Dietary Patterns, Socio-Demographic Predictors Thereof, and Associations of Dietary Patterns with Stunting and Overweight/Obesity in 1–<10-Year-Old Children in Two Economically Active Provinces in South Africa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Structure of the Sample and the Sampling Procedure
- N = the number of households per sampling stratum, taking non-response into account was calculated to be N (=175);
- Deft (=1.3) is the design effect;
- P (=0.21) is the estimated proportion of children classified as stunted;
- a (=0.2) is the desired relative standard error;
- R1 (=0.96) is the individual response rate;
- R2 (=0.89) is the household gross response rate;
- d (=1.06) is the number of eligible individuals per households [7].
2.3. Selection of Households and Children within Households
2.4. Fieldwork Teams
2.5. Measures
2.5.1. Socio-Demographic Questionnaire
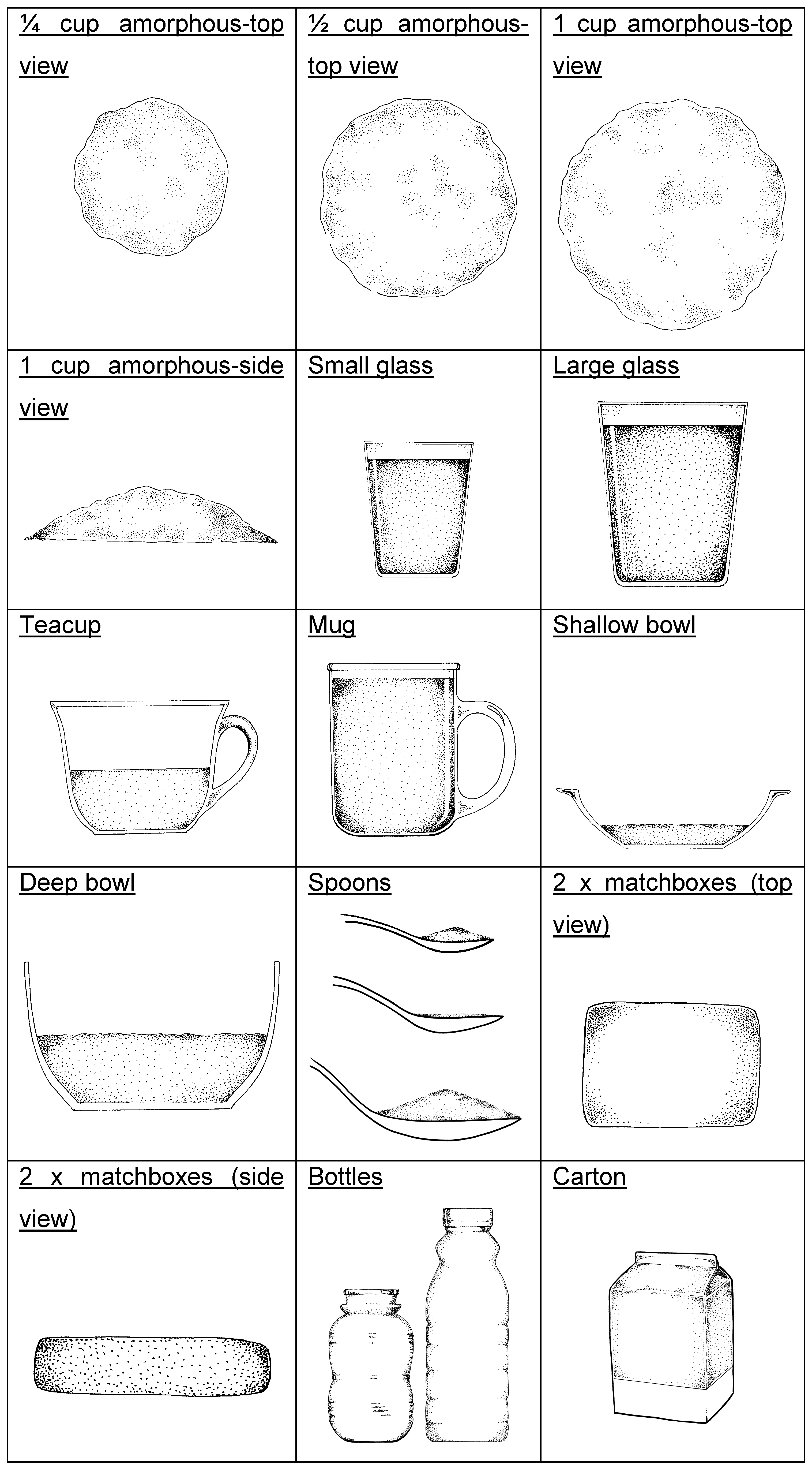
2.5.2. Dietary Intake
2.6. Data Management and Analysis
3. Results
3.1. Results for Sociodemographic Profile of HHs
3.2. Results for 1–<3-Year-Old Children
3.3. Results for 3–<6-Year-Old Children
3.4. Results for 6–<10-Year-Old Children
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ten Hove, H.; Guo, X.; Bakker, S.; Herens, M. Addressing Overweight and Obesity in LMICs in Rural Development and Food Systems: A Comprehensive Literature Review; Wageningen Centre for Development Innovation: Wageningen, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Shisana, O.; Labadarios, D.; Rehle, T.; Simbayi, L.; Zuma, K.; Dhansay, A.; Reddy, P.; Parker, W.; Hoosain, E.; Naidoo, P.; et al. The South African National Health and Nutrition Examination Survey, 2012: SANHANES-1: The Health and Nutritional Status of the Nation; HSRC Press: Cape Town, South Africa, 2014; Available online: http://repository.hsrc.ac.za/handle/20.500.11910/2864 (accessed on 20 January 2022).
- Vorster, H.H.; Kruger, A. Poverty, malnutrition, underdevelopment and cardiovascular disease: A South African perspective. Cardiovasc. J. Afr. 2007, 18, 321–324. [Google Scholar]
- Liberali, E.; Kupek, E.; Altenburg de Assis, M.A. Dietary patterns and childhood obesity risk: A systematic review. Child. Obes. 2020, 16, 70–85. [Google Scholar] [CrossRef]
- Umer, A.; Kelley, G.A.; Cottrell, L.E.; Giacobbi, P.; Innes, K.E.; Lilly, C. Childhood obesity and adult cardiovascular disease risk factors: A systematic review with meta-analysis. BMC Public Health 2017, 17, 683. [Google Scholar] [CrossRef] [PubMed]
- Katzmarzyk, P.; Barlow, S.; Bouchard, C.; Catalano, P.M.; Hsia, D.S.; Inge, T.H.; Lovelady, C.; Raynor, H.; Redman, L.M.; Staino, A.E.; et al. An evolving scientific basis for the prevention and treatment of pediatric obesity. Int. J. Obes. 2014, 38, 887–905. [Google Scholar] [CrossRef] [PubMed]
- Senekal, M.; Nel, J.H.; Malczyk, S.; Drummond, L.; Harbron, J.; Steyn, N.P. Provincial Dietary Intake Study (PDIS): Prevalence and Sociodemographic Determinants of the Double Burden of Malnutrition in A Representative Sample of 1 to Under 10-Year-Old Children from Two Urbanized and Economically Active Provinces in South Africa. Int. J. Environ. Res. Public Health 2019, 16, 3334. [Google Scholar] [CrossRef]
- Steyn, N.P.; Nel, J.H.; Drummond, L.; Malczyk, S.; Senekal, M. Has Food Security and Nutritional Status Improved in Children 1–<10 Years in two Provinces of South Africa between 1999 (National Food Consumption Survey) and 2018 (Provincial Dietary Intake Study (PDIS)). Int. J. Environ. Res. Public Health 2022, 19, 1038. [Google Scholar] [CrossRef]
- Lobstein, T.; Brinsden, H.; Neveux, M. World Obesity Atlas; World Obesity Federation: London, UK, 2022. [Google Scholar]
- WHO; FAO. Diet, Nutrition and the Prevention of Chronic Diseases; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- WHO. Good Maternal Nutrition the Best Start in Life; WHO: Geneva, Switzerland, 2016; Available online: https://iris.who.int/handle/10665/329459 (accessed on 1 May 2023).
- Mikkelsen, B.; Williams, J.; Rakovac, I.; Wickramasing, K.; Hennis, A.; Hai-Rim Shin, H.R.; Breda, J.; Huber, M.; Borges, C.; Berdzuli, N.; et al. Life course approach to prevention and control of non-communicable diseases. BMJ 2019, 364, l257. [Google Scholar] [CrossRef]
- GBD Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Popkin, B.M.; Ng, S.W. The nutrition transition to a stage of high obesity and noncommunicable disease prevalence dominated by ultra-processed foods is not inevitable. Obes. Rev. 2022, 23, e13366. [Google Scholar] [CrossRef]
- Popkin, B. Nutrition transition and the global diabetes epidemic. Curr. Diabetes Rep. 2015, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Li, Y.; Liu, A.; Zhang, Q.; Hu, X.; Du, S.; Ma, J.; Xu, G.; Ling, Y.; Guo, H.; et al. Dietary pattern and its association with the prevalence of obesity and related cardiometabolic risk factors among Chinese children. PLoS ONE 2012, 7, e43183. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The UN decade of nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, K.; Schulze, M.; Boeing, H.; Altenburg, H.P. Dietary patterns: Report of an International Workshop. Public Health Nutr. 2018, 5, 89–90. [Google Scholar] [CrossRef]
- Schwerin, H.S.; Stanton, J.L.; Smith, J.L.; Riley, A.M., Jr.; Brett, B.E. Food, eating habits, and health: A further examination of the relationship between food eating patterns and nutritional health. Am. J. Clin. Nutr. 1982, 35, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH collaborative research group. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef]
- Olinto, M.T.; Willett, W.C.; Gigante, D.P.; Victora, C.G. Sociodemographic and lifestyle characteristics in relation to dietary patterns among young Brazilian adults. Public Health Nutr. 2010, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Marques-Vidal, P.; Waeber, G.; Vollenweider, P.; Guessous, I. Socio-demographic and lifestyle determinants of dietary patterns in French-speaking Switzerland, 2009–2012. BMC Public Health 2018, 18, 131. [Google Scholar] [CrossRef]
- Melaku, Y.A.; Gill, T.K.; Taylor, A.W.; Adams, R.; Shi, Z.; Worku, A. Associations of childhood, maternal and household dietary patterns with childhood stunting in, E.thiopia: Proposing an alternative and plausible dietary analysis method to dietary diversity scores. Nutr. J. 2018, 17, 1–15. [Google Scholar] [CrossRef]
- Johnson, L.; Toumpakari, Z.; Papadaki, A. Social gradients and physical activity trends in an obesogenic dietary pattern: Cross-sectional analysis of the UK National Diet and Nutrition Survey 2008–2014. Nutrients 2018, 10, 388. [Google Scholar] [CrossRef]
- Nel, J.; Steyn, N.P. The Nutrition Transition and the Double Burden of Malnutrition in Sub-Saharan African Countries: How Do These Countries Compare with the Recommended Lancet Commission Global Diet? Int. J. Environ. Res. Public Health 2022, 19, 16791. [Google Scholar] [CrossRef]
- FAO. Food-Based Dietary Guidelines. 2018. Available online: www.fao.org/nutrition/education/food-dietaryguidelines/home/en/ (accessed on 30 January 2018).
- Bourne, L. South African paediatric food-based dietary guidelines. Matern. Child Nutr. 2007, 3, 3227–3229. [Google Scholar] [CrossRef] [PubMed]
- Vorster, H.H.; Badham, J.B.; Venter, C.S. An introduction to the revised food-based dietary guidelines for South Africa. S. Afr. J. Clin. Nutr. 2013, 3, S5–S12. [Google Scholar]
- Statistics South Africa. Mid-Year Population Estimates 2018. Available online: http://www.statssa.gov.za/?p=11341 (accessed on 6 March 2019).
- Statistics South Africa Census 2011 Metadata. Available online: http://www.statssa.gov.za/census/census_2011/census_products/Census_2011_Metadata.pdf (accessed on 30 January 2022).
- Labadarios, D.; Steyn, N.P.; Maunder, E.; MacIntryre, U.; Gericke, G.; Swart, R.; Huskisson, J.; Dannhauser, A.; Vorster, H.H.; Nesamvuni, A.E. The National Food Consumption Survey (NFCS): South Africa, 1999. Public Health Nutr. 2005, 8, 533–543. [Google Scholar] [CrossRef]
- Filmer, D.; Pritchett, L. Estimating wealth effects without expenditure data—Or tears: With an application to educational enrollments in the states of India. In The World Bank Development Research Group; The World Bank: Washington, DC, USA, 1998. [Google Scholar]
- South African Medical Research Council. South African Demographic and Health Survey: 2016; South Africa Medical Research Council: Pretoria, South Africa, 2017. [Google Scholar]
- Wehler, C.; Scott, R.; Anderson, J. The community childhood hunger identification project: A model of domestic hunger-demonstration. J. Nutr. Educ. 1992, 24, 295–355. [Google Scholar] [CrossRef]
- Moshfegh, A.J.; Rhodes, D.G.; Baer, D.J.; Muray, T.; Clemens, J.C.; Rumpler, W.V.; Paul, D.R.; Sebastian, R.S.; Kuczynski, K.J.; Ingwersen, L.A.; et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am. J. Clin. Nutr. 2008, 88, 324–332. [Google Scholar] [CrossRef]
- Steyn, N.P.; Nel, J.H.; Malczyk, S.; Drummond, L.; Senekal, M. Provincial Dietary Intake Study (PDIS): Energy and macronutrient intakes of children in a representative/random sample of 1–<10-year-old children in two economically active and urbanized provinces in South Africa. Int. J. Environ. Res. Public Health 2020, 17, 1717. [Google Scholar] [CrossRef]
- Burrows, T.L.; Martin, R.J.; Collins, C.E. A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labelled water. J. Am. Diet Assoc. 2010, 110, 1501–1510. [Google Scholar] [CrossRef]
- Steyn, N.P.; Senekal, M. The Dietary Assessment and Education Kit (DAEK) The Chronic Diseases of Lifestyle Unit of the South African Medical Research Council; MRC: Cape Town, South Africa, 2004. [Google Scholar]
- Steyn, N.P.; Senekal, M.; Norris, S.A.; Whati, L.; Mackeown, J.M.; Nel, J.H. How well do adolescents determine portion sizes of foods and beverages? Asia Pac. J. Clin. Nutr. 2006, 15, 35–42. [Google Scholar] [PubMed]
- Neville, M.C.; Allen, J.C.; Archer, P.C.; Casey, C.E.; Seacat, J.; Keller, R.P.; Lutes, V.; Rasbach, J.; Neifert, M. Studies in human lactation: Milk volume and nutrient composition during weaning and lactogenesis. Am. J. Clin. Nutr. 1991, 54, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Tooze, J.A.; Kipnis, V.; Buckman, D.W.; Carroll, R.J.; Freedman, L.S.; Guenther, P.M.; Krebs-Smith, S.M.; Subar, A.F.; Dodd, K.W. A mixed-effects model approach for estimating the distribution of usual intake of nutrients: The NCI method. Stat. Med. 2010, 29, 2857–2868. [Google Scholar] [CrossRef]
- Herrick, K.A.; Rossen, L.M.; Parsons, R.; Dodd, K.W. Estimating usual dietary intake from National Health and Nutrition Examination 5. Survey data using the National Cancer Institute method. National Center for Health Statistics. Vital Health Stat. 2018, 2, 1–63. [Google Scholar]
- Van Graan, A.E.; Chetty, J.M.; Links, M.R. Food Composition Tables for South Africa, 5th ed.; South African Medical Research Council: Cape Town, South Africa, 2017. [Google Scholar]
- O’Rourke, N.; Hatcher, L. A Step by Step Approach to Using SAS System for Factor Analysis and Structural Equation Modelling, 2nd ed.; SAS Press: Cary, NC, USA, 2013. [Google Scholar]
- Steyn, N.P.; Maunder, E.M.; Labadarios, D.; Nel, J.H. Foods and beverages that make a significant contribution to macro- and micronutrient intakes of children in South Africa- do they meet the food-based dietary guidelines? S. Afr. J. Clin. Nutr. 2006, 19, 66–76. [Google Scholar] [CrossRef]
- Maunder, E.M.W.; Nel, J.H.; Steyn, N.P.; Kruger, H.S.; Labadarios, D. Added Sugar, Macro- and Micronutrient Intakes and Anthropometry of Children in a Developing World Context. PLoS ONE 2015, 10, e0142059. [Google Scholar] [CrossRef] [PubMed]
- Okeyo, A.; Seekoe, E.; de Villiers, A.; Faber, M.; Nel, J.H.; Steyn, N.P. Dietary Practices and Adolescent Obesity in Secondary School Learners at Disadvantaged Schools in South Africa: Urban–Rural and Gender Differences. Int. J. Environ. Res. Public Health 2020, 17, 5864. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, H.F. A second generation little jiffy. Psychometrika 1970, 35, 401–415. [Google Scholar] [CrossRef]
- Blom, G. Statistical Estimates and Transformed Beta-Variables; Wiley: New York, NY, USA, 1958. [Google Scholar]
- Ricci, C.; Baumgartner, J.; Wentzel-viljoen, E.; Smuts, C.M. Food and nutrient pattern assessment using principal component analysis applied to food questionnaires. Pitfalls, tips and tricks. Int. J. Food Sci.Nutr. 2019, 70, 738–748. [Google Scholar] [CrossRef]
- Faber, M.; Rothman, M.; Laubscher, R.; Smuts, C.M. Dietary patterns of 6–24-month-old children are associated with nutrient content and quality of the diet. Matern. Child Nutr. 2019, 16, e12901. [Google Scholar] [CrossRef] [PubMed]
- Keding, G. Nutrition Transition in Rural Tanzania and Kenya. Hidden Hunger. Malnutrition and the First 1000 Days of Life: Causes, Consequences and Solutions. In World Review of Nutrition and Dietetics; Biesalski, H.K., Black, R.E., Eds.; Karger: Basel, Switzerland, 2016; Volume 115, pp. 68–81. [Google Scholar] [CrossRef]
- Hooper, R.; Calvert, J.; Thompson, R.L.; Deetlefs, M.E.; Burney, P. Urban/rural differences in diet and atopy in South Africa. Allergy 2008, 63, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Department of Health. Government Notice: Foodstuffs, Cosmetics and Disinfectants Act, No. R 2003. (Act No. 54 of 1972). Regulations Relating to the Fortification of Certain Foodstuffs; Department of Health: Pretoria, South Africa, 2003. [Google Scholar]
- O’Halloran, S.A.; Eksteen, G.; Polayya, N.; Ropertz, M.; Senekal, M. The food environment of primary school learners in a low-to-middle-income area in Cape Town, South Africa. Nutrients 2021, 13, 2043. [Google Scholar] [CrossRef] [PubMed]
- Temple, N.J.; Steyn, N.P.; De Villiers, A. Price and availability of healthy food: A study in rural South Africa. Nutr. J. 2011, 27, 55–58. [Google Scholar] [CrossRef]
- Headey, D.D.; Alderman, H.A. The relative caloric prices of healthy and unhealthy foods differ systematically across income levels and continents. J. Nutr. 2019, 149, 2020–2033. [Google Scholar] [CrossRef]
- National Treasury. Policy Paper and Proposals on the Taxation of Sugar Sweetened Beverages-8 July 2016. Available online: https://www.gov.za/documents/taxation-sugar-sweetened-beverages-policy-paper-8-jul-2016-0000 (accessed on 3 May 2023).
- Abizari, A.R.; Ali, Z. Dietary patterns and associated factors of schooling Ghanaian adolescents. J. Health Popul. Nutr. 2019, 38, 5. [Google Scholar] [CrossRef] [PubMed]
- Okeyo, A.P.; Seekoe, E.; de Villiers, A.; Faber, M.; Nel, J.H.; Steyn, N.P. The food and nutrition environment at secondary schools in the Eastern Cape, South Africa as reported by learners. Int. J. Environ. Res. Public Health 2020, 17, 4038. [Google Scholar] [CrossRef] [PubMed]
- Vorster, H.H. “Make starchy foods part of most meals”: A food-based dietary guideline for South Africa. S. Afr. J. Clin. Nutr. 2013, 3, S28–S35. [Google Scholar]
- Heady, D.; Hirvonen, K.; Hoddinott Stifel, D. Rural food markets and child nutrition. Am. J. Agri. Econ. 2019, 101, 1311–1327. [Google Scholar] [CrossRef] [PubMed]
- Fitchen, K.M. Hunger, malnutrition, and poverty in the contemporary United States: Some observations on their social and cultural context. Food Foodways 1998, 2, 309–333. [Google Scholar] [CrossRef]
- Venter, C.S.; Ochse, R.; Swart, R. “Eat dry beans, split peas, lentils and soya regularly”: A food-based dietary guideline for South Africa. S. Afr. J. Clin. Nutr. 2013, 3, S36–S45. [Google Scholar]
- Naude, C.E. “Eat plenty of vegetables and fruit every day”: A food-based dietary guideline for South Africa. S. Afr. J. Clin. Nutr. 2013, 3, S46–S56. [Google Scholar]
- Pisa, P.T.; Pedro, T.M.; Kahn, K.; Tollman, S.M.; Pettifor, J.M.; Norris, S.A. Nutrient patterns and their association with socio-demographic, lifestyle factors and obesity risk in rural South African adolescents. Nutrients 2015, 7, 3464–3482. [Google Scholar] [CrossRef]
- Senekal, M.; Nel, J.H.; Malczyk, S.; Drummond, L.; Steyn, N.P. Provincial Dietary Intake Study (PDIS): Micronutrient intakes of children in a representative/random sample of 1- to <10 year old children in two economically active and urbanised provinces in South Africa. Int. J. Environ. Res. Public Health 2020, 17, 5924. [Google Scholar] [CrossRef]
- Mikkilä, V.; Vepsäläinen, H.; Saloheimo, T.; Gonzalez, S.A.; Meisel, J.D.; Hu, G.; Champagne, C.M.; Chaput, J.P.; Church, T.S.; Katzmarzyk, P.T.; et al. International comparison of dietary patterns in 9-11-year-old children. Int. J. Obes. Suppl. 2015, 5 (Suppl. 2), S17–S21. [Google Scholar] [CrossRef]
- Mank, I.; Vandormael, A.; Traore, I.; Quedraogo, W.A.; Sauerborn, R.; Danquah, I. Dietary habits associated with growth development of children aged < 5 years in the Nouna Health and Demographic Surveillance System, Burkina Faso. Nutr. J. 2020, 19, 1–14. [Google Scholar]
- Tanaka, J.; Yoshizawa, K.; Hirayama, K.; Karama, M.; Wanjihia, V.; Changoma, M.S.; Kaneko, S. Relationship between dietary patterns and stunting in preschool children: A cohort analysis from Kwale, Kenya. Public Health 2019, 173, 58–68. [Google Scholar] [CrossRef]
- Adeomi, A.A.; Fatusi, A.; Klipstein-Grobusch, K. Food security, dietary diversity, dietary patterns and the double burden of malnutrition among school-aged children and adolescents in two Nigerian States. Nutrients 2022, 14, 789. [Google Scholar] [CrossRef]
- Holmes, M.D.; Dalal, S.; Sewram, V.; Diamond, M.B.; Adebamowo, S.N.; Ajayi, I.O.; Adebamowo, C.; Chiwanga, F.S.; Njelekela, M.; Laurence, C.; et al. Consumption of processed food dietary patterns in four African populations. Public Health Nutr. 2018, 21, 1529–1537. [Google Scholar] [CrossRef]
- Malik, V.S.; Hu, F.B. The role of sugar-sweetened beverages in the global epidemics of obesity and chronic diseases. Nat. Rev Endocrinol. 2022, 18, 205–218. [Google Scholar] [CrossRef]
- Kwansa, A.L.; Akparibo, R.; Cecil, J.E.; Infield, S.G.; Caton, S.J. Risk factors for overweight and obesity within the home environment of preschool children in Sub-Saharan Africa: A systematic review. Nutrients 2022, 14, 1706. [Google Scholar] [CrossRef]
| Food Parameters | Terminology in Result Tables | Allocated Foods |
|---|---|---|
| Infant food | Infant food | Breast milk, breast milk substitutes, infant cereals |
| White bread | Bread white | White bread or rolls |
| Brown bread | Bread brown | Brown and whole wheat bread or rolls |
| Unrefined cereals | UCs | Hi-fibre breakfast cereals, e.g., All-Bran, Weetbix |
| Refined cereals | RCs | Refined breakfast cereals, sweetened and unsweetened |
| Maize porridge | Maize pap | Soft, stiff, and crumbly |
| Other refined carbohydrates | Rcarb-other | Rice, pasta, samp, mabella, mageu |
| Cheese | Cheese | Cheddar, gouda |
| Dairy | Dairy | Milk, yoghurt, and maas (sour milk) |
| Poultry | Poultry | With or without skin, any preparation |
| Red meat | Red meat | Beef, mutton, lamb and organ meat, any preparation |
| Processed meat | Proc meat | Cold meats, sausages, canned meat, dried meat |
| Eggs | Eggs | Any preparation |
| Fish | Fish | Fresh, canned, any preparation |
| Legumes | Legumes | Beans, lentils—soup and other preparations, soy mince |
| Vegetables: starchy | Veg-starchy | Potatoes, sweet potato, corn, sweet corn |
| Vegetables: starchy + fat | Veg-starchy + fat | “Slap chips” 1, potato roasted in fat, candied sweet potato |
| Vegetables: non starchy | Veg-non starchy | All vegetables except for starchy vegetables |
| Fruit | Fruit | Any fresh, canned or dried fruit, juice |
| Fats and oils: saturated | Fats-oils-sat | Butter, lard, hard margarine, coconut oil, non-dairy creamer |
| Fats and oils: unsaturated | Fats-oils-unsat | Soft margarine, plant oils, avocado, nuts, salad dressing |
| Refined carbohydrate + fat | Rcarb + fat | Savoury snacks—crisps, crackers |
| Refined carbohydrate + fat + sugar | Rcarb + fat + sugar | Cake, tarts, doughnuts, ice-cream, chocolates |
| Refined carbohydrate +protein + fat | Rcarb + prot + fat | Samoosas, pies, vetkoek, pizza, pasta dishes, fish cake |
| Refined carbohydrates + sugar | Rcarb + sug | Sweets: boiled, jelly-like |
| Sugar-sweetened beverages | SSBs | Fizzy drinks, squash, sport drinks |
| Sugar or syrup | Sugar | Granulated sugar, syrup, jam |
| Tea-coffee | Tea-coffee | Rooibos tea, Ceylon tea, coffee (no milk/sugar added) |
| Soup-sauces | Soup-sauces | Commercial soups, tomato sauce, chutney |
| Miscellaneous | Misc. | Condiments, Marmite, Bovril, fish paste |
| Gauteng N = 733 % (95% CI) | Western Cape N = 593 % (95% CI) | Rao–Scott Chi-Sq Values | All N = 1326 % (95% CI) | |
|---|---|---|---|---|
| Primary caregiver | ||||
| Mother | 70.1 (65.6–74.6) | 71.0 (64.7–77.2) | 0.045 * | 70.4 (66.8–74.0) |
| Father | 6.6 (3.4–9.7) | 1.8 (0.2–3.3) | 5.0 (2.8–7.1) | |
| Grandparent | 16.7 (12.9–20.4) | 21.0 (15.5–26.4) | 18.1 (15.0–21.2) | |
| Other (e.g., sibling, aunt) | 6.7 (4.0–9.5) | 6.3 (2.1–10.4) | 6.6 (4.3–8.8) | |
| Age in years | ||||
| 1–<3 years | 26.3 (22.1–30.6) | 25.3 (19.4–31.2) | 0.923 | 26.0 (22.6–29.4) |
| 3–<6 years | 35.4 (31.0–39.8) | 35.1 (30.7–39.5) | 35.3 (32.1–38.5) | |
| 6–<10 years | 38.3 (34.1–42.4) | 39.6 (33.1–46.1) | 38.7 (35.2–42.2) | |
| Gender | ||||
| Male | 50.2 (45.5–54.9) | 47.5 (43.1–51.9) | 0.391 | 49.3 (45.9–52.7) |
| Female | 49.8 (45.1–54.5) | 52.5 (48.1–56.9) | 50.7 (47.3–54.1) | |
| Head of household | ||||
| Father | 40.2 (33.8–46.6) | 38.8 (34.6–43.0) | 0.132 | 39.7 (35.3–44.1) |
| Mother | 16.8 (13.8–19.9) | 10.8 (7.0–14.5) | 14.8 (12.5–17.2) | |
| Grandmother | 21.9 (15.5–28.3) | 28.3 (21.8–34.9) | 24.0 (19.3–28.8) | |
| Grandfather | 11.7 (8.3–15.1) | 14.0 (10.0–18.0) | 12.5 (9.9–15.0) | |
| Other (e.g., aunt, uncle) | 9.4 (5.7–13.1) | 8.1 (4.9–11.4) | 9.0 (6.3–11.7) | |
| Marital status of mother | ||||
| Unmarried | 41.1 (34.9–47.2) | 34.8 (28.4–41.1) | <0.001 *** | 39.0 (34.4–43.5) |
| Married | 24.9 (20.5–29.4) | 41.3 (33.3–49.2) | 30.4 (26.4–34.3) | |
| Divorced/widowed | 4.8 (2.5–7.0) | 2.4 (0.7–4.2) | 4.0 (2.4–5.6) | |
| Living together | 27.8 (22.0–33.6) | 20.8 (15.9–25.7) | 25.5 (21.4–29.6) | |
| Other | 1.4 (0.2–2.6) | 0.8 (0.0–1.8) | 1.2 (0.3–2.1) | |
| Mother’s highest education | ||||
| Not completing Gr. 12 | 51.2 (44.9–57.4) | 57.7 (47.1–68.3) | 0.183 | 53.3 (47.9–58.7) |
| Completion of Gr. 12 | 33.9 (28.4–39.4) | 24.7 (17.6–31.8) | 30.8 (26.5–35.2) | |
| Qualification after Gr.12 | 12.2 (8.7–15.7) | 15.6 (7.6–23.6) | 13.3 (9.9–16.8) | |
| Do not know | 2.8 (1.4–4.1) | 2.0 (0.5–3.5) | 2.5 (1.5–3.5) | |
| Father’s highest education | ||||
| Not completing Gr. 12 | 26.9 (22.0–31.7) | 33.8 (29.0–38.5) | 0.323 | 29.1 (25.6–32.7) |
| Completion of Gr. 12 | 32.6 (26.9–38.3) | 30.4 (25.2–35.6) | 31.9 (27.8–36.0) | |
| Qualification after Gr.12 | 13.1 (9.4–16.9) | 10.7 (5.7–15.7) | 12.3 (9.4–15.3) | |
| Do not know | 27.4 (22.4–32.4) | 25.2 (19.7–30.6) | 26.7 (22.9–30.4) | |
| Mother’s employment status | ||||
| Yes | 22.4 (17.8–26.9) | 38.4 (31.0–45.9) | <0.001 ** | 27.7 (23.9–31.5) |
| No | 74.6 (69.6–79.6) | 60.2 (53.0–67.5) | 69.8 (65.8–73.9) | |
| Do not know/not applicable | 3.0 (1.3–4.7) | 1.3 (0.3–2.4) | 2.5 (1.3–3.6) | |
| Father’s employment status | ||||
| Yes | 64.8 (60.6–69.1) | 65.3 (59.7–70.9) | 0.953 | 65.0 (61.6–68.4) |
| No | 21.4 (17.5–25.3) | 20.5 (15.1–25.9) | 21.1 (18.0–24.2) | |
| Do not know/not applicable | 13.8 (11.1–16.4) | 14.1 (10.2–18.1) | 13.9 (11.7–16.1) | |
| Wealth index quintiles | ||||
| One | 21.1 (14.6–27.6) | 17.7 (10.7–24.7) | 0.263 | 20.0 (15.1–24.8) |
| Two | 17.8 (12.0–23.6) | 24.3 (20.0–28.6) | 20.0 (15.9–24.0) | |
| Three | 21.3 (17.0–25.7) | 17.0 (12.6–21.4) | 19.9 (16.7–23.1) | |
| Four | 21.5 (16.7–26.3) | 17.5 (12.4–22.6) | 20.2 (16.6–23.7) | |
| Five | 18.3 (11.6–25.0) | 23.5 (14.5–32.5) | 20.0 (14.7–25.3) | |
| Ethnicity | ||||
| Black African | 97.8 (96.0–99.6) | 27.6 (12.9–42.3) | <0.001 ** | 74.5 (69.5–79.4) |
| Mixed ancestry | 2.2 (0.3–4.0) | 68.0 (53.7–82.4) | 24.1 (19.2–28.9) | |
| Other | 0.0 (0.0–0.1) | 4.4 (0.6–8.2) | 1.5 (0.3–2.7) | |
| Type of residence | ||||
| Rural | 2.4 (0.7–4.1) | 6.6 (1.6–11.5) | 0.194 | 3.8 (1.9–5.7) |
| Urban formal | 88.9 (82.3–95.4) | 86.8 (79.1–94.5) | 88.2 (83.2–93.2) | |
| Urban informal | 8.7 (2.7–14.7) | 6.6 (1.7–11.5) | 8.0 (3.7–12.3) | |
| Mother’s BMI [39] | ||||
| Underweight/normal BMI = <18.5 and 18.5–24.9 kgm2 | 33.3 (28.0–38.5) | 29.1 (23.6–34.5) | 0.002 ** | 32.0 (28.0–35.9) |
| Overweight BMI = 25–29.9 kgm2 | 27.7 (23.6–31.8) | 20.4 (16.5–24.3) | 25.4 (22.4–28.5) | |
| Obese BMI ≥ 30 kgm2 | 39.1 (35.8–42.3) | 50.6 (43.0–58.1) | 42.6 (39.4–45.8) | |
| Hunger scale [25] | ||||
| Total score = 0: No risk | 57.9 (49.5–66.3) | 48.8 (38.9–58.7) | 0.1483 | 54.9 (48.5–61.3) |
| 1–4: At risk of hunger | 22.1 (17.2–27.0) | 28.9 (23.0–34.9) | 24.4 (20.6–28.2) | |
| 5–8: Food shortage in house | 20.0 (14.8–25.1) | 22.3 (16.5–28.0) | 20.7 (16.8–24.6) |
| Pap Soup/Sauce Pattern | Tea/Coffee & Sugar Pattern | Mostly Unhealthy Snack Pattern | White Bread & Topping Pattern | Healthy Pattern | |||||
| Food Parameters | PL | Food Parameters | PL | Food Parameters | PL | Food Parameters | PL | Food Parameters | PL |
| Maize pap | 0.84 | Tea and/or coffee | 0.74 | RC-Fat | 0.52 | Bread White | 0.65 | Fats-oils-Unsat | 0.60 |
| Soup-sauces | 0.44 | Sugar | 0.72 | RC-Fat-sugar | 0.50 | Processed meat | 0.53 | Veg non-starchy | 0.41 |
| Dairy | −0.39 | Fats-oils-Sat | 0.59 | Bread Brown | 0.42 | Miscellaneous | 0.36 | Fish | 0.31 |
| RC-Other | −0.55 | Legumes | 0.33 | SSB | 0.41 | Eggs | 0.32 | Poultry | −0.38 |
| URC | −0.59 | Fruit | 0.36 | RC-Sugar | −0.55 | ||||
| Baby food | −0.52 | ||||||||
| % Variance explained | 2.16 | % Variance explained | 2.1 | % Variance explained | 2.0 | % Variance explained | 1.66 | % Variance explained | 1.6 |
| Pattern Predictors 1 | PE (SE) p-Value 2 | Pattern Predictors 1 | PE (SE) p-Value 2 | Pattern Predictors 1 | PE (SE) p-Value 2 | Pattern Predictors 1 | PE (SE) p-Value 2 | Pattern Predictors 1 | PE (SE) p-Value 2 |
| HHH Other -lesser adherence | −0.29 (0.13) 0.034 | Higher WI -greater adherence | 0.04 (0.01) 0.013 | PCG: Grandmother -lesser adherence | −0.38 (0.15) 0.015 | Gauteng -lesser adherence | −0.27 (0.11) 0.015 | HHH: Grandparent -lesser adherence | −0.29 (0.11) 0.009 |
| Mother has Gr 12 -lesser adherence | −0.31 (0.09) <0.001 | PCG: Other -lesser adherence | −0.4 (0.19) 0.035 | Mother has Gr 12 -greater adherence | 0.37 (0.12) 0.002 | Mother obese -lesser adherence | −0.24 (0.11) 0.026 | Gauteng -greater adherence | 0.45 (0.11) <0.001 |
| Father has Gr12+ -less adherence | −0.27 (0.14) 0.049 | Hunger risk -greater adherence | 0.35 (0.11) 0.002 | Father has Gr12+ - greater adherence | 0.4 (0.18) 0.028 | PCG: Other -lesser adherence | −0.35 (0.18) 0.06 | Mother overweight -greater adherence | 0.31 (0.14) 0.023 |
| Higher WI -lesser adherence | −0.03 (0.01) 0.016 | Mother obese -greater adherence | 0.33 (0.12) 0.007 | ||||||
| Gauteng -greater adherence | 1.23 (0.09) <0.001 | Greater WI -greater adherence | 0.03 (0.02) 0.04 | ||||||
| Hunger risk -greater adherence | 0.25 (0.1) 0.009 | ||||||||
| Hunger present -greater adherence | 0.33 (0.12) 0.008 | ||||||||
| Tea/Coffee, Sugar, & Sandwich Pattern | Unhealthy Food & Snack Pattern | Starch & Poultry Pattern | Breakfast Item Pattern | Vegetable & Legume Pattern | |||||
| Food Parameters | PL | Food Parameters | PL | Food Parameters | PL | Food Parameters | PL | Food Parameters | PL |
| Tea/coffee | 0.85 | Bread-White | 0.65 | RC-Other | 0.71 | Dairy | 0.62 | Legumes | 0.41 |
| Sugar-syrup | 0.82 | Veg-Starchy-F | 0.55 | Veg starchy | 0.48 | Fruit | 0.57 | Veg non-starchy | 0.41 |
| Fats-oils-Unsat | 0.49 | RC- Prot-Fat | 0.41 | Poultry | 0.43 | Cheese | 0.46 | Miscellaneous | 0.40 |
| Bread Brown | 0.33 | RC-Fat-sugar | 0.41 | Maize pap | −0.53 | RC-Fort-Cereal | 0.46 | URC | −0.62 |
| Fats-oils-Sat | 0.31 | Processed meat | 0.36 | ||||||
| % Variance explained | 2.2 | % Variance explained | 1.81 | % Variance explained | 1.74 | % Variance explained | 1.72 | % Variance explained | 1.68 |
| Pattern Predictors 1 | PE (SE) p-Value 2 | Pattern Predictors 1 | PE (SE) p-Value 2 | Pattern Predictors 1 | PE (SE) p-Value 2 | Pattern Predictors 1 | PE (SE p-Value 2 | Pattern Predictors 1 | PE (SE) p-Value 2 |
| None | HHH: Grandparent -greater adherence | 0.27 (0.1) 0.008 | PCG: Other -lesser adherence | −0.37 (0.14) 0.009 | PCG: Grandmother -lesser adherence | −0.32 (0.12) 0.008 | Gauteng -greater adherence | 0.66 (0.1) <0.001 | |
| Gauteng -lesser adherence | −0.63 (0.09) <0.001 | Gauteng -lesser adherence | −0.74 (0.09) <0.001 | Gender: Female -greater adherence | 0.24 (0.09) 0.006 | Hunger present -greater adherence | 0.25 (0.11) 0.022 | ||
| Greater WI -greater adherence | 0.03 (0.01) 0.016 | Mother overweight -lesser adherence | −0.30 (0.09) 0.001 | Mother has Gr12+ -greater adherence | 0.28 (0.14) 0.045 | ||||
| Mother employed -greater adherence | 0.22 (0.1) 0.026 | Father has Gr12 -greater adherence | 0.37 (0.1) <0.001 | ||||||
| Father has Gr12+ -greater adherence | 0.42 (0.15) 0.006 | ||||||||
| Mother employed -greater adherence | 0.41 (0.1) <0.001 | ||||||||
| Father employed -lesser adherence | −0.25 (0.1) 0.009 | ||||||||
| Greater WI -greater adherence | 0.05 (0.01) <0.001 | ||||||||
| Gauteng -lesser adherence | −0.24 (0.1) 0.012 | ||||||||
| Mother obese -greater adherence | 0.25 (0.09) 0.005 | ||||||||
| Mostly Unhealthy Pattern 1 | Tea/Coffee, Sugar, & Milk Pattern | Mostly Unhealthy Pattern 2 | White Bread & Topping Pattern | Starchy Pattern | |||||
| Food Parameters | PL | Food Parameters | PL | Food Parameters | PL | Food Parameters | PL | Food Parameters | PL |
| RC-Fat | 0.47 | Sugar | 0.85 | RC-BF cereal | 0.53 | Bread White | 0.84 | RC-Other | 0.68 |
| SSB | 0.44 | Tea or coffee | 0.82 | Red meat | 0.44 | Fats-oils-UnSat | 0.48 | Veg starchy | 0.46 |
| Fruit | 0.41 | Dairy | 0.56 | RC-Prot-Fat | 0.34 | Processed meat | 0.42 | ||
| URC | 0.40 | RC-Fat-Sugar | 0.32 | Bread Brown | −0.51 | ||||
| RC Sugar | 0.36 | Veg non-starchy | −0.32 | ||||||
| Fish | −0.33 | Fats oils Sat | −0.33 | ||||||
| Legumes | −0.39 | Poultry | −0.46 | ||||||
| Maize pap | −0.50 | ||||||||
| Variance explained | 2.2% | Variance explained | 2.1% | Variance explained | 1.83% | Variance explained | 1.67% | Variance explained | 1.61% |
| Pattern Predictors 1 | PE (SE) p-Value 2 | Pattern Predictors 1 | PE (SE) p-Value 2 | Pattern Predictors 1 | PE (SE) p-Value 2 | Pattern Predictors 1 | PE(SE) p-Value 2 | Pattern predictors 1 | PE(SE) p-Value 2 |
| Father has Gr12 -greater adherence | 0.19(0.09) 0.040 | HHH: Mother -less adherence | −0.41(0.11) <0.001 | Higher WI -greater adherence | 0.05(0.01) <0.001 | Gauteng -less adherence | −0.32(0.09) <0.001 | HHH: Other -greater adherence | 0.35(0.16) 0.3 |
| Higher WI -greater adherence | 0.03(0.01) 0.017 | PCG: Other -less adherence | −0.28(0.12) 0.022 | Hunger present -less adherence | −0.34(0.1) <0.001 | PCG: Grandmother -greater adherence | 0.41(0.11) <0.001 | ||
| Gauteng -less adherence | −0.43(0.09) <0.001 | Female -less adherence | −0.28(0.09) 0.001 | Gauteng -less adherence | −0.34(0.09) <0.001 | Father employed -less adherence | −0.25(0.09) 0.006 | ||
| Mother obese -greater adherence | 0.21(0.09) 0.018 | Gauteng -less adherence | −0.34(0.09) <0.001 | Mother has Gr12+ -greater adherence | 0.21(0.13) 0.1 | Gauteng -less adherence | −0.91(0.09) <0.001 | ||
| Hunger risk -less adherence | −0.33(0.11) 0.002 | Hunger risk -greater adherence | 0.35(0.11) 0.001 | ||||||
| Hunger present -less adherence | −0.7(0.11) <0.001 | Hunger present -greater adherence | 0.28(0.11) 0.01 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senekal, M.; Nel, J.H.; Eksteen, G.; Steyn, N.P. Dietary Patterns, Socio-Demographic Predictors Thereof, and Associations of Dietary Patterns with Stunting and Overweight/Obesity in 1–<10-Year-Old Children in Two Economically Active Provinces in South Africa. Nutrients 2023, 15, 4136. https://doi.org/10.3390/nu15194136
Senekal M, Nel JH, Eksteen G, Steyn NP. Dietary Patterns, Socio-Demographic Predictors Thereof, and Associations of Dietary Patterns with Stunting and Overweight/Obesity in 1–<10-Year-Old Children in Two Economically Active Provinces in South Africa. Nutrients. 2023; 15(19):4136. https://doi.org/10.3390/nu15194136
Chicago/Turabian StyleSenekal, Marjanne, Johanna H. Nel, Gabriel Eksteen, and Nelia P. Steyn. 2023. "Dietary Patterns, Socio-Demographic Predictors Thereof, and Associations of Dietary Patterns with Stunting and Overweight/Obesity in 1–<10-Year-Old Children in Two Economically Active Provinces in South Africa" Nutrients 15, no. 19: 4136. https://doi.org/10.3390/nu15194136
APA StyleSenekal, M., Nel, J. H., Eksteen, G., & Steyn, N. P. (2023). Dietary Patterns, Socio-Demographic Predictors Thereof, and Associations of Dietary Patterns with Stunting and Overweight/Obesity in 1–<10-Year-Old Children in Two Economically Active Provinces in South Africa. Nutrients, 15(19), 4136. https://doi.org/10.3390/nu15194136







