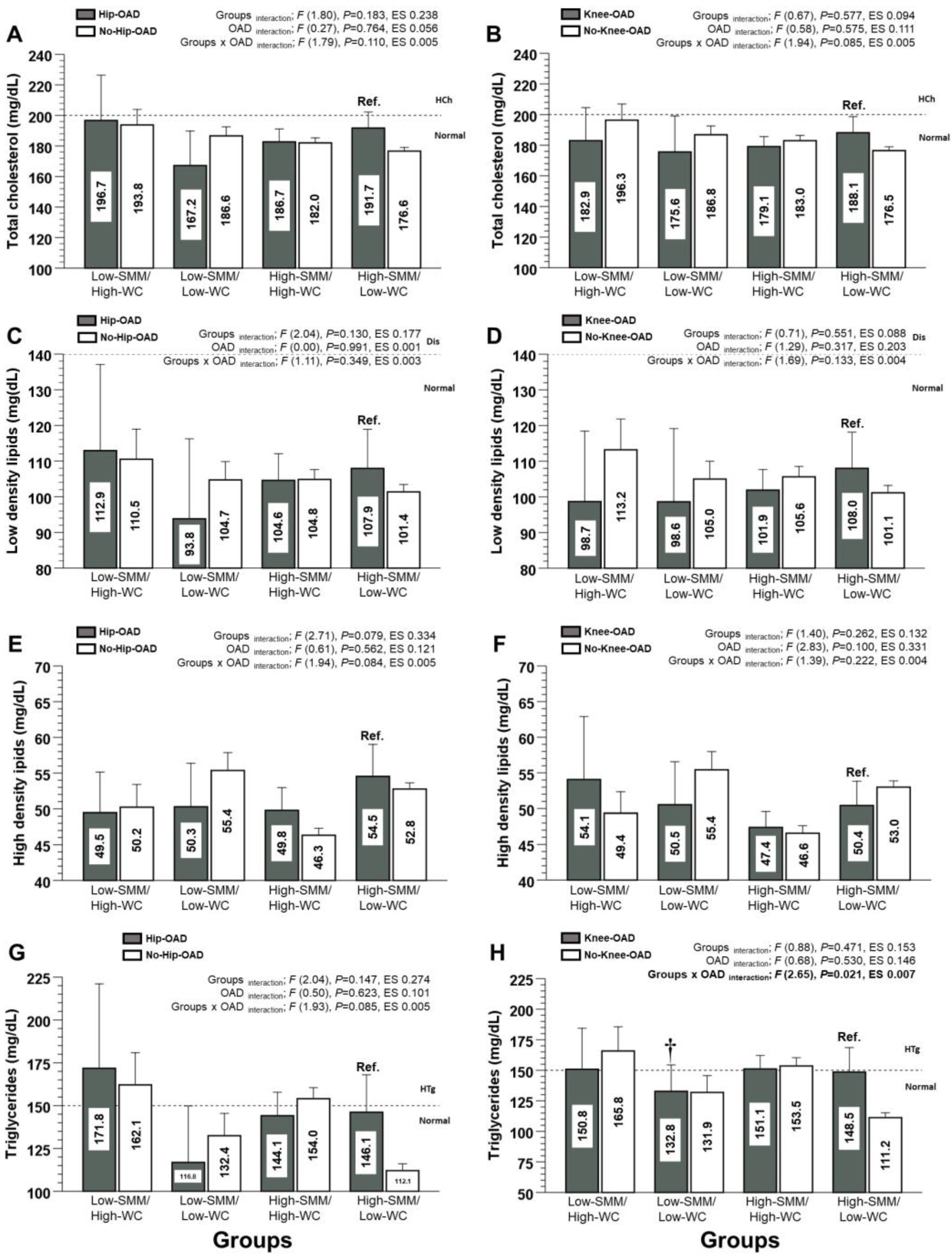
3.3. Lipid Profile/Dyslipidaemia Markers (Secondary Outcomes)
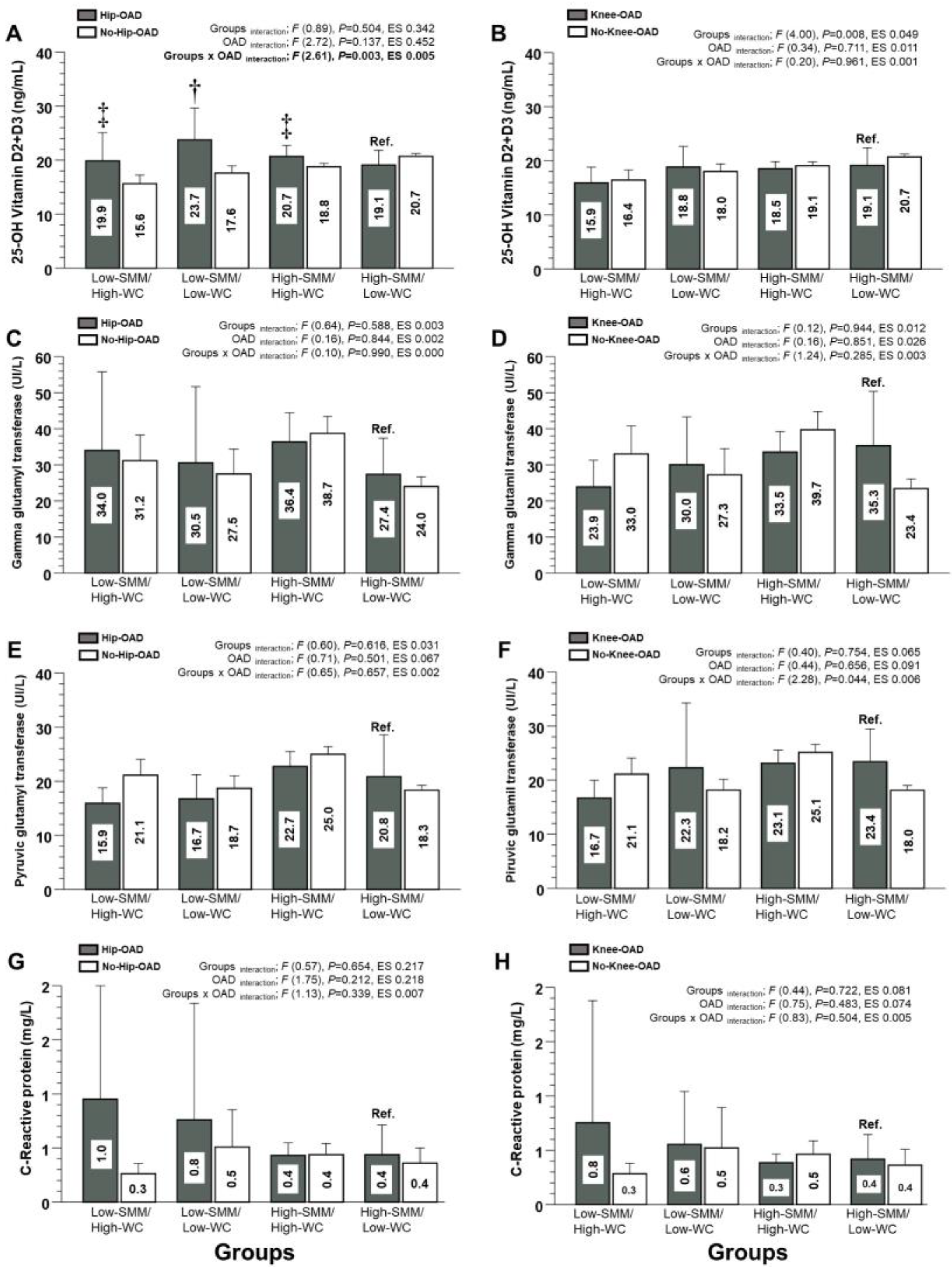
3.4. Mineral Content, Non-Alcoholic Fatty Liver Disease, and Inflammation Markers (Secondary Outcomes)
3.5. Muscleness and Fatness Phenotypes for Predicting Plasma Glucose, and Blood Pressure Control
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Long, H.; Liu, Q.; Yin, H.; Wang, K.; Diao, N.; Zhang, Y.; Lin, J.; Guo, A. Prevalence trends of site-specific osteoarthritis from 1990 to 2019: Findings from the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022, 74, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.; Thoma, L.; Golightly, Y. Epidemiology of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Dell’Isola, A.; Allan, R.; Smith, S.; Marreiros, S.; Steultjens, M. Identification of clinical phenotypes in knee osteoarthritis: A systematic review of the literature. BMC Musculoskelet Disord. 2016, 17, 425. [Google Scholar] [CrossRef] [PubMed]
- Pegreffi, F.; Balestra, A.; De Lucia, O.; Smith, L.; Barbagallo, M.; Veronese, N. Prevalence of sarcopenia in knee osteoarthritis: A systematic review and meta-analysis. J. Clin. Med. 2023, 12, 1532. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ro, H.J.; Chung, S.G.; Kang, S.H.; Seo, K.M.; Kim, D.-K. Low skeletal muscle mass in the lower limbs is independently associated to knee osteoarthritis. PLoS ONE 2016, 11, e0166385. [Google Scholar] [CrossRef]
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, diagnosis, and treatment options. Med. Clin. 2020, 104, 293–311. [Google Scholar]
- Valenzuela, P.L.; Carrera-Bastos, P.; Castillo-García, A.; Lieberman, D.E.; Santos-Lozano, A.; Lucia, A. Obesity and the risk of cardiometabolic diseases. Nat. Rev. Cardiol. 2023, 20, 475–494. [Google Scholar] [CrossRef]
- Mathieu, S.; Couderc, M.; Tournadre, A.; Soubrier, M. Cardiovascular profile in osteoarthritis: A meta-analysis of cardiovascular events and risk factors. Jt. Bone Spine Rev. Du Rhum. 2019, 86, 679–684. [Google Scholar] [CrossRef]
- Alvarez, C.; Campos-Jara, C.; Ciolac, E.G.; Vega-Guimaraes, G.; Andrade-Mayorga, O.; Cano-Montoya, J.; Andrade, D.C.; Delgado-Floody, P.; Alonso-Martínez, A.; Izquierdo, M.; et al. Pacientes hipertensos muestran una mayor respuesta de la frecuencia cardíaca durante el ejercicio progre-sivo en relación con pares adultos normotensos: PROYECTO VASCU-HEALTH (Hypertensive patients show higher heart rate response during incremental exercise and elevated arterial age estimation than normotensive adult peers: VASCU-HEALTH PROJECT). Retos 2023, 50, 25–32. [Google Scholar]
- Pagotto, V.; dos Santos, K.F.; Malaquias, S.G.; Bachion, M.M.; Silveira, E.A. Calf circumference: Clinical validation for evaluation of muscle mass in the elderly. Rev. Bras. De Enferm. 2018, 71, 322–328. [Google Scholar] [CrossRef]
- Pieńkowska, J.; Brzeska, B.; Kaszubowski, M.; Kozak, O.; Jankowska, A.; Szurowska, E. The correlation between the MRI-evaluated ectopic fat accumulation and the incidence of diabetes mellitus and hypertension depends on body mass index and waist circumference ratio. PLoS ONE 2020, 15, e0226889. [Google Scholar] [CrossRef] [PubMed]
- Dégano, I.R.; Marrugat, J.; Grau, M.; Salvador-González, B.; Ramos, R.; Zamora, A.; Martí, R.; Elosua, R. The association between education and cardiovascular disease incidence is mediated by hypertension, diabetes, and body mass index. Sci. Rep. 2017, 7, 12370. [Google Scholar] [CrossRef] [PubMed]
- Wåhlin-Larsson, B.; Wilkinson, D.J.; Strandberg, E.; Hosford-Donovan, A.; Atherton, P.J.; Kadi, F. Mechanistic links underlying the impact of C-reactive protein on muscle mass in elderly. Cell. Physiol. Biochem. 2018, 44, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Cigarroa, I.; Zapata-Lamana, R.; Leiva-Gajardo, G.; Vasquez, E.; Parrado-Romero, E.; Vásquez-Gomez, J.; Álvarez, C.; Petermann-Rocha, F.; Reyes-Molina, D. Características de la adherencia y motivos del abandono de las intervenciones basadas en el ejercicio físico en adultos mayores en América Latina: Una revisión de alcance (Adherence characteristics and reasons for abandonment of physical exercise-based in: Una revisión de alcance. Retos 2022, 44, 10–26. [Google Scholar]
- Rolland, Y.; Lauwers-Cances, V.; Cournot, M.; Nourhashémi, F.; Reynish, W.; Rivière, D. Sarcopenia, calf circumference, and physical function of elderly women: A cross-sectional study. J. Am. Geriatr. Soc. 2003, 51, 1120–1124. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Floody, P.; Lepin, C.G.; Ramirez, R.; Fuentes, C.M.; Saavedra, P.I.; Campos, C.; Cristi-Montero, C.; Sotomayor, E.M.; Caparrós, C. Lifestyle and cardiometabolic risk factors in the ethnic and non-ethnic population> 15 years of age: Results from the National Chilean Health Survey 2016–2017. Nutr. Hosp. 2023, 40, 400–411. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, 1269–1324. [Google Scholar]
- Petermann, F.; Duran, E.; Labraña, A.M.; Martínez, M.A.; Leiva, A.M.; Garrido-Mendez, A.; Poblete-Valderrama, F.; Díaz-Martínez, X.; Salas, C.; Celis-Morales, C. Risk factors associated with hypertension. Analysis of the 2009–2010 Chilean health survey. Rev. Medica Chile 2017, 145, 996–1004. [Google Scholar] [CrossRef]
- NCEP. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation 2002, 106, 3143. [Google Scholar] [CrossRef]
- Concha-Cisternas, Y.; Vásquez-Gómez, J.; Castro-Piñero, J.; Petermann-Rocha, F.; Parra-Soto, S.; Matus-Castillo, C.; Garrido-Méndez, Á.; Poblete-Valderrama, F.; Celis-Morales, C. Niveles de actividad física y tiempo sedente en personas mayores con fragilidad: Resultados de la Encuesta Nacional de Salud 2016–2017. Nutr. Hosp. 2023, 40, 28–34. [Google Scholar]
- WHO. Obesity: Preventing and Managing the Global Epidemic; WHO: Geneva, Switzerland, 2000; Volume 894, pp. i–xii,1–253. [Google Scholar]
- MINSAL. Informe de Encuesta Nacional de Salud 2016–2017; Riesgo Cardiovascular Santiago de Chile; Ministerio de Salud de Chile: Santiago de Chile, Chile, 2018.
- Lo, K.; Au, M.; Ni, J.; Wen, C. Association between hypertension and osteoarthritis: A systematic review and meta-analysis of observational studies. J. Orthop. Transl. 2022, 32, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.H.; Nielsen, J.J.; Saltin, B.; Hellsten, Y. Exercise training normalizes skeletal muscle vascular endothelial growth factor levels in patients with essential hypertension. J. Hypertens. 2010, 28, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E.; Sato, Y.; Felig, P.; Wahren, J. Synergistic interaction between exercise and insulin on peripheral glucose uptake. J. Clin. Investig. 1981, 68, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Ruiz, M.E.; Guarner-Lans, V.; Pérez-Torres, I.; Soto, M.E. Mechanisms underlying metabolic syndrome-related sarcopenia and possible therapeutic measures. Int. J. Mol. Sci. 2019, 20, 647. [Google Scholar] [CrossRef]
- Niu, J.; Clancy, M.; Aliabadi, P.; Vasan, R.; Felson, D.T. Metabolic syndrome, its components, and knee osteoarthritis: The Framingham Osteoarthritis Study. Arthritis Rheumatol. 2017, 69, 1194–1203. [Google Scholar] [CrossRef]
- Veronese, N.; Stubbs, B.; Solmi, M.; Smith, T.O.; Noale, M.; Schofield, P.; Maggi, P. Knee osteoarthritis and risk of hypertension: A longitudinal cohort study. Rejuvenation Res. 2018, 21, 15–21. [Google Scholar] [CrossRef]
- Leiva, A.M.; Petermann-Rocha, F.; Martínez-Sanguinetti, M.A.; Troncoso-Pantoja, C.; Concha, Y.; Garrido-Méndez, A.; Díaz-martínez, X.; Lanuza-Rilling, F.; Ulloa, N.; Martorell, M.; et al. Asociación de un índice de estilos de vida saludable con factores de riesgo cardiovascular en población chilena. Rev. Medica De Chile 2018, 146, 1405–1414. [Google Scholar] [CrossRef]
- dos Santos, L.L.; Pinto de Castro, J.B.; Gama Linhares, D.; Barros dos Santos, A.O.; de Souza Cordeiro, L.; Borba-Pinheiro, C.J.; Gómez de souza, V. Efectos del ejercicio físico sobre los biomarcadores hepáticos en adultos: Revisión sistemática y metanálisis (Effects of Physical Exercise on Hepatic Biomarkers in Adult Individuals: A Systematic Review and Meta-Analysis). Retos 2023, 49, 762–774. [Google Scholar] [CrossRef]
- Shin, D. Association between metabolic syndrome, radiographic knee osteoarthritis, and intensity of knee pain: Results of a national survey. J. Clin. Endocrinol. Metab. 2014, 99, 3177–3183. [Google Scholar] [CrossRef]
| Low-SMM/High-WC | Low-SMM/Low-WC | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcomes | Hip-OAD | No-Hip-OAD | Knee-OAD | No-Knee-OAD | Hip-OAD | No-Hip-OAD | Knee-OAD | No-Knee-OAD |
| Age (y) | 76.9 ± 9.1 | 71.8 ± 8.3 | 73.4 ± 9.0 | 72.3 ± 8.5 | 76.9 ± 9.1 | 73.9 ± 9.2 | 74.7 ± 8.6 | 73.9 ± 9.2 |
| Height (cm) | 149.9 ± 6.8 | 152.9 ± 9.2 | 151.5 ± 7.4 | 152.8 ± 9.1 | 149.2 ± 6.2 | 151.2 ± 8.0 | 148.4 ± 5.3 | 151.8 ± 8.0 |
| Weight (kg) | 63.7 ± 8.7 | 66.4 ± 11.2 | 63.2 ± 8.9 | 66.4 ± 10.8 | 53.5 ± 5.3 | 53.2 ± 7.6 | 53.3 ± 6.3 | 53.3 ± 7.3 |
| Body mass index (kg/m2) | 28.4 ± 3.8 | 28.4 ± 3.7 | 27.7 ± 3.6 | 28.5 ± 3.7 | 24.4 ± 2.3 | 23.3 ± 2.9 | 24.3 ± 2.1 | |
| Waist circumference (cm) | 97.1 ± 9.1 | 98.0 ± 7.8 | 95.0 ± 4.8 | 98.2 ± 8.0 | 82.1 ± 5.1 | 78.9 ± 8.4 | 82.3 ± 6.6 | 78.8 ± 8.2 |
| Calf circumference (cm) | 29.2 ± 2.8 | 30.1 ± 2.5 | 29.2 ± 3.3 | 30.1 ± 2.3 | 29.5 ± 2.9 | 29.2 ± 2.6 | 29.8 ± 2.2 | 29.2 ± 2.6 |
| Fasting plasma glucose (mg/dL) | 98.8 ± 29.1 | 122.1 ± 68.9 | 114.3 ± 41.5 | 119.3 ± 68.9 | 104.4 ± 62.9 | 101.1 ± 39.2 | 104.3 ± 52.1 | 100.5 ± 39.3 |
| HbA1c (%) | 5.8 ± 0.5 | 7.2 ± 2.2 | 6.5 ± 1.0 | 7.2 ± 2.3 | 7.0 ± 2.7 | 6.6 ± 1.9 | 6.8 ± 2.1 | 6.6 ± 2.0 |
| Systolic blood pressure (mmHg) | 145 ± 17 | 147 ± 28 | 141 ± 21 | 147 ± 28 | 144 ± 22 | 140 ± 24 | 134 ± 25 | 140 ± 23 |
| Diastolic blood pressure (mmHg) | 70 ± 10 | 76 ± 11 | 72 ± 11 | 75 ± 11 | 73 ± 9 | 71 ± 11 | 71 ± 9 | 71 ± 10 |
| Total cholesterol (mg/dL) | 196.7 ± 49.2 | 193.8 ± 47.3 | 182.9 ± 40.5 | 196.3 ± 48.5 | 167.2 ± 33.7 | 186.6 ± 38.5 | 175.5 ± 51.7 | 186.8 ± 36.2 |
| Low-density lipids (mg/dL) | 112.9 ± 40.0 | 110.5 ± 39.2 | 98.7 ± 37.0 | 113.2 ± 39.3 | 93.8 ± 33.4 | 104.7 ± 33.6 | 98.6 ± 45.0 | 104.9 ± 31.6 |
| High-density lipids (mg/dL) | 49.5 ± 9.4 | 50.2 ± 14.8 | 54.1 ± 16.6 | 49.4 ± 13.7 | 50.3 ± 9.1 | 55.4 ± 16.3 | 50.5 ± 13.2 | 55.4 ± 16.1 |
| Triglycerides (mg/dL) | 171.8 ± 81.5 | 162.1 ± 287.8 | 150.8 ± 63.0 | 165.8 ± 90.6 | 116.8 ± 49.2 | 132.4 ± 85.0 | 132.7 ± 47.5 | 131.8 ± 87.0 |
| 25-OH Vitamin D2 + D3 (ng/mL) | 19.8 ± 10.4 | 15.6 ± 7.6 | 15.9 ± 6.3 | 16.4 ± 8.6 | 23.7 ± 10.2 | 17.6 ± 8.7 | 18.8 ± 8.3 | 18.0 ± 9.0 |
| Gamma glutamyl transaminase (UI/L) | 34.0 ± 36.1 | 31.2 ± 33.0 | 23.9 ± 14.0 | 33.0 ± 35.6 | 30.5 ± 31.5 | 27.5 ± 44.6 | 30.0 ± 29.0 | 27.2 ± 45.5 |
| Pyruvic glutamyl transaminase (UI/L) | 15.9 ± 4.7 | 21.1 ± 13.4 | 16.7 ± 5.9 | 21.1 ± 13.5 | 16.7 ± 6.7 | 18.7 ± 15.2 | 22.2 ± 26.3 | 18.1 ± 12.5 |
| C-Reactive protein (mg/L) | 0.95 ± 2.17 | 0.26 ± 0.28 | 0.75 ± 1.95 | 0.28 ± 0.27 | 0.76 ± 1.1 | 0.51 ± 1.31 | 0.55 ± 0.84 | 0.52 ± 1.36 |
| PAVI (min/week) | I.A.P | 8.0 ± 13.0 | I.A.P | 7.5 ± 15.0 | I.A.P | 1.3 ± 3.5 | I.A.P | 1.4 ± 3.7 |
| PAMI (min/week) | I.A.P. | 4.4 ± 10.0 | I.A.P | 4.6 ± 10.2 | 10.0 ± 17.3 | 4.3 ± 8.6 | 15.0 ± 21.2 | 4.6 ± 9.2 |
| PALI (min/week) | 13.5 ± 14.3 | 14.5 ± 15.3 | 11.4 ± 17.3 | 14.8 ± 14.6 | 19.3 ± 14.3 | 12.4 ± 13.2 | 15.5 ± 11.3 | 12.5 ± 13.4 |
| High-SMM/High-WC | High-SMM/Low-WC | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcomes | Hip-OAD | No-Hip-OAD | Knee-OAD | No-Knee-OAD | Hip-OAD | No-Hip-OAD | Knee-OAD | No-Knee-OAD |
| Age (y) | 72.1 ± 7.8 | 69.6 ± 7.3 | 71.5 ± 8.1 | 69.5 ± 7.2 | 62.4 ± 11.7 | 41.3 ± 18.3 | 62.7 ± 10.6 | 40.9 ± 18.2 |
| Height (cm) | 153.2 ± 8.9 | 158.1 ± 9.3 | 154.8 ± 9.0 | 158.6 ± 9.3 | 151.5 ± 6.0 | 156.6 ± 6.6 | 152.9 ± 6.0 | 157.0 ± 6.6 |
| Weight (kg) | 76.9 ± 13.6 | 78.0 ± 12.2 | 78.2 ± 13.1 | 77.9 ± 12.1 | 61.0 ± 8.8 | 61.1 ± 8.2 | 61.6 ± 7.9 | 61.0 ± 8.3 |
| Body mass index (kg/m2) | 32.7 ± 5.0 | 31.3 ± 4.7 | 32.8 ± 5.3 | 31.1 ± 4.6 | 26.6 ± 3.4 | 24.9 ± 3.1 | 26.5 ± 3.0 | 24.9 ± 3.1 |
| Waist circumference (cm) | 103.8 ± 10.5 | 103.1 ± 9.4 | 104.4 ± 10.3 | 103.0 ± 9.7 | 80.1 ± 10.8 | 80.0 ± 7.3 | 82.4 ± 8.8 | 79.8 ± 7.3 |
| Calf circumference (cm) | 37.0 ± 3.8 | 37.0 ± 3.5 | 37.3 ± 3.6 | 36.9 ± 3.6 | 35.0 ± 2.2 | 34.5 ± 4.1 | 34.9 ± 2.0 | 34.5 ± 4.3 |
| Fasting plasma glucose (mg/dL) | 110.5 ± 43.1 | 111.9 ± 41.5 | 111.0 ± 38.0 | 112.0 ± 42.6 | 92.6 ± 13.3 | 90.3 ± 25.8 | 96.6 ± 25.4 | 90.0 ± 25.5 |
| HbA1c (%) | 6.8 ± 1.8 | 6.6 ± 1.5 | 6.6 ± 1.5 | 6.6 ± 1.6 | 6.1 ± 1.0 | 6.0 ± 1.58 | 6.6 ± 1.5 | 6.0 ± 1.5 |
| Systolic blood pressure (mmHg) | 141 ± 22 | 144 ± 22.0 | 141 ± 21 | 144 ± 22.1 | 126 ± 19 | 116 ± 19 | 134 ± 22 | 115.8 ± 18.5 |
| Diastolic blood pressure (mmHg) | 75 ± 9 | 78 ± 10 | 75 ± 9 | 78 ± 10 | 73 ± 10 | 70 ± 10 | 76 ± 12 | 70 ± 9 |
| Total cholesterol (mg/dL) | 182.6 ± 40.0 | 182.0 ± 41.2 | 179.0 ± 37.5 | 182.9 ± 41.8 | 191.7 ± 29.1 | 176.6 ± 37.4 | 188.1 ± 38.8 | 176.4 ± 37.0 |
| Low-density lipids (mg/dL) | 104.5 ± 35.3 | 104.8 ± 34.7 | 101.8 ± 33.4 | 105.6 ± 35.2 | 107.9 ± 30.3 | 101.3 ± 31.4 | 108.0 ± 37.4 | 101.1 ± 31.0 |
| High-density lipids (mg/dL) | 49.7 ± 15.1 | 46.3 ± 12.3 | 47.3 ± 12.7 | 46.5 ± 12.8 | 54.5 ± 12.4 | 52.7 ± 13.1 | 50.4 ± 12.6 | 52.9 ± 13.1 |
| Triglycerides (mg/dL) | 144.1 ± 64.9 | 154.0 ± 80.9 | 151.0 ± 63.7 | 153.5 ± 82.2 | 146.1 ± 60.5 | 112.0 ± 61.4 | 148.5 ± 74.0 | 111.2 ± 60.1 |
| 25-OH Vitamin D2 + D3 (ng/mL) | 20.6 ± 10.0 | 18.7 ± 8.2 | 18.5 ± 8.3 | 19.0 ± 8.6 | 19.0 ± 6.0 | 20.7 ± 8.3 | 19.1 ± 9.4 | 20.7 ± 8.2 |
| Gamma glutamyl transaminase (UI/L) | 36.3 ± 38.4 | 38.7 ± 58.5 | 33.5 ± 33.1 | 39.7 ± 60.5 | 27.3 ± 27.8 | 23.9 ± 41.1 | 35.3 ± 55.6 | 23.4 ± 39.6 |
| Pyruvic glutamyl transaminase (UI/L) | 22.7 ± 13.1 | 24.9 ± 17.8 | 23.1 ± 13.9 | 25.1 ± 18.0 | 20.8 ± 21.4 | 18.3 ± 13.3 | 23.4 ± 22.2 | 18.1 ± 12.9 |
| C-Reactive protein (mg/L) | 0.43 ± 0.46 | 0.43 ± 0.80 | 0.38 ± 0.41 | 0.46 ± 0.87 | 0.43 ± 0.64 | 0.35 ± 0.93 | 0.41 ± 0.64 | 0.36 ± 0.95 |
| PAVI (min/week) | 6.5 ± 11.0 | 6.4 ± 12.2 | 6.8 ± 11.7 | 6.3 ± 12.2 | 3.7 ± 7.5 | 5.6 ± 11.8 | 0.2 ± 0.7 | 5.8 ± 11.9 |
| PAMI (min/week) | 7.7 ± 12.4 | 5.5 ± 10.4 | 8.2 ± 12.4 | 5.2 ± 10.3 | 5.0 ± 10.8 | 3.8 ± 9.7 | 7.4 ± 13.8 | 3.7 ± 9.5 |
| PALI (min/week) | 14.7 ± 13.7 | 13.2 ± 14.0 | 14.8 ± 13.9 | 13.0 ± 13.9 | 13.1 ± 15.6 | 13.5 ± 14.1 | 12.8 ± 14.8 | 13.5 ± 14.1 |
| Outcomes | β | SE | Wald | McFadden Pseudo R2 | OR (95%CI) | p-Value |
|---|---|---|---|---|---|---|
| Suspected of ‘Diabetes’ | ||||||
| Model 1: Low-SMM/High-WC | 1.055 | 0.237 | 19.883 | 0.146 | 2.87 (1.80; 4.56) | p < 0.0001 |
| Model 2: Low-SMM/Low-WC | 0.662 | 0.220 | 9.076 | 1.93 (1.26; 2.98) | p = 0.003 | |
| Model 3: High-SMM/High-WC | 0.882 | 0.182 | 23.488 | 2.41 (1.69; 3.45) | p < 0.0001 | |
| Model 4: High-SMM/Low-WC | - | - | - | 1.00 (Ref.) | - | |
| Suspected of ‘Arterial Hypertension’ | ||||||
| Model 1: Low-SMM/High-WC | 0.994 | 0.224 | 19.703 | 0.322 | 2.70 (1.74; 4.19) | p < 0.0001 |
| Model 2: Low-SMM/Low-WC | 0.341 | 0.170 | 3.996 | 1.40 (1.00; 1.96) | p = 0.046 | |
| Model 3: High-SMM/High-WC | 1.139 | 0.120 | 89.502 | 3.12 (2.46; 3.95) | p < 0.0001 | |
| Model 4: High-SMM/Low-WC | - | - | - | 1.00 (Ref.) | - | |
| Suspected of ‘Metabolic Syndrome’ | ||||||
| Model 1: Low-SMM/High-WC | 1.514 | 0.258 | 34.342 | 0.237 | 4.54 (2.74; 7.54) | p < 0.0001 |
| Model 2: Low-SMM/Low-WC | −0.113 | 0.211 | 0.285 | 0.89 (0.59; 1.35) | p = 0.594 | |
| Model 3: High-SMM/High-WC | 1.953 | 0.157 | 154.583 | 7.04 (5.18; 9.59) | p < 0.0001 | |
| Model 4: High-SMM/Low-WC | - | - | - | 1.00 (Ref.) | - | |
| Suspected of ‘Moderate Cardiovascular Risk’ | ||||||
| Model 1: Low-SMM/High-WC | 1.016 | 0.400 | 6.457 | 0.221 | 2.76 (1.26; 6.04) | p = 0.011 |
| Model 2: Low-SMM/Low-WC | −0.076 | 0.306 | 0.062 | 0.92 (0.50; 1.68) | p = 0.803 | |
| Model 3: High-SMM/High-WC | 0.629 | 0.241 | 6.798 | 1.87 (1.16; 3.01) | p = 0.009 | |
| Model 4: High-SMM/Low-WC | - | - | - | 1.00 (Ref.) | - | |
| Suspected of ‘High Cardiovascular Risk’ | ||||||
| Model 1: Low-SMM/High-WC | 1.711 | 0.352 | 23.705 | 0.221 | 5.53 (2.78; 11.0) | p < 0.0001 |
| Model 2: Low-SMM/Low-WC | 0.873 | 0.227 | 14.766 | 2.39 (1.53; 3.73) | p < 0.0001 | |
| Model 3: High-SMM/High-WC | 1.279 | 0.211 | 36.566 | 3.59 (2.37; 5.43) | p < 0.0001 | |
| Model 4: High-SMM/Low-WC | - | - | - | 1.00 (Ref.) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guede-Rojas, F.; Ibacache-Saavedra, P.; Leal, M.I.; Tuesta, M.; Durán-Marín, C.; Carrasco-Marín, F.; Cigarroa, I.; Alvarez, C.; Izquierdo, M.; Delgado-Floody, P. A Higher Skeletal Muscle Mass and Lower Adiposity Phenotype Is Associated with Better Cardiometabolic Control in Adults with Hip and Knee Osteoarthritis: Results from the Chilean National Health Survey 2016–2017. Nutrients 2023, 15, 4263. https://doi.org/10.3390/nu15194263
Guede-Rojas F, Ibacache-Saavedra P, Leal MI, Tuesta M, Durán-Marín C, Carrasco-Marín F, Cigarroa I, Alvarez C, Izquierdo M, Delgado-Floody P. A Higher Skeletal Muscle Mass and Lower Adiposity Phenotype Is Associated with Better Cardiometabolic Control in Adults with Hip and Knee Osteoarthritis: Results from the Chilean National Health Survey 2016–2017. Nutrients. 2023; 15(19):4263. https://doi.org/10.3390/nu15194263
Chicago/Turabian StyleGuede-Rojas, Francisco, Paulina Ibacache-Saavedra, María Inés Leal, Marcelo Tuesta, Cristóbal Durán-Marín, Fernanda Carrasco-Marín, Igor Cigarroa, Cristian Alvarez, Mikel Izquierdo, and Pedro Delgado-Floody. 2023. "A Higher Skeletal Muscle Mass and Lower Adiposity Phenotype Is Associated with Better Cardiometabolic Control in Adults with Hip and Knee Osteoarthritis: Results from the Chilean National Health Survey 2016–2017" Nutrients 15, no. 19: 4263. https://doi.org/10.3390/nu15194263
APA StyleGuede-Rojas, F., Ibacache-Saavedra, P., Leal, M. I., Tuesta, M., Durán-Marín, C., Carrasco-Marín, F., Cigarroa, I., Alvarez, C., Izquierdo, M., & Delgado-Floody, P. (2023). A Higher Skeletal Muscle Mass and Lower Adiposity Phenotype Is Associated with Better Cardiometabolic Control in Adults with Hip and Knee Osteoarthritis: Results from the Chilean National Health Survey 2016–2017. Nutrients, 15(19), 4263. https://doi.org/10.3390/nu15194263










