The Effect of Dietary Pattern on Metabolic Syndrome in a Suburban Population in Shanghai, China
Abstract
1. Introduction
2. Subjects and Methods
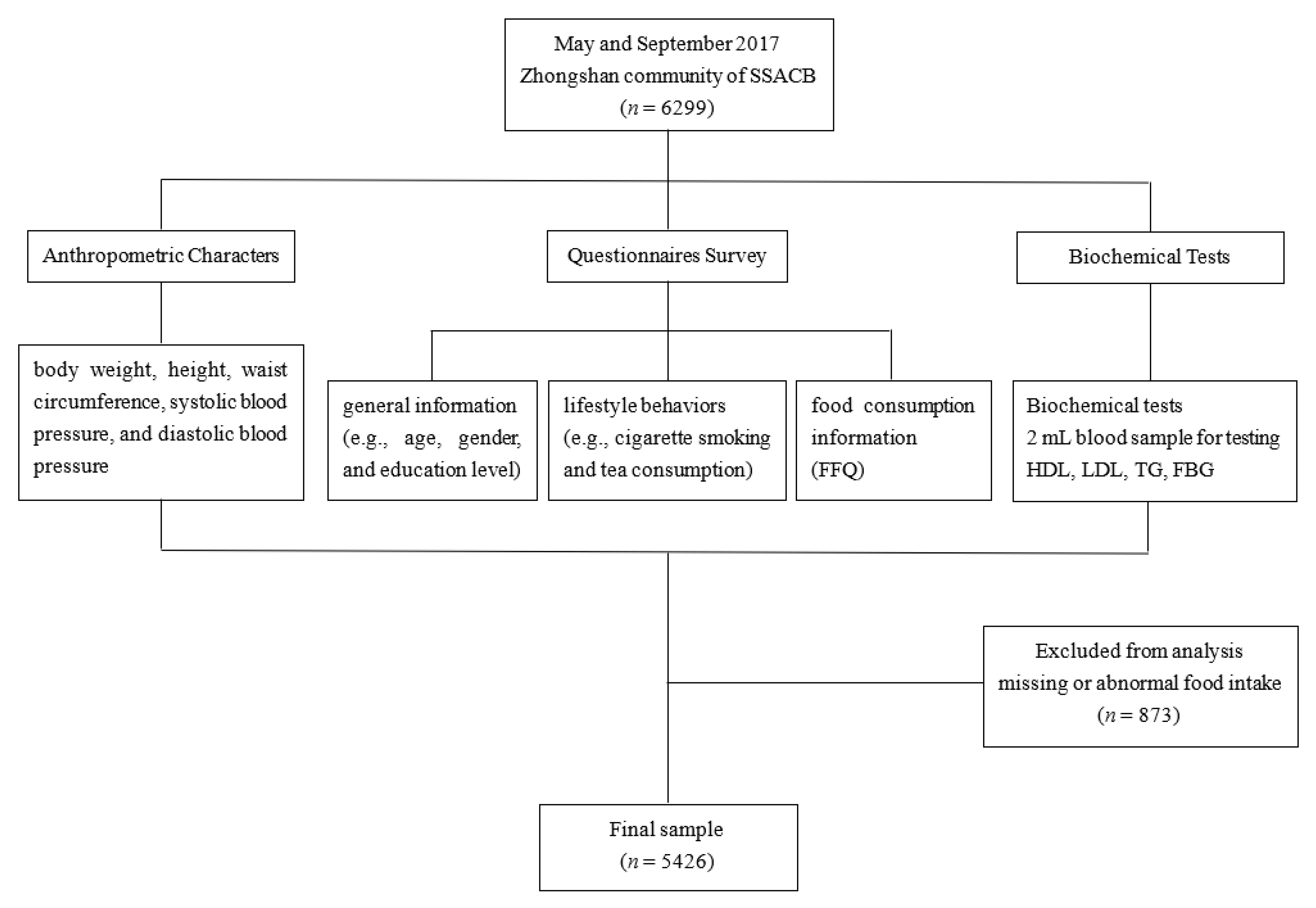
2.1. Study Population
2.2. Questionnaire Survey
2.3. Anthropometric Characters
2.4. Biochemical Tests
2.5. Covariate Assessment
2.6. Analysis of Dietary Patterns
2.6.1. A posteriori Dietary Pattern Analysis
2.6.2. A priori Dietary Pattern Analysis
2.7. Statistical Analysis
3. Results
3.1. Personal Characteristics and Prevalence of MetS
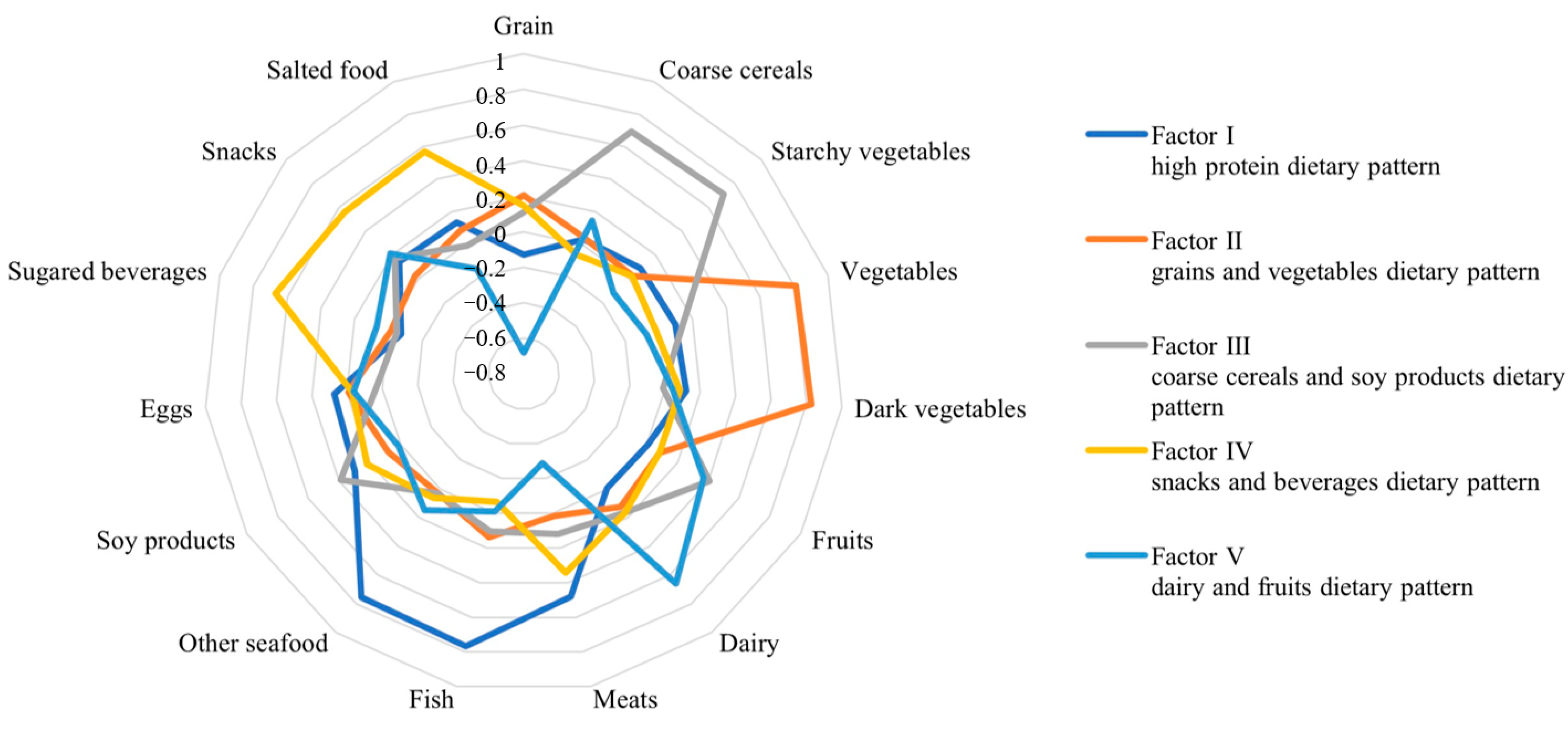
3.2. A posteriori Dietary Pattern Analysis
3.3. A priori Dietary Pattern Analysis
3.4. Association between Dietary Patterns and MetS
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Won, K.B.; Chang, H.J.; Niinuma, H.; Niwa, K.; Jeon, K.; Cho, I.J.; Shim, C.Y.; Hong, G.R.; Chung, N. Inverse association between central obesity and arterial stiffness in Korean subjects with metabolic syndrome: A cross-sectional cohort study. Diabetol. Metab. Syndr. 2015, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.B.; Zhao, Y.; Liu, Y.Q.; Zhang, J.; Xin, S.J.; Dong, G.H. Sex difference in the prevalence of metabolic syndrome and cardiovascular-related risk factors in urban adults from 33 communities of China: The CHPSNE study. Diabetes Vasc. Dis. Res. 2015, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, L.; Yu, D.; Wang, Z.; Ding, G. Metabolic syndrome prevalence and its risk factors among adults in China: A nationally representative cross-sectional study. PLoS ONE 2018, 13, e0199293. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Sidorenkov, O.; Nilssen, O.; Brenn, T.; Martiushov, S.; Arkhipovsky, V.L.; Grjibovski, A.M. Prevalence of the metabolic syndrome and its components in Northwest Russia: The Arkhangelsk study. BMC Public Health 2010, 10, 23. [Google Scholar] [CrossRef]
- Hadaegh, F.; Ghasemi, A.; Padyab, M.; Tohidi, M.; Azizi, F. The metabolic syndrome and incident diabetes: Assessment of alternative definitions of the metabolic syndrome in an Iranian urban population. Diabetes Res. Clin. Pract. 2008, 80, 328–334. [Google Scholar] [CrossRef]
- Sacco, S.; Comelli, M.; Molina, V.; Montrasio, P.L.; Giani, E.; Cavanna, F. A simplified indication of metabolic syndrome to recognize subjects with a moderate risk to develop type 2 diabetes mellitus in a large Italian sample. Acta Diabetol. 2014, 51, 35–41. [Google Scholar] [CrossRef]
- Lorenzo, C.; Williams, K.; Hunt, K.J.; Haffner, S.M. The National Cholesterol Education Program—Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care 2007, 30, 8–13. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.; Adams, H.; Kubena, K.; Guo, S. Etiology of Metabolic Syndrome and Dietary Intervention. Int. J. Mol. Sci. 2018, 20, 128. [Google Scholar] [CrossRef]
- Song, P.; Yu, J.; Chang, X.; Wang, M.; An, L. Prevalence and Correlates of Metabolic Syndrome in Chinese Children: The China Health and Nutrition Survey. Nutrients 2017, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, Z.; Whiting, S.J.; Vatanparast, H. Current evidence on the association of the metabolic syndrome and dietary patterns in a global perspective. Nutr. Res. Rev. 2016, 29, 152–162. [Google Scholar] [CrossRef]
- Calton, E.K.; James, A.P.; Pannu, P.K.; Soares, M.J. Certain dietary patterns are beneficial for the metabolic syndrome: Reviewing the evidence. Nutr. Res. 2014, 34, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.J.; Eisenmann, J.C.; Norman, G.J.; Ortiz, K.A.; Young, P.C. Dietary fiber and nutrient density are inversely associated with the metabolic syndrome in US adolescents. J. Am. Diet Assoc. 2011, 111, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Ros, E.; Valderas-Martinez, P.; Casas, R.; Arranz, S.; Guillén, M.; Lamuela-Raventós, R.M.; Llorach, R.; Andres-Lacueva, C.; et al. Effects of red wine polyphenols and alcohol on glucose metabolism and the lipid profile: A randomized clinical trial. Clin. Nutr. 2013, 32, 200–206. [Google Scholar] [CrossRef]
- Godos, J.; Zappalà, G.; Bernardini, S.; Giambini, I.; Bes-Rastrollo, M.; Martinez-Gonzalez, M. Adherence to the Mediterranean diet is inversely associated with metabolic syndrome occurrence: A meta-analysis of observational studies. Int. J. Food Sci. Nutr. 2017, 68, 138–148. [Google Scholar] [CrossRef]
- Siervo, M.; Lara, J.; Chowdhury, S.; Ashor, A.; Oggioni, C.; Mathers, J.C. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: A systematic review and meta-analysis. Br. J. Nutr. 2015, 113, 1–15. [Google Scholar] [CrossRef]
- He, D.H.; Yang, M.; Zhang, R.H.; Ma, X.G.; Huang, L.C.; Huang, E.S.; Gu, W.; Zhu, Y.B.; Zhao, D.; Zhu, X.H.; et al. Dietary Patterns Associated Metabolic Syndrome in Chinese Adults. Biomed. Environ. Sci. 2015, 28, 370–373. [Google Scholar] [CrossRef]
- Gadgil, M.D.; Anderson, C.A.; Kandula, N.R.; Kanaya, A.M. Dietary patterns are associated with metabolic risk factors in South Asians living in the United States. J. Nutr. 2015, 145, 1211–1217. [Google Scholar] [CrossRef]
- Shab-Bidar, S.; Golzarand, M.; Hajimohammadi, M.; Mansouri, S. A posteriori dietary patterns and metabolic syndrome in adults: A systematic review and meta-analysis of observational studies. Public Health Nutr. 2018, 21, 1681–1692. [Google Scholar] [CrossRef]
- Rodríguez-Monforte, M.; Sánchez, E.; Barrio, F.; Costa, B.; Flores-Mateo, G. Metabolic syndrome and dietary patterns: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2017, 56, 925–947. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, B.; Wang, R.; Zhu, M.; Shao, Y.; Wang, N.; Liu, X.; Zhang, T.; Jiang, F.; Wang, W.; et al. Cohort profile: Protocol and baseline survey for the Shanghai Suburban Adult Cohort and Biobank (SSACB) study. BMJ Open 2020, 10, e035430. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Y.; Wu, H.; He, G.; Li, S.; Chen, B. Association between Dietary Patterns and Frailty Prevalence in Shanghai Suburban Elders: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18, 852. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R., Jr.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of physical activities: An update of activity codes and MET intensities. Med. Sci. Sport. Exerc. 2000, 32, S498–S504. [Google Scholar] [CrossRef]
- Chiness Diabetes Society. Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition). Chin. J. Diabetes Mellit. 2021, 13, 315–409. [Google Scholar]
- Mellen, P.B.; Gao, S.K.; Vitolins, M.Z.; Goff, D.C., Jr. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988-1994 and 1999-2004. Arch. Intern. Med. 2008, 168, 308–314. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Kouris-Blazos, A.; Wahlqvist, M.L.; Gnardellis, C.; Lagiou, P.; Polychronopoulos, E.; Vassilakou, T.; Lipworth, L.; Trichopoulos, D. Diet and overall survival in elderly people. BMJ 1995, 311, 1457–1460. [Google Scholar] [CrossRef]
- Fung, T.T.; Rexrode, K.M.; Mantzoros, C.S.; Manson, J.E.; Willett, W.C.; Hu, F.B. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009, 119, 1093–1100. [Google Scholar] [CrossRef]
- Li, R.; Li, W.; Lun, Z.; Zhang, H.; Sun, Z.; Kanu, J.S.; Qiu, S.; Cheng, Y.; Liu, Y. Prevalence of metabolic syndrome in Mainland China: A meta-analysis of published studies. BMC Public Health 2016, 16, 296. [Google Scholar] [CrossRef]
- Vidigal Fde, C.; Ribeiro, A.Q.; Babio, N.; Salas-Salvadó, J.; Bressan, J. Prevalence of metabolic syndrome and pre-metabolic syndrome in health professionals: LATINMETS Brazil study. Diabetol. Metab. Syndr. 2015, 7, 6. [Google Scholar] [CrossRef]
- Bovolini, A.; Garcia, J.; Andrade, M.A.; Duarte, J.A. Metabolic Syndrome Pathophysiology and Predisposing Factors. Int. J. Sport. Med. 2021, 42, 199–214. [Google Scholar] [CrossRef]
- Fabiani, R.; Naldini, G.; Chiavarini, M. Dietary Patterns and Metabolic Syndrome in Adult Subjects: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 2056. [Google Scholar] [CrossRef]
- Bu, T.; Tang, D.; Liu, Y.; Chen, D. Trends in Dietary Patterns and Diet-related Behaviors in China. Am. J. Health Behav. 2021, 45, 371–383. [Google Scholar] [CrossRef]
- Tian, Y.; Su, L.; Wang, J.; Duan, X.; Jiang, X. Fruit and vegetable consumption and risk of the metabolic syndrome: A meta-analysis. Public Health Nutr. 2018, 21, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Alissa, E.M.; Ferns, G.A. Dietary fruits and vegetables and cardiovascular diseases risk. Crit. Rev. Food Sci. Nutr. 2017, 57, 1950–1962. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.; Shen, M.; Yang, Z.; Wang, N.; Zhu, Z.; Wu, Z.; Xie, D. Association between egg consumption and metabolic syndrome in Chinese population: A cross-sectional study. BMJ Open 2021, 11, e050317. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, Y. Relationship between Egg Consumption and Metabolic Syndrome. A Meta-Analysis of Observational Studies. J. Nutr. Health Aging 2022, 26, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Lee, H.W.; Kim, C.E.; Lim, J.; Lee, J.K.; Lee, S.A.; Kang, D. Egg Consumption and Risk of Metabolic Syndrome in Korean Adults: Results from the Health Examinees Study. Nutrients 2017, 9, 687. [Google Scholar] [CrossRef] [PubMed]
- Blesso, C.N.; Andersen, C.J.; Bolling, B.W.; Fernandez, M.L. Egg intake improves carotenoid status by increasing plasma HDL cholesterol in adults with metabolic syndrome. Food Funct. 2013, 4, 213–221. [Google Scholar] [CrossRef]
- Blesso, C.N.; Andersen, C.J.; Barona, J.; Volek, J.S.; Fernandez, M.L. Whole egg consumption improves lipoprotein profiles and insulin sensitivity to a greater extent than yolk-free egg substitute in individuals with metabolic syndrome. Metabolism 2013, 62, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.C.; Szeto, I.M.; Chen, L.H.; Han, S.F.; Li, Y.J.; van Hekezen, R.; Qin, L.Q. Dairy products consumption and metabolic syndrome in adults: Systematic review and meta-analysis of observational studies. Sci. Rep. 2015, 5, 14606. [Google Scholar] [CrossRef]
- Jin, S.; Je, Y. Dairy Consumption and Risk of Metabolic Syndrome: Results from Korean Population and Meta-Analysis. Nutrients 2021, 13, 1574. [Google Scholar] [CrossRef] [PubMed]
- Babio, N.; Becerra-Tomás, N.; Martínez-González, M.; Corella, D.; Estruch, R.; Ros, E.; Sayón-Orea, C.; Fitó, M.; Serra-Majem, L.; Arós, F.; et al. Consumption of Yogurt, Low-Fat Milk, and Other Low-Fat Dairy Products Is Associated with Lower Risk of Metabolic Syndrome Incidence in an Elderly Mediterranean Population. J. Nutr. 2015, 145, 2308–2316. [Google Scholar] [CrossRef] [PubMed]
- Rice, B.H.; Cifelli, C.J.; Pikosky, M.A.; Miller, G.D. Dairy components and risk factors for cardiometabolic syndrome: Recent evidence and opportunities for future research. Adv. Nutr. 2011, 2, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.; Lorenzen, J.K.; Svith, C.R.; Bartels, E.M.; Melanson, E.L.; Saris, W.H.; Tremblay, A.; Astrup, A. Effect of calcium from dairy and dietary supplements on faecal fat excretion: A meta-analysis of randomized controlled trials. Obes. Rev. 2009, 10, 475–486. [Google Scholar] [CrossRef]
- Zemel, M.B. Calcium modulation of hypertension and obesity: Mechanisms and implications. J. Am. Coll. Nutr. 2001, 20, 428S–435S, discussion 440S–442S. [Google Scholar] [CrossRef] [PubMed]
- Young, D.B.; Lin, H.; McCabe, R.D. Potassium’s cardiovascular protective mechanisms. Am. J. Physiol. 1995, 268, R825–R837. [Google Scholar] [CrossRef]
- Cheng, M.; Wang, H.; Wang, Z.; Du, W.; Ouyang, Y.; Zhang, B. Relationship between dietary factors and the number of altered metabolic syndrome components in Chinese adults: A cross-sectional study using data from the China Health and Nutrition Survey. BMJ Open 2017, 7, e014911. [Google Scholar] [CrossRef]
- Ren, G.; Fan, X.; Teng, C.; Li, Y.; Everaert, N.; Blecker, C. The Beneficial Effect of Coarse Cereals on Chronic Diseases through Regulating Gut Microbiota. Foods 2021, 10, 2891. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, Y.; Hu, Y.; Zhao, G.; Tang, Y.; Zou, L. Concise review: Coarse cereals exert multiple beneficial effects on human health. Food Chem. 2020, 325, 126761. [Google Scholar] [CrossRef]
- Ruscica, M.; Pavanello, C.; Gandini, S.; Gomaraschi, M.; Vitali, C.; Macchi, C.; Morlotti, B.; Aiello, G.; Bosisio, R.; Calabresi, L.; et al. Effect of soy on metabolic syndrome and cardiovascular risk factors: A randomized controlled trial. Eur. J. Nutr. 2018, 57, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Mohammadifard, N.; Sajjadi, F.; Haghighatdoost, F. Effects of soy consumption on metabolic parameters in patients with metabolic syndrome: A systematic review and meta-analysis. EXCLI J. 2021, 20, 665–685. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Yamori, Y. Potential Effects of Soy Isoflavones on the Prevention of Metabolic Syndrome. Molecules 2021, 26, 5863. [Google Scholar] [CrossRef]
- Glisic, M.; Kastrati, N.; Musa, J.; Milic, J.; Asllanaj, E.; Portilla Fernandez, E.; Nano, J.; Ochoa Rosales, C.; Amiri, M.; Kraja, B.; et al. Phytoestrogen supplementation and body composition in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Maturitas 2018, 115, 74–83. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Mirrahimi, A.; Srichaikul, K.; Berryman, C.E.; Wang, L.; Carleton, A.; Abdulnour, S.; Sievenpiper, J.L.; Kendall, C.W.; Kris-Etherton, P.M. Soy protein reduces serum cholesterol by both intrinsic and food displacement mechanisms. J. Nutr. 2010, 140, 2302s–2311s. [Google Scholar] [CrossRef] [PubMed]
- Nasca, M.M.; Zhou, J.R.; Welty, F.K. Effect of soy nuts on adhesion molecules and markers of inflammation in hypertensive and normotensive postmenopausal women. Am. J. Cardiol. 2008, 102, 84–86. [Google Scholar] [CrossRef]
- Li, S.H.; Liu, X.X.; Bai, Y.Y.; Wang, X.J.; Sun, K.; Chen, J.Z.; Hui, R.T. Effect of oral isoflavone supplementation on vascular endothelial function in postmenopausal women: A meta-analysis of randomized placebo-controlled trials. Am. J. Clin. Nutr. 2010, 91, 480–486. [Google Scholar] [CrossRef]
- Guo, F.; Zhang, Q.; Jiang, H.; He, Y.; Li, M.; Ran, J.; Lin, J.; Tian, L.; Ma, L. Dietary potato intake and risks of type 2 diabetes and gestational diabetes mellitus. Clin. Nutr. 2021, 40, 3754–3764. [Google Scholar] [CrossRef]
- Borgi, L.; Rimm, E.B.; Willett, W.C.; Forman, J.P. Potato intake and incidence of hypertension: Results from three prospective US cohort studies. BMJ 2016, 353, i2351. [Google Scholar] [CrossRef]
- Borch, D.; Juul-Hindsgaul, N.; Veller, M.; Astrup, A.; Jaskolowski, J.; Raben, A. Potatoes and risk of obesity, type 2 diabetes, and cardiovascular disease in apparently healthy adults: A systematic review of clinical intervention and observational studies. Am. J. Clin. Nutr. 2016, 104, 489–498. [Google Scholar] [CrossRef]
- Li, Z.; Wang, D.; Ruiz-Narváez, E.A.; Peterson, K.E.; Campos, H.; Baylin, A. Starchy Vegetables and Metabolic Syndrome in Costa Rica. Nutrients 2021, 13, 1639. [Google Scholar] [CrossRef] [PubMed]
- Nahab, F.; Pearson, K.; Frankel, M.R.; Ard, J.; Safford, M.M.; Kleindorfer, D.; Howard, V.J.; Judd, S. Dietary fried fish intake increases risk of CVD: The REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Public Health Nutr. 2016, 19, 3327–3336. [Google Scholar] [CrossRef]
- Provido, S.M.P.; Abris, G.P.; Hong, S.; Yu, S.H.; Lee, C.B.; Lee, J.E. Association of fried food intake with prehypertension and hypertension: The Filipino women’s diet and health study. Nutr. Res. Pract. 2020, 14, 76–84. [Google Scholar] [CrossRef]
- King, J.C.; Slavin, J.L. White potatoes, human health, and dietary guidance. Adv. Nutr. 2013, 4, 393s–401s. [Google Scholar] [CrossRef] [PubMed]
- Robert, L.; Narcy, A.; Rock, E.; Demigne, C.; Mazur, A.; Rémésy, C. Entire potato consumption improves lipid metabolism and antioxidant status in cholesterol-fed rat. Eur. J. Nutr. 2006, 45, 267–274. [Google Scholar] [CrossRef]
- Phillips, C.M.; Harrington, J.M.; Perry, I.J. Relationship between dietary quality, determined by DASH score, and cardiometabolic health biomarkers: A cross-sectional analysis in adults. Clin. Nutr. 2019, 38, 1620–1628. [Google Scholar] [CrossRef]
- Mozaffari, H.; Ajabshir, S.; Alizadeh, S. Dietary Approaches to Stop Hypertension and risk of chronic kidney disease: A systematic review and meta-analysis of observational studies. Clin. Nutr. 2020, 39, 2035–2044. [Google Scholar] [CrossRef]
- Chiavaroli, L.; Viguiliouk, E.; Nishi, S.K.; Blanco Mejia, S.; Rahelić, D.; Kahleová, H.; Salas-Salvadó, J.; Kendall, C.W.; Sievenpiper, J.L. DASH Dietary Pattern and Cardiometabolic Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Nutrients 2019, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Saneei, P.; Fallahi, E.; Barak, F.; Ghasemifard, N.; Keshteli, A.H.; Yazdannik, A.R.; Esmaillzadeh, A. Adherence to the DASH diet and prevalence of the metabolic syndrome among Iranian women. Eur. J. Nutr. 2015, 54, 421–428. [Google Scholar] [CrossRef]
- Ghorabi, S.; Salari-Moghaddam, A.; Daneshzad, E.; Sadeghi, O.; Azadbakht, L.; Djafarian, K. Association between the DASH diet and metabolic syndrome components in Iranian adults. Diabetes Metab. Syndr. 2019, 13, 1699–1704. [Google Scholar] [CrossRef]
- Lari, A.; Sohouli, M.H.; Fatahi, S.; Cerqueira, H.S.; Santos, H.O.; Pourrajab, B.; Rezaei, M.; Saneie, S.; Rahideh, S.T. The effects of the Dietary Approaches to Stop Hypertension (DASH) diet on metabolic risk factors in patients with chronic disease: A systematic review and meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2766–2778. [Google Scholar] [CrossRef]
- Joyce, B.T.; Wu, D.; Hou, L.; Dai, Q.; Castaneda, S.F.; Gallo, L.C.; Talavera, G.A.; Sotres-Alvarez, D.; Van Horn, L.; Beasley, J.M.; et al. DASH diet and prevalent metabolic syndrome in the Hispanic Community Health Study/Study of Latinos. Prev. Med. Rep. 2019, 15, 100950. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Sánchez-Villegas, A. The emerging role of Mediterranean diets in cardiovascular epidemiology: Monounsaturated fats, olive oil, red wine or the whole pattern? Eur. J. Epidemiol. 2004, 19, 9–13. [Google Scholar] [CrossRef]
- Velázquez-López, L.; Santiago-Díaz, G.; Nava-Hernández, J.; Muñoz-Torres, A.V.; Medina-Bravo, P.; Torres-Tamayo, M. Mediterranean-style diet reduces metabolic syndrome components in obese children and adolescents with obesity. BMC Pediatr. 2014, 14, 175. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Nolen-Doerr, E.; Mantzoros, C.S. The Effect of the Mediterranean Diet on Metabolic Health: A Systematic Review and Meta-Analysis of Controlled Trials in Adults. Nutrients 2020, 12, 3342. [Google Scholar] [CrossRef]
- Montemayor, S.; Mascaró, C.M.; Ugarriza, L.; Casares, M.; Llompart, I.; Abete, I.; Zulet, M.; Martínez, J.A.; Tur, J.A.; Bouzas, C. Adherence to Mediterranean Diet and NAFLD in Patients with Metabolic Syndrome: The FLIPAN Study. Nutrients 2022, 14, 3186. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Marfella, R.; Ciotola, M.; Di Palo, C.; Giugliano, F.; Giugliano, G.; D’Armiento, M.; D’Andrea, F.; Giugliano, D. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. Jama 2004, 292, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Veissi, M.; Anari, R.; Amani, R.; Shahbazian, H.; Latifi, S.M. Mediterranean diet and metabolic syndrome prevalence in type 2 diabetes patients in Ahvaz, southwest of Iran. Diabetes Metab. Syndr. 2016, 10, S26–S29. [Google Scholar] [CrossRef]
| Variables | With MetS (n = 1219) | Without MetS (n = 4207) | p-Value |
|---|---|---|---|
| Gender a | < 0.001 | ||
| Male | 602(49.38) | 1404(33.40) | |
| Female | 617(50.62) | 2803(66.63) | |
| Retirement status a | 0.002 | ||
| Yes | 831(68.17) | 2667(63.39) | |
| No | 388(31.83) | 1540(36.61) | |
| Marital status a | 0.126 | ||
| Married | 1141(93.60) | 3883(92.30) | |
| Other | 78(6.40) | 324(7.70) | |
| Education level a | < 0.001 | ||
| Primary school and below | 593(48.65) | 1632(38.79) | |
| Junior high school | 419(34.37) | 1624(38.60) | |
| High school and above | 207(16.98) | 951(22.61) | |
| Cigarette smoking a | < 0.001 | ||
| No | 829(68.01) | 3354(79.72) | |
| Yes | 390(31.99) | 853(20.28) | |
| Alcohol drinking a | < 0.001 | ||
| No | 1014(83.18) | 3741(88.92) | |
| Yes | 205(16.82) | 466(11.08) | |
| Tea drinking a | < 0.001 | ||
| No | 710(58.24) | 2858(67.93) | |
| Yes | 509(41.76) | 1349(32.07) | |
| Age b | 58.31 ± 8.77 | 55.39 ± 10.22 | < 0.001 |
| BMI (kg/m2) b | 26.93 ± 3.09 | 23.95 ± 3.00 | < 0.001 |
| Energy intake (Kcal/d) b | 1378.17 ± 416.41 | 1393.72 ± 434.84 | 0.267 |
| Physical activity (MET min/week) b | 3458.72 ± 2207.95 | 3670.39 ± 2157.09 | 0.003 |
| Waist circumference (cm) b | 86.73 ± 8.29 | 77.28 ± 7.96 | < 0.001 |
| Fasting blood glucose (mmol/L) b | 6.64 ± 2.06 | 5.46 ± 1.08 | < 0.001 |
| Systolic blood pressure (mmHg) b | 146.38 ± 20.85 | 132.42 ± 21.57 | < 0.001 |
| Diastolic blood pressure (mmHg) b | 88.34 ± 11.02 | 81.10 ± 10.87 | < 0.001 |
| Triglycerides (mmol/L) b | 2.94 ± 1.89 | 1.50 ± 0.83 | < 0.001 |
| HDL cholesterol (mmol/L) b | 1.06 ± 0.27 | 1.43 ± 0.29 | < 0.001 |
| LDL cholesterol (mmol/L) b | 2.71 ± 0.98 | 2.80 ± 0.78 | 0.001 |
| Food Groups | Cluster I (n = 291) | Cluster II (n = 1051) | Cluster III (n = 2722) | Cluster IV (n = 1362) |
|---|---|---|---|---|
| Grain | 341.84 | 402.63 | 311.99 | 277.44 |
| Coarse cereals | 19.01 | 14.83 | 10.92 | 32.72 |
| Starchy vegetables | 20.23 | 19.14 | 14.41 | 32.56 |
| Vegetables | 220.41 | 378.58 | 165.81 | 235.99 |
| Dark vegetables | 129.09 | 244.55 | 65.91 | 115.80 |
| Fruits | 137.86 | 109.17 | 95.11 | 168.65 |
| Dairy | 125.05 | 56.24 | 56.04 | 149.97 |
| Meats | 80.79 | 81.39 | 48.75 | 78.19 |
| Fish | 41.88 | 46.52 | 27.12 | 57.66 |
| Other seafood | 20.04 | 16.41 | 10.14 | 25.17 |
| Soy products | 6.58 | 5.25 | 3.18 | 8.15 |
| Eggs | 32.52 | 37.25 | 25.45 | 41.28 |
| Sugared beverages | 212.81 | 8.90 | 6.20 | 7.57 |
| Snacks | 42.18 | 27.84 | 18.94 | 42.19 |
| Salted food | 17.55 | 18.01 | 8.56 | 14.24 |
| Dietary Pattern Score, Mean ± SD | Total (n = 5426) | With MetS (n = 1219) | Without MetS (n = 4207) | p-Value a |
|---|---|---|---|---|
| DASH | 1.48 ± 1.14 | 1.46 ± 1.13 | 1.48 ± 1.14 | 0.488 |
| MD | 3.93 ± 1.59 | 3.89 ± 1.57 | 3.95 ± 1.59 | 0.248 |
| Method | Dietary Pattern | Model 1 | Model 2 | ||
|---|---|---|---|---|---|
| OR (95 % CI) | p-Value | OR (95 % CI) | p-Value | ||
| Cluster analysis | Balanced pattern | Reference | Reference | ||
| Snacks and beverages pattern | 0.92 (0.69, 1.23) | 0.580 | 1.06 (0.77, 1.46) | 0.718 | |
| Grains and vegetables pattern | 1.03 (0.88, 1.22) | 0.699 | 0.97 (0.81, 1.17) | 0.764 | |
| Dairy and fruits pattern | 0.66 (0.56, 0.78) | <0.001 | 0.81 (0.66, 0.98) | 0.032 | |
| Factor analysis a | High protein pattern | ||||
| Q1 | Reference | Reference | |||
| Q2 | 0.99 (0.82, 1.18) | 0.864 | 1.02 (0.85, 1.22) | 0.838 | |
| Q3 | 0.85 (0.70, 1.01) | 0.070 | 0.91 (0.75, 1.10) | 0.318 | |
| Q4 | 0.99 (0.83, 1.19) | 0.936 | 1.12 (0.93, 1.36) | 0.239 | |
| p-value for trend b | 0.660 | 0.382 | |||
| Grains and vegetables pattern | |||||
| Q1 | Reference | Reference | |||
| Q2 | 0.94 (0.78, 1.12) | 0.467 | 0.95 (0.79, 1.15) | 0.616 | |
| Q3 | 1.05 (0.88, 1.26) | 0.579 | 1.07 (0.89, 1.29) | 0.453 | |
| Q4 | 0.93 (0.78, 1.12) | 0.433 | 0.92 (0.76, 1.12) | 0.406 | |
| p-value for trend b | 0.622 | 0.553 | |||
| Coarse cereals and soy products pattern | |||||
| Q1 | Reference | Reference | |||
| Q2 | 0.81 (0.68, 0.97) | 0.022 | 0.86 (0.72, 1.03) | 0.098 | |
| Q3 | 0.79 (0.66, 0.94) | 0.009 | 0.86 (0.71, 1.03) | 0.105 | |
| Q4 | 0.67 (0.56, 0.80) | <0.001 | 0.74 (0.61, 0.91) | 0.005 | |
| p-value for trend b | <0.001 | 0.007 | |||
| Snacks and beverages pattern | |||||
| Q1 | Reference | Reference | |||
| Q2 | 1.09 (0.91, 1.31) | 0.333 | 1.08 (0.90, 1.30) | 0.417 | |
| Q3 | 0.99 (0.82, 1.18) | 0.890 | 1.02 (0.85, 1.24) | 0.822 | |
| Q4 | 0.99 (0.82, 1.19) | 0.898 | 1.09 (0.88, 1.34) | 0.422 | |
| p-value for trend b | 0.593 | 0.537 | |||
| Dairy and fruits pattern | |||||
| Q1 | Reference | Reference | |||
| Q2 | 0.82 (0.69, 0.98) | 0.025 | 0.97 (0.80, 1.16) | 0.707 | |
| Q3 | 0.69 (0.57, 0.82) | <0.001 | 0.95 (0.78, 1.15) | 0.598 | |
| Q4 | 0.60 (0.50, 0.72) | <0.001 | 0.97 (0.79, 1.19) | 0.753 | |
| p-value for trend b | <0.001 | 0.722 | |||
| Dietary Pattern Score | Model 1 | Model 2 | ||
|---|---|---|---|---|
| OR (95 % CI) | p-Value | OR (95 % CI) | p-Value | |
| DASH | 0.98 (0.93, 1.04) | 0.488 | 1.02 (0.96, 1.08) | 0.541 |
| MD | 0.98 (0.94, 1.02) | 0.247 | 1.01 (0.97, 1.06) | 0.675 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, L.; Fan, J.; Dong, R.; Zhang, M.; Jiang, Y.; Zhao, Q.; Zhao, G.; Chen, B.; Li, J.; Liu, S. The Effect of Dietary Pattern on Metabolic Syndrome in a Suburban Population in Shanghai, China. Nutrients 2023, 15, 2185. https://doi.org/10.3390/nu15092185
Wei L, Fan J, Dong R, Zhang M, Jiang Y, Zhao Q, Zhao G, Chen B, Li J, Liu S. The Effect of Dietary Pattern on Metabolic Syndrome in a Suburban Population in Shanghai, China. Nutrients. 2023; 15(9):2185. https://doi.org/10.3390/nu15092185
Chicago/Turabian StyleWei, Lanxin, Jing Fan, Ruihua Dong, Mei Zhang, Yonggen Jiang, Qi Zhao, Genming Zhao, Bo Chen, Jing Li, and Shaojie Liu. 2023. "The Effect of Dietary Pattern on Metabolic Syndrome in a Suburban Population in Shanghai, China" Nutrients 15, no. 9: 2185. https://doi.org/10.3390/nu15092185
APA StyleWei, L., Fan, J., Dong, R., Zhang, M., Jiang, Y., Zhao, Q., Zhao, G., Chen, B., Li, J., & Liu, S. (2023). The Effect of Dietary Pattern on Metabolic Syndrome in a Suburban Population in Shanghai, China. Nutrients, 15(9), 2185. https://doi.org/10.3390/nu15092185







