Soy Protein Containing Isoflavones Improves Facial Signs of Photoaging and Skin Hydration in Postmenopausal Women: Results of a Prospective Randomized Double-Blind Controlled Trial
Abstract
:1. Introduction
2. Methods and Materials
2.1. Materials
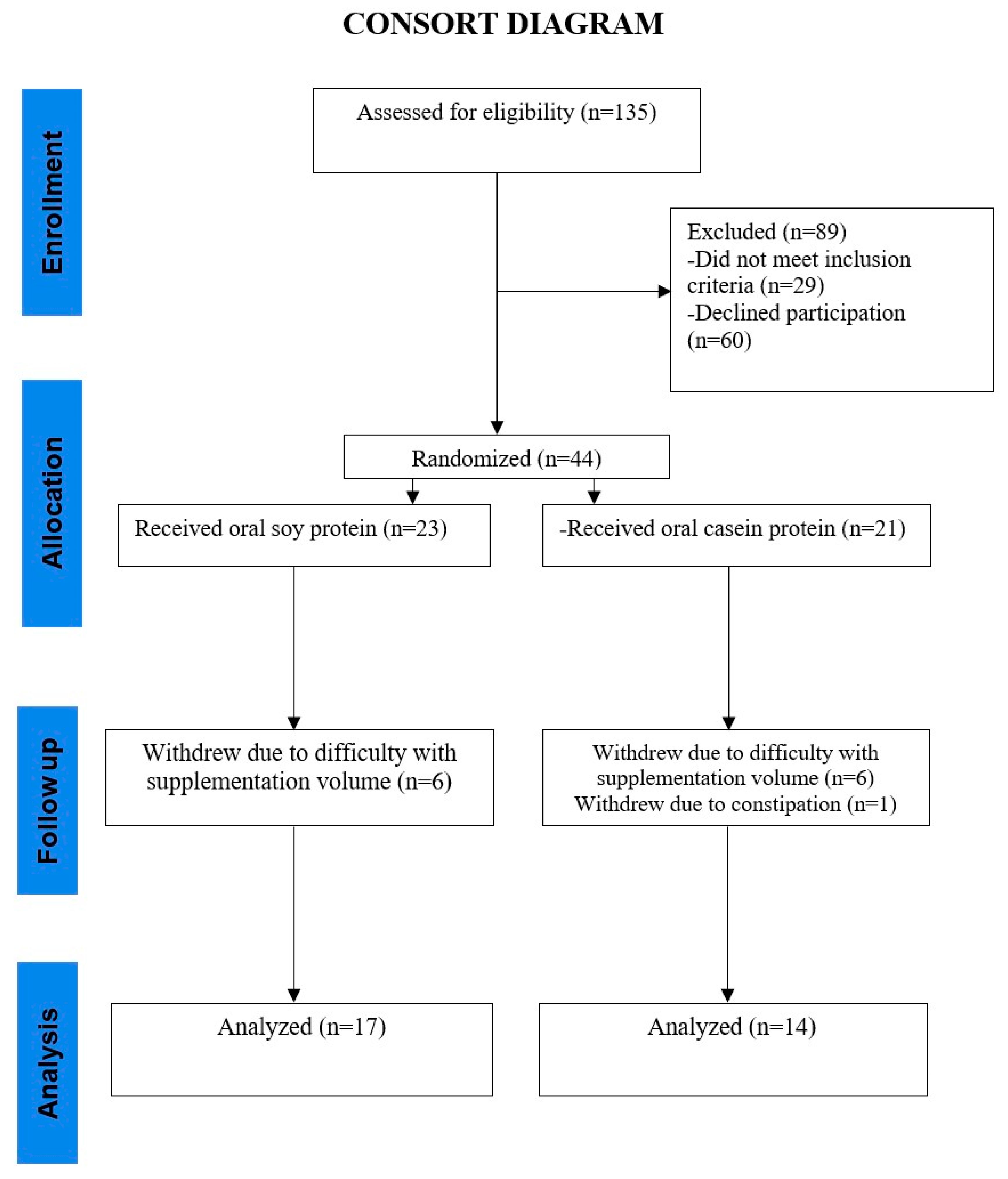
2.2. Study Design, Recruitment, and Randomization
2.3. Inclusion and Exclusion Criteria
2.4. Facial Imaging and Skin Biophysical Measurements
2.5. Statistical Analysis
3. Results
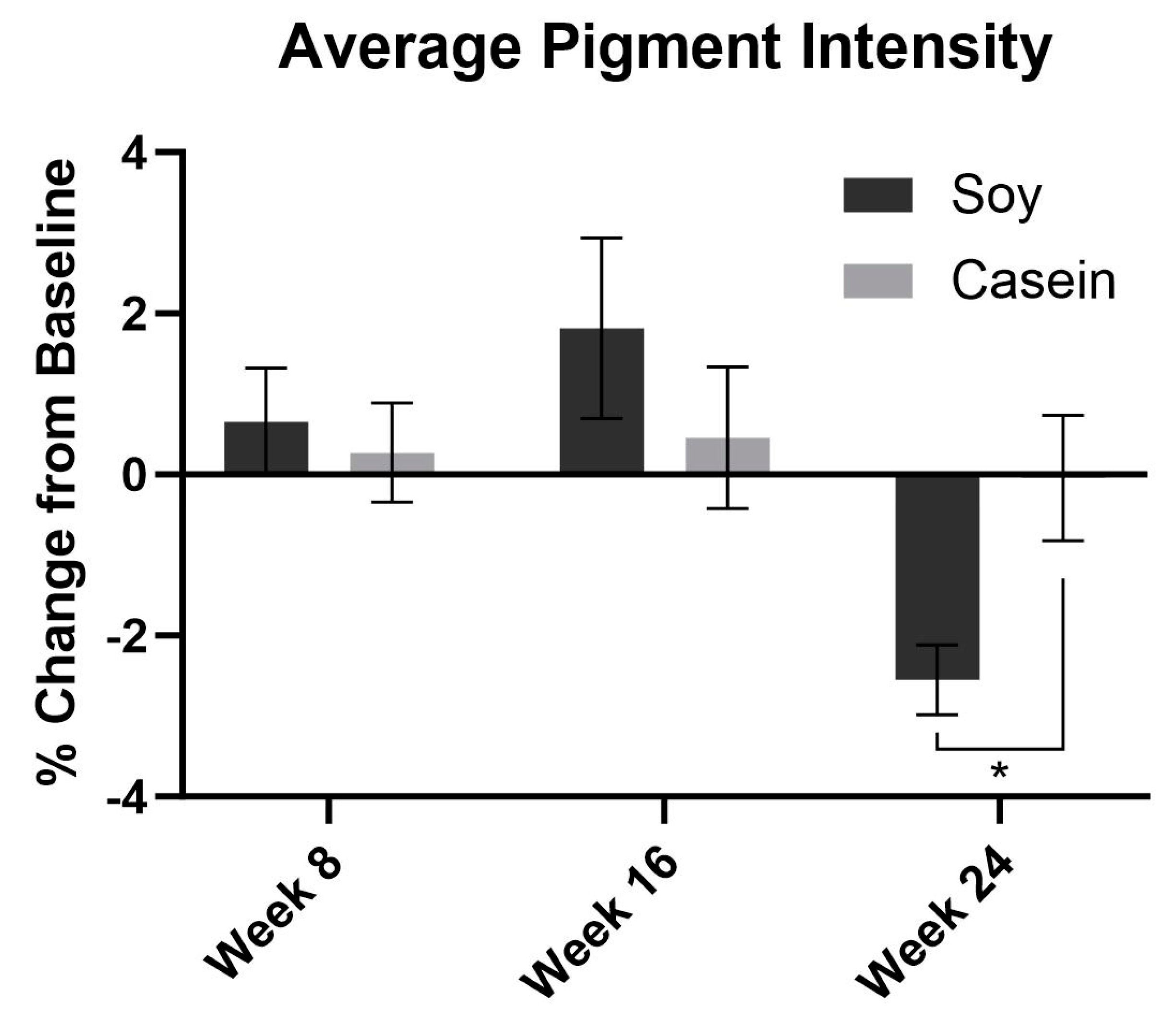
3.1. Imaging System-Based Photographic Analysis of Wrinkle Severity and Pigment Intensity
3.2. Skin Hydration
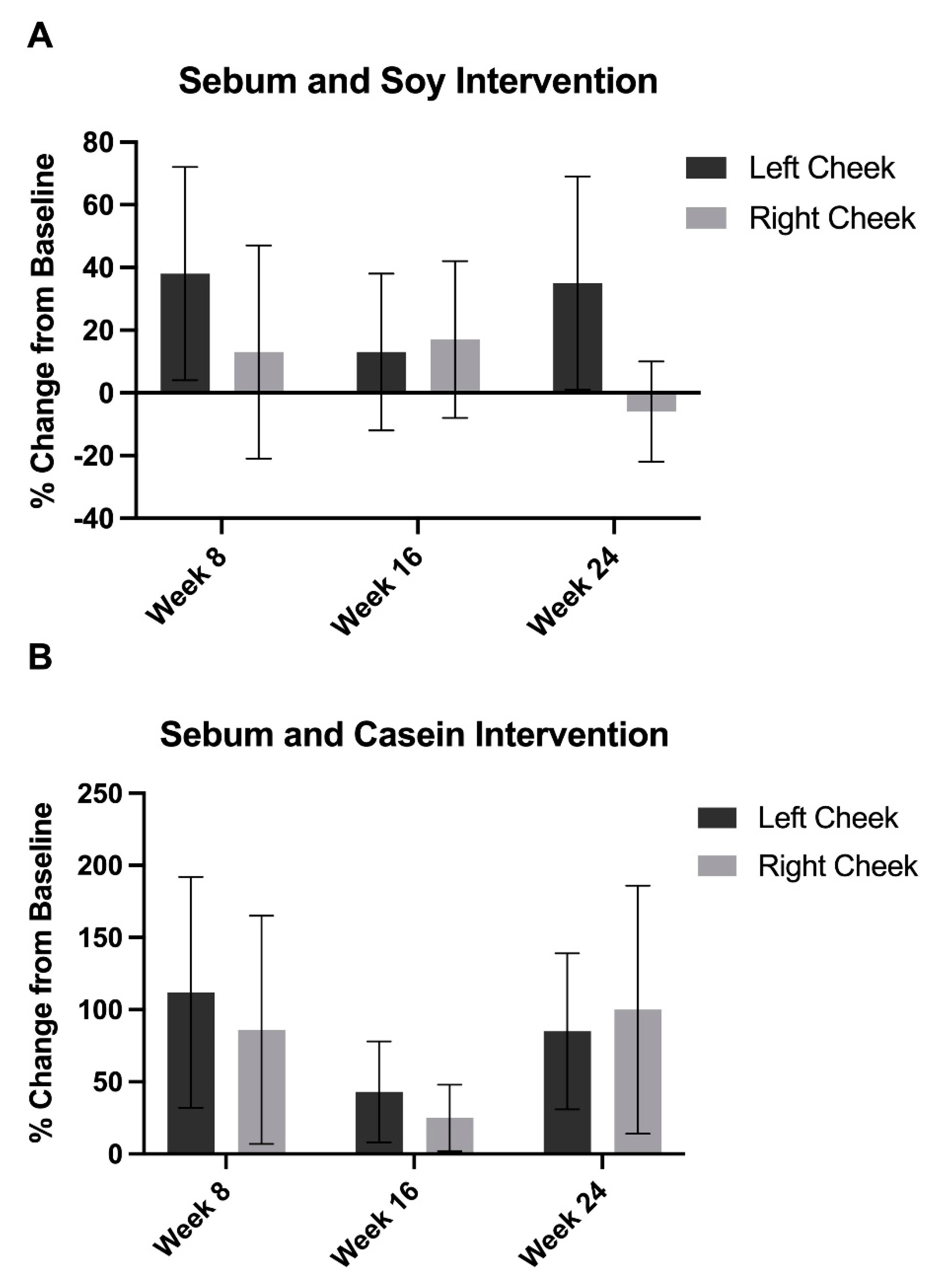
3.3. Sebum Excretion
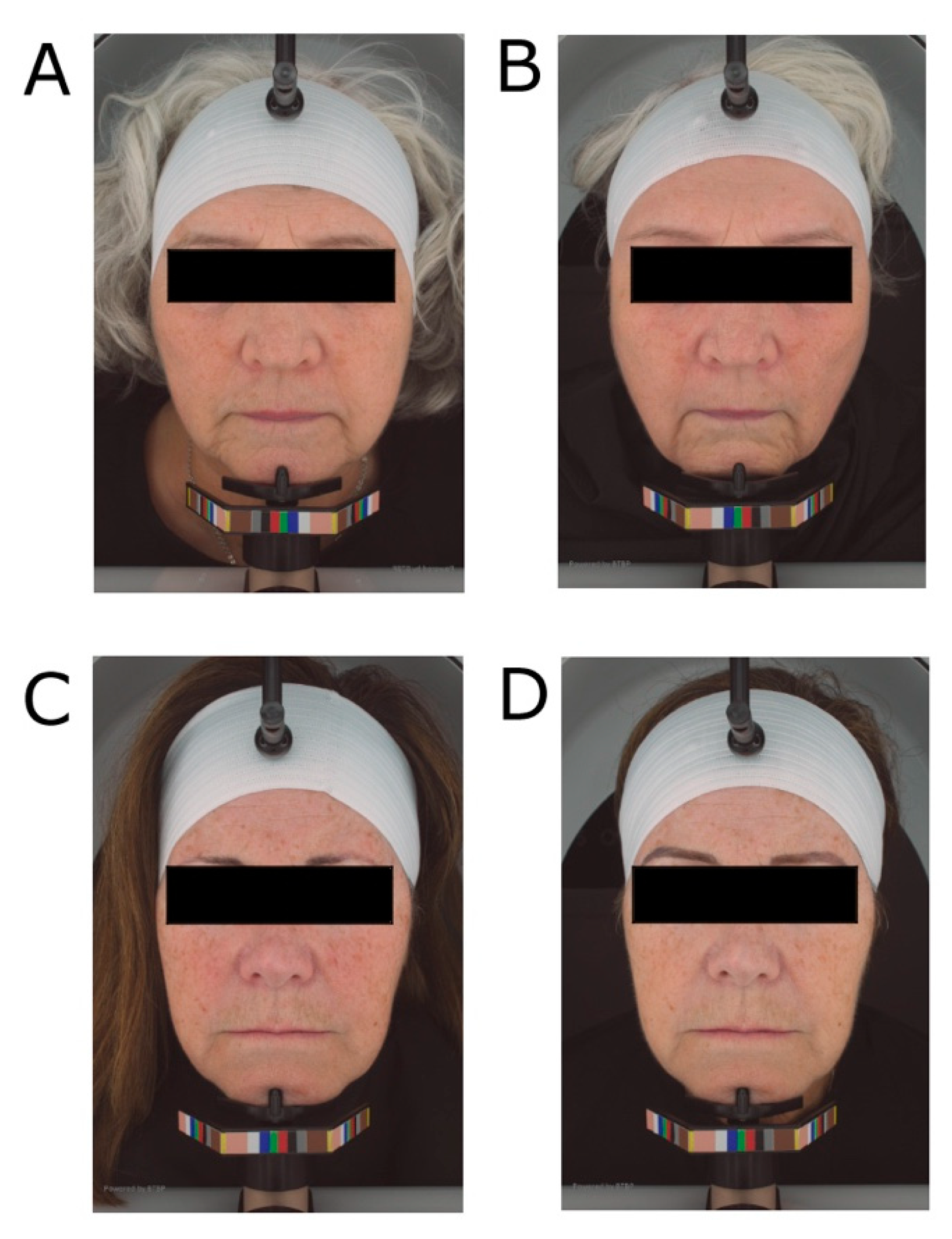
3.4. Facial Photography
3.5. Adverse Events
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krizova, L.; Dadakova, K.; Kasparovska, J.; Kasparovsky, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed]
- Nakai, S.; Fujita, M.; Kamei, Y. Health Promotion Effects of Soy Isoflavones. J. Nutr. Sci. Vitaminol. 2020, 66, 502–507. [Google Scholar] [CrossRef]
- Lephart, E.D. Phytoestrogens (Resveratrol and Equol) for Estrogen-Deficient Skin-Controversies/Misinformation versus Anti-Aging In Vitro and Clinical Evidence via Nutraceutical-Cosmetics. Int. J. Mol. Sci. 2021, 22, 1218. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D.; Naftolin, F. Menopause and the Skin: Old Favorites and New Innovations in Cosmeceuticals for Estrogen-Deficient Skin. Dermatol. Ther. 2021, 11, 53–69. [Google Scholar] [CrossRef]
- Rietjens, I.M.; Sotoca, A.M.; Vervoort, J.; Louisse, J. Mechanisms underlying the dualistic mode of action of major soy isoflavones in relation to cell proliferation and cancer risks. Mol. Nutr. Food Res. 2013, 57, 100–113. [Google Scholar] [CrossRef]
- Evsen, M.S.; Ozler, A.; Gocmez, C.; Varol, S.; Tunc, S.Y.; Akil, E.; Uzar, E.; Kaplan, I. Effects of estrogen, estrogen/progesteron combination and genistein treatments on oxidant/antioxidant status in the brain of ovariectomized rats. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1869–1873. [Google Scholar]
- Jia, Z.; Babu, P.V.; Si, H.; Nallasamy, P.; Zhu, H.; Zhen, W.; Misra, H.P.; Li, Y.; Liu, D. Genistein inhibits TNF-alpha-induced endothelial inflammation through the protein kinase pathway A and improves vascular inflammation in C57BL/6 mice. Int. J. Cardiol. 2013, 168, 2637–2645. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Yang, W.; Bosland, M.C. Soy isoflavones and prostate cancer: A review of molecular mechanisms. J. Steroid Biochem. Mol. Biol. 2014, 140, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Patriarca, M.T.; Barbosa de Moraes, A.R.; Nader, H.B.; Petri, V.; Martins, J.R.; Gomes, R.C.; Soares, J.M., Jr. Hyaluronic acid concentration in postmenopausal facial skin after topical estradiol and genistein treatment: A double-blind, randomized clinical trial of efficacy. Menopause 2013, 20, 336–341. [Google Scholar] [CrossRef]
- Chua, L.S.; Lee, S.Y.; Abdullah, N.; Sarmidi, M.R. Review on Labisia pumila (Kacip Fatimah): Bioactive phytochemicals and skin collagen synthesis promoting herb. Fitoterapia 2012, 83, 1322–1335. [Google Scholar] [CrossRef]
- Gopaul, R.; Knaggs, H.E.; Lephart, E.D. Biochemical investigation and gene analysis of equol: A plant and soy-derived isoflavonoid with antiaging and antioxidant properties with potential human skin applications. Biofactors 2012, 38, 44–52. [Google Scholar] [CrossRef]
- Messina, M. Soy foods, isoflavones, and the health of postmenopausal women. Am. J. Clin. Nutr. 2014, 100 (Suppl. 1), 423S–430S. [Google Scholar] [CrossRef] [PubMed]
- Accorsi-Neto, A.; Haidar, M.; Simoes, R.; Simoes, M.; Soares, J., Jr.; Baracat, E. Effects of isoflavones on the skin of postmenopausal women: A pilot study. Clinics 2009, 64, 505–510. [Google Scholar] [CrossRef]
- Duchnik, E.; Kruk, J.; Baranowska-Bosiacka, I.; Pilutin, A.; Maleszka, R.; Marchlewicz, M. Effects of the soy isoflavones, genistein and daidzein, on male rats’ skin. Postępy Dermatol. Alergol. 2019, 36, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Leyden, J.; Wallo, W. The mechanism of action and clinical benefits of soy for the treatment of hyperpigmentation. Int. J. Dermatol. 2011, 50, 470–477. [Google Scholar] [CrossRef]
- Khapre, S.; Deshmukh, U.; Jain, S. The Impact of Soy Isoflavone Supplementation on the Menopausal Symptoms in Perimenopausal and Postmenopausal Women. J. Midlife Health 2022, 13, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.R.; Chen, K.H. Utilization of Isoflavones in Soybeans for Women with Menopausal Syndrome: An Overview. Int. J. Mol. Sci. 2021, 22, 3212. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.R.; Ko, N.Y.; Chen, K.H. Isoflavone Supplements for Menopausal Women: A Systematic Review. Nutrients 2019, 11, 2649. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.N.T.; Hu, L.M.; Jeppesen, P.B. A systematic review and meta-analysis of the effects of isoflavone formulations against estrogen-deficient bone resorption in peri- and postmenopausal women. Am. J. Clin. Nutr. 2017, 106, 801–811. [Google Scholar] [CrossRef]
- Mueller, M.; Jungbauer, A. Red clover extract: A putative source for simultaneous treatment of menopausal disorders and the metabolic syndrome. Menopause 2008, 15, 1120–1131. [Google Scholar] [CrossRef]
- Riyanto, P.; Subchan, P.; Lelyana, R. Advantage of soybean isoflavone as antiandrogen on acne vulgaris. Dermato-endocrinology 2015, 7, e1063751. [Google Scholar] [CrossRef] [PubMed]
- Youn, C.S.; Kwon, O.S.; Won, C.H.; Hwang, E.J.; Park, B.J.; Eun, H.C.; Chung, J.H. Effect of pregnancy and menopause on facial wrinkling in women. Acta Derm.-Venereol. 2003, 83, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Xiao, Z.; Wu, Y.; Ge, C. Diet and Skin Aging-From the Perspective of Food Nutrition. Nutrients 2020, 12, 870. [Google Scholar] [CrossRef]
- Gupta, V.; Sharma, V.K. Skin typing: Fitzpatrick grading and others. Clin. Dermatol. 2019, 37, 430–436. [Google Scholar] [CrossRef]
- Sharma, A.N.; Patel, B.C. Laser Fitzpatrick Skin Type Recommendations. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Roberts, W.E. Skin type classification systems old and new. Dermatol. Clin. 2009, 27, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Pappas, A.; Liakou, A.; Zouboulis, C.C. Nutrition and skin. Rev. Endocr. Metab. Disord. 2016, 17, 443–448. [Google Scholar] [CrossRef]
- Grether-Beck, S.; Marini, A.; Jaenicke, T.; Stahl, W.; Krutmann, J. Molecular evidence that oral supplementation with lycopene or lutein protects human skin against ultraviolet radiation: Results from a double-blinded, placebo-controlled, crossover study. Br. J. Dermatol. 2017, 176, 1231–1240. [Google Scholar] [CrossRef]
- Fam, V.W.; Holt, R.R.; Keen, C.L.; Sivamani, R.K.; Hackman, R.M. Prospective Evaluation of Mango Fruit Intake on Facial Wrinkles and Erythema in Postmenopausal Women: A Randomized Clinical Pilot Study. Nutrients 2020, 12, 3381. [Google Scholar] [CrossRef] [PubMed]
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Scali, E.; Patruno, C.; Nistico, S.P. Role of Vitamins in Skin Health: A Systematic Review. Curr. Nutr. Rep. 2020, 9, 226–235. [Google Scholar] [CrossRef]
- Rybak, I.; Carrington, A.E.; Dhaliwal, S.; Hasan, A.; Wu, H.; Burney, W.; Maloh, J.; Sivamani, R.K. Prospective Randomized Controlled Trial on the Effects of Almonds on Facial Wrinkles and Pigmentation. Nutrients 2021, 13, 785. [Google Scholar] [CrossRef]
- Foolad, N.; Vaughn, A.R.; Rybak, I.; Burney, W.A.; Chodur, G.M.; Newman, J.W.; Steinberg, F.M.; Sivamani, R.K. Prospective randomized controlled pilot study on the effects of almond consumption on skin lipids and wrinkles. Phytother. Res. 2019, 33, 3212–3217. [Google Scholar] [CrossRef]
- Mitsuishi, T.; Shimoda, T.; Mitsui, Y.; Kuriyama, Y.; Kawana, S. The effects of topical application of phytonadione, retinol and vitamins C and E on infraorbital dark circles and wrinkles of the lower eyelids. J. Cosmet. Dermatol. 2004, 3, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Katta, R.; Huang, S. Skin, Hair and Nail Supplements: An Evidence-Based Approach. Ski. Ther. Lett. 2019, 24, 7–13. [Google Scholar]
- Kao, T.H.; Chen, B.H. Functional components in soybean cake and their effects on antioxidant activity. J. Agric. Food Chem. 2006, 54, 7544–7555. [Google Scholar] [CrossRef]
- Kao, T.H.; Wu, W.M.; Hung, C.F.; Wu, W.B.; Chen, B.H. Anti-inflammatory effects of isoflavone powder produced from soybean cake. J. Agric. Food Chem. 2007, 55, 11068–11079. [Google Scholar] [CrossRef]
- Pabich, M.; Materska, M. Biological Effect of Soy Isoflavones in the Prevention of Civilization Diseases. Nutrients 2019, 11, 1660. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Hsu, B.Y.; Wu, N.L.; Tsui, W.H.; Lin, T.J.; Su, C.C.; Hung, C.F. Anti-photoaging effects of soy isoflavone extract (aglycone and acetylglucoside form) from soybean cake. Int. J. Mol. Sci. 2010, 11, 4782–4795. [Google Scholar] [CrossRef]
- Natarelli, N.; Gahoonia, N.; Maloh, J.; Sivamani, R.K. Clinical Efficacy of Topical or Oral Soy Supplementation in Dermatology: A Systematic Review. J. Clin. Med. 2023, 12, 4171. [Google Scholar] [CrossRef]
- Tokudome, Y.; Nakamura, K.; Kage, M.; Todo, H.; Sugibayashi, K.; Hashimoto, F. Effects of soybean peptide and collagen peptide on collagen synthesis in normal human dermal fibroblasts. Int. J. Food Sci. Nutr. 2012, 63, 689–695. [Google Scholar] [CrossRef]
- Uyar, B.; Sivrikoz, O.N.; Ozdemir, U.; Dasbasi, T.; Sacar, H. Histological investigation of the effect of soybean (Glycine max) extracts on the collagen layer and estrogen receptors in the skin of female rats. Clinics 2014, 69, 854–861. [Google Scholar] [CrossRef]
- Jenkins, G.; Wainwright, L.J.; Holland, R.; Barrett, K.E.; Casey, J. Wrinkle reduction in post-menopausal women consuming a novel oral supplement: A double-blind placebo-controlled randomized study. Int. J. Cosmet. Sci. 2014, 36, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Foolad, N.; Prakash, N.; Shi, V.Y.; Kamangar, F.; Wang, Q.; Li, C.S.; Sivamani, R.K. The use of facial modeling and analysis to objectively quantify facial redness. J. Cosmet. Dermatol. 2016, 15, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, D.; Ahmad, A.; Bao, B.; Sarkar, F.H. Antioxidant function of isoflavone and 3,3′-diindolylmethane: Are they important for cancer prevention and therapy? Antioxid. Redox Signal. 2013, 19, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Callender, V.D.; St Surin-Lord, S.; Davis, E.C.; Maclin, M. Postinflammatory hyperpigmentation: Etiologic and therapeutic considerations. Am. J. Clin. Dermatol. 2011, 12, 87–99. [Google Scholar] [CrossRef]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef]
- Kim, M.A.; Kim, M.J. Isoflavone profiles and antioxidant properties in different parts of soybean sprout. J. Food Sci. 2020, 85, 689–695. [Google Scholar] [CrossRef]
- Izumi, T.; Saito, M.; Obata, A.; Arii, M.; Yamaguchi, H.; Matsuyama, A. Oral intake of soy isoflavone aglycone improves the aged skin of adult women. J. Nutr. Sci. Vitaminol. 2007, 53, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Skovgaard, G.R.; Jensen, A.S.; Sigler, M.L. Effect of a novel dietary supplement on skin aging in post-menopausal women. Eur. J. Clin. Nutr. 2006, 60, 1201–1206. [Google Scholar] [CrossRef]
- Lin, V.C.; Ding, H.Y.; Tsai, P.C.; Wu, J.Y.; Lu, Y.H.; Chang, T.S. In vitro and in vivo melanogenesis inhibition by biochanin A from Trifolium pratense. Biosci. Biotechnol. Biochem. 2011, 75, 914–918. [Google Scholar] [CrossRef]
- Chang, T.-S. Natural Melanogenesis Inhibitors Acting Through the Down-Regulation of Tyrosinase Activity. Materials 2012, 5, 1661–1685. [Google Scholar] [CrossRef]
- Paulson, K.G.; Iyer, J.G.; Nghiem, P. Asymmetric lateral distribution of melanoma and Merkel cell carcinoma in the United States. J. Am. Acad. Dermatol. 2011, 65, 35–39. [Google Scholar] [CrossRef]
- Butler, S.T.; Fosko, S.W. Increased prevalence of left-sided skin cancers. J. Am. Acad. Dermatol. 2010, 63, 1006–1010. [Google Scholar] [CrossRef]
- Gordon, J.R.; Brieva, J.C. Images in clinical medicine. Unilateral dermatoheliosis. N. Engl. J. Med. 2012, 366, e25. [Google Scholar] [CrossRef]
- Joshi, R.S.; Phadke, V.A.; Khopkar, U.S.; Wadhwa, S.L. Unilateral solar purpura as a manifestation of asymmetrical photodamage in taxi drivers. Arch. Dermatol. 1996, 132, 715–716. [Google Scholar] [CrossRef] [PubMed]
- Widgerow, A.D.; Napekoski, K. New approaches to skin photodamage histology-Differentiating ‘good’ versus ‘bad’ Elastin. J. Cosmet. Dermatol. 2021, 20, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Kotlus, B.S. Effect of sleep position on perceived facial aging. Dermatol. Surg. 2013, 39, 1360–1362. [Google Scholar] [CrossRef]
- Wa, C.V.; Maibach, H.I. Mapping the human face: Biophysical properties. Ski. Res. Technol. 2010, 16, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D.; Diaz, I.; Namkoong, J.; Wu, J.; Boyd, T. Efficacy Evaluation of a Topical Hyaluronic Acid Serum in Facial Photoaging. Dermatol. Ther. 2021, 11, 1385–1394. [Google Scholar] [CrossRef]
- Stevenson, S.; Thornton, J. Effect of estrogens on skin aging and the potential role of SERMs. Clin. Interv. Aging 2007, 2, 283–297. [Google Scholar] [CrossRef]
- Kim, J.; Ko, Y.; Park, Y.K.; Kim, N.I.; Ha, W.K.; Cho, Y. Dietary effect of lactoferrin-enriched fermented milk on skin surface lipid and clinical improvement of acne vulgaris. Nutrition 2010, 26, 902–909. [Google Scholar] [CrossRef]
- Prokopidis, K.; Mazidi, M.; Sankaranarayanan, R.; Tajik, B.; McArdle, A.; Isanejad, M. Effects of whey and soy protein supplementation on inflammatory cytokines in older adults: A systematic review and meta-analysis. Br. J. Nutr. 2023, 129, 759–770. [Google Scholar] [CrossRef]
- Gholami, A.; Baradaran, H.R.; Hariri, M. Can soy isoflavones plus soy protein change serum levels of interlukin-6? A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2021, 35, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Gholami, A.; Mollanoroozy, E.; Reza Baradaran, H.; Hariri, M. The efficacy of soy isoflavones combined with soy protein on serum concentration of interleukin-6 and tumour necrosis factor-alpha among post-menopausal women? A systematic review and meta-analysis of randomized controlled trials. Clin. Exp. Pharmacol. Physiol. 2022, 49, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Ho, S.C.; Lam, S.S.; Ho, S.S.; Woo, J.L. Soy isoflavones have a favorable effect on bone loss in Chinese postmenopausal women with lower bone mass: A double-blind, randomized, controlled trial. J. Clin. Endocrinol. Metab. 2003, 88, 4740–4747. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.C.; Woo, J.; Lam, S.; Chen, Y.; Sham, A.; Lau, J. Soy protein consumption and bone mass in early postmenopausal Chinese women. Osteoporos. Int. 2003, 14, 835–842. [Google Scholar] [CrossRef]
- Belobrajdic, D.P.; James-Martin, G.; Jones, D.; Tran, C.D. Soy and Gastrointestinal Health: A Review. Nutrients 2023, 15, 1959. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Isoflavone Content of Selected Foods, Release 2.0; US Department of Agriculture: Washington, DC, USA, 2008. [Google Scholar]
- Boutas, I.; Kontogeorgi, A.; Dimitrakakis, C.; Kalantaridou, S.N. Soy Isoflavones and Breast Cancer Risk: A Meta-analysis. In Vivo 2022, 36, 556–562. [Google Scholar] [CrossRef]
- Messina, M. Impact of Soy Foods on the Development of Breast Cancer and the Prognosis of Breast Cancer Patients. Forsch. Komplement. 2016, 23, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, M.; Li, Z.; Jiang, H.; Shi, J.; Shi, X.; Liu, S.; Zhao, J.; Kong, L.; Zhang, W.; et al. Intake of Soy, Soy Isoflavones and Soy Protein and Risk of Cancer Incidence and Mortality. Front. Nutr. 2022, 9, 847421. [Google Scholar] [CrossRef]
- Chi, F.; Wu, R.; Zeng, Y.C.; Xing, R.; Liu, Y.; Xu, Z.G. Post-diagnosis soy food intake and breast cancer survival: A meta-analysis of cohort studies. Asian Pac. J. Cancer Prev. 2013, 14, 2407–2412. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzo, J.; Min, M.; Adnan, S.; Afzal, N.; Maloh, J.; Chambers, C.J.; Fam, V.; Sivamani, R.K. Soy Protein Containing Isoflavones Improves Facial Signs of Photoaging and Skin Hydration in Postmenopausal Women: Results of a Prospective Randomized Double-Blind Controlled Trial. Nutrients 2023, 15, 4113. https://doi.org/10.3390/nu15194113
Rizzo J, Min M, Adnan S, Afzal N, Maloh J, Chambers CJ, Fam V, Sivamani RK. Soy Protein Containing Isoflavones Improves Facial Signs of Photoaging and Skin Hydration in Postmenopausal Women: Results of a Prospective Randomized Double-Blind Controlled Trial. Nutrients. 2023; 15(19):4113. https://doi.org/10.3390/nu15194113
Chicago/Turabian StyleRizzo, Julianne, Mildred Min, Sarah Adnan, Nasima Afzal, Jessica Maloh, Cindy J. Chambers, Vivien Fam, and Raja K. Sivamani. 2023. "Soy Protein Containing Isoflavones Improves Facial Signs of Photoaging and Skin Hydration in Postmenopausal Women: Results of a Prospective Randomized Double-Blind Controlled Trial" Nutrients 15, no. 19: 4113. https://doi.org/10.3390/nu15194113
APA StyleRizzo, J., Min, M., Adnan, S., Afzal, N., Maloh, J., Chambers, C. J., Fam, V., & Sivamani, R. K. (2023). Soy Protein Containing Isoflavones Improves Facial Signs of Photoaging and Skin Hydration in Postmenopausal Women: Results of a Prospective Randomized Double-Blind Controlled Trial. Nutrients, 15(19), 4113. https://doi.org/10.3390/nu15194113







