Beyond Insoluble Dietary Fiber: Bioactive Compounds in Plant Foods
Abstract
:1. Introduction
1.1. A Shift from a Chemical Definition of Fiber to a Physiological Definition
1.2. Dietary Recommendations Are for Total Fiber, Not Soluble or Insoluble Fiber
1.3. Human Plant Foods and Protective Health Properties
1.4. Whole Grains
1.5. Fruits and Vegetables
1.6. Legumes/Pulses
1.7. Regulations on Insoluble Fiber
1.8. Purpose/Aim
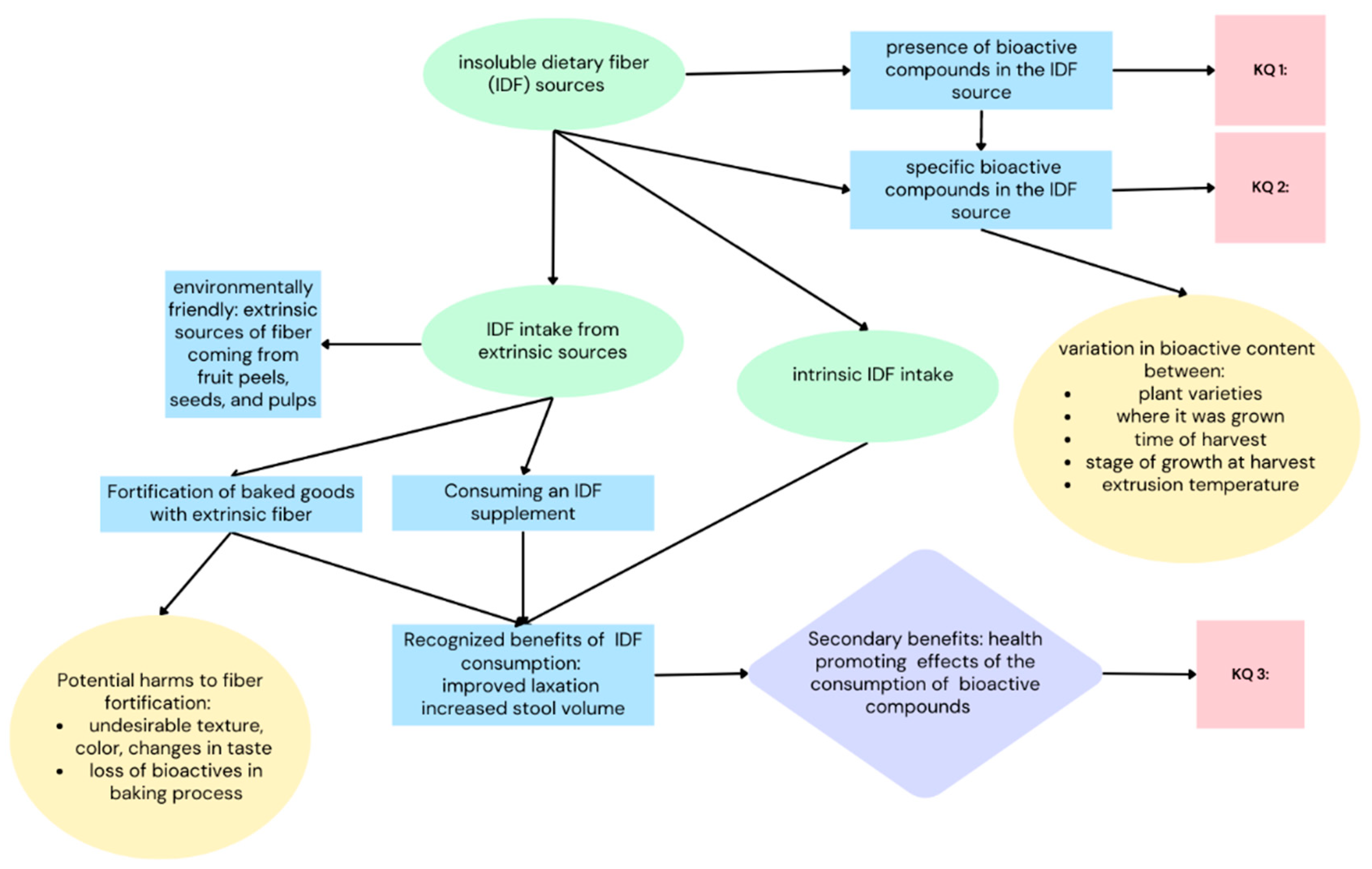
1.9. Key Questions (Figure 1)
- What IDF sources have been examined in regard to their bioactive content?
- What bioactive compounds are present in IDF?
- Do these bioactive compounds exhibit health-promoting effects?
2. Materials and Methods
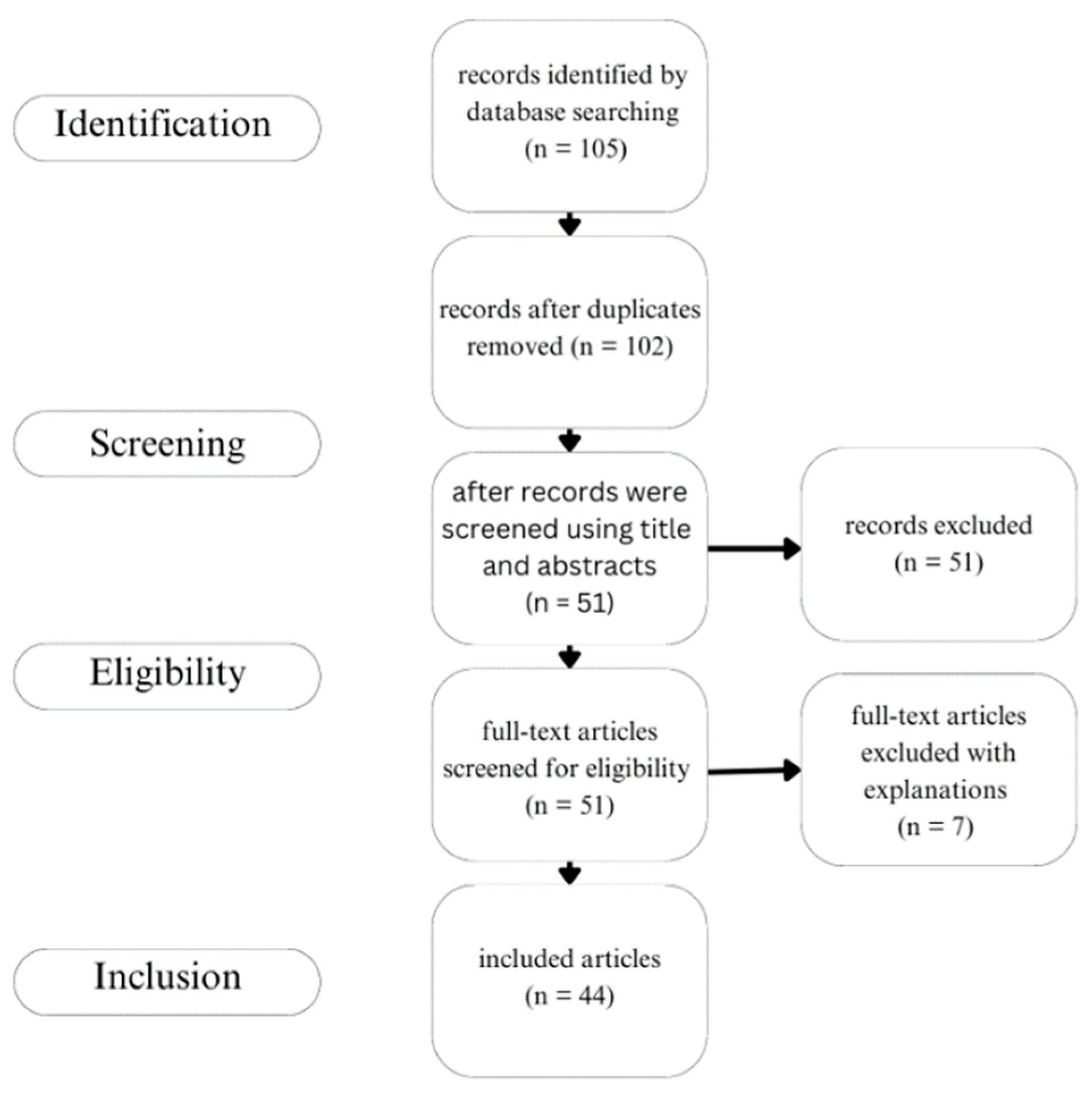
2.1. Search Strategy
2.2. Study Selection Process
3. Results
3.1. Bioactive Sources in Insoluble Dietary Fiber
3.2. Bioactive Compounds in Sources of IDF
4. Results and Discussion
4.1. Sources of Insoluble Dietary Fiber and Bioactives
4.2. TPC, TFC, and AA
4.3. Extraction, Processing, and Maintenance
4.4. Food Applications
4.5. Factors That Influence Bioactivity
4.6. Health Benefits of Bioactive Compounds
4.7. Applicability
4.8. Research Recommendations/Future
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A. Excluded Article Bibliography at Full-Text Screen
- -
- Not originally published in English (n = 1) (Delgado-Nieblas, et al. [62]);
- -
- Extracted insoluble fiber but only examined the antioxidant effects/phytochemical; content of the soluble fiber (n = 1) (Multari, et al. [63]);
- -
- -
- Did not measure outcomes relevant to this article (n = 3);
- ⚬
- Surface tension and solvent diffusional properties (Verdú, et al. [66]);
- ⚬
- Effect of enzymatic pretreatment of fruit pomaces (Alberici, et al. [67]);
- ■
- Could be valuable source in the future research section, if fortification is going to occur how can we optimize/maintain the nutritive quality of the extracted IDF;
- ⚬
- Created an extract that did not have IDF in it (Cairone, et al. [68]);
- ⚬
- No discussion/testing of phytochemicals (Liu, et al. [69]).
References
- Korczak, R.; Slavin, J.L. Definitions, Regulations, and New Frontiers for Dietary Fiber and Whole Grains. Nutr. Rev. 2020, 78 (Suppl. S1), 6–12. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L. Dietary Fiber: Classification, Chemical Analyses, and Food Sources. J. Am. Diet. Assoc. 1987, 87, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Dietary Reference Intakes: Proposed Definition of Dietary Fiber; Institute of Medicine (U.S.) (Ed.) The Compass Series; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Slavin, J.L. Position of the American Dietetic Association: Health Implications of Dietary Fiber. J. Am. Diet. Assoc. 2008, 108, 1716–1731. [Google Scholar] [CrossRef]
- McKeown, N.M.; Fahey, G.C.; Slavin, J.; Van Der Kamp, J.-W. Fibre Intake for Optimal Health: How Can Healthcare Professionals Support People to Reach Dietary Recommendations? BMJ 2022, 378, e054370. [Google Scholar] [CrossRef]
- Hojsak, I.; Benninga, M.A.; Hauser, B.; Kansu, A.; Kelly, V.B.; Stephen, A.M.; Morais Lopez, A.; Slavin, J.; Tuohy, K. Benefits of Dietary Fibre for Children in Health and Disease. Arch. Dis. Child. 2022, 107, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Timm, M.; Slavin, J. Dietary Fiber: Classification and Physiological Role. In Encyclopedia of Human Nutrition; Elsevier: Amsterdam, The Netherlands, 2023; pp. 209–216. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health Benefits of Fruits and Vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Roe, D.A. History of Promotion of Vegetable Cereal Diets. J. Nutr. 1986, 116, 1355–1363. [Google Scholar] [CrossRef]
- Southgate. Nature and Variability of Human Food Consumption. Phil. Trans. R. Soc. Lond. B 1991, 334, 281–288. [Google Scholar] [CrossRef]
- Liener, I.E. The Nutritional Significance of Plant Protease Inhibitors. Proc. Nutr. Soc. 1979, 38, 109–113. [Google Scholar] [CrossRef]
- Smith, S.A.; Campbell, D.R.; Elmer, P.J.; Martini, M.C.; Slavin, J.L.; Potter, J.D. The University of Minnesota Cancer Prevention Research Unit Vegetable and Fruit Classification Scheme (United States). Cancer Causes Control 1995, 6, 292–302. [Google Scholar] [CrossRef]
- Havemeier, S.; Erickson, J.; Slavin, J. Dietary Guidance for Pulses: The Challenge and Opportunity to Be Part of Both the Vegetable and Protein Food Groups: Dietary Guidance for Pulses. Ann. N. Y. Acad. Sci. 2017, 1392, 58–66. [Google Scholar] [CrossRef]
- CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=101.9 (accessed on 3 October 2022).
- Mai, T.H.A.; Tran, T.T.T.; Le, V.V.M. Use of Pitaya Peel Powder for Partial Replacement of Wheat Flour in Cookie Making: Effects of Particle Size of Pitaya Peel Powder on the Product Quality. J. Food Process. Preserv. 2022, 46, e16214. [Google Scholar] [CrossRef]
- Espinales, C.; Cuesta, A.; Tapia, J.; Palacios-Ponce, S.; Peñas, E.; Martínez-Villaluenga, C.; Espinoza, A.; Cáceres, P.J. The Effect of Stabilized Rice Bran Addition on Physicochemical, Sensory, and Techno-Functional Properties of Bread. Foods 2022, 11, 3328. [Google Scholar] [CrossRef] [PubMed]
- Nugraheni, M.; Windarwati, W.; Palupi, S. Gluten-Free Noodles Made Based on Germinated Organic Red Rice: Chemical Composition, Bioactive Compounds, Antioxidant Activity and Sensory Evaluation. Food Res. 2022, 6, 354–363. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, R.; Dong, L.; Huang, F.; Liu, L.; Deng, Y.; Ma, Y.; Zhang, Y.; Wei, Z.; Xiao, J.; et al. A Comparison of the Chemical Composition, In Vitro Bioaccessibility and Antioxidant Activity of Phenolic Compounds from Rice Bran and Its Dietary Fibres. Molecules 2018, 23, 202. [Google Scholar] [CrossRef] [PubMed]
- Kuan, C.-Y.; Yuen, K.-H.; Liong, M.-T. Physical, Chemical and Physicochemical Characterization of Rice Husk. Br. Food J. 2012, 114, 853–867. [Google Scholar] [CrossRef]
- Lin, P.-Y.; Lai, H.-M. Bioactive Compounds in Rice during Grain Development. Food Chem. 2011, 127, 86–93. [Google Scholar] [CrossRef]
- Asghar, A.; Afzaal, M.; Nosheen, F.; Saeed, F.; Nayik, G.A.; AL-Farga, A.; Alansari, W.S.; Eskandrani, A.A.; Shamlan, G. Isolation and Molecular Characterization of Processed Soybean Waste for the Development of Synbiotic Yogurt. Fermentation 2022, 8, 622. [Google Scholar] [CrossRef]
- Wang, A.; Zhu, Y.; Zou, L.; Zhao, G.; Wu, J. Development of Protein-Enriched Biscuit Based on Oat-Milk Byproduct Fortified with Chickpea Flour. LWT 2023, 177, 114594. [Google Scholar] [CrossRef]
- Caporizzi, R.; Schönlechner, R.; D’amico, S.; Severini, C.; Derossi, A. Novel Gluten-Free Breakfast Cereals Produced by Extrusion Cooking from Rice and Teff: Effects on Microstructural, Physical and Nutritional Properties. Foods 2023, 12, 609. [Google Scholar] [CrossRef]
- Lau, T.; Harbourne, N.; Oruña-Concha, M.J. Valorisation of Sweet Corn (Zea Mays) Cob by Extraction of Valuable Compounds. Int. J. Food Sci. Technol. 2019, 54, 1240–1246. [Google Scholar] [CrossRef]
- Ciudad-Mulero, M.; Barros, L.; Fernandes, Â.; Ferreira, I.C.F.R.; Callejo, M.J.; Matallana-González, M.C.; Fernández-Ruiz, V.; Morales, P.; Carrillo, J.M. Potential Health Claims of Durum and Bread Wheat Flours as Functional Ingredients. Nutrients 2020, 12, 504. [Google Scholar] [CrossRef]
- Kumari, A.; Sharma, S.; Sharma, N.; Chunduri, V.; Kapoor, P.; Kaur, S.; Goyal, A.; Garg, M. Influence of Biofortified Colored Wheats (Purple, Blue, Black) on Physicochemical, Antioxidant and Sensory Characteristics of Chapatti (Indian Flatbread). Molecules 2020, 25, 5071. [Google Scholar] [CrossRef]
- Durazzo, A.; Turfani, V.; Narducci, V.; Azzini, E.; Maiani, G.; Carcea, M. Nutritional characterization and bioactive components of commercial carobs flours. Food Chem. 2014, 153, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, I.U.D.; Aquino, J.S.; Cavalcanti, N.S.H.; Campos, A.R.N.; Cordeiro, A.M.T.M.; Damasceno, K.S.F.S.C.; Hoskin, R.T. Characterization and Functionality of Fibre-Rich Pomaces from the Tropical Fruit Pulp Industry. Br. Food J. 2020, 122, 813–826. [Google Scholar] [CrossRef]
- Betrouche, A.; Estivi, L.; Colombo, D.; Pasini, G.; Benatallah, L.; Brandolini, A.; Hidalgo, A. Antioxidant Properties of Gluten-Free Pasta Enriched with Vegetable By-Products. Molecules 2022, 27, 8993. [Google Scholar] [CrossRef] [PubMed]
- Göncü, A.; Çelik, İ. Investigation of Some Properties of Gluten-Free Tarhanas Produced by Red, Green and Yellow Lentil Whole Flour. Food Sci. Technol. 2020, 40, 574–581. [Google Scholar] [CrossRef]
- Di Stefano, V.; Pagliaro, A.; Del Nobile, M.A.; Conte, A.; Melilli, M.G. Lentil Fortified Spaghetti: Technological Properties and Nutritional Characterization. Foods 2021, 10, 4. [Google Scholar] [CrossRef]
- Mattila, P.H.; Pihlava, J.-M.; Hellström, J.; Nurmi, M.; Eurola, M.; Mäkinen, S.; Jalava, T.; Pihlanto, A. Contents of Phytochemicals and Antinutritional Factors in Commercial Protein-Rich Plant Products. Food Qual. Saf. 2018, 2, 213–219. [Google Scholar] [CrossRef]
- Singh, J.P.; Kaur, A.; Shevkani, K.; Singh, N. Composition, Bioactive Compounds and Antioxidant Activity of Common Indian Fruits and Vegetables. J. Food Sci. Technol. 2016, 53, 4056–4066. [Google Scholar] [CrossRef]
- Sudha, M.L.; Indumathi, K.; Sumanth, M.S.; Rajarathnam, S.; Shashirekha, M.N. Mango Pulp Fibre Waste: Characterization and Utilization as a Bakery Product Ingredient. J. Food Meas. Charact. 2015, 9, 382–388. [Google Scholar] [CrossRef]
- Abdul Aziz, N.A.; Wong, L.M.; Bhat, R.; Cheng, L.H. Evaluation of Processed Green and Ripe Mango Peel and Pulp Flours (Mangifera Indica Var. Chokanan) in Terms of Chemical Composition, Antioxidant Compounds and Functional Properties. J. Sci. Food Agric. 2012, 92, 557–563. [Google Scholar] [CrossRef]
- Diaz, L.D.; Dorta, E.; Mahre, S.; Morales, P.; Fernandez-Ruiz, V.; Camara, M.; Sanchez-Mata, M.-C. Potential nutrition and health claims in deastringed persimmon fruits. Nutrients 2020, 12, 1397. [Google Scholar] [CrossRef]
- Chouaibi, M.; Rezig, L.; Boussaid, A.; Hamdi, S. Insoluble Tomato-Fiber Effect on Wheat Dough Rheology and Cookies’ Quality. Ital. J. Food Sci. 2019, 31, 1–18. [Google Scholar]
- Isik, F.; Topkaya, C. Effects of Tomato Pomace Supplementation on Chemical and Nutritional Properties of Crackers. Ital. J. Food Sci. 2016, 28, 525–535. [Google Scholar]
- Asadi, S.Z.; Khan, M.A. The Effect of Beetroot (Beta Vulgaris L.) Leaves Powder on Nutritional, Textural, Sensorial and Antioxidant Properties of Cookies. J. Culin. Sci. Technol. 2021, 19, 424–438. [Google Scholar] [CrossRef]
- Zagury, Y.; David, S.; Edelman, R.; Hazan Brill, R.; Livney, Y.D. Sugar Beet Pectin as a Natural Carrier for Curcumin, a Water-Insoluble Bioactive for Food and Beverage Enrichment: Formation and Characterization. Innov. Food Sci. Emerg. Technol. 2021, 74, 102858. [Google Scholar] [CrossRef]
- Bainsal, N.; Kaur, S.; Mallan, S. Pharmacognostical, Physicochemical and Phytochemical Studies of Different Varieties of Beet Root Grown in Punjab. Res. J. Pharm. Technol. 2021, 14, 1689–1692. [Google Scholar] [CrossRef]
- Gouw, V.P.; Jung, J.; Zhao, Y. Functional properties, bioactive compounds, and in vitro gastrointestinal digestion study of dried fruit pomace powders as functional food ingredients. LWT Food Sci. Technol. 2017, 80, 136–144. [Google Scholar] [CrossRef]
- Cerda-Tapia, A.; Pérez-Chabela, M.L.; Pérez-Álvarez, J.Á.; Fernández-López, J.; Viuda-Martos, M. Valorization of Pomace Powder Obtained from Native Mexican Apple (Malus Domestica Var. Rayada): Chemical, Techno-Functional and Antioxidant Properties. Plant Foods Hum. Nutr. 2015, 70, 310–316. [Google Scholar] [CrossRef]
- Rana, S.; Rana, A.; Gupta, S.; Bhushan, S. Varietal Influence on Phenolic Constituents and Nutritive Characteristics of Pomace Obtained from Apples Grown in Western Himalayas. J. Food Sci. Technol. 2021, 58, 166–174. [Google Scholar] [CrossRef]
- Wang, X.; Kristo, E.; LaPointe, G. The Effect of Apple Pomace on the Texture, Rheology and Microstructure of Set Type Yogurt. Food Hydrocoll. 2019, 91, 83–91. [Google Scholar] [CrossRef]
- Mzoughi, Z.; Chahdoura, H.; Chakroun, Y.; Cámara, M.; Fernández-Ruiz, V.; Morales, P.; Mosbah, H.; Flamini, G.; Snoussi, M.; Majdoub, H. Wild Edible Swiss Chard Leaves (Beta vulgaris L. Var. Cicla): Nutritional, Phytochemical Composition and Biological Activities. Food Res. Int. 2019, 119, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Yu, C.-C.; Chen, G.-W.; Chen, C.-H.; Sinaki, N.Y.; Lin, J.; Koksel, F. Butterfly Pea Flower as a Novel Ingredient to Produce Antioxidant-Enriched Yellow Pea-Based Breakfast Cereals. Foods 2022, 11, 3447. [Google Scholar] [CrossRef]
- Saenz, C.; Yoong, M.; Figuerola, F.; Chiffelle, I.; María Estevez, A. Cactus Pear Cladodes Powders as a Source of Dietary Fibre: Purification and Properties. Int. J. Food Sci. Nutr. 2012, 63, 283–289. [Google Scholar] [CrossRef]
- Saikia, S.; Mahanta, C.L. In Vitro Physicochemical, Phytochemical and Functional Properties of Fiber Rich Fractions Derived from by-Products of Six Fruits. J. Food Sci. Technol. 2016, 53, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Nagarajaiah, S.B.; Ramakrishna, M.G.; Prakash, J. Formulation of Nutrient Dense Chapatti Premix Suitable for Diabetics. Indian J. Tradit. Knowl. 2021, 20, 852–859. [Google Scholar] [CrossRef]
- Russo, M.; Bonaccorsi, I.L.; Arigò, A.; Cacciola, F.; De Gara, L.; Dugo, P.; Mondello, L. Blood Orange (Citrus Sinensis) as a Rich Source of Nutraceuticals: Investigation of Bioactive Compounds in Different Parts of the Fruit by HPLC-PDA/MS. Nat. Prod. Res. 2021, 35, 4606–4610. [Google Scholar] [CrossRef]
- Larrauri, J.A.; Ruperez, P.; Calixto, F.S. Pineapple shell as a source of dietary fiber with associated polyphenols. J. Agric. Food Chem. 1997, 45, 4028–4031. [Google Scholar] [CrossRef]
- Gumul, D.; Korus, A.; Ziobro, R. Extruded Preparations with Sour Cherry Pomace Influence Quality and Increase the Level of Bioactive Components in Gluten-Free Breads. Int. J. Food Sci. 2020, 2020, 8024398. [Google Scholar] [CrossRef]
- Salgado, J.M.; Rodrigues, B.S.; Donado-Pestana, C.M.; dos Santos Dias, C.T.; Morzelle, M.C. Cupuassu (Theobroma Grandiflorum) Peel as Potential Source of Dietary Fiber and Phytochemicals in Whole-Bread Preparations. Plant Foods Hum. Nutr. 2011, 66, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Feng, X.; Wu, Z.; Li, S.; Bai, X.; Zhao, C.; Ameer, K. Development of Wheat Bread Added with Insoluble Dietary Fiber from Ginseng Residue and Effects on Physiochemical Properties, In Vitro Adsorption Capacities and Starch Digestibility. LWT 2021, 149, 111855. [Google Scholar] [CrossRef]
- Krawęcka, A.; Sobota, A.; Pankiewicz, U.; Zielińska, E.; Zarzycki, P. Stinging Nettle (Urtica Dioica l.) as a Functional Component in Durum Wheat Pasta Production: Impact on Chemical Composition, In Vitro Glycemic Index, and Quality Properties. Molecules 2021, 26, 6909. [Google Scholar] [CrossRef] [PubMed]
- Li, B.W.; Andrews, K.W.; Pehrsson, P.R. Individual Sugars, Soluble, and Insoluble Dietary Fiber Contents of 70 High Consumption Foods. J. Food Compos. Anal. 2002, 15, 715–723. [Google Scholar] [CrossRef]
- Xu, J.; Bai, M.; Song, H.; Yang, L.; Zhu, D.; Liu, H. Hemp (Cannabis Sativa Subsp. Sativa) Chemical Composition and the Application of Hempseeds in Food Formulations. Plant Foods Hum. Nutr. 2022, 77, 504–513. [Google Scholar] [CrossRef]
- Cerino, P.; Buonerba, C.; Cannazza, G.; D’Auria, J.; Ottoni, E.; Fulgione, A.; Di Stasio, A.; Pierri, B.; Gallo, A. A Review of Hemp as Food and Nutritional Supplement. Cannabis Cannabinoid Res. 2021, 6, 19–27. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Fang, Z. Lignanamides: Sources, Biosynthesis and Potential Health Benefits—A Minireview. Crit. Rev. Food Sci. Nutr. 2021, 61, 1404–1414. [Google Scholar] [CrossRef]
- Bolster, D.; Chae, L.; Klinken, J.-W.V.; Kalgaonkar, S. Impact of Selected Novel Plant Bioactives on Improvement of Impaired Gut Barrier Function Using Human Primary Cell Intestinal Epithelium. JFB 2022, 20. [Google Scholar] [CrossRef]
- Delgado-Nieblas, C.I.; Zazueta-Morales, J.J.; Gallegos-Infante, J.A.; Aguilar-Palazuelos, E.; Camacho-Hernandez, I.L.; Ordorica-Falomir, C.A.; Pires de Melo, M.; Carrillo-Lopez, A. Elaboration of functional snack foods using raw materials rich in carotenoids and dietary fiber: Effects of extrusion processing. CyTA—J. Food 2015, 13, 69–79. [Google Scholar] [CrossRef]
- Multari, S.; Neacsu, M.; Scobbie, L.; Cantlay, L.; Duncan, G.; Vaughan, N.; Stewart, D.; Russell, W.R. Nutritional and phytochemical content of high-protein crops. J. Agric. Food Chem. 2016, 64, 7800–7811. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, R.; Tin, L.; Zhang, N. Novel blasting extrusion processing improved the physiochemical properties of soluble dietary fiber from soybean residue and in vivo evaluation. J. Food Eng. 2014, 120, 1–8. [Google Scholar] [CrossRef]
- Garcia, D.; You, S.W.; Aleman, R.S.; King, J.M.; Komarnytsky, S.; Toskin, R.T.; Moncada, M. Total utilization-upcycling of mushroom protein by-product: Characterization and assessment as an alternative batter ingredient for fried shrimp. Foods 2023, 12, 763. [Google Scholar] [CrossRef] [PubMed]
- Verdu, S.; Barat, M.J.; Alava, C.; Grau, R. Effect of tiger-nut (Cyperus esculentus) milk co-product on the surface and diffusional properties of a wheat-based matrix. Food Chem. 2017, 224, 69–77. [Google Scholar] [CrossRef]
- Alberici, N.; Fiorentini, C.; House, A.; Dordoni, R.; Bassani, A.; Spigno, G. Enzymatic pre-treatment of fruit pomace for fibre hydrolysis and antioxidants release. Chem. Eng. Trans. 2020, 97, 175–180. [Google Scholar]
- Cairone, F.; Fraschetti, C.; Menghini, L.; Zengin, G.; Filippi, A.; Casadei, M.A.; Case, S. Effects of processing on chemical composition of extracts from sour cherry fruits, a neglected functional food. Antioxidants 2023, 12, 445. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, W.; Chen, W.; Yang, J.; Zheng, L. Feasibility in multispectral imaging for predict the content of bioactive compounds in intact tomato fruit. Food Chem. 2015, 173, 482–488. [Google Scholar] [CrossRef] [PubMed]
| Database | Search String |
|---|---|
| Ovid Medline/Ovid Agricola | [insoluble fiber.mp.] AND [“phytochemical.mp. or Phytochemicals/” OR bioactive.mp. OR phytonutrient.mp.] AND [“apple.mp. or exp Malus/” OR “beet.mp. or Beta vulgaris/” OR “blueberry.mp. or exp Blueberry plants/” OR carob.mp. OR “carrot.mp. or exp Daucus carota/” OR “chickpea.mo. or exp Cicer/” OR “citrus.mp. or exp Citrus/” OR “cranberry.mp. or Vaccinium macrocarpon/” OR “kiwi.mp or exp Actinidia/” OR “pea.mp. or exp Peas/” OR “potato.mp or exp Solanum tuberosum/” OR chia.mp OR “flax.mp or exp Flax/” OR “hemp.mp. or Cannabis/” OR “corn.mp. or exp Zea mays/” OR “oat.mp. or exp Zea mays/” OR “rice.mp. or exp Oryza/” OR soy.mp. OR “wheat.mp or exp Triticum”] |
| Scopus | [“insoluble” AND “fiber”] AND [“phytochemical” OR “bioactive” OR “phytonutrient”] AND [“apple” OR “beet” OR “blueberry” OR “carob” OR “carrot” OR “chickpea” OR “citrus” OR “cranberry” OR “kiwi” OR “pea” OR “potato” OR “chia” OR “flax” OR “hemp” OR “corn” OR “oat” OR “rice” OR “soy” OR “wheat”] |
| Insoluble Fiber Type Examined | Bioactive Content Measured | Plant Forms | Total Phenolic Content (TPC) | Antioxidant Activity (aa) | Article | |
|---|---|---|---|---|---|---|
| Total Flavenoid Content (TFC) | ||||||
| GRAIN | ||||||
| RICE | n = 5 | stabilized rice bran | X free | Espinales et al. (2022) [16] | ||
| germinated organic red rice | X | X | X | Nugraheni et al. (2022) [17] | ||
| defatted rice bran | X | X | X | Zhao et al. (2018) [18] | ||
| rice husk | X | Kuan et al. (2012) [19] | ||||
| KFSW and TK16 mature and immature rice grains | X | X | X | Lin et al. (2011) [20] | ||
| SOYBEAN | n = 1 | Okara | X | X | Asghar et al. (2022) [21] | |
| OAT | n = 1 | oat milk byproduct | X | X | X | Wang (2023) [22] |
| TEFF | n = 1 | teff | X free bound | X free bound | Caporizzi et al. (2023) [23] | |
| CORN | n = 2 | Corncob | X free, bound, esterified | X free, bound, esterified | Lau et al. (2019) [24] | |
| corncob nanofibers | X | Kuan et al. (2012) [19] | ||||
| WHEAT | n = 2 | durum and bread wheat flours | X | X | Cuidad-Mulero et al. (2020) [25] | |
| blue, black, and purple biofortified wheats | X free bound | X | Kumari et al. (2020) [26] | |||
| SEEDS/LEGUMES | ||||||
| CAROB | n = 1 | carob | X | X | Durazzo et al. (2014) [27] | |
| CASHEW | n = 1 | pomace | X | X | X | Medeiros et al. (2020) [28] |
| CHICKPEA | n = 1 | X | X | X | Wang et al. (2023) [22] | |
| LINSEED | n = 1 | meal | X | Betrouche et al. (2022) [29] | ||
| LENTILS | n = 2 | yellow, red, and green commercial and local lentils hull | X | X | Goncu et al. (2020) [30] | |
| X | Stefano et al. (2021) [31] | |||||
| HEMP | X | X | Matilla et al. (2018) [32] | |||
| FRUITS/VEGETABLES | ||||||
| MANGO | n = 3 | mango peel, pulp, and seeds | X | X | X | Singh et al. (2016) [33] |
| n/a | Sudha et al. (2015) [34] | |||||
| X | X | Abdul et al. (2012) [35] | ||||
| TOMATO/PERSIMMON | n = 5 | persimmon, tomato byproduct (seeds, peels, and pulp) | n/a | Diaz et al. (2020) [36] | ||
| X | X | Chouaibi et al. (2019) [37] | ||||
| X | X | Isik et al. (2016) [38] | ||||
| X | X | Isik et al. (2016) [38] | ||||
| X | Betrouche et al. (2022) [29] | |||||
| BEETS | n = 4 | sugar beet pectin, beetroot leaf, beetroot | X | X | X | Singh et al. (2016) [33] |
| X | X | Asadi et al. (2021) [39] | ||||
| n/a | Zagury et al. (2021) [40] | |||||
| X | X | Bainsal et al. (2021) [41] | ||||
| APPLE | n = 4 | pomace (seeds, peel, core) | X | X | Gouw et al. (2017) [42] | |
| X | X | X | Cerda-Tapia et al. (2015) [43] | |||
| X | X | Rana et al. (2021) [44] | ||||
| X | Wang et al. (2019) [45] | |||||
| SWISS CHARD | n = 1 | leaves | n/a | Mzoughi et al. (2019) [46] | ||
| PEA | n = 1 | butterfly pea and yellow pea | X | X | Singh et al. (2022) [47] | |
| PEAR | n = 1 | cladode | X | Saenz et al. (2012) [48] | ||
| ORANGE | n = 4 | blood orange, kinnow, Khasi mandarin | X | X | X | Saikia et al. (2016) [49] |
| X | X | X | Nagarajaiah et al. (2021) [50] | |||
| X | X | X | Singh et al. (2016) [33] | |||
| n/a | Russo et al. (2021) [51] | |||||
| PINEAPPLE | n = 3 | pomace, shell pomace | Larrauri et al. (1997) [52] | |||
| X | X | X | Saikia et al. (2016) [49] | |||
| X | X | X | Nagarajaiah et al (2021) [50] | |||
| GUAVA | n = 2 | pomace | X | X | X | Medeiros et al. (2020) [28] |
| CHERRY | n = 2 | pomace, sour cherry | X | X | X | Medeiros et al. (2020) [28] |
| X | X | X | Gumul et al. (2020) [53] | |||
| PITAYA | n = 1 | peel | X | X | Mai et al. (2022) [15] | |
| CAPUASSU | n = 1 | peel | X | Salgado et al. (2011) [54] | ||
| POMEGRANATE | n = 1 | pomace | X | X | X | Singh et al. (2016) [33] |
| BANANA | n = 2 | pomace, blossom of seeded banana | X | X | X | Saikia et al. (2016) [49] |
| X | X | X | Singh et al. (2016) [33] | |||
| PLUM | n = 1 | pomace | X | X | X | Singh et al. (2016) [33] |
| GRAPE | n = 3 | pomace, Burmese grape, blue grape pomace | X | X | X | Saikia et al. (2016) [49] |
| X | X | X | Nagarajaiah et al. (2021) [50] | |||
| X | X | X | Singh et al. (2016) [33] | |||
| SAPODILLA | n = 1 | X | X | X | Singh et al. (2016) [33] | |
| EGGPLANT (BRINJAL) | n = 1 | X | X | X | Singh et al(2016) [33] | |
| CARROT | n = 1 | orange carrot | X | X | X | Singh et al. (2016) [33] |
| GOURD | n = 1 | bitter gourd | X | X | X | Singh et al. (2016) [33] |
| MENTHA | n = 1 | X | X | X | Singh et al. (2016) [33] | |
| GINSENG | n = 1 | ginseng residue | X | X | X | Jiang et al. (2021) [55] |
| SPINACH | n = 1 | X | X | X | Singh et al. (2016) [33] | |
| BLUEBERRY | n = 1 | pomace | X | X | Gouw et al. (2017) [42] | |
| RASPBERRY | n = 1 | red raspberry pomace | X | X | Gouw et al. (2017) [42] | |
| CRANBERRY | n = 1 | pomace | X | X | Gouw et al. (2017) [42] | |
| LEMON | n = 1 | sweet lemon | X | X | X | Nagarajaiah et al. (2021) [50] |
| CARAMBOLA | n = 1 | pomace | X | X | X | Saikia et al. (2016) [49] |
| WATERMELON | n = 1 | peel | X | X | X | Saikia et al. (2016) [49] |
| STINGING NETTLE | n = 1 | n/a | Krawecka et al. (2021) [56] | |||
| Measurement of AA | Definition |
|---|---|
| ABTS assay | free radical scavenging assay |
| DPPH assay | free radical scavenging assay |
| FRAP | ferric reducing antioxidant power assay |
| CAA | cellular antioxidant activity |
| TEAC | Trolox equivalent antioxidant assay |
| beta-carotene bleaching assay | |
| PCL | photochemiluminescence assay |
| Bioactive Compound | IDF Fraction Found in | Form of IDF Fraction | Number of IDF Sources Bioactive WAS Found in | Sources |
|---|---|---|---|---|
| PHENOLIC ACIDS: | ||||
| FERULIC ACID | Rice | KFSW and TK16 mature and immature rice grains | n = 17 | Lin et al. (2011) [20] |
| corn | sweet corn cob | Lau et al. (2019) [24] | ||
| Pineapple | pineapple pomace | Saikia et al. (2016) [49] | ||
| grape | Burmese grape peel | Saikia et al. (2016) [49] | ||
| carambola | Saikia et al. (2016) [49] | |||
| banana | banana blossom | Saikia et al. (2016) [49] | ||
| fava bean | Betrouche et al. (2022) [29] | |||
| tomato | tomato byproduct | Betrouche et al. (2022) [29] | ||
| mango | peel and pulp | Singh et al. (2016) [33] | ||
| kinnow | peel and pulp | Singh et al. (2016) [33] | ||
| banana | peel and pulp | Singh et al. (2016) [33] | ||
| orange | Singh et al. (2016) [33] | |||
| carrot | black carrot | Singh et al. (2016) [33] | ||
| mango | mango pulp fiber waste (wet and dried) | Sudha et al. (2015) [34] | ||
| ginseng | ginseng residue | Jiang et al. (2021) [55] | ||
| cherry | sour cherry pomace | Gumul et al. (2020) [53] | ||
| rice | defatted rice bran | Zhao et al. (2018) [18] | ||
| FERULIC ACID METHYL-ESTER | rice | defatted rice bran | n = 1 | Zhao et al. (2018) [18] |
| CAFFEIC ACID | Rice | KFSW and TK16 mature and immature rice grains | n = 17 | Lin et al. (2010) [20] |
| pineapple | pineapple pomace | Saikia et al. (2016) [49] | ||
| orange | Khasi mandarin orange peel | Saikia et al. (2016) [49] | ||
| pomegranate | peel | Singh et al. (2016) [33] | ||
| kinnow | peel and pulp | Singh et al. (2016) [33] | ||
| mango | peel | Singh et al. (2016) [33] | ||
| banana | peel and pulp | Singh et al. (2016) [33] | ||
| grapes | Singh et al. (2016) [33] | |||
| jambolana | Singh et al. (2016) [33] | |||
| beetroot | Singh et al. (2016) [33] | |||
| brinjal | Singh et al. (2016) [33] | |||
| mentha | Singh et al. (2016) [33] | |||
| bitter gourd | Singh et al. (2016) [33] | |||
| carrot | black and orange | Singh et al. (2016) [33] | ||
| mango | mango pulp fiber waste (wet and dried) | Sudha et al. (2015) [34] | ||
| apple | Mexican apple | Cerdia-Tapia et al. (2015) [43] | ||
| rice | defatted rice bran | Zhao et al. (2018) [18] | ||
| CAFFEIC ACID METHYL-ESTER | rice | defatted rice bran | n = 1 | Zhao et al. (2018) [18] |
| CHLOROGENIC ACID | watermelon | n = 6 | Saikia et al. (2016) [49] | |
| mango | mango pulp fiber waste (wet and dried) | Sudha et al. (2015) [34] | ||
| apple | royal delicious, golden delicious, red delicious, red chief, red gold | Rana et al. (2021) [44] | ||
| apple | Mexican apple | Cerdia-Tapia et al. (2015) [43] | ||
| ginseng | ginseng residue | Jiang et al. (2021) [55] | ||
| rice | defatted rice bran | Zhao et al. (2018) [18] | ||
| P-COUMARIC ACID | corn | sweet corn cob | n = 9 | Lau et al. (2019) [24] |
| pineapple | pineapple shell | Larrauri et al. (1997) [52] | ||
| grape | Burmese grape peel | Saikia et al. (2016) [49] | ||
| watermelon | Saikia et al. (2016) [49] | |||
| fava bean | Betrouche et al. (2022) [29] | |||
| tomato | tomato byproduct | Betrouche et al. (2022) [29] | ||
| mango | mango pulp fiber waste (wet and dried) | Sudha et al. (2015) [34] | ||
| ginseng | ginseng residue | Jiang et al. (2021) [55] | ||
| rice | defatted rice bran | Zhao et al. (2018) [18] | ||
| GALLIC ACID | pineapple | pineapple pomace | n = 18 | Saikia et al. (2016) [49] |
| grape | Burmese grape peel | Saikia et al. (2016) [49] | ||
| orange | Khasi mandarin peel | Saikia et al. (2016) [49] | ||
| carambola | Saikia et al. (2016) [49] | |||
| watermelon | Saikia et al. (2016) [49] | |||
| banana | banana blossom | Saikia et al. (2016) [49] | ||
| banana | peel and pulp | Singh et al. (2016) [33] | ||
| jambolan | peel and pulp | Singh et al. (2016) [33] | ||
| pomegranate | peel and pulp | Singh et al. (2016) [33] | ||
| mango | peel and pulp | Singh et al. (2016) [33] | ||
| sapodilla | Singh et al. (2016) [33] | |||
| grapes | Singh et al. (2016) [33] | |||
| beetroot | Singh et al. (2016) [33] | |||
| bitter gourd | Singh et al. (2016) [33] | |||
| mentha | Singh et al. (2016) [33] | |||
| carrot | black carrot | Singh et al. (2016) [33] | ||
| mango | mango pulp fiber waste (wet and dried) | Sudha et al. (2015) [34] | ||
| rice | defatted rice bran | Zhao et al. (2018) [18] | ||
| SYRINGIC ACID | fava bean | n = 8 | Betrouche et al. (2022) [29] | |
| tomato | tomato byproduct | Betrouche et al. (2022) [29] | ||
| pineapple | pineapple shell | Saikia et al. (2016) [49] | ||
| orange | Khasi mandarin peel | Saikia et al. (2016) [49] | ||
| watermelon | Saikia et al. (2016) [49] | |||
| banana | banana blossom | Saikia et al. (2016) [49] | ||
| ginseng | ginseng residue | Jiang et al. (2021) [55] | ||
| rice | defatted rice bran | Zhao et al. (2018) [55] | ||
| TRANS CINNAMIC ACID | pineapple | pineapple shell | n = 2 | Larrauri et al. (1997) [52] |
| mango | mango pulp fiber waste | Sudha et al. (2015) [34] | ||
| CINNAMIC ACID | ginseng | ginseng residue | n = 1 | Jiang et al. (2021) [55] |
| SINAPIC ACID | pomegranate | peel and pulp | n = 4 | Singh et al. (2016) [33] |
| kinnow | peel and pulp | Singh et al. (2016) [33] | ||
| grapes | Singh et al. (2016) [33] | |||
| jambolan | Singh et al. (2016) [33] | |||
| 4-HYDROXYBENZOIC ACID | fava bean | n = 3 | Betrouche et al. (2022) [29] | |
| tomato | tomato byproduct | Betrouche et al. (2022) [29] | ||
| rice | defatted rice bran | Zhao et al. (20118) [18] | ||
| SALICYLIC ACID | pineapple | pineapple shell | n = 4 | Larrauri et al. (1997) [52] |
| acerola | cherry | Medeiros et al. (2020) [28] | ||
| guava | Medeiros et al. (2020) [28] | |||
| cashew | Medeiros et al. (2020) [28] | |||
| VANILLIN | rice | defatted rice bran | n = 1 | Zhao et al. (2018) [18] |
| VANILLIC ACID | acerola | cherry | n = 4 | Medeiros et al. (2020) [28] |
| guava | Medeiros et al. (2020) [28] | |||
| cashew | Medeiros et al. (2020) [28] | |||
| Rice | defatted rice bran | Zhao et al. (2018) [18] | ||
| FLAVONOIDS: | ||||
| FLAVANOLS | ||||
| QUERCETIN | carambola | n = 19 | Saikia et al. (2016) [49] | |
| grape | Burmese grape peel | Saikia et al. (2016) [49] | ||
| banana | banana blossom | Saikia et al. (2016) [49] | ||
| tomato | tomato byproduct | Betrouche et al. (2022) [29] | ||
| pomegranate | pulp | Singh et al. (2016) [33] | ||
| mango | peel and pulp | Singh et al. (2016) [33] | ||
| banana | peel | Singh et al. (2016) [33] | ||
| sapodilla | peel and pulp | Singh et al. (2016) [33] | ||
| jambolan | Singh et al. (2016) [33] | |||
| grapes | Singh et al. (2016) [33] | |||
| beetroot | Singh et al. (2016) [33] | |||
| carrot | black carrot | Singh et al. (2016) [33] | ||
| spinach | Singh et al. (2016) [33] | |||
| acerola | cherry | Medeiros et al. (2020) [28] | ||
| guava | Medeiros et al. (2020) [28] | |||
| cashew | Medeiros et al. (2020) [28] | |||
| apple | royal delicious, golden delicious, red delicious, red chief, red gold | Rana et al. (2021) [44] | ||
| apple | Mexican apple | Cerdia-Tapia et al. (2015) [43] | ||
| rice | defatted rice bran | Zhao et al. (2018) [18] | ||
| QUERCITRIN | apple | royal delicious, golden delicious, red delicious, red chief, red gold | n = 2 | Rana et al. (2021) [44] |
| apple | Mexican apple | Cerdia-Tapia et al. (2015) [43] | ||
| QUERCETIN DERIVATIVE | fava bean | n = 2 | Betrouche et al. (2022) [29] | |
| tomato | tomato byproduct | |||
| ISOQUERCITRIN | ginseng | ginseng residue | n = 3 | Jiang et al. (2021) [55] |
| rice | defatted rice bran | Zhao et al. (2018) [18] | ||
| apple | royal delicious, golden delicious, red delicious, red chief, red gold | Rana et al. (2021) [44] | ||
| MYRICETIN | pineapple | pineapple shell | n = 4 | Larrauri et al. (1997) [52] |
| acerola | cherry | Medeiros et al. (2020) [28] | ||
| guava | Medeiros et al. (2020) [28] | |||
| cashew | Medeiros et al. (2020) [28] | |||
| KAEMPFEROL | kinnow | pulp | n = 9 | Singh et al. (2016) [33] |
| mango | peel and pulp | Singh et al. (2016) [33] | ||
| banana | peel | Singh et al. (2016) [33] | ||
| sapodilla | peel and pulp | Singh et al. (2016) [33] | ||
| jambolan | Singh et al. (2016) [33] | |||
| grapes | Singh et al. (2016) [33] | |||
| beetroot | Singh et al. (2016) [33] | |||
| carrot | black carrot | Singh et al. (2016) [33] | ||
| spinach | Singh et al. (2016) [33] | |||
| RUTIN | tomato | tomato byproduct | n = 3 | Betrouche et al. (2022) [29] |
| apple | Mexican apple | Cerdia-Tapia et al. (2015) [43] | ||
| cherry | sour cherry pomace | Gumul et al. (2020) [53] | ||
| RUTIN HYDRATE | orange | Khasi mandarin | n = 3 | Saikia et al. (2016) [49] |
| carambola | Saikia et al. (2016) [49] | |||
| banana | banana blossom | Saikia et al. (2016) [49] | ||
| AVICULARIN | apple | Mexican apple | n = 1 | Cerdia-Tapia et al. (2015) [43] |
| HYPERIN | apple | Mexican apple | n = 1 | Cerdia-Tapia et al. (2015) [43] |
| FLAVAN-3-OLS | ||||
| CATECHIN | grape | Burmese grape peel | n = 13 | Saikia et al. (2016) [49] |
| carambola | Saikia et al. (2016) [49] | |||
| watermelon | Saikia et al. (2016) [49] | |||
| fava bean | Betrouche et al. (2022) [29] | |||
| pomegranate | peel and pulp | Singh et al. (2016) [33] | ||
| mango | peel and pulp | Singh et al. (2016) [33] | ||
| banana | peel and pulp | Singh et al. (2016) [33] | ||
| sapodilla | peel and pulp | Singh et al. (2016) [33] | ||
| bitter gourd | Singh et al. (2016) [33] | |||
| grapes | whole | Singh et al. (2016) [33] | ||
| mango | mango pulp fiber waste (wet and dried) | Sudha et al. (2015) [34] | ||
| ginseng | ginseng residue | Jiang et al. (2021) [55] | ||
| rice | defatted rice bran | Zhao et al. (2018) [18] | ||
| EPICATECHIN | mango | mango pulp fiber waste (wet and dried) | n = 5 | Sudha et al. (2015) [34] |
| apple | royal delicious, golden delicious, red delicious | Rana et al. (2021) [44] | ||
| apple | Mexican apple | Cerdia-Tapia et al. (2015) [43] | ||
| ginseng | ginseng residue | Jiang et al. (2021) [55] | ||
| rice | defatted rice bran | Zhao et al. (2018) [18] | ||
| PROTOCATECHUIC ACID | rice | defatted rice bran | n = 1 | Zhao et al. (2018) [18] |
| PYROCATECHINIC ACID | fava bean | n = 4 | Betrouche et al. (2022) [29] | |
| tomato | tomato byproduct | Betrouche et al. (2022) [29] | ||
| kinnow | peel | Singh et al. (2016) [33] | ||
| banana | pulp | Singh et al. (2016) [33] | ||
| ANTHOCYANINS | ||||
| TOTAL ANTHOCYANINS | wheat | durum and bread wheat flour | n = 4 | Cuidad-Murelo et al. (2020) [25] |
| wheat | black, blue, and purple | Kumari et al. (2020) [26] | ||
| mango | green peel, green pulp, ripe peel and ripe pulp flour | Abdul et al. (2015) [35] | ||
| cherry | sour cherry pomace | Gumul et al. (2020) [53] | ||
| FLAVANONES | ||||
| NARINGENIN | tomato | tomato byproduct | n = 1 | Betrouche et al. (2022) [29] |
| HESPERITIN | acerola | cherry | n = 3 | Medeiros et al. (2020) [28] |
| guava | Medeiros et al. (2020) [28] | |||
| cashew | Medeiros et al. (2020) [28] | |||
| FLAVONES | ||||
| APIGENIN | tomato | tomato byproduct | n = 1 | Betrouche et al. (2022) [29] |
| CHRYSIN | acerola | cherry | n = 3 | Medeiros et al. (2020) [28] |
| guava | Medeiros et al. (2020) [28] | |||
| cashew | Medeiros et al. (2020) [28] | |||
| OTHER | ||||
| PHLORIDZIN | apple | royal delicious, golden delicious, red delicious, red chief, red gold | n = 2 | Rana et al. (2021) [44] |
| apple | Mexican apple | Cerdia-Tapia et al. (2015) [43] | ||
| NON-FLAVONOID COMPOUNDS: | ||||
| TANNINS | ||||
| TANNIC ACID | pineapple | pineapple shell | n = 1 | Larrauri et al. (1997) [52] |
| TOTAL TANNINS | pineapple | n = 5 | Nagarajaiah et al. (2021) [50] | |
| lemon | sweet lemon | Nagarajaiah et al. (2021) [50] | ||
| grapes | blue grapes | Nagarajaiah et al. (2021) [50] | ||
| orange | Nagarajaiah et al. (2021) [50]. | |||
| capuassu | capuassu peel | Salgado et al. (2011) [54] | ||
| STILLBENES | ||||
| RESVERATROL | rice | rice flour | n = 10 | Betrouche et al. (2022) [29] |
| fava bean | Betrouche et al. (2022) [29] | |||
| linseed | linseed meal | Betrouche et al. (2022) [29] | ||
| pomegranate | pulp | Singh et al. (2016) [33] | ||
| banana | peel and pulp | Singh et al. (2016) [33] | ||
| grapes | Singh et al. (2016) [33] | |||
| sapodilla | peel | Singh et al. (2016) [33] | ||
| spinach | Singh et al. (2016) [33] | |||
| mentha | Singh et al. (2016) [33] | |||
| ginseng | ginseng residue | Jiang et al. (2021) [55] | ||
| RESVERATROL DERIVATIVE | rice | rice flour | n = 3 | Betrouche et al. (2022) [29] |
| fava bean | ||||
| linseed | linseed meal | |||
| TOTAL STILLBENES | rice | rice flour | n = 3 | Betrouche et al. (2022) [29] |
| fava bean | ||||
| linseed | linseed meal | |||
| TOCOPHEROLS AND TOCOTRIENOLS | ||||
| ALPHA-TOCOPHEROL | Rice | KFSW and TK16 mature and immature rice grains | n = 2 | Lin et al. (2011) [20] |
| wheat | durum and bread wheat flour | Cuidad-Murelo et al. (2020) [25] | ||
| BETA-TOCOPHEROL | Rice | KFSW and TK16 mature and immature rice grains | n = 2 | Lin et al. (2011) [20] |
| wheat | durum and bread wheat flour | Cuidad-Murelo et al. (2020) [25] | ||
| GAMMA-TOCOPHEROL | Rice | KFSW and TK16 mature and immature rice grains | n = 1 | Lin et al. (2011) [20] |
| DELTA-TOCOPHEROL | Rice | KFSW and TK16 mature and immature rice grains | n = 1 | Lin et al. (2011) [20] |
| ALPHA-TOCOTRIENOL | Rice | KFSW and TK16 mature and immature rice grains | n = 1 | Lin et al. (2011) [20] |
| BETA-TOCOTRIENOL | Rice | KFSW and TK16 mature and immature rice grains | n = 1 | Lin et al. (2011) [20] |
| GAMMA-TOCOTRIENOL | Rice | KFSW and TK16 mature and immature rice grains | n = 1 | Lin et al. (2011) [20] |
| VITAMIN C | ||||
| ASCORBIC ACID | lemon | sweet lemon | n = 5 | Nagarajaiah et al. (2021) [50] |
| grapes | blue grapes | Nagarajaiah et al. (2021) [50] | ||
| orange | Nagarajaiah et al. (2021) [50] | |||
| pineapple | Nagarajaiah et al. (2021) [50] | |||
| mango | green peel, green pulp, ripe peel and ripe pulp flour | Abdul et al. (2012) [35] | ||
| RETINOL EQUIVALENTS | persimmon | destringed | n = 1 | Diaz et al. (2020) [36] |
| CAROTENOIDS | ||||
| TOTAL CAROTENOIDS | stinging nettle | n = 10 | Krawecka et al. (2021) [56] | |
| lemon | sweet lemon | Nagarajaiah et al. (2021) [50] | ||
| pineapple | Nagarajaiah et al (2021) [50] | |||
| orange | Nagarajaiah et al. (2021) [50] | |||
| rice | rice flour | Betrouche et al. (2022) [29] | ||
| fava bean | Betrouche et al. (2022) [29] | |||
| tomato | tomato byproduct | Betrouche et al. (2022) [29] | ||
| linseed | linseed meal | Betrouche et al. (2022) [29] | ||
| persimmon | destringed | Diaz et al. (2020) [36] | ||
| mango | green peel, green pulp, ripe peel and ripe pulp flour | Abdul et al. (2012) [35] | ||
| BETA-CAROTENE | corn | sweet corn cob | n = 7 | Lau et al. (2019) [24] |
| rice | rice flour | Betrouche et al. (2022) [29] | ||
| fava bean | Betrouche et al. (2022) [29] | |||
| tomato | tomato byproduct | Betrouche et al. (2022) [29] | ||
| linseed | linseed meal | Betrouche et al. (2022) [29] | ||
| persimmon | destringed | Diaz et al. (2020) [36] | ||
| mango | mango pulp fiber waste (wet and dried) | Sudha et al. (2015) [34] | ||
| BETA-CRYPTOXANTHIN | fava bean | n = 4 | Betrouche et al. (2022) [29] | |
| tomato | tomato byproduct | Betrouche et al. (2022) [29] | ||
| linseed | linseed meal | Betrouche et al. (2022) [29] | ||
| persimmon | destringed | Diaz et al. (2020) [36] | ||
| NEOXANTHIN | persimmon | destringed | n = 1 | Diaz et al. (2020) [36] |
| VIOLAXANTHIN | persimmon | destringed | n = 1 | Diaz et al. (2020) [36] |
| ZEAXANTHIN | corn | sweet corn cob | n = 5 | Lau et al. (2019) [24] |
| rice | rice flour | Betrouche et al. (2022) [29] | ||
| fava bean | Betrouche et al. (2022) [29] | |||
| tomato | tomato byproduct | Betrouche et al. (2022) [29] | ||
| linseed | linseed meal | Betrouche et al. (2022) [29] | ||
| LUTEIN | corn | sweet corn cob | n = 5 | Lau et al. (2019) [24] |
| fava bean | Betrouche et al. (2022) [29] | |||
| tomato | tomato byproduct | Betrouche et al. (2022) [29] | ||
| linseed | linseed meal | Betrouche et al. (2022) [29] | ||
| mango | mango pulp fiber waste (wet and dried) | Sudha et al. (2015) [34] | ||
| LYCOPENE | rice | rice flour | n = 5 | Betrouche et al. (2022) [29] |
| fava bean | Betrouche et al. (2022) [29] | |||
| tomato | tomato byproduct | Betrouche et al. (2022) [29] | ||
| linseed | linseed meal | Betrouche et al. (2022) [29] | ||
| persimmon | destringed | Diaz et al. (2020) [36] | ||
| CHLOROPHYLLS | ||||
| CHLOROPHYLL A | stinging nettle | n = 1 | Krawecka et al. (2021) [56] | |
| CHLOROPHYLL B | stinging nettle | n = 1 | Krawecka et al. (2021) [56] | |
| BETALAINS | ||||
| BETACYANIN | pitaya | pitaya peel powder | n = 1 | Mai et al. (2022) [15] |
| OTHER | ||||
| TYROSOL | fava bean | n = 1 | Betrouche et al. (2022) [29] | |
| tomato | tomato byproduct | n = 1 | Betrouche et al. (2022) [29] | |
| γ-ORYZANOL | Rice | KFSW and TK16 mature and immature rice grains | n = 2 | Lin et al. (2011) [20] |
| rice | stabilized rice bran | Espinales et al. (2022) [16] | ||
| BETA-GLUCAN | rice | stabilized rice bran | n = 2 | Espinales et al. (2022) [16] |
| oat | oat milk byproduct | Wang et al. (2023) [22] | ||
| γ-AMINOBUTYRIC ACID (GABA) | Rice | stabilized rice bran | n = 1 | Espinales et al. (2022) [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timm, M.; Offringa, L.C.; Van Klinken, B.J.-W.; Slavin, J. Beyond Insoluble Dietary Fiber: Bioactive Compounds in Plant Foods. Nutrients 2023, 15, 4138. https://doi.org/10.3390/nu15194138
Timm M, Offringa LC, Van Klinken BJ-W, Slavin J. Beyond Insoluble Dietary Fiber: Bioactive Compounds in Plant Foods. Nutrients. 2023; 15(19):4138. https://doi.org/10.3390/nu15194138
Chicago/Turabian StyleTimm, Madeline, Lisa C. Offringa, B. Jan-Willem Van Klinken, and Joanne Slavin. 2023. "Beyond Insoluble Dietary Fiber: Bioactive Compounds in Plant Foods" Nutrients 15, no. 19: 4138. https://doi.org/10.3390/nu15194138
APA StyleTimm, M., Offringa, L. C., Van Klinken, B. J.-W., & Slavin, J. (2023). Beyond Insoluble Dietary Fiber: Bioactive Compounds in Plant Foods. Nutrients, 15(19), 4138. https://doi.org/10.3390/nu15194138






