Siraitia grosvenorii Extract Attenuates Airway Inflammation in a Mouse Model of Respiratory Disease Induced by Particulate Matter 10 Plus Diesel Exhaust Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction of SGE
2.2. Mouse Model
2.3. Collection of Mouse Lung Cells from Bronchoalveolar Lavage Fluid
2.4. Measurement of Cytokines
2.5. Histopathological Examination of Lung Tissue
2.6. Quantitative Reverse-Transcription–Polymerase Chain Reaction
2.7. Flow Cytometry Analysis
2.8. Immunoblot
2.9. Statistical Analysis
3. Results
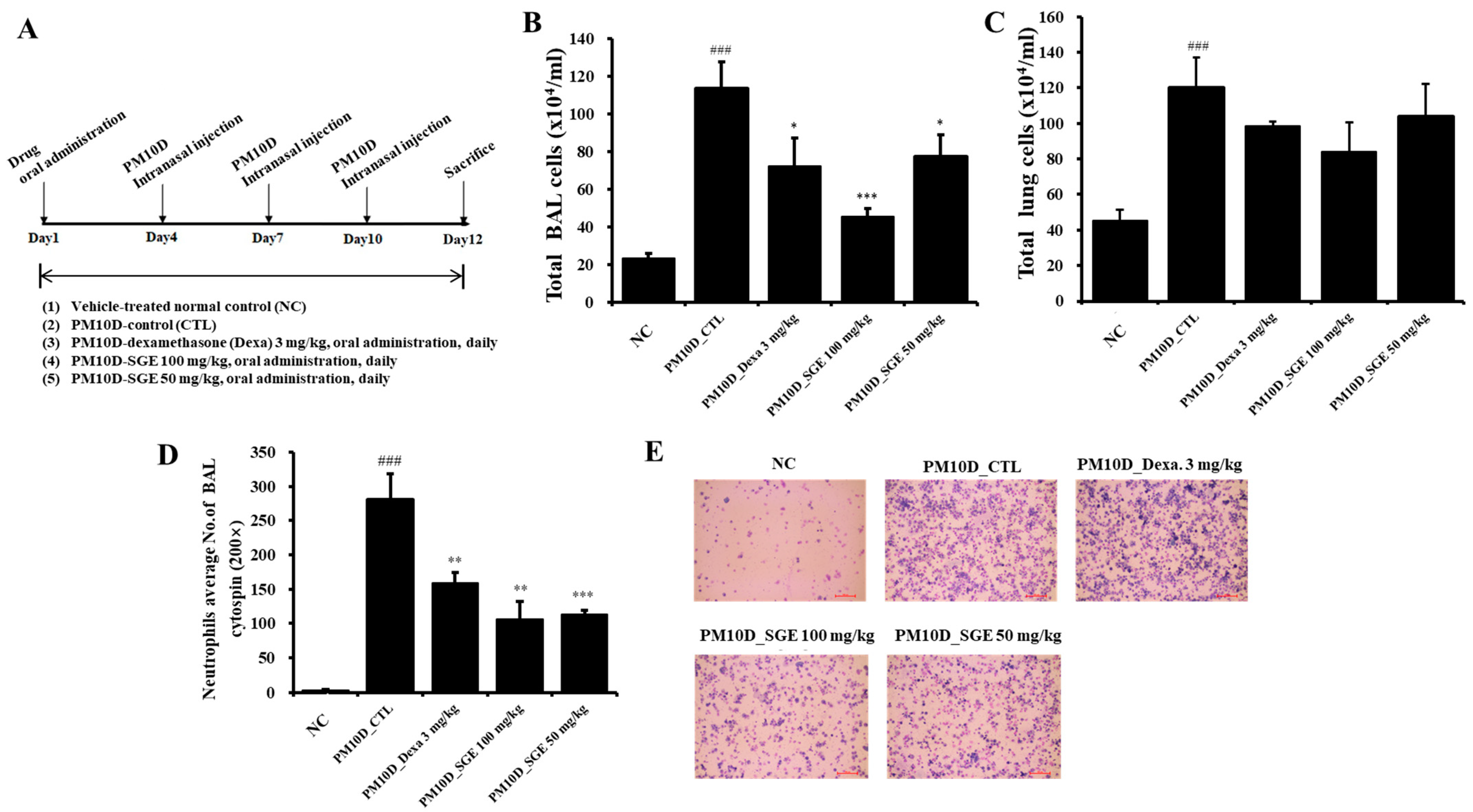
3.1. Effect of SGE on Neutrophil Infiltration in PM10D-Exposed Mice
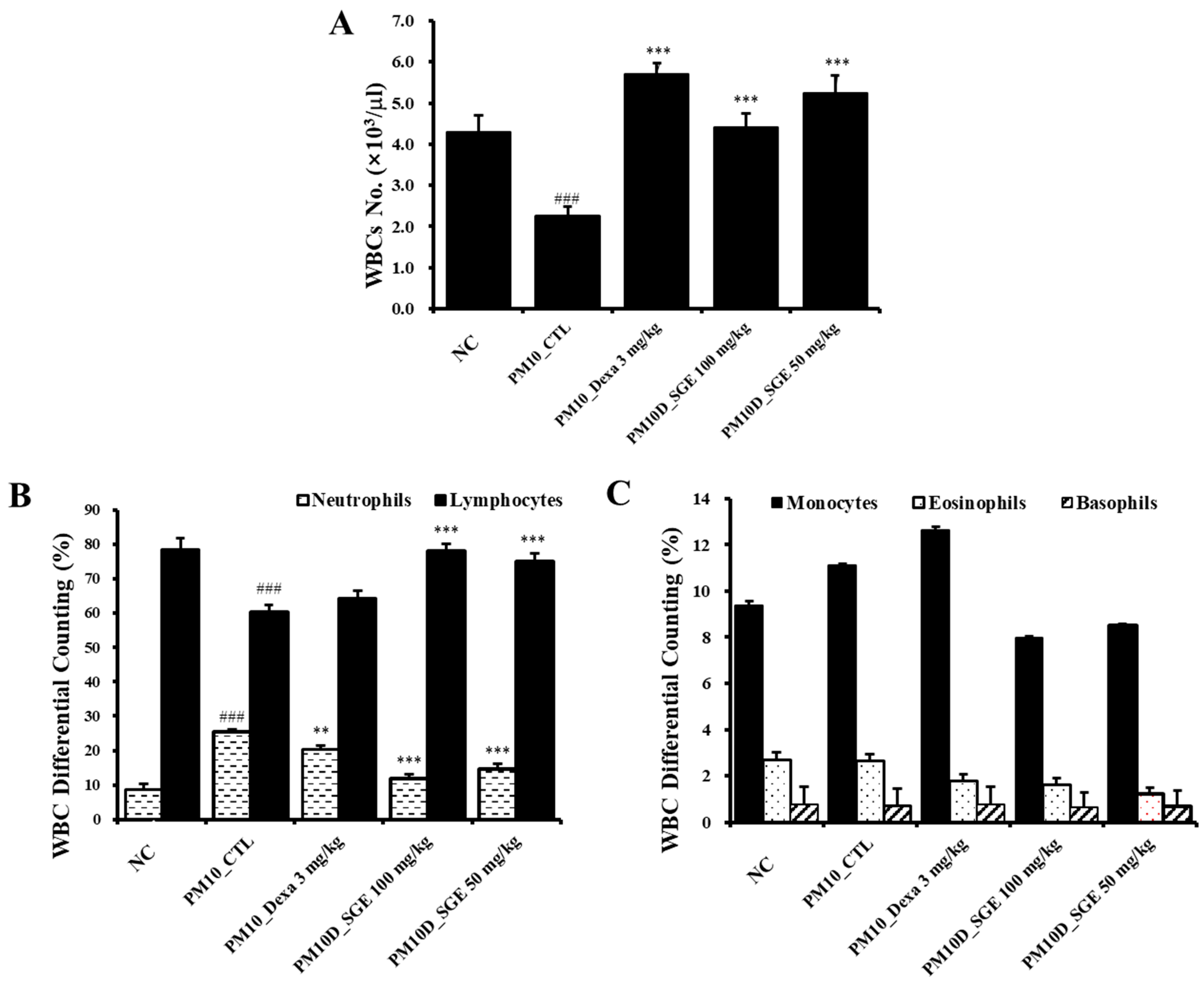
3.2. Effect of SGE on White Blood Cells in the Blood
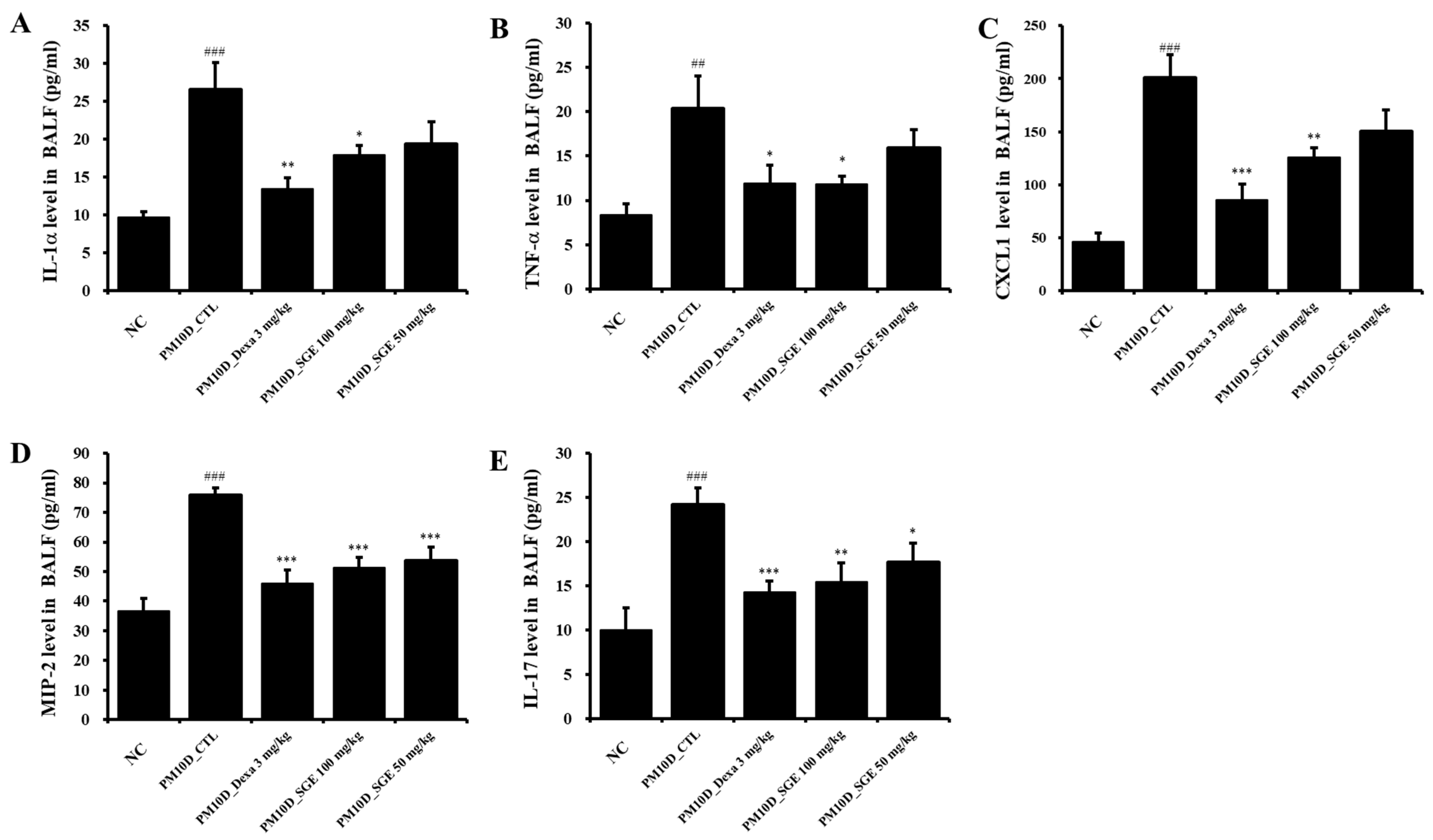
3.3. Effect of SGE on the Release of Inflammatory Mediators in BALF
3.4. Lung Histopathology
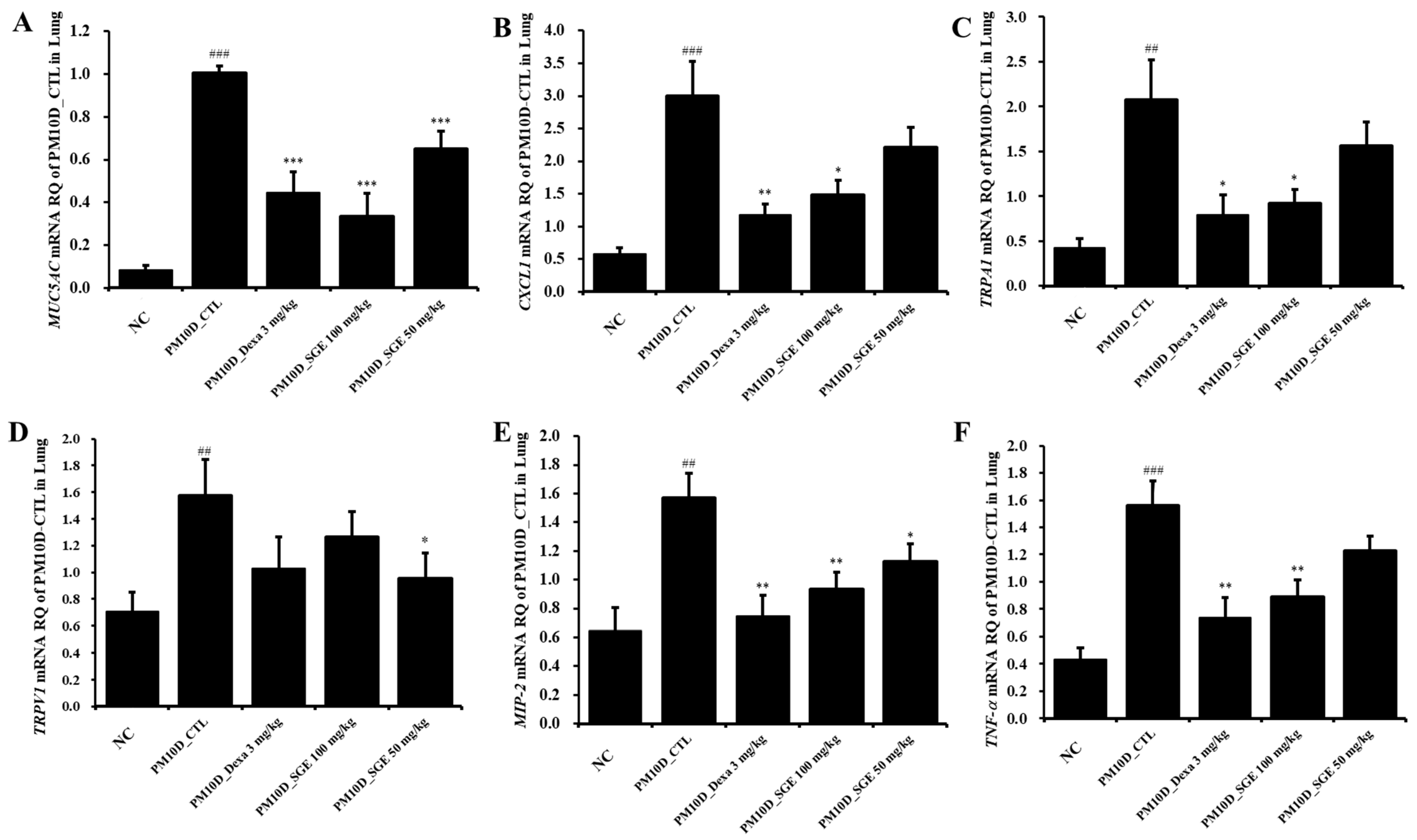
3.5. Effect of SGE on the Expression of Inflammatory Mediators in the Lung Tissue
3.6. Effect of SGE on Immune Cell Numbers in the Lung Tissue and BALF
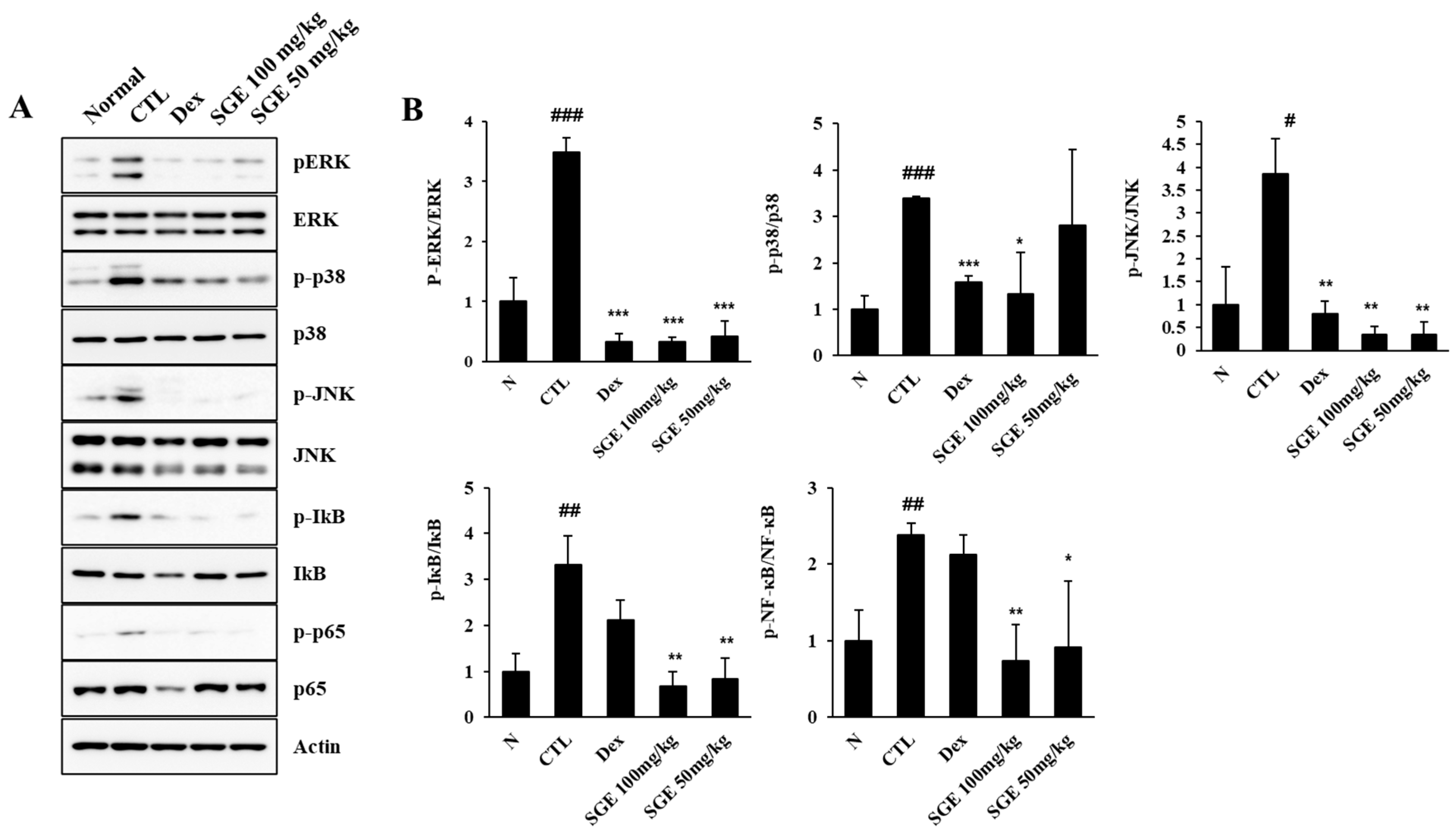
3.7. Effect of SGE on the MAPK/NF-κB Signaling Pathway in the Lungs
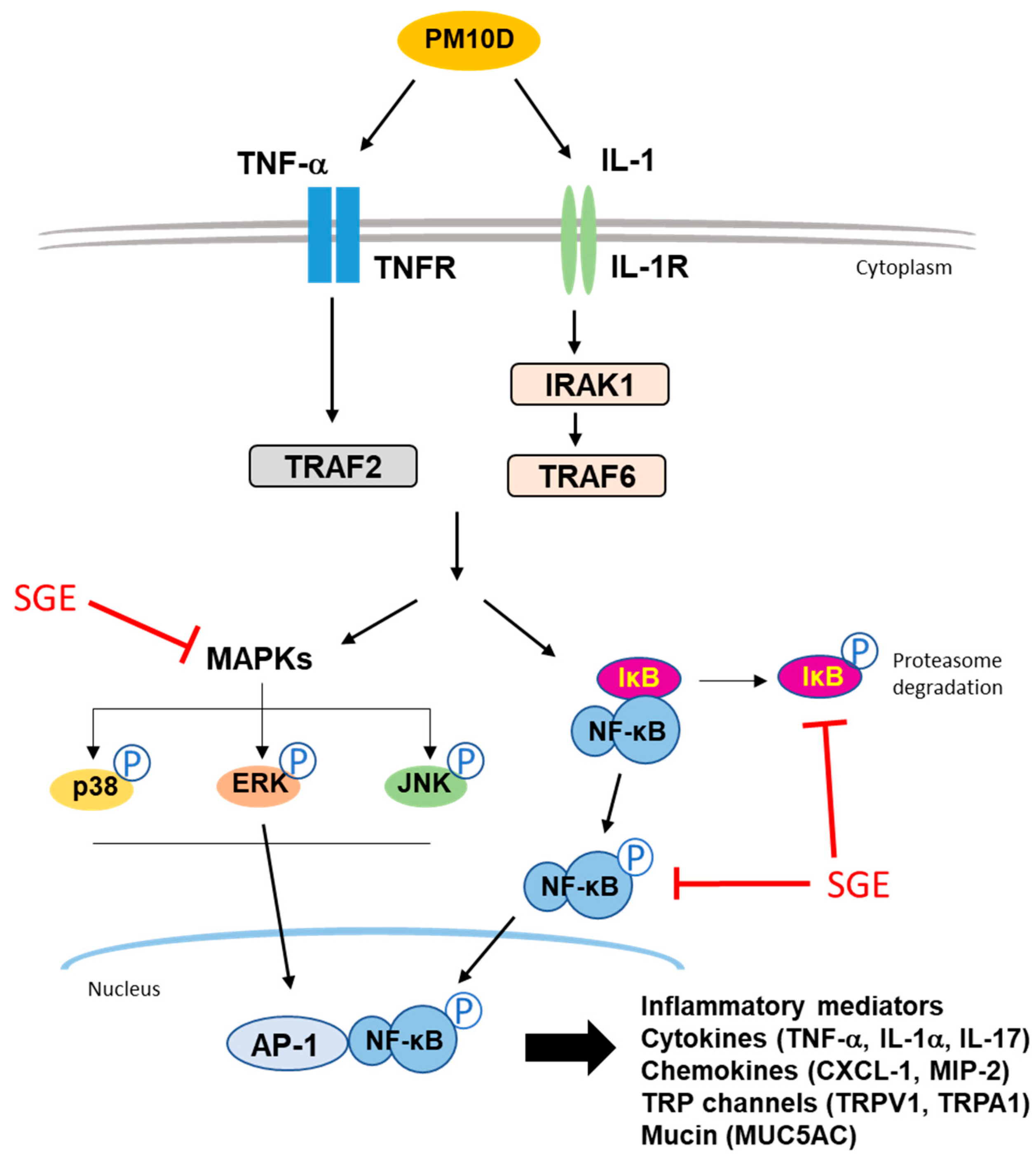
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kyung, S.Y.; Jeong, S.H. Particulate-Matter related respiratory diseases. Tuberc. Respir. Dis. 2020, 83, 116–121. [Google Scholar] [CrossRef]
- Tangavel, P.; Park, D.; Lee, Y.C. Recent insights into Particulate Matter (PM2.5)-mediated toxicity in humans: An overview. Int. J. Environ. Res. Public Health 2022, 19, 7511. [Google Scholar] [CrossRef]
- Leikauf, G.D.; Kim, S.H.; Jang, A.S. Mechanisms of ultrafine particle-induced respiratory health effects. Exp. Mol. Med. 2020, 52, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A.; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 2002, 287, 1132–1141. [Google Scholar] [CrossRef]
- Zhang, S.; Li, G.; Tian, L.; Guo, Q.; Pan, X. Short-term exposure to air pollution and morbidity of COPD and asthma in East Asian area: A systematic review and meta-analysis. Environ. Res. 2016, 148, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, H.E. Diesel exhaust particles. Inhal. Toxicol. 2007, 19, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Jung, C.J.; Lee, D.G.; Choi, B.R.; Ku, T.H.; La, I.J.; Cho, I.J.; Ku, S.K. Adenophora stricta root extract protects lung injury from exposure to Particulate Matter 2.5 in mice. Antioxidants 2022, 11, 1376. [Google Scholar] [CrossRef]
- Lee, Y.S.; Yang, W.K.; Park, Y.R.; Park, Y.C.; Park, I.J.; Lee, G.J.; Kang, H.S.; Kim, B.K.; Kim, S.H. Opuntia ficus-indica alleviates Particulate Matter 10 Plus Diesel Exhaust Particles (PM10D)-induced airway inflammation by suppressing the expression of inflammatory cytokines and chemokines. Plants 2022, 11, 520. [Google Scholar] [CrossRef]
- Zhao, X.; Fu, X.; Wang, T.; Xu, R.; Shayiranbieke, A.; Zheng, X.; Jia, X.; Xiao, C.; Zhao, X. Screening of bioactive flavour compounds targeting muscarinic-3 acetylcholine receptor from Siraitia grosvenorii and evaluation of their synergistic anti-asthmatic activity. Food Chem. 2022, 395, 133593. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Qi, X.; Zou, J.; Sun, Z. Antiglycation and antioxidant activities of mogroside extract from Siraitia grosvenorii (Swingle) fruits. J. Food Sci. Technol. 2018, 55, 1880–1888. [Google Scholar] [CrossRef]
- Chen, G.; Liu, C.; Meng, G.; Zhang, C.; Chen, F.; Tang, S.; Hong, H.; Zhang, C. Neuroprotective effect of mogrol against Aβ1–42-induced memory impairment neuroinflammation and apoptosis in mice. J. Pharm. Pharmacol. 2019, 71, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, M.; Romeih, E.; Huang, Z.; Enomoto, T.; Huang, L.; Li, L. Bioactive properties of probiotic set-yogurt sup-plemented with Siraitia grosvenorii fruit extract. Food Chem. 2020, 303, 125400. [Google Scholar] [CrossRef] [PubMed]
- Thakur, K.; Partap, M.; Kumar, P.; Sharma, R.; Warghat, A.R. Understandings of bioactive composition, molecular regulation, and biotechnological interventions in the development and usage of specialized metabolites as health-promoting substances in Siraitia grosvenorii (Swingle) C. Jeffrey. J. Food Compost. Anal. 2023, 116, 105070. [Google Scholar] [CrossRef]
- Sung, Y.Y.; Kim, S.H.; Yuk, H.J.; Yang, W.K.; Lee, Y.M.; Son, E.; Kim, D.S. Siraitia grosvenorii residual extract attenuates ovalbumin-induced lung inflammation by down-regulating IL-4, IL-5, IL-13, IL-17, and MUC5AC expression in mice. Phytomedicine 2019, 61, 152835. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.Y.; Yuk, H.J.; Yang, W.K.; Kim, S.H.; Kim, D.S. Siraitia grosvenorii residual extract attenuates atopic dermatitis by regulating immune dysfunction and skin barrier abnormality. Nutrients 2020, 12, 3638. [Google Scholar] [CrossRef]
- Song, J.L.; Qian, B.; Pan, C.; Lv, F.; Wang, H.; Gao, Y.; Zhou, Y. Protective activity of mogroside V against ovalbumin-induced experimental allergic asthma in Kunming mice. J. Food Biochem. 2019, 43, e12973. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, D.S.; Yuk, H.J.; Kim, S.H.; Yang, W.K.; Park, G.D.; Kim, K.S.; Ham, W.J.; Sung, Y.Y. Siraitia grosvenorii extract attenuates airway inflammation in a murine model of chronic obstructive pulmonary disease induced by cigarette smoke and lipopolysaccharide. Nutrients 2023, 15, 468. [Google Scholar] [CrossRef]
- Montgomery, S.T.; Dittrich, A.S.; Garratt, L.W.; Turkovic, L.; Frey, D.L.; Stick, S.M.; Mall, M.A.; Kicic, A.; Arest, C.F. Interleukin-1 is associated with inflammation and structural lung disease in young children with cystic fibrosis. J. Cyst. Fibros. 2018, 17, 715–722. [Google Scholar] [CrossRef]
- Schaller, M.A.; Lundy, S.K.; Huffnagle, G.B.; Lukacs, N.W. CD8+ T cell contributions to allergen induced pulmonary inflammation and airway hyperreactivity. Eur. J. Immunol. 2005, 35, 2061–2070. [Google Scholar] [CrossRef]
- Wang, Y.H.; Voo, K.S.; Liu, B.; Chen, C.Y.; Uygungil, B.; Spoede, W.; Bernstein, J.A.; Huston, D.P.; Liu, Y.J. A novel subset of CD4+ Th2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J. Exp. Med. 2010, 207, 2479–2491. [Google Scholar] [CrossRef]
- Tsiganov, E.N.; Verbina, E.M.; Radaeva, T.V.; Sosunov, V.V.; Kosmiadi, G.A.; Nikitina, I.Y.; Lyadova, I.V. Gr-1dimCD11b+ Immature Myeloid-Derived Suppressor Cells, but not Neutrophils, are Markers of Lethal Tuberculosis Infection in Mice. J. Immunol. 2014, 192, 4718–4727. [Google Scholar] [CrossRef] [PubMed]
- Kwok, I.; Becht, E.; Xia, Y.; Ng, M.; The, Y.C.; Tan, L.; Evrard, M.; Li, J.L.Y.; Tran, H.T.N.; Tan, Y.; et al. Combinatorial single-cell analyses of granulocyte-monocyte progenitor heterogeneity reveals an early uni-potent neutrophil progenitor. Immunity 2020, 53, 303–318.e5. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, E.; Maverakis, E.; Sarin, R.; Bouchareychas, L.; Kuchroo, V.K.; Nestle, F.O.; Adamopoulos, I.E. T Cell-independent mechanisms associated with neutrophil extracellular trap formation and selective autophagy in IL-17A-mediated epidermal hyperplasia. J. Immunol. 2016, 197, 4403–4412. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Natarajan, S.; Vaickus, L.J.; Bouchard, J.C.; Beal, D.; Cruikshank, W.W.; Remick, D.G. Diesel exhaust particulates exacerbate asthma-like inflammation by increasing CXC chemokines. Am. J. Pathol. 2011, 179, 2730–2739. [Google Scholar] [CrossRef]
- Bonser, L.R.; Erle, D.J. Airway mucus and asthma: The role of MUC5AC and MUC5B. J. Clin. Med. 2017, 6, 112. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ye, Z. The Potential Role and Regulatory Mechanisms of MUC5AC in Chronic Obstructive Pulmonary Disease. Molecules 2020, 25, 4437. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, Y.; Wang, M.; Zhang, H.; Chen, Y.; Adcock, I.M.; Chung, K.F.; Mo, J.; Zhang, Y.; Li, F. TRPV1 and TRPA1 in Lung Inflammation and Airway Hyperresponsiveness Induced by Fine Particulate Matter (PM2.5). Oxid. Med. Cell. Longev. 2019, 2019, 7450151. [Google Scholar] [CrossRef]
- Grace, M.S.; Baxter, M.; Dubuis, E.; Birrell, M.A.; Belvisi, M.G. Transient receptor potential (TRP) channels in the airway: Role in airway disease. Br. J. Pharmacol. 2014, 171, 2593–2607. [Google Scholar] [CrossRef]
- Dietrich, A.; Steinritz, D.; Gudermann, T. Transient receptor potential (TRP) channels as molecular targets in lung toxicology and associated diseases. Cell Calcium 2017, 67, 123–137. [Google Scholar] [CrossRef]
- Bonvini, S.J.; Belvisi, M.G. Cough and airway disease: The role of ion channels. Pulm. Pharmacol. Ther. 2017, 47, 21–28. [Google Scholar] [CrossRef]
- Batra, S.; Balamayooran, G.; Sahoo, M.K. Nuclear Factor-κB: A key regulator in health and disease of lungs. Arch. Immunol. Ther. Exp. (Warsz.) 2011, 59, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.R.; Simms, B.T.; Lupa, M.M.; Kogan, M.S.; Mizgerd, J.P. Lung NF-κB activation and neutrophil recruitment require IL-1 and TNF receptor signaling during pneumococcal pneumonia. J. Immunol. 2005, 175, 7530–7535. [Google Scholar] [CrossRef] [PubMed]
- Cabello, N.; Mishra, V.; Sinha, U.; DiAngelo, S.L.; Chroneos, Z.C.; Ekpa, N.A.; Cooper, T.K.; Caruso, C.R.; Silveyra, P. Sex differences in the expression of lung inflammatory mediators in response to ozone. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L1150–L1163. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer Direction | Oligonucleotide Sequence (5′-3′) |
|---|---|---|
| β-actin | F | TGGAATCCTGTGGCATCCAT |
| R | TAAAACGCAGCTCGTAACAG | |
| TNF-α | F | CCTGTAGCCCACGTCGTAGC |
| R | TTGACCTCAGCGCTGAGTTG | |
| MIP-2 | F | ATGCCTGAAGACCCTGCCAAG |
| R | GGTCAGTTAGCCTTGCCTTTG | |
| CXCL1 | F | CCGAAGTCATAGCCACAC |
| R | GTGCCATCAGAGCAGTCT | |
| MUC5AC | F | AGAATATCTTTCAGGACCCCT |
| R | ACACCAGTGCTGAGCATACTT | |
| TRPV1 | F | CATCTTCACCACGGCTGCTTAC |
| R | CAGACAGGATCTCTCCAGTGAC | |
| TRPA1 | F | TGAGATCGACCGGAGT |
| R | TGCTGAAGGCATCTTG |
| Cell Types | Absolute No. | ||||
|---|---|---|---|---|---|
| NC | PM10D-CTL | PM10D-Dexa 3 3 mg/kg | PM10D-SGE 100 mg/kg | PM10D-SGE 50 mg/kg | |
| BALF | |||||
| Lymphocytes (×104 cells) | 2.73 ± 0.68 | 7.45 ± 1.76 # | 4.20 ± 1.02 | 3.39 ± 0.65 * | 4.90 ± 1.94 |
| Neutrophils (×104 cells) | 5.41 ± 1.14 | 53.09 ± 6.44 ### | 21.19 ± 5.56 ** | 11.53 ± 2.39 *** | 23.18 ± 5.37 ** |
| Eosinophils (×104 cells) | 11.74 ± 3.89 | 48.90 ± 12.93 ## | 44.74 ± 16.68 | 28.97 ± 3.16 | 47.26 ± 9.15 |
| CD4+ (×104 cells) | 0.55 ± 0.25 | 31.56 ± 8.11 ## | 10.51 ± 2.32 * | 7.71 ± 1.00 ** | 15.90 ± 4.20 |
| CD8+ (×104 cells) | 0.10 ± 0.06 | 17.01 ± 2.15 ### | 3.98 ± 1.01 ** | 6.69 ± 2.30 * | 10.39 ± 3.02 |
| CD62L−/CD44high+ (×104 cells) | 1.64 ± 0.39 | 89.75 ± 15.92 ### | 55.28 ± 15.35 | 25.55 ± 3.39 * | 56.39 ± 10.65 |
| Gr-1+SiglecF− (×104 cells) | 1.09 ± 0.53 | 53.18 ± 9.61 ## | 14.35 ± 4.32 * | 7.63 ± 2.02 ** | 20.40 ± 4.99 * |
| Lung | |||||
| Lymphocytes (×104 cells) | 14.46 ± 1.96 | 24.06 ± 4.26 # | 33.31 ± 2.46 | 29.20 ± 9.50 | 37.82 ± 12.24 |
| Neutrophils (×104 cells) | 24.15 ± 5.70 | 81.34 ± 17.00 ## | 50.86 ± 2.19 | 40.57 ± 10.67 * | 51.09 ± 10.56 |
| Eosinophils (×104 cells) | 5.96 ± 0.73 | 12.20 ± 2.48 # | 12.53 ± 0.55 | 12.55 ± 4.09 | 12.70 ± 3.01 |
| CD4+ (×104 cells) | 15.94 ± 3.51 | 34.79 ± 5.47 ## | 33.93 ± 1.28 | 30.73 ± 9.48 | 32.90 ± 7.49 |
| CD8+ (×104 cells) | 6.68 ± 1.49 | 21.77 ± 4.24 ## | 17.45 ± 0.79 | 14.79 ± 3.55 | 15.70 ± 4.68 |
| CD4+ CD69+ (×104 cells) | 1.38 ± 0.44 | 4.06 ± 0.67 ## | 2.41 ± 0.34 * | 2.13 ± 0.59 * | 2.66 ± 0.59 |
| CD62L−/CD44high+ (×104 cells) | 3.88 ± 0.76 | 17.88 ± 1.47 ### | 9.00 ± 1.01 ** | 9.68 ± 2.79 * | 11.82 ± 3.51 |
| Gr-1+SiglecF− (×104 cells) | 9.49 ± 2.76 | 41.83 ± 3.15 ## | 19.26 ± 2.26 ** | 13.45 ± 2.52 ** | 21.31 ± 3.69 * |
| Gr-1+CD11b+ (×104 cells) | 15.58 ± 3.13 | 56.92 ± 7.92 ## | 28.78 ± 2.15 * | 21.39 ± 3.97 * | 30.03 ± 6.30 |
| CD21+/CD35+B220+ (×104 cells) | 4.33 ± 2.15 | 21.08 ± 4.84 ## | 8.09 ± 2.14 * | 10.40 ± 3.27 | 14.78 ± 5.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, Y.-Y.; Kim, M.; Yuk, H.J.; Kim, S.-H.; Yang, W.-K.; Park, G.D.; Kim, K.S.; Ham, W.J.; Kim, D.-S. Siraitia grosvenorii Extract Attenuates Airway Inflammation in a Mouse Model of Respiratory Disease Induced by Particulate Matter 10 Plus Diesel Exhaust Particles. Nutrients 2023, 15, 4140. https://doi.org/10.3390/nu15194140
Sung Y-Y, Kim M, Yuk HJ, Kim S-H, Yang W-K, Park GD, Kim KS, Ham WJ, Kim D-S. Siraitia grosvenorii Extract Attenuates Airway Inflammation in a Mouse Model of Respiratory Disease Induced by Particulate Matter 10 Plus Diesel Exhaust Particles. Nutrients. 2023; 15(19):4140. https://doi.org/10.3390/nu15194140
Chicago/Turabian StyleSung, Yoon-Young, Misun Kim, Heung Joo Yuk, Seung-Hyung Kim, Won-Kyung Yang, Geum Duck Park, Kyung Seok Kim, Woo Jung Ham, and Dong-Seon Kim. 2023. "Siraitia grosvenorii Extract Attenuates Airway Inflammation in a Mouse Model of Respiratory Disease Induced by Particulate Matter 10 Plus Diesel Exhaust Particles" Nutrients 15, no. 19: 4140. https://doi.org/10.3390/nu15194140
APA StyleSung, Y.-Y., Kim, M., Yuk, H. J., Kim, S.-H., Yang, W.-K., Park, G. D., Kim, K. S., Ham, W. J., & Kim, D.-S. (2023). Siraitia grosvenorii Extract Attenuates Airway Inflammation in a Mouse Model of Respiratory Disease Induced by Particulate Matter 10 Plus Diesel Exhaust Particles. Nutrients, 15(19), 4140. https://doi.org/10.3390/nu15194140








