A Posteriori Dietary Patterns and Coronary Artery Disease in a Greek Case–Control Study
Abstract
:1. Introduction
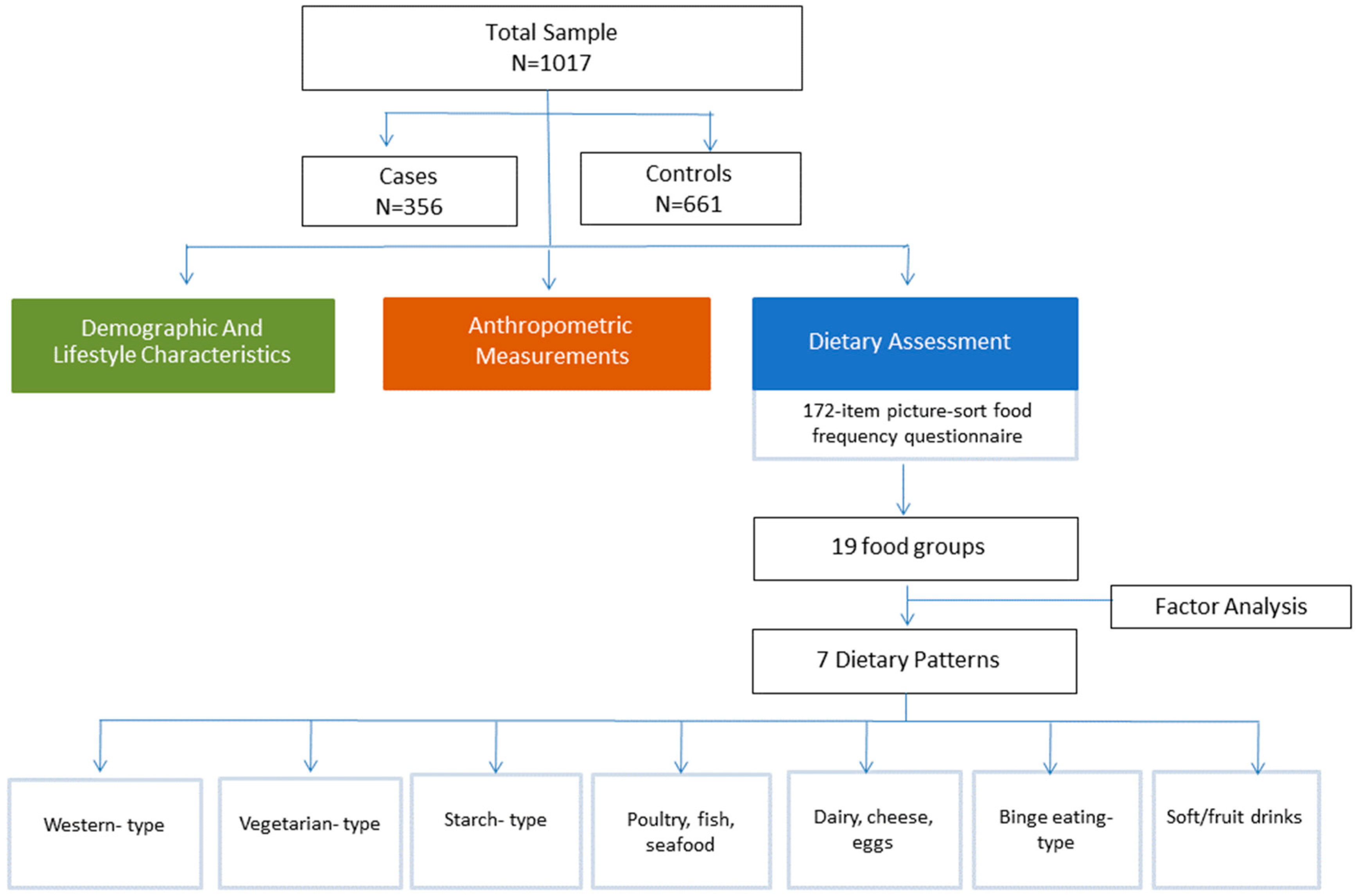
2. Materials and Methods
2.1. Study Design and Population
2.2. Demographic and Lifestyle Characteristics
2.3. Anthropometric Measurements
2.4. Dietary Assessment
2.5. Statistical Analysis
3. Results
- (1)
- Component 1, a Western-type pattern, which included red meat, processed meat, fried potatoes and fast foods;
- (2)
- Component 2, a vegetarian-type pattern, which is mainly characterized by vegetables, legumes and potatoes (boiled, baked or smashed);
- (3)
- Component 3, a starch pattern, which was loaded with refined and unrefined starch (with the unrefined starch prevailing)l
- (4)
- Component 4, a pattern that was characterized by the consumption of poultry, fish and seafood;
- (5)
- Component 5, a pattern that included dairy and eggs,
- (6)
- Component 6, a binge eating-type pattern that included the intake of sweets and nuts;
- (7)
- Component 7, a pattern that included soft drinks and fruit drinks. Fruits and fresh fruit juice were not loaded.
| Component a | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Red meat | 0.584 | 0.300 | −0.117 | 0.043 | 0.087 | 0.033 | −0.122 |
| Processed meat | 0.671 | −0.076 | 0.097 | −0.006 | 0.031 | 0.066 | 0.082 |
| Potatoes, fried | 0.549 | 0.135 | −0.175 | 0.007 | 0.189 | −0.189 | 0.039 |
| Fast foods | 0.630 | −0.083 | −0.165 | 0.037 | 0.152 | 0.149 | 0.173 |
| Vegetables | −0.105 | 0.625 | 0.164 | 0.268 | 0.089 | −0.097 | 0.198 |
| Legumes | −0.036 | 0.666 | −0.147 | −0.010 | −0.046 | 0.297 | −0.145 |
| Potatoes, boiled/baked/smashed | 0.208 | 0.615 | −0.054 | −0.034 | 0.082 | −0.031 | −0.010 |
| Refined starch | 0.139 | 0.256 | −0.645 | −0.057 | 0.056 | 0.056 | 0.124 |
| Unrefined starch | −0.030 | 0.096 | 0.813 | 0.020 | 0.054 | −0.041 | −0.092 |
| Fish | −0.007 | 0.208 | 0.031 | 0.606 | −0.048 | −0.004 | −0.168 |
| Seafood | 0.204 | −0.108 | −0.059 | 0.688 | 0.065 | −0.028 | 0.135 |
| Poultry | 0.537 | 0.082 | 0.098 | 0.431 | −0.331 | −0.026 | −0.099 |
| Dairy, full fat | 0.096 | 0.298 | 0.003 | 0.089 | 0.565 | −0.193 | 0.041 |
| Dairy, semi/non fat | −0.033 | −0.020 | 0.443 | −0.201 | −0.440 | −0.214 | 0.292 |
| Eggs | 0.121 | −0.026 | 0.019 | −0.084 | 0.624 | 0.193 | −0.011 |
| Sweets | 0.214 | −0.038 | 0.032 | 0.132 | 0.336 | 0.498 | 0.292 |
| Nuts | 0.024 | 0.095 | 0.024 | −0.029 | −0.029 | 0.739 | −0.070 |
| Soft drinks | 0.180 | 0.112 | −0.023 | −0.255 | −0.058 | −0.225 | 0.701 |
| Fruit drinks | −0.02 | 0.081 | −0.158 | 0.224 | −0.037 | 0.184 | 0.590 |
| Fruits | −0.219 | 0.243 | 0.0201 | 0.366 | −0.020 | 0.279 | 0.215 |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Atlas on Cardiovascular Diseases Prevention and Control; World Health Organization: Geneva, Switzerland, 2011; Available online: http://www.who.int/cardiovascular_diseases/publications/atlas_cvd/en/ (accessed on 12 November 2017).
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.S.; Kris-Etherton, P.M. Diet Quality Assessment and the Relationship between Diet Quality and Cardiovascular Disease Risk. Nutrients 2021, 13, 4305. [Google Scholar] [CrossRef] [PubMed]
- Grech, E.D. Pathophysiology and investigation of coronary artery disease. BMJ 2003, 326, 1027–1030. [Google Scholar] [CrossRef]
- Sesso, H.D.; Manson, J.E.; Aragaki, A.K.; Rist, P.M.; Johnson, L.G.; Friedenberg, G.; Copeland, T.; Clar, A.; Mora, S.; Moorthy, M.V.; et al. Effect of cocoa flavanol supplementation for the prevention of cardiovascular disease events: The COcoa Supplement and Multivitamin Outcomes Study (COSMOS) randomized clinical trial. Am. J. Clin. Nutr. 2022, 115, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, J.K.; Nurmi, T.; Aro, A.; Bertone-Johnson, E.R.; Hyppönen, E.; Kröger, H.; Lamberg-Allardt, C.; Manson, J.E.; Mursu, J.; Mäntyselkä, P.; et al. Vitamin D supplementation and prevention of cardiovascular disease and cancer in the Finnish Vitamin D Trial: A randomized controlled trial. Am. J. Clin. Nutr. 2022, 115, 1300–1310. [Google Scholar] [CrossRef]
- Dehghan, M.; Mente, A.; Rangarajan, S.; Mohan, V.; Lear, S.; Swaminathan, S.; Wielgosz, A.; Seron, P.; Avezum, A.; Lopez-Jaramillo, P.; et al. Association of egg intake with blood lipids, cardiovascular disease, and mortality in 177,000 people in 50 countries. Am. J. Clin. Nutr. 2020, 111, 795–803. [Google Scholar] [CrossRef]
- Tindall, A.M.; McLimans, C.J.; Petersen, K.S.; Kris-Etherton, P.M.; Lamendella, R. Walnuts and Vegetable Oils Containing Oleic Acid Differentially Affect the Gut Microbiota and Associations with Cardiovascular Risk Factors: Follow-up of a Randomized, Controlled, Feeding Trial in Adults at Risk for Cardiovascular Disease. J. Nutr. 2020, 150, 806–817. [Google Scholar] [CrossRef]
- Trautwein, E.A.; McKay, S. The Role of Specific Components of a Plant-Based Diet in Management of Dyslipidemia and the Impact on Cardiovascular Risk. Nutrients 2020, 12, 2671. [Google Scholar] [CrossRef]
- Tucker, K. Dietary patterns, approaches, and multicultural perspective. Appl. Physiol. Nutr. Metab. 2010, 35, 211–218. [Google Scholar] [CrossRef]
- Nestel, P.J.; Mori, T.A. Dietary patterns, dietary nutrients and cardiovascular disease. Rev. Cardiovasc. Med. 2022, 23, 17. [Google Scholar] [CrossRef]
- Shan, Z.; Li, Y.; Baden, M.Y.; Bhupathiraju, S.N.; Wang, D.D.; Sun, Q.; Rexrode, K.M.; Rimm, E.B.; Qi, L.; Willett, W.C.; et al. Association between Healthy Eating Patterns and Risk of Cardiovascular Disease. JAMA Intern. Med. 2020, 180, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, M.; Rallidis, L.S.; Theodoraki, E.V.; Kalafati, I.P.; Kolovou, G.; Dedoussis, G.V. Exclusive olive oil consumption has a protective effect on coronary artery disease; overview of the THISEAS study. Public Health Nutr. 2016, 19, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Kavouras, S.A.; Maraki, M.I.; Kollia, M.; Gioxari, A.; Jansen, L.; Sidossis, L. Development, reliability and validity of a physical activity questionnaire for estimating energy expenditure in Greek adults. Sci. Sport. 2016, 31, e47–e53. [Google Scholar] [CrossRef]
- Supreme Scientific Health Council & Ministry of Health and Welfare of Greece. Dietary guidelines for adults in Greece. Arch. Hell. Med. 1999, 16, 516–524. [Google Scholar]
- Zhao, Y.; Wu, K.; Zheng, J.; Zuo, R.; Li, D. Association of coffee drinking with all-cause mortality: A systematic review and meta-analysis. Public Health Nutr. 2015, 18, 1282–1291. [Google Scholar] [CrossRef]
- Hill, J.A. In vino veritas: Alcohol and heart disease. Am. J. Med. Sci. 2005, 329, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Wirfalt, E.; Drake, I.; Wallstrom, P. What do review papers conclude about food and dietary patterns? Food Nutr. Res. 2013, 57, 20523. [Google Scholar] [CrossRef]
- Mente, A.; de Koning, L.; Shannon, H.S.; Anand, S.S. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch. Intern. Med. 2009, 169, 659–669. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Skoumas, Y.; Stefanadis, C. The association between food patterns and the metabolic syndrome using principal components analysis: The ATTICA Study. J. Am. Diet. Assoc. 2007, 107, 979–987. [Google Scholar] [CrossRef]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota-Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef]
- Chen, X.; Chu, J.; Hu, W.; Sun, N.; He, Q.; Liu, S.; Feng, Z.; Li, T.; Han, Q.; Shen, Y. Associations of ultra-processed food consumption with cardiovascular disease and all-cause mortality: UK Biobank. Eur. J. Public Health 2022, 32, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Juul, F.; Vaidean, G.; Lin, Y.; Deierlein, A.L.; Parekh, N. Ultra-Processed Foods and Incident Cardiovascular Disease in the Framingham Offspring Study. J. Am. Coll. Cardiol. 2021, 77, 1520–1531. [Google Scholar] [CrossRef] [PubMed]
- Srour, B.; Fezeu, L.K.; Kesse-Guyot, E.; Allès, B.; Méjean, C.; Andrianasolo, R.M.; Chazelas, E.; Deschasaux, M.; Hercberg, S.; Galan, P.; et al. Ultra-processed food intake and risk of cardiovascular disease: Prospective cohort study (NutriNet-Santé). BMJ 2019, 365, l1451. [Google Scholar] [CrossRef] [PubMed]
- Rees, K.; Al-Khudairy, L.; Takeda, A.; Stranges, S. Vegan dietary pattern for the primary and secondary prevention of cardiovascular diseases. Cochrane Database Syst. Rev. 2021, 2, CD013501. [Google Scholar] [CrossRef]
- Satija, A.; Hu, F.B. Plant-based diets and cardiovascular health. Trends Cardiovasc. Med. 2018, 28, 437–441. [Google Scholar] [CrossRef]
- Lockheart, M.S.K.; Steffen, L.M.; Møklebust Rebnord, H.; Fimreite, R.L.; Ringstad, J.; Thelle, D.S.; Pedersen, J.I.; Jacobs, D.R. Dietary patterns, food groups and myocardial infarction: A case-control study. Br. J. Nutr. 2007, 98, 380–387. [Google Scholar] [CrossRef]
- Jeppesen, J.; Schaaf, P.; Jones, C.; Zhou, M.Y.; Chen, Y.D.; Reaven, G.M. Effects of low-fat, high-carbohydrate diets on risk factors for ischemic heart disease in postmenopausal women. Am. J. Clin. Nutr. 1997, 65, 1027–1033. [Google Scholar] [CrossRef]
- Fung, T.T.; Malik, V.; Rexrode, K.M.; Manson, J.E.; Willett, W.C.; Hu, F.B. Sweetened beverage consumption and risk of coronary heart disease in women. Am. J. Clin. Nutr. 2009, 89, 1037–1042. [Google Scholar] [CrossRef]
- Wong, M.M.H.; Louie, J.C.Y. A priori dietary patterns and cardiovascular disease incidence in adult population-based studies: A review of recent evidence. Crit. Rev. Food Sci. Nutr. 2022, 62, 6153–6168. [Google Scholar] [CrossRef]
- Critselis, E.; Kontogianni, M.D.; Georgousopoulou, E. Comparison of the Mediterranean diet and the Dietary Approach Stop Hypertension in reducing the risk of 10-year fatal and non-fatal CVD events in healthy adults: The ATTICA Study (2002–2012). Public Health Nutr. 2021, 24, 2746–2757. [Google Scholar] [CrossRef]
- Fillipatos, T.D.; Panagiotakos, D.P.; Georgousopoulo, E.N. Mediterranean Diet and 10-year (2002–2012) Incidence of Diabetes and Cardiovascular Disease in Participants with Prediabetes: The ATTICA study. Rev. Diabet. Stud. 2016, 13, 226–235. [Google Scholar] [CrossRef] [PubMed]
| Controls (N = 661) | Cases (N = 356) | ||||
|---|---|---|---|---|---|
| Mean or Frequency | ±SD * | Mean or Frequency | ±SD | p-Value | |
| Demographic & Lifestyle characteristics | |||||
| Age (years) | 54.1 | ±14.1 | 62.5 | ±10.1 | <0.001 |
| Male sex (%) | 49.5 | 82.1 | 0.000 | ||
| Years of education | 12.3 | ±4.6 | 11.5 | ±4.9 | 0.007 |
| Relative Frequency (%) | Relative Frequency (%) | p-Value | |||
| Physical inactivity | 79.5% | 90.9% | 0.000 | ||
| Current smokers | 26.4% | 46.7% | 0.000 | ||
| Clinical characteristics | |||||
| Mean | ±SD | Mean | ±SD | p-Value | |
| Body mass index | 28.4 | ±4.9 | 27.8 | ±3.8 | 0.040 |
| Systolic blood pressure (mmHg) | 134 | ±18 | 134 | ±20 | 0.944 |
| Diastolic blood pressure (mmHg) | 80 | ±11 | 80 | ±13 | 0.658 |
| Total cholesterol (mg/dL) | 210 | ±39 | 192 | ±48 | 0.000 |
| Low-density lipoprotein cholesterol (mg/dL) | 133 | ±35 | 123 | ±42 | 0.000 |
| Triglyceride (mg/dL) | 114 | ±64 | 148 | ±103 | 0.000 |
| Blood glucose (mg/dL) | 98 | ±23 | 113 | ±35 | 0.000 |
| Relative Frequency (%) | Relative Frequency (%) | p-Value | |||
| Prevalence of hypertension | 47.5% | 90.3% | 0.000 | ||
| Use of antihypertensive medication | 29.0% | 85.1% | 0.000 | ||
| Prevalence of hypercholesterolemia | 69.5% | 88.4% | 0.000 | ||
| Use of lipid lowering medication | 21.5% | 79.4% | 0.000 | ||
| Prevalence of diabetes mellitus | 10.6% | 35.0% | 0.000 | ||
| Use of anti-diabetic medication | 5.7% | 21.8% | 0.000 | ||
| Odds Ratio | 95% CI | p-Value | |
|---|---|---|---|
| Component 1: a Western-type dietary pattern (DP) | |||
| * Model 1 | 1.10 | 1.01–1.10 | 0.034 |
| ** Model 2 | 1.20 | 1.09–1.32 | <0.001 |
| *** Model 3 | 1.13 | 1.02–1.24 | 0.017 |
| Component 2: b Vegetarian-type DP | |||
| Model 1 | 0.97 | 0.90–1.05 | 0.48 |
| Model 2 | 0.95 | 0.84–1.04 | 0.26 |
| Model 3 | 0.94 | 0.85–1.04 | 0.22 |
| Component 3: c Starch-type DP | |||
| Model 1 | 0.95 | 0.91–0.99 | 0.007 |
| Model 2 | 0.98 | 0.94–1.03 | 0.45 |
| Model 3 | 1.00 | 0.95–1.05 | 0.97 |
| Component 4: Poultry, fish and seafood DP | |||
| Model 1 | 0.75 | 0.61–0.92 | 0.005 |
| Model 2 | 0.85 | 0.67–1.07 | 0.15 |
| Model 3 | 0.80 | 0.61–1.04 | 0.10 |
| Component 5: Dairy, cheese and eggs DP | |||
| Model 1 | 1.09 | 1.03–1.16 | 0.003 |
| Model 2 | 1.06 | 1.00–1.14 | 0.06 |
| Model 3 | 1.06 | 0.98–1.15 | 0.12 |
| Component 6: d Binge eating-type DP | |||
| Model 1 | 0.79 | 0.71–0.89 | <0.001 |
| Model 2 | 0.84 | 0.75–0.95 | 0.005 |
| Model 3 | 0.88 | 0.77–1.01 | 0.07 |
| Component 7: e Soft/fruit drinks DP | |||
| Model 1 | 0.66 | 0.52–0.83 | 0.001 |
| Model 2 | 0.77 | 0.77–1.00 | 0.052 |
| Model 3 | 0.83 | 0.62–1.12 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitriou, M.; Kalafati, I.P.; Rallidis, L.S.; Kolovou, G.; Dedoussis, G.V. A Posteriori Dietary Patterns and Coronary Artery Disease in a Greek Case–Control Study. Nutrients 2023, 15, 4733. https://doi.org/10.3390/nu15224733
Dimitriou M, Kalafati IP, Rallidis LS, Kolovou G, Dedoussis GV. A Posteriori Dietary Patterns and Coronary Artery Disease in a Greek Case–Control Study. Nutrients. 2023; 15(22):4733. https://doi.org/10.3390/nu15224733
Chicago/Turabian StyleDimitriou, Maria, Ioanna Panagiota Kalafati, Loukianos S. Rallidis, Genovefa Kolovou, and George V. Dedoussis. 2023. "A Posteriori Dietary Patterns and Coronary Artery Disease in a Greek Case–Control Study" Nutrients 15, no. 22: 4733. https://doi.org/10.3390/nu15224733
APA StyleDimitriou, M., Kalafati, I. P., Rallidis, L. S., Kolovou, G., & Dedoussis, G. V. (2023). A Posteriori Dietary Patterns and Coronary Artery Disease in a Greek Case–Control Study. Nutrients, 15(22), 4733. https://doi.org/10.3390/nu15224733






