Effect of Intake of Extra Virgin Olive Oil on Mortality in a South Italian Cohort with and without NAFLD
Abstract
:1. Introduction
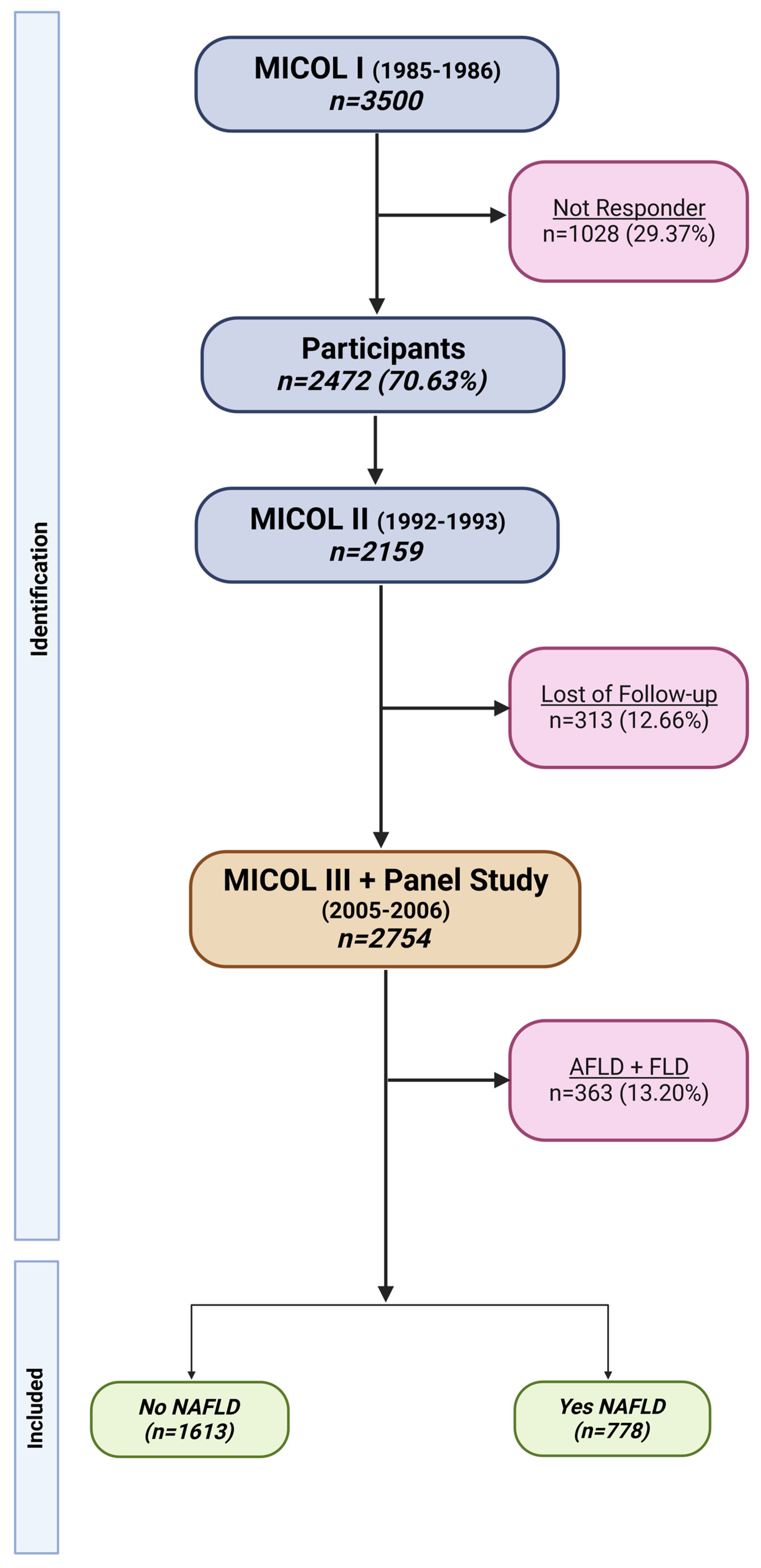
2. Materials and Methods
2.1. Data Collection
2.2. Exposure Assessment
2.3. Statistical Analyses
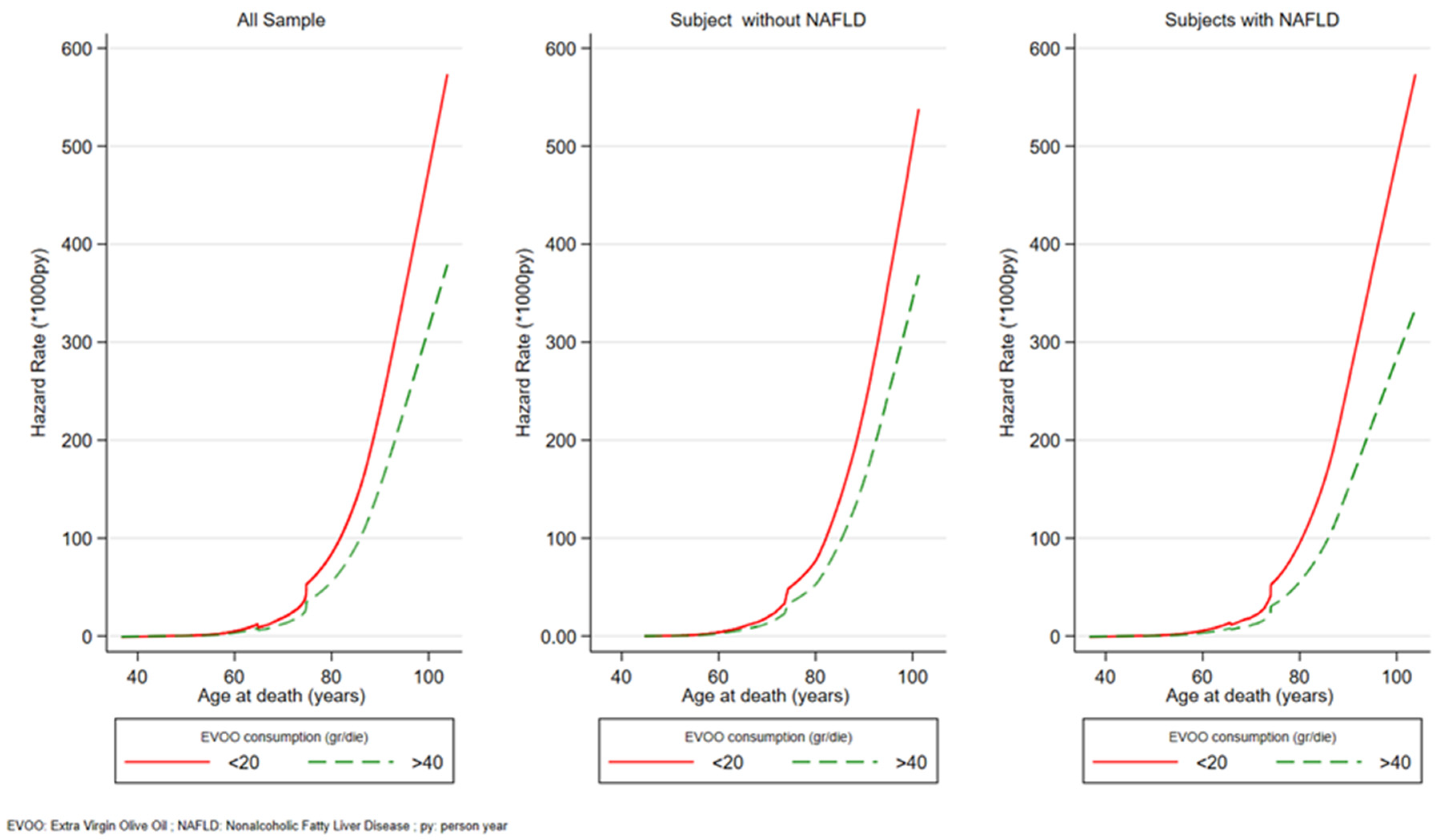
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing Evidence on Benefits of Adherence to the Mediterranean Diet on Health: An Updated Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [CrossRef]
- Willett, W.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean Diet Pyramid: A Cultural Model for Healthy Eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet; A Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef]
- D’Alessandro, A.; De Pergola, G. Mediterranean Diet Pyramid: A Proposal for Italian People. Nutrients 2014, 6, 4302–4316. [Google Scholar] [CrossRef]
- Keys, A.; Menotti, A.; Aravanis, C.; Blackburn, H.; Djordevič, B.S.; Buzina, R.; Dontas, A.S.; Fidanza, F.; Karvonen, M.J.; Kimura, N.; et al. The Seven Countries Study: 2289 Deaths in 15 Years. Prev. Med. 1984, 13, 141–154. [Google Scholar] [CrossRef]
- Serra-Majem, L.; Román-Viñas, B.; Sanchez-Villegas, A.; Guasch-Ferré, M.; Corella, D.; La Vecchia, C. Benefits of the Mediterranean Diet: Epidemiological and Molecular Aspects. Mol. Asp. Med. 2019, 67, 1–55. [Google Scholar] [CrossRef] [PubMed]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive Polyphenols: Anti-Oxidant and Anti-Inflammatory Properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- Gerber, M.; Hoffman, R. The Mediterranean Diet: Health, Science and Society. Br. J. Nutr. 2015, 113, S4–S10. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean Diet and Health Status: Meta-Analysis. BMJ 2008, 337, a1344. [Google Scholar] [CrossRef] [PubMed]
- Lăcătușu, C.-M.; Grigorescu, E.-D.; Floria, M.; Onofriescu, A.; Mihai, B.-M. The Mediterranean Diet: From an Environment-Driven Food Culture to an Emerging Medical Prescription. Int. J. Environ. Res. Public Health 2019, 16, 942. [Google Scholar] [CrossRef] [PubMed]
- Sotos-Prieto, M.; Bhupathiraju, S.N.; Mattei, J.; Fung, T.T.; Li, Y.; Pan, A.; Willett, W.C.; Rimm, E.B.; Hu, F.B. Association of Changes in Diet Quality with Total and Cause-Specific Mortality. N. Engl. J. Med. 2017, 377, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Milanović, M.; Milić, N.; Luzza, F.; Giuffrè, A.M. Olive Oil Antioxidants and Non-Alcoholic Fatty Liver Disease. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 739–749. [Google Scholar] [CrossRef]
- Piroddi, M.; Albini, A.; Fabiani, R.; Giovannelli, L.; Luceri, C.; Natella, F.; Rosignoli, P.; Rossi, T.; Taticchi, A.; Servili, M.; et al. Nutrigenomics of Extra-Virgin Olive Oil: A Review: Nutri-genomics of Extra-Virgin Olive Oil. BioFactors 2017, 43, 17–41. [Google Scholar] [CrossRef]
- Jakobušić Brala, C.; Barbarić, M.; Karković Marković, A.; Uršić, S. Biomedicinal Aspects and Activities of Olive Oil Phenolic Compounds. In Handbook of Olive Oil: Phenolic Compounds, Production and Health Benefits; Nova Science Publishers: Hauppauge, NY, USA, 2017. [Google Scholar]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- Fabiani, R. Anti-Cancer Properties of Olive Oil Secoiridoid Phenols: A Systematic Review of In Vivo Studies. Food Funct. 2016, 7, 4145–4159. [Google Scholar] [CrossRef]
- Siracusa, R.; Scuto, M.; Fusco, R.; Trovato, A.; Ontario, M.L.; Crea, R.; Di Paola, R.; Cuzzocrea, S.; Calabrese, V. Anti-Inflammatory and Anti-Oxidant Activity of Hidrox® in Rotenone-Induced Parkinson’s Disease in Mice. Antioxidants 2020, 9, 824. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Prior, Á.; Bermúdez-Oria, A.; Millán-Linares, M.D.C.; Fernández-Bolaños, J.; Espejo-Calvo, J.A.; Rodríguez-Gutiérrez, G. Anti-Inflammatory and Antioxidant Activity of Hydroxytyrosol and 3,4-Dihydroxyphenyglycol Purified from Table Olive Effluents. Foods 2021, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Lucas, L.; Keast, R. Biological Activities of Phenolic Compounds Present in Virgin Olive Oil. Int. J. Mol. Sci. 2010, 11, 458–479. [Google Scholar] [CrossRef] [PubMed]
- Berr, C.; Portet, F.; Carriere, I.; Akbaraly, T.N.; Feart, C.; Gourlet, V.; Combe, N.; Bar-berger-Gateau, P.; Ritchie, K. Olive Oil and Cognition: Results from the Three-City Study. Dement. Geriatr. Cogn. Disord. 2009, 28, 357–364. [Google Scholar] [CrossRef]
- Rigacci, S.; Stefani, M. Nutraceuticals and Amyloid Neurodegenerative Diseases: A Focus on Natural Phenols. Expert Rev. Neurother. 2015, 15, 41–52. [Google Scholar] [CrossRef]
- Osella, A.R.; Misciagna, G.; Guerra, V.M.; Chiloiro, M.; Cuppone, R.; Cavallini, A.; Di Leo, A. Hepatitis C Virus (HCV) Infection and Liver-Related Mortality: A Population-Based Cohort Study in Southern Italy. Int. J. Epiodemiol. 2000, 29, 922–927. [Google Scholar] [CrossRef]
- Campanella, A.; Misciagna, G.; Mirizzi, A.; Caruso, M.G.; Bonfiglio, C.; Aballay, L.R.; Vas De Arruda Silveira, L.; Bianco, A.; Franco, I.; Sorino, P.; et al. The Effect of the Mediterranean Diet on Lifespan: A Treatment-Effect Survival Analysis of a Population-Based Prospective Cohort Study in Southern Italy. Int. J. Epidemiol. 2021, 50, 245–255. [Google Scholar] [CrossRef]
- ISCED-97; International Standard Classification of Education. UNESCO United Nations Educational, Scientific and Cultural Organization: Paris, France, 1997.
- ISCO-08; International Standard Classification of Occupations. International Labour Office: Geneva, Switzerland, 2008.
- Sever, P. New Hypertension Guidelines from the National Institute for Health and Clinical Excellence and the British Hypertension Society. J. Renin Angiotensin Aldosterone Syst. 2006, 7, 61–63. [Google Scholar] [CrossRef]
- Riboli, E.; Hunt, K.; Slimani, N.; Ferrari, P.; Norat, T.; Fahey, M.; Charrondière, U.; Hémon, B.; Casagrande, C.; Vignat, J.; et al. European Prospective Investigation into Cancer and Nutrition (EPIC): Study Populations and Data Collection. Public Health Nutr. 2002, 5, 1113–1124. [Google Scholar] [CrossRef]
- Buckland, G.; Gonzalez, C.A.; Agudo, A.; Vilardell, M.; Berenguer, A.; Amiano, P.; Ardanaz, E.; Arriola, L.; Barricarte, A.; Basterretxea, M.; et al. Adherence to the Mediterranean Diet and Risk of Coronary Heart Disease in the Spanish EPIC Cohort Study. Am. J. Epidemiol. 2009, 170, 1518–1529. [Google Scholar] [CrossRef] [PubMed]
- Cortese, G.; Scheike, T.H.; Martinussen, T. Flexible Survival Regression Modelling. Stat. Methods Med. Res. 2010, 19, 5–28. [Google Scholar] [CrossRef]
- Ikemoto, K.; Takahashi, K.; Ozawa, T.; Isobe, H. Akaike’s Information Criterion for Stoichiometry Inference of Supramolecular Complexes. Angew. Chem. Int. Ed. 2023, 62, e202219059. [Google Scholar] [CrossRef]
- Covas, M.-I.; Konstantinidou, V.; Fitó, M. Olive Oil and Cardiovascular Health. J. Cardiovasc. Pharmacol. 2009, 54, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Canela, M.; Martínez-González, M.A. Olive Oil in the Primary Prevention of Cardio-vascular Disease. Maturitas 2011, 68, 245–250. [Google Scholar] [CrossRef]
- Bendinelli, B.; Masala, G.; Saieva, C.; Salvini, S.; Calonico, C.; Sacerdote, C.; Agnoli, C.; Grioni, S.; Frasca, G.; Mattiello, A.; et al. Fruit, Vegetables, and Olive Oil and Risk of Coronary Heart Disease in Italian Women: The EPICOR Study. Am. J. Clin. Nutr. 2011, 93, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Zhong, Y.; Peng, Y.; Qian, C. Olive Oil Consumption and Risk of Cardiovascular Disease and All-Cause Mortality: A Meta-Analysis of Prospective Cohort Studies. Front. Nutr. 2022, 9, 1041203. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, E.; De La Torre-Carbot, K.; Lamuela-Raventós, R.M.; Castellote, A.I.; Fitó, M.; De La Torre, R.; Covas, M.-I.; Carmen López-Sabater, M. Changes in the Phenolic Content of Low Density Lipoprotein after Olive Oil Consumption in Men. A Randomized Crossover Controlled Trial. Br. J. Nutr. 2007, 98, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Lukić, I.; Da Ros, A.; Guella, G.; Camin, F.; Masuero, D.; Mulinacci, N.; Vrhovsek, U.; Mattivi, F. Lipid Profiling and Stable Isotopic Data Analysis for Differentiation of Extra Virgin Olive Oils Based on Their Origin. Molecules 2019, 25, 4. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Jayedi, A.; Shab-Bidar, S.; Becerra-Tomás, N.; Salas-Salvadó, J. Adherence to the Mediterranean Diet in Relation to All-Cause Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Adv. Nutr. 2019, 10, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Gantenbein, K.V.; Kanaka-Gantenbein, C. Mediterranean Diet as an Antioxidant: The Impact on Metabolic Health and Overall Wellbeing. Nutrients 2021, 13, 1951. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Sayón-Orea, C.; Bullón-Vela, V.; Bes-Rastrollo, M.; Rodríguez-Artalejo, F.; Yusta-Boyo, M.J.; García-Solano, M. Effect of Olive Oil Consumption on Cardiovascular Disease, Cancer, Type 2 Diabetes, and All-Cause Mortality: A Systematic Review and Meta-Analysis. Clin. Nutr. 2022, 41, 2659–2682. [Google Scholar] [CrossRef]
- Perez-Martinez, P.; Garcia-Rios, A.; Delgado-Lista, J.; Perez-Jimenez, F.; Lopez-Miranda, J. Mediterranean Diet Rich in Olive Oil and Obesity, Metabolic Syndrome and Diabetes Mellitus. Curr. Pharm. Des. 2011, 17, 769–777. [Google Scholar] [CrossRef]
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Mediterranean Diet and Multiple Health Outcomes: An Umbrella Review of Meta-Analyses of Observational Studies and Randomised Trials. Eur. J. Clin. Nutr. 2018, 72, 30–43. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Morze, J.; Hoffmann, G. Mediterranean Diet and Health Status: Active Ingredients and Pharmacological Mechanisms. Br. J. Pharmacol. 2020, 177, 1241–1257. [Google Scholar] [CrossRef]
- Fontana, L. Calorie Restriction and Cardiometabolic Health. Eur. J. Cardiovasc. Prev. Rehabil. 2008, 15, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Partridge, L. Promoting Health and Longevity through Diet: From Model Organisms to Humans. Cell 2015, 161, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gómez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with Diet, Physical Activity and Exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Donghia, R.; Pesole, P.L.; Castellaneta, A.; Coletta, S.; Squeo, F.; Bonfiglio, C.; De Pergola, G.; Rinaldi, R.; De Nucci, S.; Giannelli, G.; et al. Age-Related Dietary Habits and Blood Biochemical Parameters in Patients with and without Steatosis-MICOL Cohort. Nutrients 2023, 15, 4058. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Li, Y.; Willett, W.C.; Sun, Q.; Sampson, L.; Salas-Salvadó, J.; Mar-tínez-González, M.A.; Stampfer, M.J.; Hu, F.B. Consumption of Olive Oil and Risk of Total and Cause-Specific Mortality among U.S. Adults. J. Am. Coll. Cardiol. 2022, 79, 101–112. [Google Scholar] [CrossRef] [PubMed]
| N *** (2754) | EVOO Oil Categories (g/Die) | ||||
|---|---|---|---|---|---|
| <20 | 21–30 | 31–40 | >40 | p–Value | |
| 645 (23.42) | 635 (23.06) | 595 (21.60) | 879 (31.92) | ||
| Gender ** | |||||
| Female | 305 (25.6) | 282 (23.6) | 249 (20.9) | 357 (29.9) | 0.055 |
| Male | 340 (21.8) | 353 (22.6) | 346 (22.2) | 522 (33.4) | |
| Age enrolment (yrs) | 46.38 (13.00) | 52.15 (13.99) | 56.9 (14.77) | 61.77 (13.54) | <0.001 |
| Age categories (yrs) | |||||
| <40 | 256 (42.6) | 153 (25.5) | 103 (17.1) | 89 (14.8) | <0.001 |
| 40–49 | 209 (37.0) | 167 (29.6) | 109 (19.3) | 80 (14.2) | |
| 50–59 | 84 (15.6) | 130 (24.1) | 145 (26.9) | 181 (33.5) | |
| 60–69 | 42 (8.3) | 103 (20.4) | 112 (22.2) | 248 (49.1) | |
| 70–79 | 37 (8.7) | 65 (15.3) | 87 (20.5) | 235 (55.4) | |
| ≥80 | 17 (14.3) | 17 (14.3) | 39 (32.8) | 46 (38.7) | |
| DBP (mmHg) * | 116.68 (18.67) | 121.41 (18.56) | 125.20 (19.55) | 129.27 (19.86) | <0.001 |
| SBP (mmHg) * | 73.41 (10.23) | 74.92 (10.77) | 75.40 (9.89) | 75.28 (9.69) | 0.001 |
| Weight (kg) * | 74.58 (15.40) | 74.55 (14.82) | 75.53 (15.20) | 75.75 (15.55) | 0.31 |
| BMI (kg/m2) * | 27.71 (4.84) | 28.34 (4.96) | 29.00 (5.26) | 29.55 (5.42) | <0.001 |
| Kcal days * | 2040.92 (712.00) | 2161.72 (732.58) | 2196.68 (692.40) | 2348.09 (687.87) | <0.001 |
| TGL (mmol/L) * | 1.39 (1.05) | 1.49 (1.20) | 1.48 (1.03) | 1.47 (0.91) | 0.23 |
| TC (mmol/L) * | 5.17 (0.95) | 5.15 (1.05) | 5.19 (1.00) | 5.14 (1.00) | 0.78 |
| HDL (mmol/L) * | 1.33 (0.38) | 1.32 (0.38) | 1.30 (0.34) | 1.33 (0.36) | 0.36 |
| LDL (mmol/L) * | 3.22 (0.80) | 3.13 (0.93) | 3.21 (0.86) | 3.13 (0.85) | 0.064 |
| Glucose (mmol/L) * | 5.90 (1.40) | 6.07 (1.48) | 6.08 (1.58) | 6.16 (1.51) | 0.008 |
| AST (μkat/L) * | 0.20 (0.10) | 0.21 (0.13) | 0.22 (0.15) | 0.23 (0.22) | 0.001 |
| ALT (μkat/L) * | 0.29 (0.18) | 0.30 (0.26) | 0.30 (0.25) | 0.30 (0.30) | 0.95 |
| Smoker *** | |||||
| Never/Former | 498 (21.9) | 516 (22.7) | 502 (22.1) | 756 (33.3) | <0.001 |
| Current | 147 (30.5) | 119 (24.7) | 93 (19.3) | 123 (25.5) | |
| Age at Death (yrs) ** | 59.45 (53.66–68.98) | 65.61 (56.95–77.96) | 72.37 (60.34–81.60) | 78.51 (70.02–85.05) | <0.001 |
| Observation time ** (yrs) | 16.91 (16.13–17.07) | 16.93 (16.12–17.26) | 16.94 (16.03–17.43) | 16.97 (13.55–17.45) | 0.033 |
| Status *** | |||||
| Alive and/or Censored | 562 (27.0) | 516 (24.8) | 436 (21.0) | 565 (27.2) | <0.001 |
| Dead | 83 (12.3) | 119 (17.6) | 159 (23.6) | 314 (46.5) | |
| Cause of death | |||||
| Colon Cancer | 2 (11.8) | 2 (11.8) | 5 (29.4) | 8 (47.1) | 0.68 |
| Liver Cancer | 2 (11.1) | 4 (22.2) | 6 (33.3) | 6 (33.3) | |
| OC | 22 (16.3) | 29 (21.5) | 34 (25.2) | 50 (37.0) | |
| CVD | 20 (10.3) | 29 (14.9) | 43 (22.2) | 102 (52.6) | |
| DSD | 4 (9.8) | 9 (22.0) | 8 (19.5) | 20 (48.8) | |
| OCD | 33 (12.2) | 46 (17.0) | 63 (23.3) | 128 (47.4) | |
| NAFLD | |||||
| No | 402 (24.9) | 371 (23.0) | 335 (20.8) | 505 (31.3) | 0.67 |
| Yes | 188 (24.2) | 182 (23.4) | 177 (22.8) | 231 (29.7) | |
| rMED | 7.00 (6.00–9.00) | 8.00 (6.00–9.00) | 8.00 (7.00–9.00) | 8.00 (7.00–10.00) | <0.001 |
| with NAFLD | without NAFLD | |||||||
|---|---|---|---|---|---|---|---|---|
| EVOO Categories (g/Die) | EVOO Categories (g/Die) | |||||||
| <20 | 21–30 | 31–40 | >40 | <20 | 21–30 | 31–40 | >40 | |
| N | 188 | 182 | 177 | 231 | 402 | 371 | 335 | 505 |
| Gender ** | ||||||||
| Female | 57 (21.4) | 63 (23.7) | 65 (24.4) | 81 (30.5) | 236 (28.8) | 198 (24.1) | 158 (19.3) | 228 (27.8) |
| Male | 131 (25.6) | 119 (23.2) | 112 (21.9) | 150 (29.3) | 166 (20.9) | 173 (21.8) | 177 (22.3) | 277 (34.9) |
| Age enrolment (yrs) | 46.80 (12.57) | 52.60 (12.40) | 55.79 (12.62) | 61.57 (13.16) | 45.50 (13.33) | 51.31 (15.12) | 55.47 (16.22) | 61.21 (14.28) |
| DBP (mmHg) * | 121.95 (18.18) | 125.05 (18.16) | 128.85 (18.50) | 131.16 (19.09) | 112.86 (18.10) | 118.82 (19.01) | 121.36 (18.91) | 128.00 (20.32) |
| SBP (mmHg) * | 76.85 (10.30) | 77.41 (12.32) | 78.15 (9.58) | 76.89 (9.99) | 71.08 (9.67) | 73.22 (10.04) | 73.40 (9.74) | 74.55 (9.53) |
| Weight (kg) * | 85.13 (14.68) | 83.24 (14.46) | 83.96 (16.52) | 83.96 (16.04) | 68.64 (12.76) | 69.09 (12.34) | 69.87 (12.50) | 70.74 (13.36) |
| BMI (kg/m2) * | 31.12 (4.60) | 31.41 (4.82) | 31.91 (5.49) | 32.23 (5.68) | 25.78 (3.99) | 26.41 (3.90) | 26.90 (4.29) | 27.80 (4.68) |
| Kcal days * | 1986.8 (723.6) | 2185.4 (762.0) | 2144.6 (726.1) | 2299.0 (683.3) | 2031.0 (680.3) | 2117.7 (708.4) | 2215.1 (680.5) | 2340.4 (685.7) |
| TGL (mmol/L) * | 1.87 (1.40) | 1.88 (1.34) | 1.90 (1.19) | 1.85 (1.08) | 1.11 (0.70) | 1.17 (0.87) | 1.16 (0.67) | 1.26 (0.73) |
| TC (mmol/L) * | 5.27 (0.96) | 5.27 (1.16) | 5.30 (1.02) | 5.25 (0.93) | 5.06 (0.89) | 5.04 (0.97) | 5.07 (0.97) | 5.07 (0.98) |
| HDL (mmol/L) * | 1.16 (0.28) | 1.18 (0.29) | 1.18 (0.28) | 1.23 (0.27) | 1.41 (0.38) | 1.40 (0.40) | 1.37 (0.36) | 1.40 (0.38) |
| LDL (mmol/L) * | 3.30 (0.82) | 3.24 (1.09) | 3.26 (0.81) | 3.18 (0.83) | 3.14 (0.76) | 3.10 (0.86) | 3.17 (0.86) | 3.08 (0.83) |
| Glucose (mmol/L) * | 6.26 (1.75) | 6.22 (1.32) | 6.34 (1.67) | 6.52 (1.71) | 5.62 (0.85) | 5.85 (1.31) | 5.80 (1.36) | 5.89 (1.27) |
| AST (μkat/L) * | 0.35 (0.18) | 0.36 (0.25) | 0.31 (0.14) | 0.30 (0.18) | 0.24 (0.13) | 0.24 (0.25) | 0.27 (0.25) | 0.28 (0.34) |
| ALT (μkat/L) * | 0.21 (0.13) | 0.22 (0.10) | 0.21 (0.08) | 0.21 (0.09) | 0.18 (0.07) | 0.19 (0.10) | 0.21 (0.16) | 0.23 (0.25) |
| Status *** | ||||||||
| Alive and/or Censored | 165 (27.5) | 145 (24.2) | 141 (23.5) | 149 (24.8) | 352 (28.6) | 307 (24.9) | 243 (19.7) | 329 (26.7) |
| Dead | 23 (12.9) | 37 (20.8) | 36 (20.2) | 82 (46.1) | 50 (13.1) | 64 (16.8) | 92 (24.1) | 176 (46.1) |
| rMED | 8 (6–9) | 8 (6–9) | 8 (7–10) | 9 (7–10) | 7 (6–9) | 8 (6–10) | 8 (7–10) | 8 (8–10) |
| EVOO Consumption Categories (gr/die) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 21–30 | 31–40 | >40 | Continuos | |||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | CI 95% | |
| All sample | 0.82 | 0.61, 1.10 | 0.73 * | 0.55; 0.97 | 0.66 * | 0.50; 0.87 | 0.99 * | 0.98; 0.99 |
| Without NAFLD | 0.76 | 0.51; 1.12 | 0.74 | 0.51; 1.07 | 0.68 * | 0.48; 0.98 | 0.99 | 0.99; 1.00 |
| With NAFLD | 0.99 | 0.59; 1.69 | 0.58 * | 0.33; 0.999 | 0.58 * | 0.35; 0.97 | 0.99 * | 0.98; 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonfiglio, C.; Cuccaro, F.; Campanella, A.; Rosso, N.; Tatoli, R.; Giannelli, G.; Donghia, R. Effect of Intake of Extra Virgin Olive Oil on Mortality in a South Italian Cohort with and without NAFLD. Nutrients 2023, 15, 4593. https://doi.org/10.3390/nu15214593
Bonfiglio C, Cuccaro F, Campanella A, Rosso N, Tatoli R, Giannelli G, Donghia R. Effect of Intake of Extra Virgin Olive Oil on Mortality in a South Italian Cohort with and without NAFLD. Nutrients. 2023; 15(21):4593. https://doi.org/10.3390/nu15214593
Chicago/Turabian StyleBonfiglio, Caterina, Francesco Cuccaro, Angelo Campanella, Natalia Rosso, Rossella Tatoli, Gianluigi Giannelli, and Rossella Donghia. 2023. "Effect of Intake of Extra Virgin Olive Oil on Mortality in a South Italian Cohort with and without NAFLD" Nutrients 15, no. 21: 4593. https://doi.org/10.3390/nu15214593
APA StyleBonfiglio, C., Cuccaro, F., Campanella, A., Rosso, N., Tatoli, R., Giannelli, G., & Donghia, R. (2023). Effect of Intake of Extra Virgin Olive Oil on Mortality in a South Italian Cohort with and without NAFLD. Nutrients, 15(21), 4593. https://doi.org/10.3390/nu15214593







