Ex Vivo Colonic Fermentation of NUTRIOSE® Exerts Immuno-Modulatory Properties and Strong Anti-Inflammatory Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. NUTRIOSE® Pre-Digestion
2.2. Colon-on-a-plate™
2.3. Caco-2/THP1-Blue™ Co-Culture Model
2.4. Microbial Metabolic Activity Analysis
2.5. Microbial Community Analysis
2.6. Statistical Methods
3. Results
3.1. Analysis of Host-Microbe Interactions
3.1.1. Transepithelial Electrical Resistance
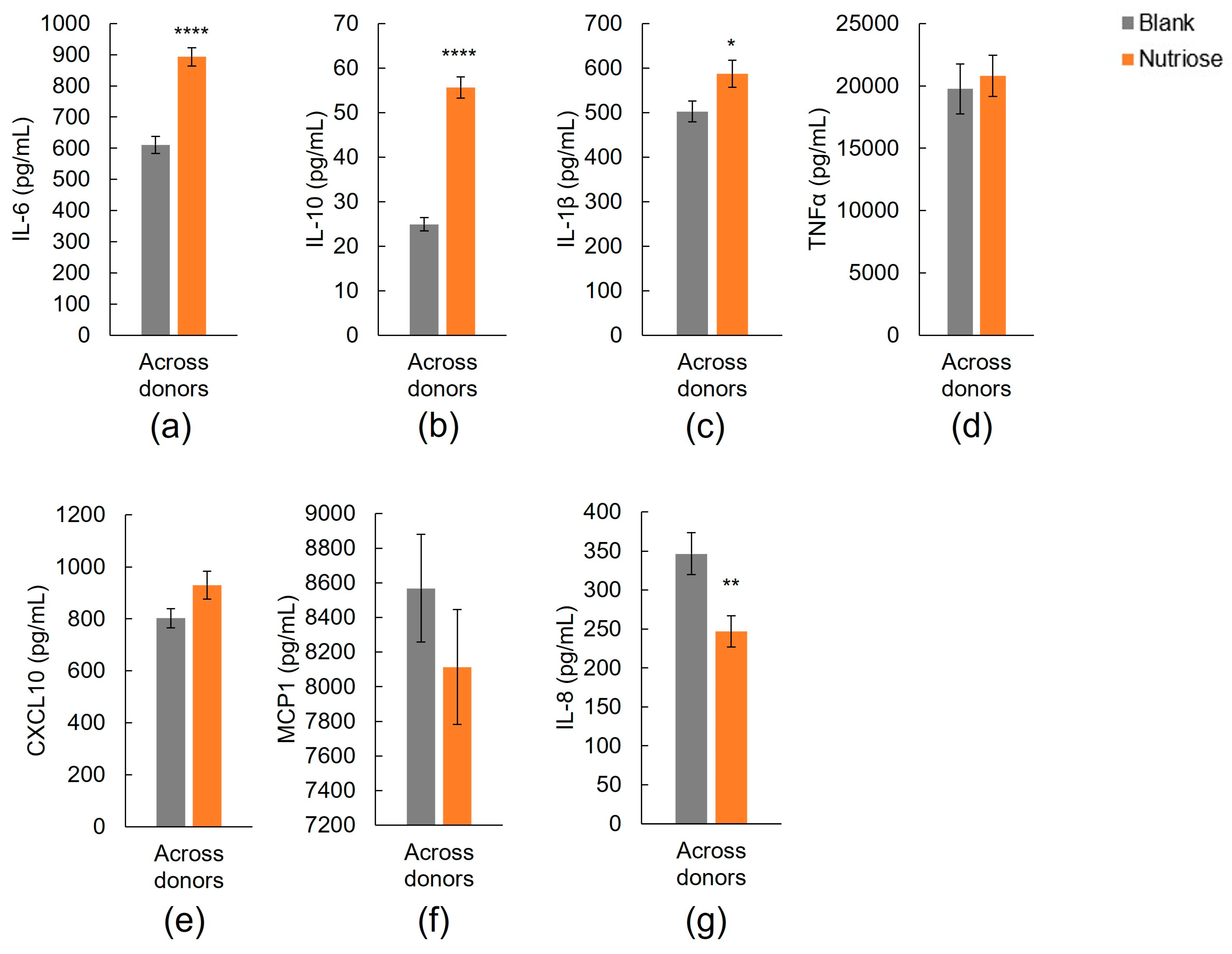
3.1.2. Immune Markers
3.2. Microbial Community Analysis
3.2.1. Fermentation Activity and Changes in Metabolite Production
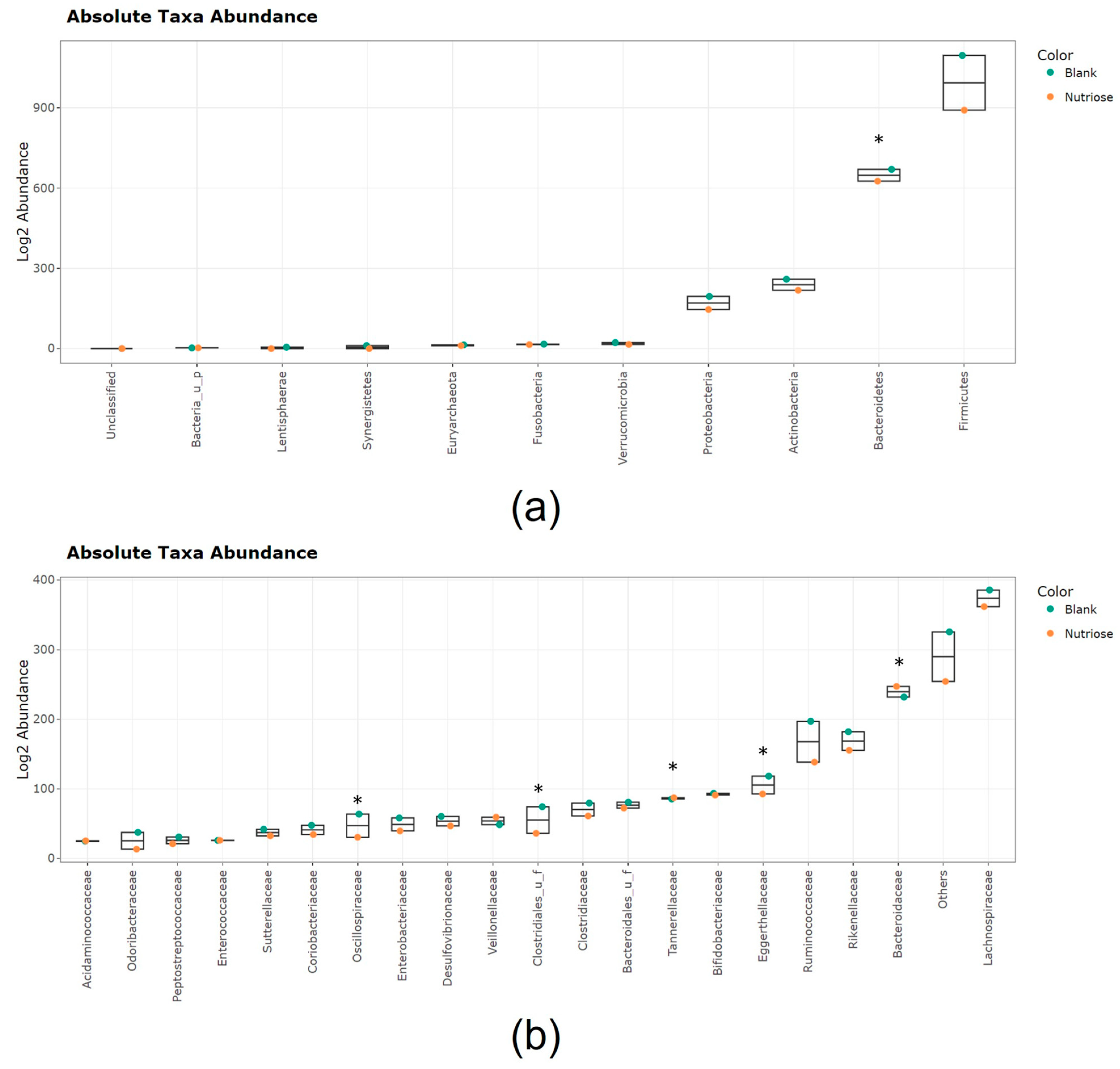
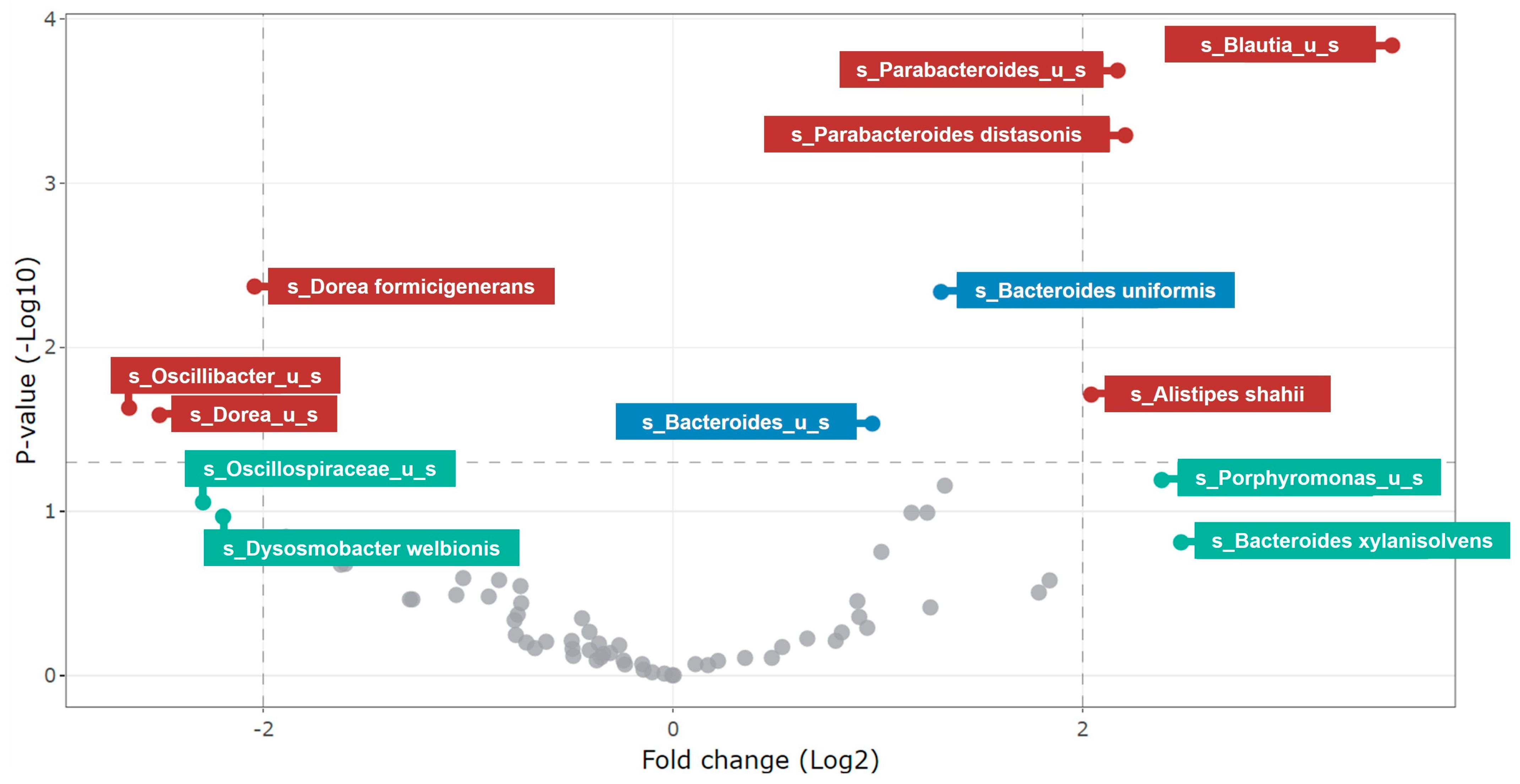
3.2.2. Changes in Microbial Community Composition
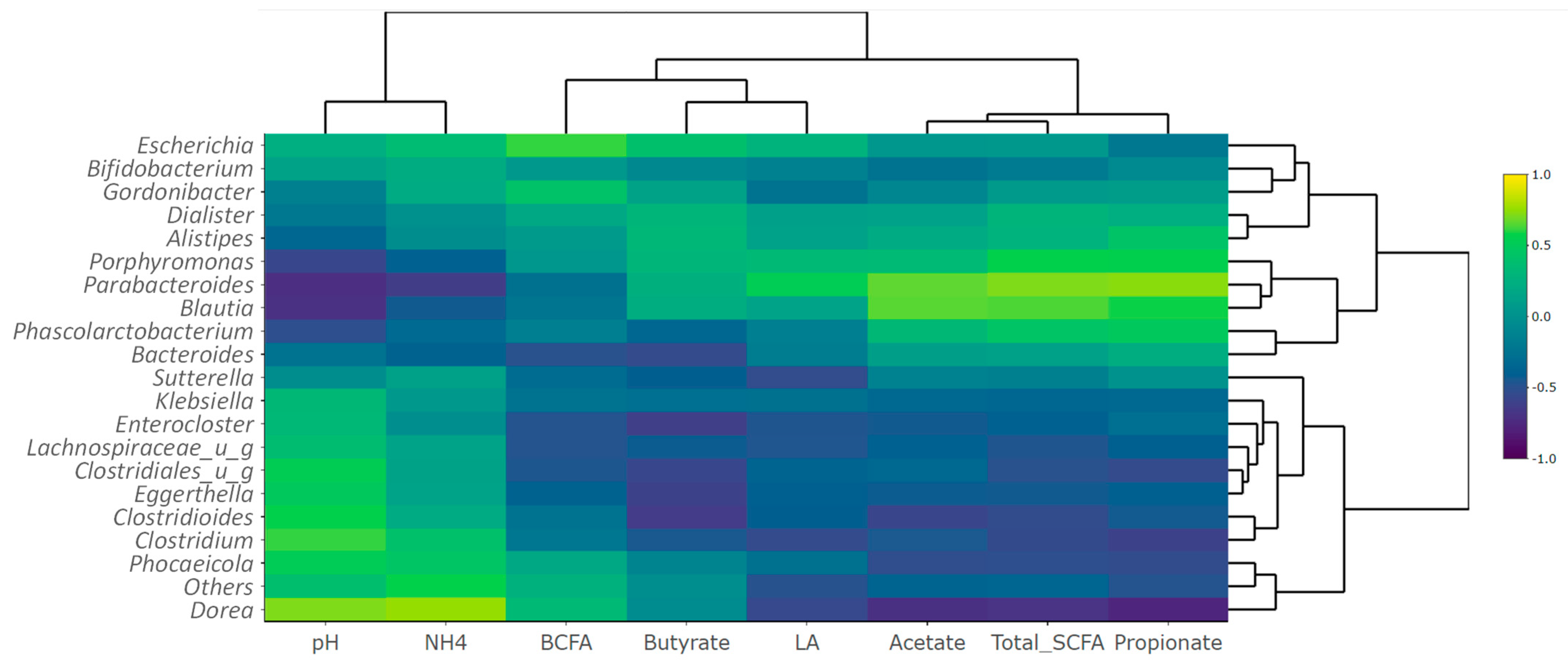
3.2.3. Metabolite-Metagenomics Correlations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vallianou, N.; Christodoulatos, G.S.; Karampela, I.; Tsilingiris, D.; Magkos, F.; Stratigou, T.; Kounatidis, D.; Dalamaga, M. Understanding the role of the gut microbiome and microbial metabolites in non-alcoholic fatty liver disease: Current evidence and perspectives. Biomolecules 2021, 12, 56. [Google Scholar] [CrossRef]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef]
- Kostic, A.D.; Gevers, D.; Siljander, H.; Vatanen, T.; Hyotylainen, T.; Hamalainen, A.M.; Peet, A.; Tillmann, V.; Poho, P.; Mattila, I.; et al. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe 2015, 17, 260–273. [Google Scholar] [CrossRef]
- Clemente, J.C.; Manasson, J.; Scher, J.U. The role of the gut microbiome in systemic inflammatory disease. BMJ 2018, 360, j5145. [Google Scholar] [CrossRef]
- Hevia, A.; Milani, C.; Lopez, P.; Cuervo, A.; Arboleya, S.; Duranti, S.; Turroni, F.; Gonzalez, S.; Suarez, A.; Gueimonde, M.; et al. Intestinal dysbiosis associated with systemic lupus erythematosus. mBio 2014, 5, e01548-14. [Google Scholar] [CrossRef]
- Dabke, K.; Hendrick, G.; Devkota, S. The gut microbiome and metabolic syndrome. J. Clin. Investig. 2019, 129, 4050–4057. [Google Scholar] [CrossRef]
- Chen, X.; Devaraj, S. Gut microbiome in obesity, metabolic syndrome, and diabetes. Curr. Diabetes Rep. 2018, 18, 129. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Health benefits of non-digestible oligosaccharides. Diet. Fiber Health Dis. 1997, 427, 211–219. [Google Scholar]
- Priyadarshini, M.; Kotlo, K.U.; Dudeja, P.K.; Layden, B.T. Role of short shain fatty acid receptors in intestinal physiology and pathophysiology. Compr. Physiol. 2018, 8, 1091–1115. [Google Scholar]
- Lefranc-Millot, C. NUTRIOSE® 06: A useful soluble dietary fibre for added nutritional value. Nutr. Bull. 2008, 33, 234–239. [Google Scholar] [CrossRef]
- Vermorel, M.; Coudray, C.; Wils, D.; Sinaud, S.; Tressol, J.C.; Montaurier, C.; Vernet, J.; Brandolini, M.; Bouteloup-Demange, C.; Rayssiguier, Y. Energy value of a low-digestible carbohydrate, NUTRIOSE FB, and its impact on magnesium, calcium and zinc apparent absorption and retention in healthy young men. Eur. J. Nutr. 2004, 43, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Van den Heuvel, E.G.; Wils, D.; Pasman, W.J.; Bakker, M.; Saniez, M.H.; Kardinaal, A.F. Short-term digestive tolerance of different doses of NUTRIOSE FB, a food dextrin, in adult men. Eur. J. Clin. Nutr. 2004, 58, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Guerin-Deremaux, L.; Ringard, F.; Desailly, F.; Wils, D. Effects of a soluble dietary fibre NUTRIOSE® on colonic fermentation and excretion rates in rats. Nutr. Res. Pract. 2010, 4, 470–476. [Google Scholar] [CrossRef][Green Version]
- Guérin-Deremaux, L.; Hobden, M.R.; Zhang, C.; Neut, C.; Thabuis, C.; Vazhappilly, R.; Gibson, G.R.; Kennedy, O.B. NUTRIOSE® soluble fiber selectively modulates gut microbiota composition in healthy volunteers. In Proceedings of the FENS 2019 13th European Nutrition Converence, Dublin, Ireland, 15–18 October 2019. [Google Scholar]
- Lefranc-Millot, C.; Guerin-Deremaux, L.; Wils, D.; Neut, C.; Miller, L.E.; Saniez-Degrave, M.H. Impact of a resistant dextrin on intestinal ecology: How altering the digestive ecosystem with NUTRIOSE®, a soluble fibre with prebiotic properties, may be beneficial for health. J. Int. Med. Res. 2012, 40, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Thirion, F.; da Silva, K.; Plaza Onate, F.; Alvarez, A.S.; Thabuis, C.; Pons, N.; Berland, M.; Le Chatelier, E.; Galleron, N.; Levenez, F.; et al. Diet supplementation with NUTRIOSE, a resistant dextrin, increases the abundance of Parabacteroides distasonis in the human gut. Mol. Nutr. Food Res. 2022, 66, e2101091. [Google Scholar] [CrossRef]
- Pouillart, P.R.; Depeint, F.; Abdelnour, A.; Deremaux, L.; Vincent, O.; Maziere, J.C.; Madec, J.Y.; Chatelain, D.; Younes, H.; Wils, D.; et al. Nutriose, a prebiotic low-digestible carbohydrate, stimulates gut mucosal immunity and prevents TNBS-induced colitis in piglets. Inflamm. Bowel Dis. 2010, 16, 783–794. [Google Scholar] [CrossRef]
- Farhangi, M.A.; Javid, A.Z.; Sarmadi, B.; Karimi, P.; Dehghan, P. A randomized controlled trial on the efficacy of resistant dextrin, as functional food, in women with type 2 diabetes: Targeting the hypothalamic-pituitary-adrenal axis and immune system. Clin. Nutr. 2018, 37, 1216–1223. [Google Scholar] [CrossRef]
- Aliasgharzadeh, A.; Dehghan, P.; Gargari, B.P.; Asghari-Jafarabadi, M. Resistant dextrin, as a prebiotic, improves insulin resistance and inflammation in women with type 2 diabetes: A randomised controlled clinical trial. Br. J. Nutr. 2015, 113, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Perreau, C.; Thabuis, C.; Sergent, J.; Bitane, V.; Scotte, A.; Desailly, F.; Ringard, F.; Herbomez, A.C.; Desreumaux, P.; Guerin-Deremaux, L. Effects of a prebiotic soluble fiber NUTRIOSE® on intestinal immune system and gut homeostasis. In Proceedings of the International Scientific Association for Probiotics and Prebiotics Annual Meeting, Barcelona, Spain, 15–17 June 2022. [Google Scholar]
- Lefranc-Millot, C.; Deremaux, L.; Rousseaux, C.; Wils, D.; Saniez-Degrave, M.H.; Desreumaux, P. Impact of a new resistant dextrin on intestinal well-being and immunity maintenance. Ann. Nutr. Metabol. 2007, 51, 154. [Google Scholar]
- Van den Abbeele, P.; Verstrepen, L.; Ghyselinck, J.; Albers, R.; Marzorati, M.; Mercenier, A. A novel non-digestible, carrot-derived polysaccharide (cRG-I) selectively modulates the human gut microbiota while promoting gut barrier integrity: An integrated in vitro approach. Nutrients 2020, 12, 1917. [Google Scholar] [CrossRef]
- Marzorati, M.; Bubeck, S.; Bayne, T.F.; Krishnan, K.; Young, A. Evaluation of the effect of food products containing prebiotics and Bacillus subtilis HU58 on the gut microbial community activity and community composition using an in vitro M-SHIME® model. Appl. Sci. 2021, 11, 11963. [Google Scholar] [CrossRef]
- Verhoeckx, K.; Cotter, P.; Lopez-Exposito, I.; Kleiveland, C.; Lea, T.; Mackie, A.; Requena, T.; Swiatecka, D.; Wichers, H. (Eds.) Part VI, In Vitro Fermentation Models: General Introduction. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Daguet, D.; Pinheiro, I.; Verhelst, A.; Possemiers, S.; Marzorati, M. Arabinogalactan and fructooligosaccharides improve the gut barrier function in distinct areas of the colon in the Simulator of the Human Intestinal Microbial Ecosystem. J. Funct. Foods 2016, 20, 369–379. [Google Scholar] [CrossRef]
- De Weirdt, R.; Possemiers, S.; Vermeulen, G.; Moerdijk-Poortvliet, T.C.; Boschker, H.T.; Verstraete, W.; Van de Wiele, T. Human faecal microbiota display variable patterns of glycerol metabolism. FEMS Microbiol. Ecol. 2010, 74, 601–611. [Google Scholar] [CrossRef] [PubMed]
- De Wiele, T.V.; Boon, N.; Possemiers, S.; Jacobs, H.; Verstraete, W. Prebiotic effects of chicory inulin in the simulator of the human intestinal microbial ecosystem. FEMS Microbiol. Ecol. 2004, 51, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Boon, N.; Top, E.M.; Verstraete, W.; Siciliano, S.D. Bioaugmentation as a tool to protect the structure and function of an activated-sludge microbial community against a 3-chloroaniline shock load. Appl. Environ. Microbiol. 2003, 69, 1511–1520. [Google Scholar] [CrossRef]
- Duysburgh, C.; van den Abbeele, P.; Krishnan, K.; Bayne, T.F.; Marzorati, M. A synbiotic concept containing spore-forming Bacillus strains and a prebiotic fiber blend consistently enhanced metabolic activity by modulation of the gut microbiome in vitro. Int. J. Pharm. X 2019, 1, 100021. [Google Scholar] [CrossRef]
- Ottesen, A.; Ramachandran, P.; Reed, E.; White, J.R.; Hasan, N.; Subramanian, P.; Ryan, G.; Jarvis, K.; Grim, C.; Daquiqan, N.; et al. Enrichment dynamics of Listeria monocytogenes and the associated microbiome from naturally contaminated ice cream linked to a listeriosis outbreak. BMC Microbiol. 2016, 16, 275. [Google Scholar] [CrossRef]
- Ponnusamy, D.; Kozlova, E.V.; Sha, J.; Erova, T.E.; Azar, S.R.; Fitts, E.C.; Kirtley, M.L.; Tiner, B.L.; Andersson, J.A.; Grim, C.J.; et al. Cross-talk among flesh-eating Aeromonas hydrophila strains in mixed infection leading to necrotizing fasciitis. Proc. Natl. Acad. Sci. USA 2016, 113, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.A.; Young, B.A.; Minard-Smith, A.T.; Saeed, K.; Li, H.; Heizer, E.M.; McMillan, N.J.; Isom, R.; Abdullah, A.S.; Bornman, D.M.; et al. Microbial community profiling of human saliva using shotgun metagenomic sequencing. PLoS ONE 2014, 9, e97699. [Google Scholar] [CrossRef] [PubMed]
- Lax, S.; Smith, D.P.; Hampton-Marcell, J.; Owens, S.M.; Handley, K.M.; Scott, N.M.; Gibbons, S.M.; Larsen, P.; Shogan, B.D.; Weiss, S.; et al. Longitudinal analysis of microbial interaction between humans and the indoor environment. Science 2014, 345, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Soneson, C.; Germain, P.L.; Schmidt, T.S.B.; Mering, C.V.; Robinson, M.D. treeclimbR pinpoints the data-dependent resolution of hierarchical hypotheses. Genome Biol. 2021, 22, 157. [Google Scholar] [CrossRef]
- Anderson, J.M.; Van Itallie, C.M. Physiology and function of the tight junction. Cold Spring Harb. Perspect. Biol. 2009, 1, a002584. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Miele, L.; Valenza, V.; la Torre, G.; Montalto, M.; Cammarota, G.; Ricci, R.; Masciana, R.; Forgione, A.; Gabrieli, M.L.; Perotti, G.; et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009, 49, 1877–1887. [Google Scholar] [CrossRef]
- Hamilton, M.K.; Boudry, G.; Lemay, D.G.; Raybould, H.E. Changes in intestinal barrier function and gut microbiota in high-fat diet-fed rats are dynamic and region dependent. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G840–G851. [Google Scholar] [CrossRef]
- Fasano, A. Leaky gut and autoimmune diseases. Clin. Rev. Allergy Immunol. 2012, 42, 71–78. [Google Scholar] [CrossRef]
- Paray, B.A.; Albeshr, M.F.; Jan, A.T.; Rather, I.A. Leaky gut and autoimmunity: An intricate balance in individuals health and the diseased state. Int. J. Mol. Sci. 2020, 21, 9770. [Google Scholar] [CrossRef]
- Perreau, C.; Albert, M.; Sergent, J.; Bitane, V.; Scotte, A.; Vazhappilly, R.; Desailly, F.; Ringard, F.; Herbomez, A.C.; Guerin-Deremaux, L.; et al. NUTRIOSE® fibre is fermented in the colon inducing a modulation of genes expression involved in glucose metabolism and membrane integrity. Proc. Nutr. Soc. 2020, 79, E249. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Taminiau, B.; Pinheiro, I.; Duysburgh, C.; Jacobs, H.; Pijls, L.; Marzorati, M. Arabinoxylo-oligosaccharides and inulin impact inter-individual variation on microbial metabolism and composition, which immunomodulates human cells. J. Agric. Food Chem. 2018, 66, 1121–1130. [Google Scholar] [CrossRef]
- Marzorati, M.; Bubeck, S.; Bayne, T.; Krishnan, K.; Giusto, M. Effects of combined prebiotic, probiotic, IgG and amino acid supplementation on the gut microbiome of patients with inflammatory bowel disease. Future Microbiol. 2022, 17, 1307–1324. [Google Scholar] [CrossRef] [PubMed]
- Lafontaine, G.M.F.; Fish, N.M.; Connerton, I.F. In vitro evaluation of the effects of commercial prebiotic GOS and FOS products on human colonic Caco-2 cells. Nutrients 2020, 12, 1281. [Google Scholar] [CrossRef]
- Lindsay, J.O.; Whelan, K.; Stagg, A.J.; Gobin, P.; Al-Hassi, H.O.; Rayment, N.; Kamm, M.A.; Knight, S.C.; Forbes, A. Clinical, microbiological, and immunological effects of fructo-oligosaccharide in patients with Crohn’s disease. Gut 2006, 55, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Vulevic, J.; Juric, A.; Walton, G.E.; Claus, S.P.; Tzortzis, G.; Toward, R.E.; Gibson, G.R. Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. Br. J. Nutr. 2015, 114, 586–595. [Google Scholar] [CrossRef]
- Watzl, B.; Girrbach, S.; Roller, M. Inulin, oligofructose and immunomodulation. Br. J. Nutr. 2005, 93 (Suppl. 1), S49–S55. [Google Scholar] [CrossRef]
- Fibbiani, M.; Ghelli Luserna, D.I.R.L.; Novelli, T.; Peroni, D.G. The impact of human milk oligosaccharides on health from infancy to childhood. Minerva Pediatr. 2022, 74, 724–732. [Google Scholar] [CrossRef]
- Vogt, L.; Meyer, D.; Pullens, G.; Faas, M.; Smelt, M.; Venema, K.; Ramasamy, U.; Schols, H.A.; De Vos, P. Immunological properties of inulin-type fructans. Crit. Rev. Food Sci. Nutr. 2015, 55, 414–436. [Google Scholar] [CrossRef]
- Lin, M.Y.; de Zoete, M.R.; van Putten, J.P.; Strijbis, K. Redirection of epithelial immune responses by short-chain fatty acids through inhibition of histone deacetylases. Front. Immunol. 2015, 6, 554. [Google Scholar] [CrossRef]
- Nielsen, D.S.G.; Jensen, B.B.; Theil, P.K.; Nielsen, T.S.; Knudsen, K.E.B.; Paurup, S. Effect of butyrate and fermentation products on epithelial integrity in a mucus-secreting human colon cell line. J. Funct. Foods 2018, 40, 9–17. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, Y.; Wang, P.; Huang, Y.; Wang, F. Short-Chain Fatty Acids Manifest Stimulative and Protective Effects on Intestinal Barrier Function Through the Inhibition of NLRP3 Inflammasome and Autophagy. Cell. Physiol. Biochem. 2018, 49, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C.; et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef] [PubMed]
- Haller, T.; Buckel, T.; Retey, J.; Gerlt, J.A. Discovering new enzymes and metabolic pathways: Conversion of succinate to propionate by Escherichia coli. Biochemistry 2000, 39, 4622–4629. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perreau, C.; Thabuis, C.; Verstrepen, L.; Ghyselinck, J.; Marzorati, M. Ex Vivo Colonic Fermentation of NUTRIOSE® Exerts Immuno-Modulatory Properties and Strong Anti-Inflammatory Effects. Nutrients 2023, 15, 4229. https://doi.org/10.3390/nu15194229
Perreau C, Thabuis C, Verstrepen L, Ghyselinck J, Marzorati M. Ex Vivo Colonic Fermentation of NUTRIOSE® Exerts Immuno-Modulatory Properties and Strong Anti-Inflammatory Effects. Nutrients. 2023; 15(19):4229. https://doi.org/10.3390/nu15194229
Chicago/Turabian StylePerreau, Caroline, Clementine Thabuis, Lynn Verstrepen, Jonas Ghyselinck, and Massimo Marzorati. 2023. "Ex Vivo Colonic Fermentation of NUTRIOSE® Exerts Immuno-Modulatory Properties and Strong Anti-Inflammatory Effects" Nutrients 15, no. 19: 4229. https://doi.org/10.3390/nu15194229
APA StylePerreau, C., Thabuis, C., Verstrepen, L., Ghyselinck, J., & Marzorati, M. (2023). Ex Vivo Colonic Fermentation of NUTRIOSE® Exerts Immuno-Modulatory Properties and Strong Anti-Inflammatory Effects. Nutrients, 15(19), 4229. https://doi.org/10.3390/nu15194229






