Sclerostin, Osteocytes, and Wnt Signaling in Pediatric Renal Osteodystrophy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Bone Biopsy, Serum Biochemistry, and Histomorphometry Measurements
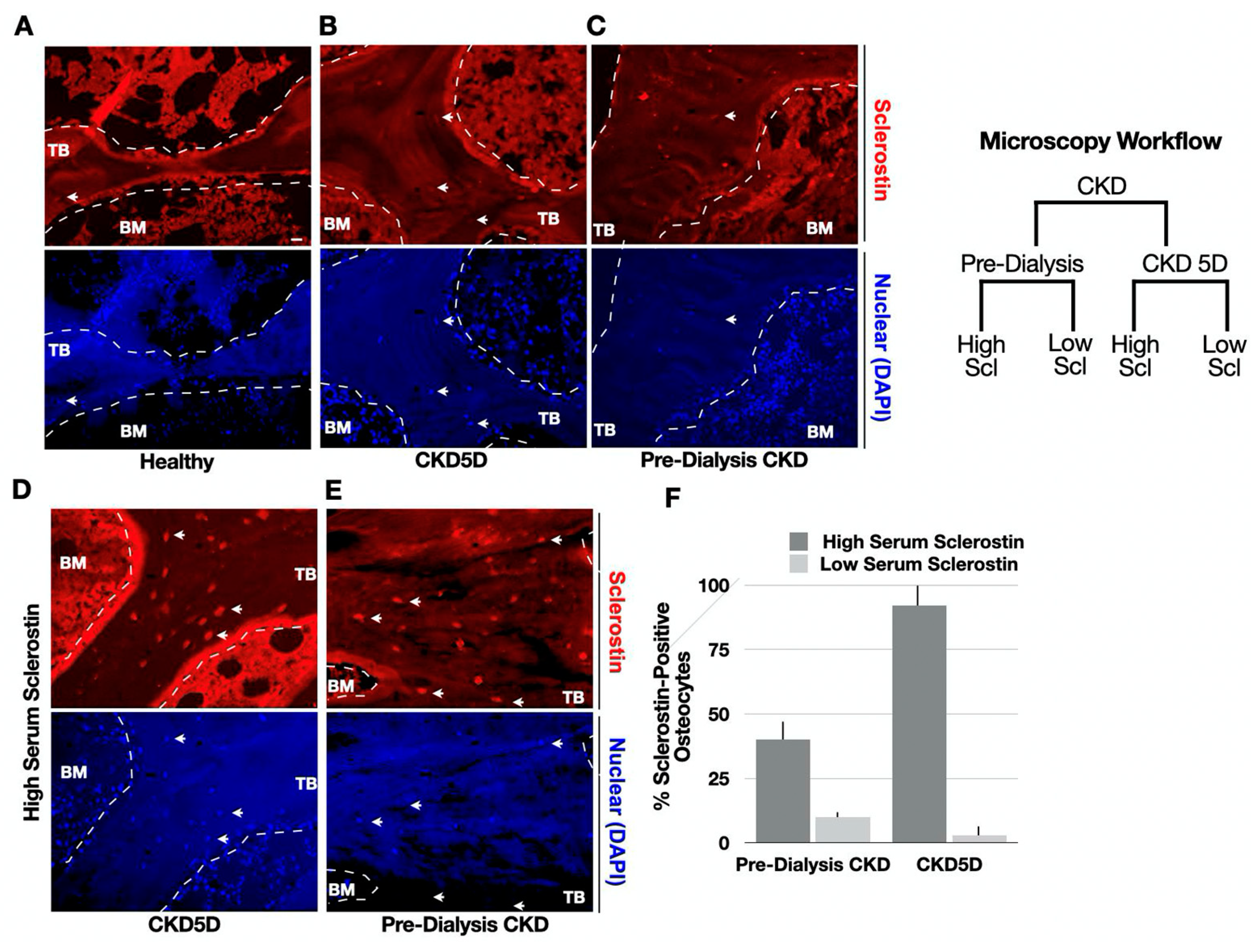
2.3. Bone Immunohistochemistry (IHC) and Quantification of Sclerostin and FGF-23
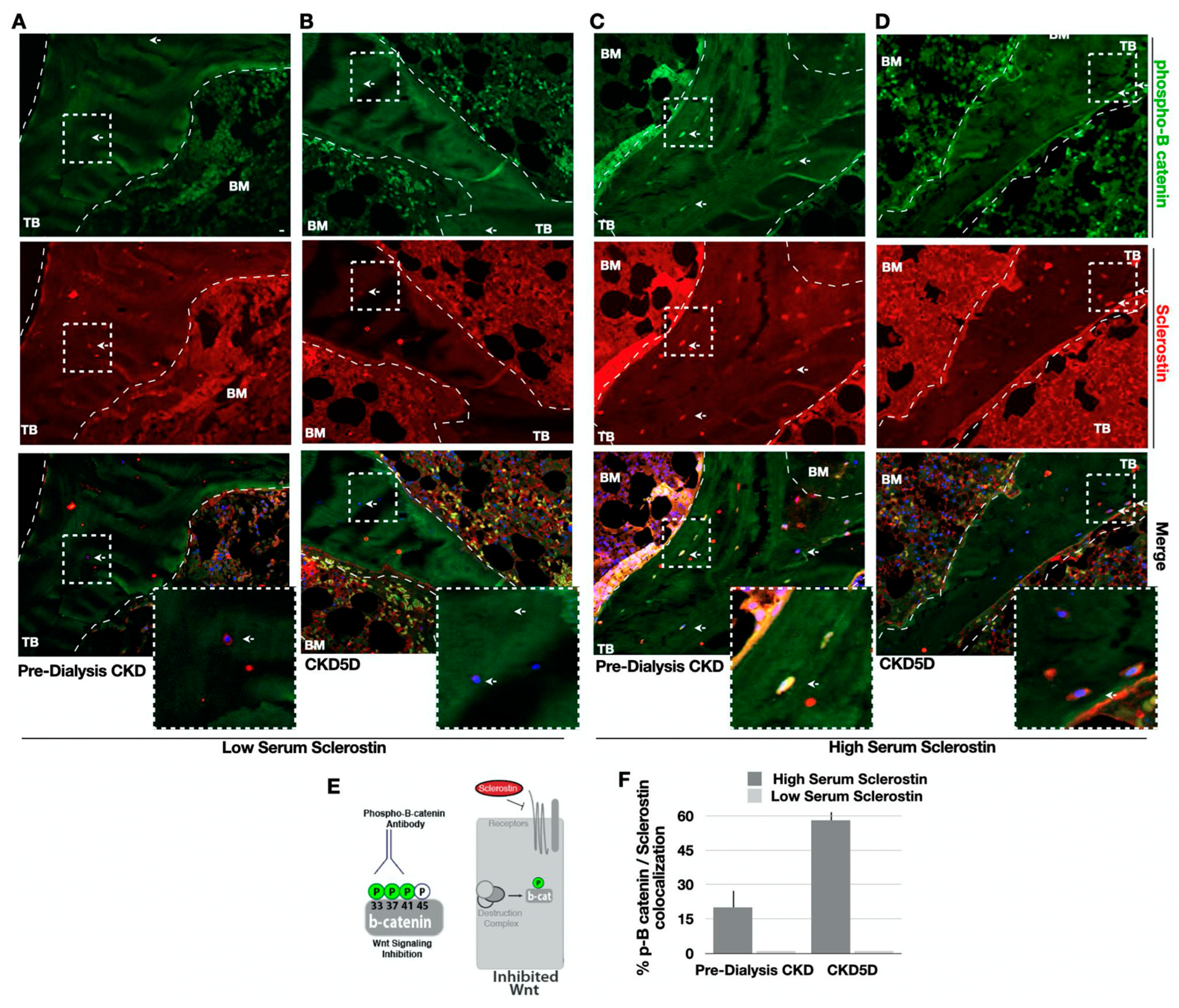
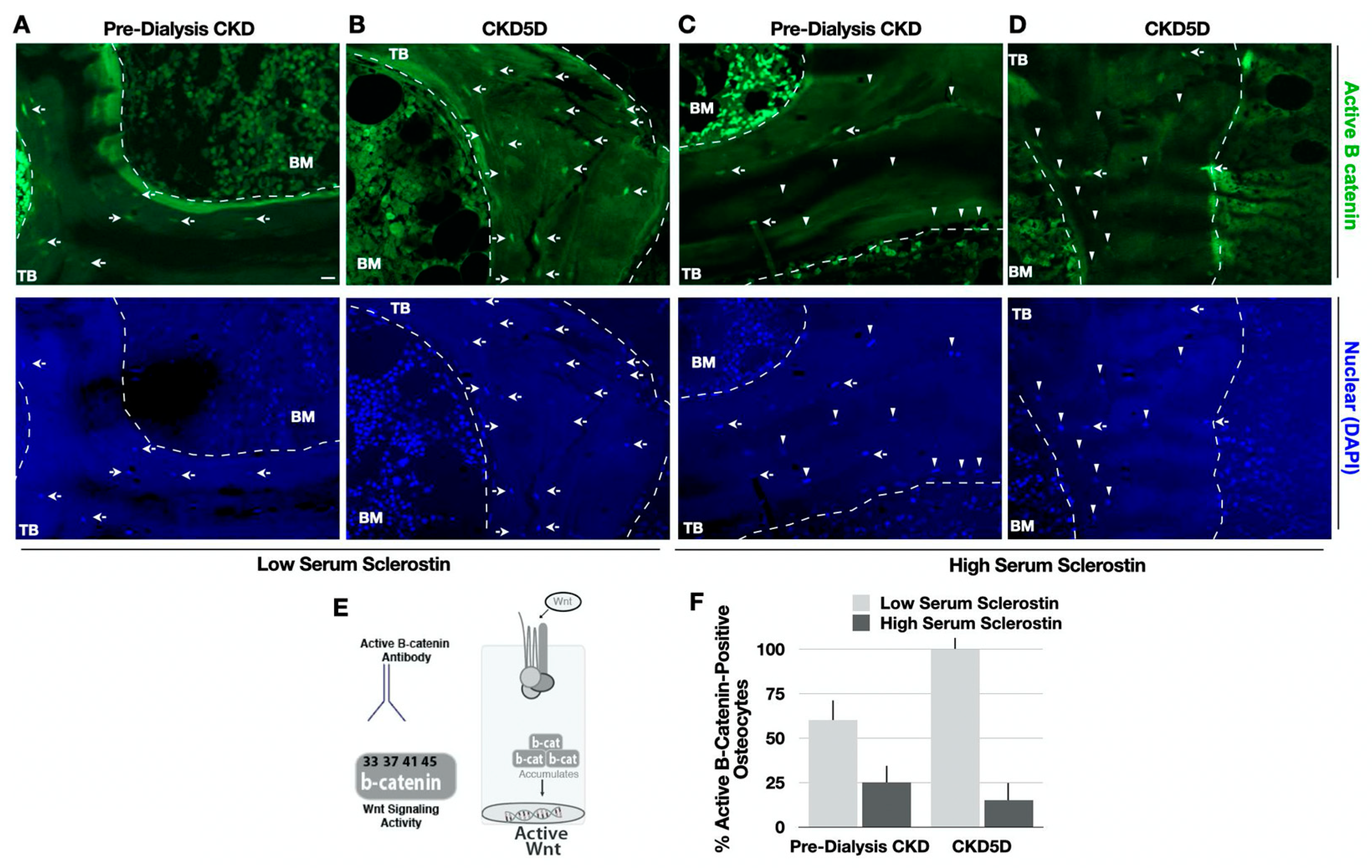
2.4. Analysis of Wnt Signaling Activity
2.5. Statistical Analysis
3. Results
3.1. Cohort Characteristics
3.2. Biochemical Characteristics
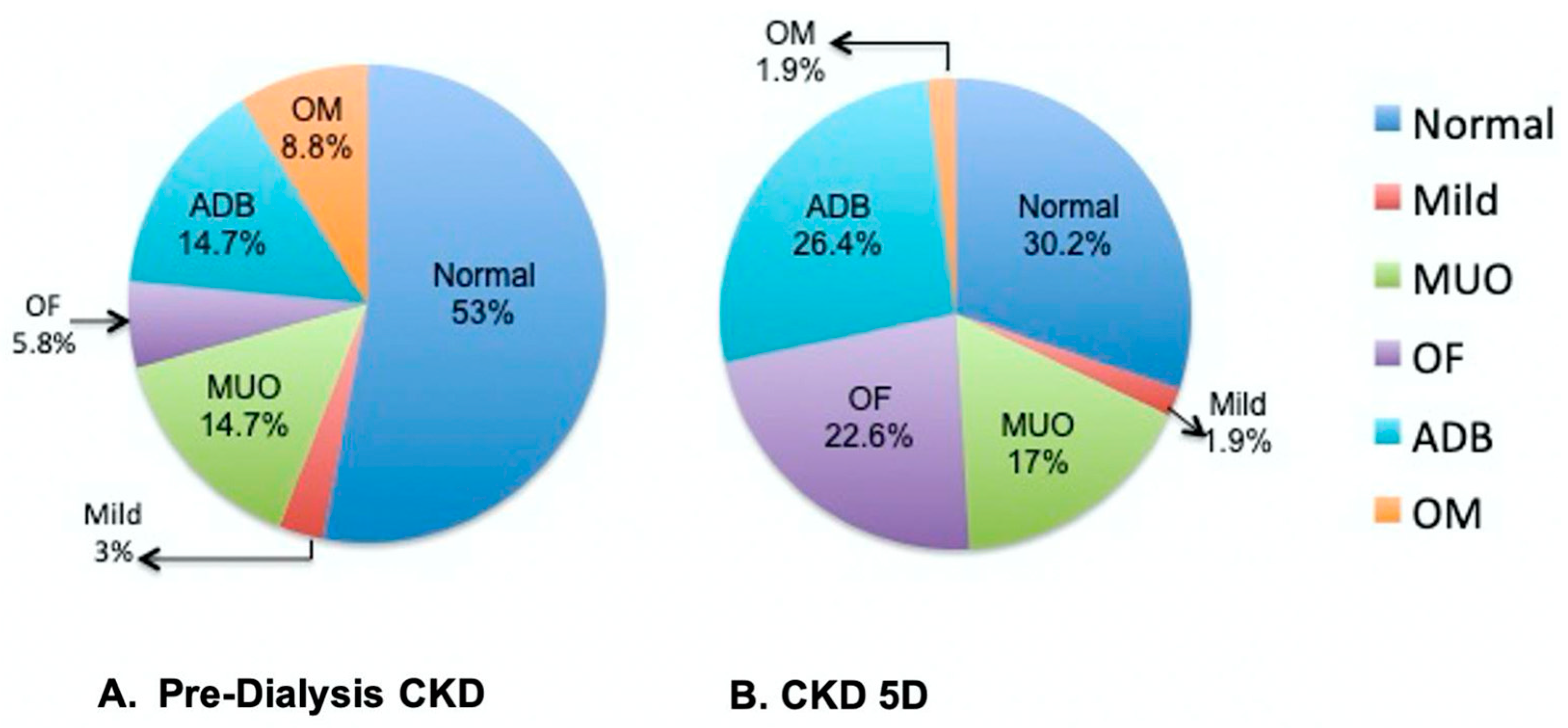
3.3. Bone Histomorphometry in Pre-Dialysis CKD and CKD 5D
3.4. Correlations of Serum and Bone Sclerostin with Indices of Bone Histomorphometry
3.5. Pre-Dialysis CKD Cohort
3.6. CKD 5D Cohort
3.7. CKD Patients with Low Bone Turnover
3.8. Serum Sclerostin Correlates with Bone Sclerostin and Wnt Signaling in CKD
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denburg, M.R.; Kumar, J.; Jemielita, T.; Brooks, E.R.; Skversky, A.; Portale, A.A.; Salusky, I.B.; Warady, B.A.; Furth, S.L.; Leonard, M.B. Fracture Burden and Risk Factors in Childhood CKD: Results from the CKiD Cohort Study. J. Am. Soc. Nephrol. 2016, 27, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Manciu, M.; Cardenas, M.; Bennet, K.E.; Maran, A.; Yaszemski, M.J.; Maldonado, T.A.; Magiricu, D.; Manciu, F.S. Assessment of Renal Osteodystrophy via Computational Analysis of Label-free Raman Detection of Multiple Biomarkers. Diagnostics 2020, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Smout, D.; Jørgensen, H.S.; Cavalier, E.; Evenepoel, P. Clinical utility of bone turnover markers in patients with chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2022, 31, 332–338. [Google Scholar]
- Wagner, J.; Jhaveri, K.D.; Rosen, L.; Sunday, S.; Mathew, A.T.; Fishbane, S. Increased bone fractures among elderly United States hemodialysis patients. Nephrol. Dial. Transplant. 2014, 29, 146–151. [Google Scholar]
- Vilaca, T.; Salam, S.; Schini, M.; Harnan, S.; Sutton, A.; Poku, E.; Allen, I.E.; Cummings, S.R.; Eastell, R. Risks of Hip and Nonvertebral Fractures in Patients with CKD G3a-G5D: A Systematic Review and Meta-analysis. Am. J. Kidney Dis. 2020, 76, 521–532. [Google Scholar] [CrossRef]
- Groothoff, J.W.; Offringa, M.; Van Eck-Smit, B.L.F.; Gruppen, M.P.; Van De Kar, N.J.; Wolff, E.D.; Lilien, M.R.; Davin, J.C.; Heymans, H.S.A.; Dekker, F.W. Severe bone disease and low bone mineral density after juvenile renal failure. Kidney Int. 2003, 63, 266–275. [Google Scholar] [CrossRef]
- Sabbagh, Y.; Graciolli, F.G.; O’Brien, S.; Tang, W.; Reis, L.M.D.; Ryan, S.; Phillips, L.; Boulanger, J.; Song, W.; Bracken, C.; et al. Repression of osteocyte Wnt/β-catenin signaling is an early event in the progression of renal osteodystrophy. J. Bone Miner. Res. 2012, 27, 1757–1772. [Google Scholar] [CrossRef]
- Pereira, R.C.; Juppner, H.; Azucena-Serrano, C.E.; Yadin, O.; Salusky, I.B.; Wesseling-Perry, K. Patterns of FGF-23, DMP1, and MEPE expression in patients with chronic kidney disease. Bone 2009, 45, 1161–1168. [Google Scholar] [CrossRef]
- Albrecht, L.V.; Pereira, R.C.; Salusky, I.B. All the Might of the Osteocyte: Emerging Roles in Chronic Kidney Disease. Kidney Int. 2023; Epub ahead of print. [Google Scholar] [CrossRef]
- Figurek, A.; Rroji, M.; Spasovski, G. Sclerostin: A new biomarker of CKD-MBD. Int. Urol. Nephrol. 2020, 52, 107–113. [Google Scholar]
- Bonewald, L.F. The Role of the Osteocyte in Bone and Nonbone Disease. Endocrinol. Metab. Clin. N. Am. 2017, 46, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.T.; Khosla, S. Hormonal and systemic regulation of sclerostin. Bone 2017, 96, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Siriopol, D.; Saglam, M.; Kurt, Y.G.; Gok, M.; Cetinkaya, H.; Karaman, M.; Unal, H.U.; Oguz, Y.; Sari, S.; et al. Serum sclerostin and adverse outcomes in nondialyzed chronic kidney disease patients. J. Clin. Endocrinol. Metab. 2014, 99, E1854–E1861. [Google Scholar] [CrossRef] [PubMed]
- De Maré, A.; Verhulst, A.; Cavalier, E.; Delanaye, P.; Behets, G.J.; Meijers, B.; Kuypers, D.; D’Haese, P.C.; Evenepoel, P. Clinical Inference of Serum and Bone Sclerostin Levels in Patients with End-Stage Kidney Disease. J. Clin. Med. 2019, 8, 2027. [Google Scholar] [CrossRef] [PubMed]
- Cadigan, K.M.; Waterman, M.L. TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb. Perspect. Biol. 2012, 4, a007906. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R.; Clevers, H.; Signaling, C. Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef]
- Semënov, M.; Tamai, K.; He, X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J. Biol. Chem. 2005, 280, 26770–26775. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Kang, H.; Liu, W.; Liu, P.; Zhang, J.; Harris, S.E.; Wu, D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J. Biol. Chem. 2005, 280, 19883–19887. [Google Scholar] [CrossRef]
- Franco, C.N.; Noe, M.M.; Albrecht, L.V. Metabolism and Endocrine Disorders: What Wnt Wrong? Front. Endocrinol. 2022, 13, 887037. [Google Scholar] [CrossRef]
- Truswell, A.S. Osteopetrosis with syndactyly: A morphological variant of Albers-Schönberg’s disease. J. Bone Jt. Surg. Br. 1958, 40-B, 209–218. [Google Scholar]
- Van Buchem, F.S.; Hadders, H.N.; Ubbens, R. An uncommon familial systemic disease of the skeleton: Hyperostosis corticalis generalisata familiaris. Acta Radiol. 1955, 44, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Saag, K.G.; Petersen, J.; Brandi, M.L.; Karaplis, A.C.; Lorentzon, M.; Thomas, T.; Maddox, J.; Fan, M.; Meisner, P.D.; Grauer, A. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N. Engl. J. Med. 2017, 377, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.J.C.L.N.; Marques, I.D.B.; Graciolli, F.G.; Fukuhara, L.; Reis, L.M.D.; Custódio, M.; Jorgetti, V.; Elias, R.M.; David-Neto, E.; Moysés, R.M.A. Comparison of serum levels with bone content and gene expression indicate a contradictory effect of kidney transplantation on sclerostin. Kidney Int. 2019, 96, 1100–1104. [Google Scholar] [CrossRef]
- Graciolli, F.G.; Neves, K.R.; Barreto, F.; Barreto, D.V.; Reis, L.M.D.; Canziani, M.E.; Sabbagh, Y.; Carvalho, A.B.; Jorgetti, V.; Elias, R.M.; et al. The complexity of chronic kidney disease-mineral and bone disorder across stages of chronic kidney disease. Kidney Int. 2017, 91, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.D.; Wesseling, K.; Pereira, R.; Gales, B.; Harrison, R.; Salusky, I.B. Technical approach to iliac crest biopsy. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. 3), S164–S169. [Google Scholar] [CrossRef]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature; symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef]
- Moe, S.M.; Chertow, G.M.; Parfrey, P.S.; Kubo, Y.; Block, G.A.; Correa-Rotter, R.; Drüeke, T.B.; Herzog, C.A.; London, G.M.; Mahaffey, K.W.; et al. Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) Trial Investigators*, Cinacalcet, Fibroblast Growth Factor-23, and Cardiovascular Disease in Hemodialysis: The Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) Trial. Circulation 2015, 132, 27–39. [Google Scholar]
- Sanchez, C.P.; Salusky, I.B.; Kuizon, B.D.; Ramirez, J.A.; Gales, B.; Ettenger, R.B.; Goodman, W.G. Bone disease in children and adolescents undergoing successful renal transplantation. Kidney Int. 1998, 53, 1358–1364. [Google Scholar] [CrossRef]
- Bakkaloglu, S.A.; Wesseling-Perry, K.; Pereira, R.C.; Gales, B.; Wang, H.-J.; Elashoff, R.M.; Salusky, I.B. Value of the new bone classification system in pediatric renal osteodystrophy. Clin. J. Am. Soc. Nephrol. 2010, 5, 1860–1866. [Google Scholar] [CrossRef]
- Laster, M.; Pereira, R.C.; Salusky, I.B. Unraveling the osteocyte in CKD-MBD post-renal transplantation. Kidney Int. 2019, 96, 1059–1061. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.C.; Salusky, I.B.; Roschger, P.; Klaushofer, K.; Yadin, O.; Freymiller, E.G.; Bowen, R.; Delany, A.M.; Fratzl-Zelman, N.; Wesseling-Perry, K. Impaired osteocyte maturation in the pathogenesis of renal osteodystrophy. Kidney Int. 2018, 94, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Lockitch, G.; Halstead, A.C.; Albersheim, S.; MacCallum, C.; Quigley, G. Age- and sex-specific pediatric reference intervals for biochemistry analytes as measured with the Ektachem-700 analyzer. Clin. Chem. 1988, 34, 1622–1625. [Google Scholar] [CrossRef] [PubMed]
- Moe, S.; Drüeke, T.; Cunningham, J.; Goodman, W.; Martin, K.; Olgaard, K.; Ott, S.; Sprague, S.; Lameire, N.; Eknoyan, G. Kidney Disease: Improving Global Outcomes (KDIGO), Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006, 69, 1945–1953. [Google Scholar] [CrossRef]
- Morena, M.; Jaussent, I.; Dupuy, A.-M.; Bargnoux, A.-S.; Kuster, N.; Chenine, L.; Leray-Moragues, H.; Klouche, K.; Vernhet, H.; Canaud, B.; et al. Osteoprotegerin and sclerostin in chronic kidney disease prior to dialysis: Potential partners in vascular calcifications. Nephrol. Dial. Transplant. 2015, 30, 1345–1356. [Google Scholar] [CrossRef]
- Dallas, S.L.; Prideaux, M.; Bonewald, L.F. The osteocyte: An endocrine cell ... and more. Endocr. Rev. 2013, 34, 658–690. [Google Scholar] [CrossRef]
- Robling, A.G.; Bonewald, L.F. The Osteocyte: New Insights. Annu. Rev. Physiol. 2020, 82, 485–506. [Google Scholar] [CrossRef]
- Isakova, T.; Wahl, P.; Vargas, G.S.; Gutiérrez, O.M.; Scialla, J.; Xie, H.; Appleby, D.; Nessel, L.; Bellovich, K.; Chen, J.; et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011, 79, 1370–1378. [Google Scholar] [CrossRef]
- Poole, K.E.S.; van Bezooijen, R.L.; Loveridge, N.; Hamersma, H.; Papapoulos, S.E.; Löwik, C.W.; Reeve, J. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005, 19, 1842–1844. [Google Scholar] [CrossRef]
- Martin, A. Bone and heart health in chronic kidney disease: Role of dentin matrix protein 1. Curr. Opin. Nephrol. Hypertens. 2019, 28, 297–303. [Google Scholar] [CrossRef]
- Dussold, C.; Gerber, C.; White, S.; Wang, X.; Qi, L.; Francis, C.; Capella, M.; Courbon, G.; Wang, J.; Li, C.; et al. DMP1 prevents osteocyte alterations, FGF23 elevation and left ventricular hypertrophy in mice with chronic kidney disease. Bone Res. 2019, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Bellido, T.; Ali, A.A.; Gubrij, I.; Plotkin, L.I.; Fu, Q.; O’Brien, C.A.; Manolagas, S.C.; Jilka, R.L. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: A novel mechanism for hormonal control of osteoblastogenesis. Endocrinology 2005, 146, 4577–4583. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Warmington, K.S.; Niu, Q.-T.; Asuncion, F.J.; Barrero, M.; Grisanti, M.; Dwyer, D.; Stouch, B.; Thway, T.M.; Stolina, M.; et al. Inhibition of sclerostin by monoclonal antibody increases bone formation, bone mass, and bone strength in aged male rats. J. Bone Miner. Res. 2010, 25, 2647–2656. [Google Scholar] [CrossRef]
- Roschger, A.; Roschger, P.; Keplingter, P.; Klaushofer, K.; Abdullah, S.; Kneissel, M.; Rauch, F. Effect of sclerostin antibody treatment in a mouse model of severe osteogenesis imperfecta. Bone 2014, 66, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Wesseling-Perry, K.; Pereira, R.C.; Tseng, C.-H.; Elashoff, R.; Zaritsky, J.J.; Yadin, O.; Sahney, S.; Gales, B.; Jüppner, H.; Salusky, I.B. Early skeletal and biochemical alterations in pediatric chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2012, 7, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Lerch, C.; Shroff, R.; Wan, M.; Rees, L.; Aitkenhead, H.; Bulut, I.K.; Thurn, D.; Bayazit, A.K.; Niemirska, A.; Canpolat, N.; et al. Effects of nutritional vitamin D supplementation on markers of bone and mineral metabolism in children with chronic kidney disease. Nephrol. Dial. Transplant. 2018, 33, 2208–2217. [Google Scholar] [CrossRef]
- Guven, S.; Gokce, I.; Cicek, N.; Yaman, A.; Vatansever, P.; Alpay, H. Sclerostin and osteoprotegerin: New markers of chronic kidney disease mediated mineral and bone disease in children. J. Pediatr. Endocrinol. Metab. 2020, 33, 1383–1390. [Google Scholar] [CrossRef]
- Evenepoel, P.; Claes, K.; Viaene, L.; Bammens, B.; Meijers, B.; Naesens, M.; Sprangers, B.; Kuypers, D. Decreased Circulating Sclerostin Levels in Renal Transplant Recipients with Persistent Hyperparathyroidism. Transplantation 2016, 100, 2188–2193. [Google Scholar] [CrossRef]
- Wesseling-Perry, K.; Harkins, G.C.; Wang, H.; Elashoff, R.; Gales, B.; Horwitz, M.J.; Stewart, A.F.; Jüppner, H.; Salusky, I.B. The calcemic response to continuous parathyroid hormone (PTH)(1-34) infusion in end-stage kidney disease varies according to bone turnover: A potential role for PTH(7-84). J. Clin. Endocrinol. Metab. 2010, 95, 2772–2780. [Google Scholar] [CrossRef]
- Delanaye, P.; Krzesinski, J.-M.; Warling, X.; Moonen, M.; Smelten, N.; Médart, L.; Bruyère, O.; Reginster, J.-Y.; Pottel, H.; Cavalier, E. Clinical and biological determinants of sclerostin plasma concentration in hemodialysis patients. Nephron. Clin. Pract. 2014, 128, 127–134. [Google Scholar] [CrossRef]
- Moysés, R.M.A.; Jamal, S.A.; Graciolli, F.G.; Reis, L.M.D.; Elias, R.M. Can we compare serum sclerostin results obtained with different assays in hemodialysis patients? Int. Urol. Nephrol. 2015, 47, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Bonani, M.; Rodriguez, D.; Fehr, T.; Mohebbi, N.; Brockmann, J.; Blum, M.; Graf, N.; Frey, D.; Wüthrich, R.P. Sclerostin blood levels before and after kidney transplantation. Kidney Blood Press. Res. 2014, 39, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Pietrzyk, B.; Wyskida, K.; Ficek, J.; Kolonko, A.; Ficek, R.; Więcek, A.; Olszanecka-Glinianowicz, M.; Chudek, J. Relationship between plasma levels of sclerostin, calcium-phosphate disturbances, established markers of bone turnover, and inflammation in haemodialysis patients. Int. Urol. Nephrol. 2019, 51, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, L.; Liabeuf, S.; Oliveira, R.B.; Louvet, L.; Kamel, S.; Lemke, H.-D.; Vanholder, R.; Choukroun, G.; Massy, Z.A. European Uremic Toxin (EUTox) Work Group, Uremic toxicity and sclerostin in chronic kidney disease patients. Nephrol. Ther. 2014, 10, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Ryan, Z.C.; Craig, T.A.; McGee-Lawrence, M.; Westendorf, J.J.; Kumar, R. Alterations in vitamin D metabolite, parathyroid hormone and fibroblast growth factor-23 concentrations in sclerostin-deficient mice permit the maintenance of a high bone mass. J. Steroid Biochem. Mol. Biol. 2015, 148, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Neto, R.; Pereira, L.; Magalhães, J.; Quelhas-Santos, J.; Martins, S.; Carvalho, C.; Frazão, J.M. Sclerostin and DKK1 circulating levels associate with low bone turnover in patients with chronic kidney disease Stages 3 and 4. Clin. Kidney J. 2021, 14, 2401–2408. [Google Scholar] [CrossRef]
- Cejka, D. Cardiovascular Safety of Anti-Sclerostin Therapy in Chronic Kidney Disease. Metabolites 2021, 11, 770. [Google Scholar] [CrossRef]
- Pelletier, S.; Dubourg, L.; Carlier, M.-C.; Hadj-Aissa, A.; Fouque, D. The relation between renal function and serum sclerostin in adult patients with CKD. Clin. J. Am. Soc. Nephrol. 2013, 8, 819–823. [Google Scholar] [CrossRef]
- Bonewald, L.F.; Wacker, M.J. FGF23 production by osteocytes. Pediatr. Nephrol. 2013, 28, 563–568. [Google Scholar] [CrossRef]
- Lima, F.; El-Husseini, A.; Monier-Faugere, M.-C.; David, V.; Mawad, H.; Quarles, D.; Malluche, H.H. FGF-23 serum levels and bone histomorphometric results in adult patients with chronic kidney disease on dialysis. Clin. Nephrol. 2014, 82, 287–295. [Google Scholar] [CrossRef]
- Lima, F.; Monier-Faugere, M.-C.; Mawad, H.; David, V.; Malluche, H.H. FGF-23 and sclerostin in serum and bone of CKD patients. Clin. Nephrol. 2023, 99, 209–218. [Google Scholar] [CrossRef] [PubMed]
- De Maré, A.; Opdebeeck, B.; Neven, E.; D’Haese, P.C.; Verhulst, A. Sclerostin Protects Against Vascular Calcification Development in Mice. J. Bone Miner. Res. 2022, 37, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Huybrechts, Y.; Evenepoel, P.; Haarhaus, M.; Cavalier, E.; Dams, G.; VanHul, W.; D’Haese, P.C.; Verhulst, A. Osteocytic Sclerostin Expression as an Indicator of Altered Bone Turnover. Nutrients 2023, 15, 598. [Google Scholar] [CrossRef] [PubMed]
| Pre-Dialysis CKD | CKD 5D | p Value | |
|---|---|---|---|
| N (%) | 24 | 29 | |
| Age, (median (IQR) | 15.0 (12.7, 17.1) | 18.3 (16.4, 19.7) | <0.0001 |
| Gender, (n (%)) | 0.8 | ||
| Male | 23 (68) | 37 (70) | |
| Female | 11 (32) | 16 (30) | |
| Race, (n (%)) | 0.01 | ||
| Black | 2 (6) | 5 (9) | |
| White | 14 (41) | 6 (11) | |
| Hispanic | 18 (53) | 39 (74) | |
| Asian | 3 (6) | ||
| Disease, (n (%)) | 0.0003 | ||
| CAKUT | 23 (68) | 15 (28) | |
| GN | 10 (29) | 21 (40) | |
| Unknown | 1 (3) | 17 (32) | |
| CKD Stage, (n (%)) | |||
| Stage 2 & 3 | 28 (82) | ||
| Stage 4 | 5 (14) | ||
| Stage 5 | 1 (3) | ||
| Dialysis, (n (%)) | |||
| HD | 25 (45) | ||
| PD | 29 (55) |
| Pre-Dialysis CKD | CKD 5D | p Value | |
|---|---|---|---|
| Ca (mg/dL) | 9.4 (9.0, 9.7) | 9.2 (8.7, 9.7) | 0.2 |
| P04 (mg/dL) | 4.8 (4.0, 5.3) | 6.3 (5.2, 7.6) | <0.0001 |
| P04 Z-score | 1.5 (0.3, 2.3) | 5.0 (2.9, 7.7) | <0.0001 |
| Alk Phos (IU/L) | 171 (110, 265) | 150 (92, 283) | 0.5 |
| 25-(OH) Vitamin D (ng/mL) | 26 (24, 30) | 21 (13, 30) | 0.1 |
| iPTH (pg/mL) | 95 (50, 159) | 411 (211, 991) | <0.0001 |
| Intact FGF-23 (pg/mL) | 95 (64, 140) | 1195 (286, 5718) | <0.0001 |
| C-terminal FGF-23 (RU/mL) | 199 (101, 344) | 1463 (705, 5577) | <0.0001 |
| Scl (pmol/L) | 40.3 (34.2, 52.6) | 66.9 (48.3, 87.2) | <0.0001 |
| Pre-Dialysis CKD | CKD 5D | p Value | Reference Range | |
|---|---|---|---|---|
| Turnover | ||||
| Bone formation rate (BFR/BS; μm3/μm2/year) | 19.4 (12.2, 44.8) | 30.9 (5.1, 61.8) | 0.8 | 8.0–73.4 |
| Eroded Surface (ES/BS; %) | 4.4 (2.5, 7) | 9.8 (5.8, 13.5) | 0.0001 | 0.5–4.3 |
| Mineralization | ||||
| Osteoid Volume (OV/BV; %) | 4.9 (1.6, 7.8) | 4.4 (1.7, 7.6) | 0.6 | 0.2–5.8 |
| Osteoid thickness (O.Th; μm) | 10.9 (7.7, 15.4) | 9.7 (6.9, 11.8) | 0.1 | 2.0–13.2 |
| Osteoid surface (OS/BS; %) | 27.2 (12.5, 39.9) | 30.4 (16.7, 43.7) | 0.5 | 4.3–37.0 |
| Osteoid maturation time (OMT; day) | 13.2 (9.3, 18.5) | 11.8 (8.5, 15) | 0.2 | 1.2–11.5 |
| Mineralization lag time (MLT; day) | 34.3 (18.5, 52.4) | 28.4 (19.5, 75.4) | 0.9 | 2.3–63.8 |
| Volume | ||||
| Bone volume (BV/TV; %) | 27.2 (22.7, 32.3) | 31.5 (26.2, 36.4) | 0.04 | 8.9–34.4 |
| Trabecular number (Tb.N; mm) | 2.2 (1.8, 2.4) | 2.2 (1.9, 2.4) | 0.3 | 1.1–2.2 |
| Trabecular Separation (Tb.Sp; μm) | 334 (307, 422) | 325 (271, 361) | 0.1 | 351–737 |
| Osteoblast Surface (ObS/BS; %) | 2.7 (1.2, 17.3) | 5.5 (2, 9.9) | 0.5 | |
| Osteoclast Surface (OcS/BS; %) | 0.8 (0.3, 1.1) | 1.9 (1.2, 3.4) | 0.0001 | |
| Pre-Dialysis CKD | CKD 5D | |||||
|---|---|---|---|---|---|---|
| PTH | Alk Phos | Bone FGF-23 | PTH | Alk Phos | Bone FGF-23 | |
| Serum Scl | −0.42 p = 0.02 | 0.11 p = 0.5 | −0.36 p = 0.1 | −0.31 p = 0.04 | −0.37 p = 0.01 | 0.12 p = 0.4 |
| Bone Scl | 0.28 p = 0.3 | −0.29 p = 0.3 | 0.18 p = 0.5 | −0.29 p = 0.06 | −0.34 p = 0.02 | 0.5 p = 0.0002 |
| Scl/PTH ratio | −0.93 p < 0.0001 | −0.23 p = 0.2 | 0.19 p = 0.4 | −0.93 p < 0.0001 | −0.44 p = 0.004 | 0.12 p = 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laster, M.; Pereira, R.C.; Noche, K.; Gales, B.; Salusky, I.B.; Albrecht, L.V. Sclerostin, Osteocytes, and Wnt Signaling in Pediatric Renal Osteodystrophy. Nutrients 2023, 15, 4127. https://doi.org/10.3390/nu15194127
Laster M, Pereira RC, Noche K, Gales B, Salusky IB, Albrecht LV. Sclerostin, Osteocytes, and Wnt Signaling in Pediatric Renal Osteodystrophy. Nutrients. 2023; 15(19):4127. https://doi.org/10.3390/nu15194127
Chicago/Turabian StyleLaster, Marciana, Renata C. Pereira, Kathleen Noche, Barbara Gales, Isidro B. Salusky, and Lauren V. Albrecht. 2023. "Sclerostin, Osteocytes, and Wnt Signaling in Pediatric Renal Osteodystrophy" Nutrients 15, no. 19: 4127. https://doi.org/10.3390/nu15194127
APA StyleLaster, M., Pereira, R. C., Noche, K., Gales, B., Salusky, I. B., & Albrecht, L. V. (2023). Sclerostin, Osteocytes, and Wnt Signaling in Pediatric Renal Osteodystrophy. Nutrients, 15(19), 4127. https://doi.org/10.3390/nu15194127






