Association between Dietary Niacin Intake and Nonalcoholic Fatty Liver Disease: NHANES 2003–2018
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Measurement of Dietary Niacin Intake
2.3. Definition of NAFLD
2.4. Assessment of Covariates
2.5. Statistical Analysis
3. Results
3.1. Selection of Study Population
3.2. Baseline Characteristics
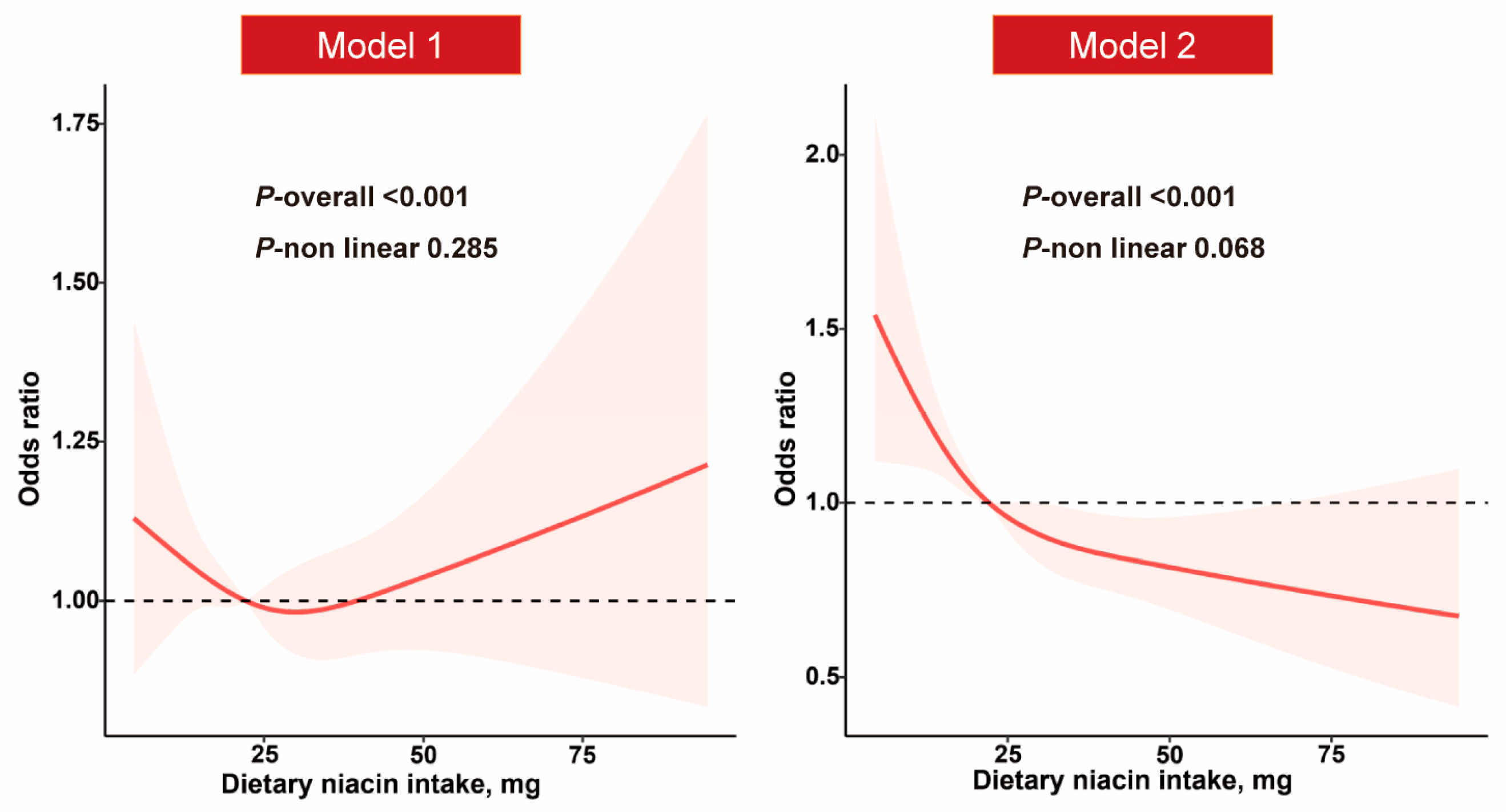
3.3. Dietary Niacin Intake and NAFLD
| Dietary Niacin Intake, mg | |||||
|---|---|---|---|---|---|
| Quartile 1 (≤16.3) | Quartile 2 (16.4–22.1) | Quartile 3 (22.2–29.1) | Quartile 4 (≥29.2) | ptrend | |
| Model 1 | 1.00 (Ref) | 0.88 (0.75, 1.04) | 0.92 (0.79, 1.07) | 0.93 (0.79, 1.09) | 0.597 |
| Model 2 | 1.00 (Ref) | 0.84 (0.68, 1.03) | 0.80 (0.65, 0.97) | 0.69 (0.55, 0.85) | 0.001 |
3.4. Stratified Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Le, M.H.; Yeo, Y.H.; Li, X.; Li, J.; Zou, B.; Wu, Y.; Ye, Q.; Huang, D.Q.; Zhao, C.; Zhang, J.; et al. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2021, 20, 2809–2817.e28. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P.; et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2017, 67, 123–133. [Google Scholar] [CrossRef]
- Meyer-Ficca, M.; Kirkland, J.B. Niacin. Adv. Nutr. 2016, 7, 556–558. [Google Scholar] [CrossRef]
- Vandamme, E.J.; Revuelta, J.L. Industrial Biotechnology of Vitamins, Biopigments, and Antioxidants; Wiley-VCH: Weinheim, Germany, 2016; pp. 41–65. [Google Scholar]
- Zhou, C.C.; Yang, X.; Hua, X.; Liu, J.; Fan, M.B.; Li, G.Q.; Song, J.; Xu, T.-Y.; Li, Z.-Y.; Guan, Y.-F.; et al. Hepatic NAD(+) deficiency as a therapeutic target for non-alcoholic fatty liver disease in ageing. Br. J. Pharmacol. 2016, 173, 2352–2368. [Google Scholar] [CrossRef]
- Ganji, S.H.; Kukes, G.D.; Lambrecht, N.; Kashyap, M.L.; Kamanna, V.S. Therapeutic role of niacin in the prevention and regression of hepatic steatosis in rat model of nonalcoholic fatty liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G320–G327. [Google Scholar] [CrossRef]
- Pirinen, E.; Auranen, M.; Khan, N.A.; Brilhante, V.; Urho, N.; Pessia, A.; Hakkarainen, A.; Kuula, J.; Heinonen, U.; Schmidt, M.S.; et al. Niacin Cures Systemic NAD(+) Deficiency and Improves Muscle Performance in Adult-Onset Mitochondrial Myopathy. Cell Metab. 2020, 31, 1078–1090. [Google Scholar] [CrossRef] [PubMed]
- Linder, K.; Willmann, C.; Kantartzis, K.; Machann, J.; Schick, F.; Graf, M.; Kümmerle, S.; Häring, H.U.; Fritsche, A.; Stefan, N.; et al. Dietary Niacin Intake Predicts the Decrease of Liver Fat Content During a Lifestyle Intervention. Sci. Rep. 2019, 9, 1303. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. About the National Health and Nutrition Examination Survey. Available online: http://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed on 1 July 2023).
- National Health and Nutrition Examination Survey. 2003–2004 Data Documentation, Codebook, and Frequencies. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2003-2004/DR1TOT_C.htm (accessed on 1 July 2023).
- Ruhl, C.E.; Everhart, J.E. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment. Pharmacol. Ther. 2015, 41, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Golabi, P.; Paik, J.M.; Harring, M.; Younossi, E.; Kabbara, K.; Younossi, Z.M. Prevalence of High and Moderate Risk Nonalcoholic Fatty Liver Disease Among Adults in the United States, 1999–2016. Clin. Gastroenterol. Hepatol. 2022, 20, 2838–2847. [Google Scholar] [CrossRef] [PubMed]
- Minhas, A.M.K.; Jain, V.; Maqsood, M.H.; Pandey, A.; Khan, S.S.; Fudim, M.; Fonarow, G.C.; Butler, J.; Khan, M.S. Non-Alcoholic Fatty Liver Disease, Heart Failure, and Long-Term Mortality: Insights From the National Health and Nutrition Examination Survey. Curr. Probl. Cardiol. 2022, 47, 101333. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Younossi, Y.; Golabi, P.; Mishra, A.; Rafiq, N.; Henry, L. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut 2020, 69, 564–568. [Google Scholar] [CrossRef]
- Zou, B.; Yeo, Y.H.; Nguyen, V.H.; Cheung, R.; Ingelsson, E.; Nguyen, M.H. Prevalence, characteristics and mortality outcomes of obese, nonobese and lean NAFLD in the United States, 1999–2016. J. Intern. Med. 2020, 288, 139–151. [Google Scholar] [CrossRef]
- Satija, A.; Bhupathiraju, S.N.; Spiegelman, D.; Chiuve, S.E.; Manson, J.E.; Willett, W.; Rexrode, K.M.; Rimm, E.B.; Hu, F.B. Healthful and Unhealthful Plant-Based Diets and the Risk of Coronary Heart Disease in U.S. Adults. J. Am. Coll. Cardiol. 2017, 70, 411–422. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; U.S. Department of Agriculture: Washington, DC, USA, December 2020. Available online: DietaryGuidelines.gov (accessed on 10 July 2023).
- Institute of Medicine; Food and Nutrition Board. Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academy Press: Washington, DC, USA, 1998. [Google Scholar]
- Ye, L.; Cao, Z.; Lai, X.; Shi, Y.; Zhou, N. Niacin Ameliorates Hepatic Steatosis by Inhibiting De Novo Lipogenesis Via a GPR109A-Mediated PKC-ERK1/2-AMPK Signaling Pathway in C57BL/6 Mice Fed a High-Fat Diet. J. Nutr. 2020, 150, 672–684. [Google Scholar] [CrossRef]
- Fang, H.; Li, Z.; Graff, E.C.; Mccafferty, K.J.; Judd, R.L. Niacin increases diet-induced hepatic steatosis in B6129 mice. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2020, 1865, 158731. [Google Scholar] [CrossRef]
- Hu, M.; Chu, W.C.W.; Yamashita, S.; Yeung, D.K.W.; Shi, L.; Wang, D.; Masuda, D.; Yang, Y.; Tomlinson, B. Liver fat reduction with niacin is influenced by DGAT-2 polymorphisms in hypertriglyceridemic patients. J. Lipid Res. 2012, 53, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, M.L.; Ganji, S.; Nakra, N.K.; Kamanna, V.S. Niacin for treatment of nonalcoholic fatty liver disease (NAFLD): Novel use for an old drug? J. Clin. Lipidol. 2019, 13, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Ganji, S.H.; Kashyap, M.L.; Kamanna, V.S. Niacin inhibits fat accumulation, oxidative stress, and inflammatory cytokine IL-8 in cultured hepatocytes: Impact on non-alcoholic fatty liver disease. Metabolism 2015, 64, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Lorbeer, R.; Bayerl, C.; Auweter, S.; Rospleszcz, S.; Lieb, W.; Meisinger, C.; Heier, M.; Peters, A.; Bamberg, F.; Hetterich, H. Association between MRI-derived hepatic fat fraction and blood pressure in participants without history of cardiovascular disease. J. Hypertens. 2017, 35, 737–744. [Google Scholar] [CrossRef]
- Ciardullo, S.; Monti, T.; Grassi, G.; Mancia, G.; Perseghin, G. Blood pressure, glycemic status and advanced liver fibrosis assessed by transient elastography in the general United States population. J. Hypertens. 2021, 39, 1621–1627. [Google Scholar] [CrossRef]
- Ma, J.; Hwang, S.-J.; Pedley, A.; Massaro, J.M.; Hoffmann, U.; Chung, R.T.; Benjamin, E.J.; Levy, D.; Fox, C.S.; Long, M.T. Bi-directional analysis between fatty liver and cardiovascular disease risk factors. J. Hepatol. 2017, 66, 390–397. [Google Scholar] [CrossRef]
- Chu, X.; Raju, R.P. Regulation of NAD(+) metabolism in aging and disease. Metabolism 2022, 126, 154923. [Google Scholar] [CrossRef]
- Radenkovic, D.; Reason; Verdin, E. Clinical Evidence for Targeting NAD Therapeutically. Pharmaceuticals 2020, 13, 247. [Google Scholar] [CrossRef]
- Rajman, L.; Chwalek, K.; Sinclair, D.A. Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence. Cell Metab. 2018, 27, 529–547. [Google Scholar] [CrossRef]
- He, Y.; Su, Y.; Duan, C.; Wang, S.; He, W.; Zhang, Y.; An, X.; He, M. Emerging role of aging in the progression of NAFLD to HCC. Ageing Res. Rev. 2023, 84, 101833. [Google Scholar] [CrossRef]
- Bogan, K.L.; Brenner, C. Nicotinic acid, nicotinamide, and nicotinamide riboside: A molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu. Rev. Nutr. 2008, 28, 115–130. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Overall N = 13,255 | Non-NAFLD N = 8877 | NAFLD N = 4378 | p Value |
|---|---|---|---|---|
| Age, years | 48.0 (34.0, 61.0) | 45.0 (31.0, 59.0) | 54.0 (39.0, 65.0) | <0.001 |
| Age (years), n (%) | <0.001 | |||
| ≤39 | 4145 (35.6) | 3212 (40.5) | 933 (25.1) | |
| 40–59 | 4357 (36.7) | 2833 (35.4) | 1524 (39.4) | |
| ≥60 | 4753 (27.7) | 2832 (24.1) | 1921 (35.5) | |
| Gender, n (%) | <0.001 | |||
| Male | 6238 (47.0) | 3912 (43.8) | 2326 (53.8) | |
| Female | 7017 (53.0) | 4965 (56.2) | 2052 (46.2) | |
| Race/ethnicity, n (%) | <0.001 | |||
| Mexican American | 2170 (8.5) | 1099 (6.3) | 1071 (13.1) | |
| Non-Hispanic white | 5992 (68.1) | 3948 (67.4) | 2044 (69.4) | |
| Non-Hispanic black | 2557 (10.3) | 2061 (12.3) | 496 (6.1) | |
| Other | 2536 (13.2) | 1769 (13.9) | 767 (11.5) | |
| Education levels, n (%) | <0.001 | |||
| <High school | 3167 (15.8) | 1836 (13.6) | 1331 (20.5) | |
| High school | 3011 (23.3) | 2009 (22.9) | 1002 (24.3) | |
| Some college or above | 7068 (60.8) | 5026 (63.4) | 2042 (55.1) | |
| Family income–poverty ratio, n (%) | 0.002 | |||
| <1.0 | 2249 (13.0) | 1448 (12.4) | 801 (14.3) | |
| 1.0–3.0 | 5280 (37.5) | 3441 (36.4) | 1839 (39.8) | |
| >3.0 | 4691 (49.6) | 3331 (51.2) | 1360 (46.0) | |
| Smoking status, n (%) | <0.001 | |||
| Never smoker | 7586 (56.9) | 5289 (59.4) | 2297 (51.5) | |
| Former smoker | 3370 (25.3) | 1989 (22.4) | 1381 (31.6) | |
| Current smoker | 2295 (17.8) | 1597 (18.2) | 698 (16.9) | |
| Physical activity, n (%) | <0.001 | |||
| Active | 4100 (62.1) | 3102 (65.1) | 998 (53.4) | |
| Inactive | 2695 (37.9) | 1874 (34.9) | 821 (46.6) | |
| BMI, kg/m2 | 27.9 (24.1, 32.6) | 25.9 (23.0, 29.2) | 33.6 (29.9, 38.5) | <0.001 |
| BMI (kg/m2), n (%) | <0.001 | |||
| <30.0 | 8097 (62.5) | 6849 (79.5) | 1248 (25.3) | |
| ≥30.0 | 5130 (37.5) | 2015 (20.5) | 3115 (74.7) | |
| Total energy, kcal | 1963.4 (1533.2, 2496.9) | 1939.5 (1524.0, 2464.5) | 2018.4 (1550.5, 2569.2) | 0.004 |
| Hypertension, n (%) | <0.001 | |||
| Yes | 4893 (32.9) | 2640 (25.0) | 2253 (50.2) | |
| No | 8337 (67.1) | 6218 (75.0) | 2119 (49.8) | |
| High cholesterol, n (%) | <0.001 | |||
| Yes | 4666 (39.3) | 2702 (34.4) | 1964 (49.5) | |
| No | 6756 (60.7) | 4820 (65.6) | 1936 (50.5) | |
| Diabetes, n (%) | <0.001 | |||
| Yes | 1761 (9.9) | 678 (4.9) | 1083 (20.7) | |
| No | 11,486 (90.1) | 8194 (95.1) | 3292 (79.3) | |
| ALT, U/L | 21.0 (16.0, 28.0) | 19.0 (15.9, 25.0) | 26.0 (20.0, 35.1) | <0.001 |
| AST, U/L | 23.0 (19.0, 27.0) | 22.0 (19.0, 26.0) | 24.1 (21.0, 29.0) | <0.001 |
| TC, mg/dL | 190.0 (164.0, 218.0) | 189.0 (164.0, 216.0) | 192.0 (165.0, 221.0) | 0.033 |
| TG, mg/dL | 105.0 (72.0, 155.0) | 90.0 (64.0, 127.0) | 148.0 (105.0, 209.0) | <0.001 |
| LDL-C, mg/dL | 111.0 (90.0, 136.0) | 111.0 (90.0, 135.0) | 113.0 (90.0, 138.0) | 0.157 |
| HDL-C, mg/dL | 51.0 (42.0, 62.0) | 55.0 (46.0, 66.0) | 44.0 (38.0, 52.0) | <0.001 |
| Dietary Niacin Intake, mg | |||||
|---|---|---|---|---|---|
| Characteristic | Quartile 1 (≤16.3) | Quartile 2 (16.4–22.1) | Quartile 3 (22.2–29.1) | Quartile 4 (≥29.2) | pinteraction |
| Age, years | |||||
| <60 | 1.00 (Ref) | 0.81 (0.62, 1.05) | 0.86 (0.66, 1.12) | 0.71 (0.54, 0.94) | 0.654 |
| ≥60 | 1.00 (Ref) | 0.88 (0.66, 1.19) | 0.72 (0.54, 0.96) | 0.62 (0.42, 0.93) | |
| Gender | |||||
| Male | 1.00 (Ref) | 0.73 (0.50, 1.08) | 0.69 (0.49, 0.99) | 0.61 (0.43, 0.88) | 0.615 |
| Female | 1.00 (Ref) | 0.83 (0.65, 1.06) | 0.80 (0.62, 1.05) | 0.70 (0.50, 0.98) | |
| Race/ethnicity | |||||
| Non-Hispanic white | 1.00 (Ref) | 0.70 (0.53, 0.94) | 0.71 (0.54, 0.94) | 0.63 (0.47, 0.83) | 0.294 |
| Other | 1.00 (Ref) | 1.05 (0.80, 1.38) | 0.99 (0.75, 1.30) | 0.82 (0.60, 1.13) | |
| Education levels | |||||
| ≤High school | 1.00 (Ref) | 0.89 (0.67, 1.20) | 0.91 (0.67, 1.23) | 0.78 (0.56, 1.07) | 0.961 |
| Some college or above | 1.00 (Ref) | 0.78 (0.59, 1.05) | 0.73 (0.54, 0.98) | 0.63 (0.45, 0.89) | |
| Family income–poverty ratio | |||||
| ≤3.0 | 1.00 (Ref) | 0.98 (0.78, 1.22) | 0.97 (0.76, 1.24) | 0.86 (0.65, 1.13) | 0.554 |
| >3.0 | 1.00 (Ref) | 0.64 (0.45, 0.93) | 0.61 (0.42, 0.89) | 0.51 (0.35, 0.72) | |
| Smoking status | |||||
| Never | 1.00 (Ref) | 0.75 (0.56, 1.00) | 0.82 (0.59, 1.14) | 0.57 (0.41, 0.80) | 0.227 |
| Former or current | 1.00 (Ref) | 0.95 (0.71, 1.29) | 0.77 (0.55, 1.06) | 0.83 (0.60, 1.15) | |
| Physical activity | |||||
| Active | 1.00 (Ref) | 0.85 (0.63, 1.15) | 0.80 (0.58, 1.09) | 0.61 (0.43, 0.85) | 0.315 |
| Inactive | 1.00 (Ref) | 0.82 (0.60, 1.11) | 0.82 (0.59, 1.14) | 0.82 (0.58, 1.16) | |
| BMI | |||||
| <30.0 | 1.00 (Ref) | 0.95 (0.70, 1.29) | 0.80 (0.58, 1.10) | 0.77 (0.56, 1.08) | 0.413 |
| ≥30.0 | 1.00 (Ref) | 0.76 (0.58, 0.99) | 0.81 (0.62, 1.07) | 0.65 (0.48, 0.88) | |
| Hypertension | |||||
| Yes | 1.00 (Ref) | 0.99 (0.72, 1.37) | 1.02 (0.74, 1.41) | 0.96 (0.64, 1.44) | 0.033 |
| No | 1.00 (Ref) | 0.70 (0.52, 0.94) | 0.66 (0.50, 0.88) | 0.54 (0.40, 0.74) | |
| High cholesterol | |||||
| Yes | 1.00 (Ref) | 0.84 (0.63, 1.13) | 0.76 (0.56, 1.02) | 0.58 (0.40, 0.85) | 0.876 |
| No | 1.00 (Ref) | 0.86 (0.65, 1.13) | 0.87 (0.66, 1.14) | 0.82 (0.60, 1.11) | |
| Diabetes | |||||
| Yes | 1.00 (Ref) | 0.61 (0.38, 1.00) | 0.76 (0.49, 1.19) | 0.88 (0.48, 1.62) | 0.552 |
| No | 1.00 (Ref) | 0.87 (0.69, 1.10) | 0.81 (0.65, 1.01) | 0.67 (0.53, 0.85) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, J.; Hu, Y.; Pang, N.; Yang, L. Association between Dietary Niacin Intake and Nonalcoholic Fatty Liver Disease: NHANES 2003–2018. Nutrients 2023, 15, 4128. https://doi.org/10.3390/nu15194128
Pan J, Hu Y, Pang N, Yang L. Association between Dietary Niacin Intake and Nonalcoholic Fatty Liver Disease: NHANES 2003–2018. Nutrients. 2023; 15(19):4128. https://doi.org/10.3390/nu15194128
Chicago/Turabian StylePan, Jie, Yuhua Hu, Nengzhi Pang, and Lili Yang. 2023. "Association between Dietary Niacin Intake and Nonalcoholic Fatty Liver Disease: NHANES 2003–2018" Nutrients 15, no. 19: 4128. https://doi.org/10.3390/nu15194128
APA StylePan, J., Hu, Y., Pang, N., & Yang, L. (2023). Association between Dietary Niacin Intake and Nonalcoholic Fatty Liver Disease: NHANES 2003–2018. Nutrients, 15(19), 4128. https://doi.org/10.3390/nu15194128





