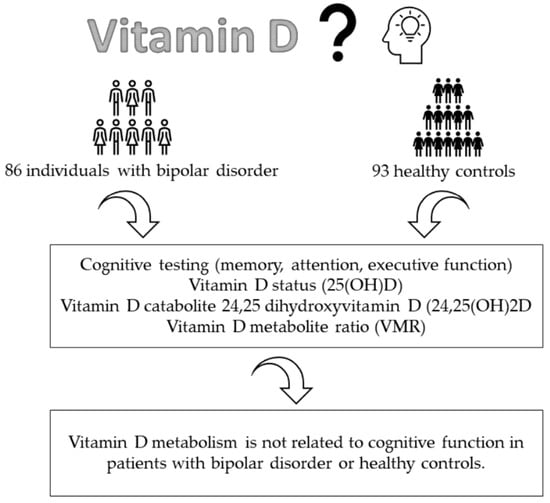
The Influence of Vitamin D Status on Cognitive Ability in Patients with Bipolar Disorder and Healthy Controls
Abstract
:1. Introduction
Aim of the Study
2. Materials and Methods
2.1. Participants and Study Design
2.2. Determination of Vitamin D Metabolites
2.3. Assessment Scales
2.4. Statistics
3. Results
3.1. Demographics and Sample Characteristics
3.2. Multiple Hierarchical Regressions
3.3. Patients with Bipolar Disorder
3.3.1. Attention
3.3.2. Memory
3.3.3. Executive Function
3.3.4. Healthy Controls
3.3.5. Multivariate Analysis of Covariance
4. Discussion
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rothenhäusler, H.B.; Täschner, K.L. Kompendium Praktische Psychiatrie, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Elias, L.R.; Miskowiak, K.W.; Vale, A.M.; Köhler, C.A.; Kjærstad, H.L.; Stubbs, B.; Kessing, L.V.; Vieta, E.; Maes, M.; Goldstein, B.I.; et al. Cognitive impairment in euthymic pediatric bipolar disorder: A systematic review and meta-analysis. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Bora, E. Neurocognitive features in clinical subgroups of bipolar disorder: A meta-analysis. J. Affect. Disord. 2018, 229, 125–134. [Google Scholar] [CrossRef]
- Martínez-Arán, A.; Vieta, E.; Reinares, M.; Colom, F.; Torrent, C.; Sánchez-Moreno, J.; Benabarre, A.; Goikolea, J.M.; Comes, M.; Salamero, M. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am. J. Psychiatry 2004, 161, 262–270. [Google Scholar] [CrossRef]
- Vrabie, M.; Marinescu, V.; Talaşman, A.; Tăutu, O.; Drima, E.; Micluţia, I. Cognitive impairment in manic bipolar patients: Important, understated, significant aspects. Ann. Gen. Psychiatry 2015, 14, 41. [Google Scholar] [CrossRef] [PubMed]
- Bora, E.; McIntyre, R.S.; Ozerdem, A. Neurocognitive and neuroimaging correlates of obesity and components of metabolic syndrome in bipolar disorder: A systematic review. Psychol. Med. 2019, 49, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Dalkner, N.; Bengesser, S.A.; Birner, A.; Fellendorf, F.T.; Fleischmann, E.; Großschädl, K.; Lenger, M.; Maget, A.; Platzer, M.; Queissner, R.; et al. Metabolic syndrome impairs executive function in bipolar disorder. Front. Neurosci. 2021, 15, 717824. [Google Scholar] [CrossRef]
- Simjanoski, M.; Patel, S.; De Boni, R.; Balanzá-Martínez, V.; Frey, B.N.; Minuzzi, L.; Kapczinski, F.; de Azevedo Cardoso, T. Lifestyle interventions for bipolar disorders: A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2023, 152, 105257. [Google Scholar] [CrossRef]
- Mayne, P.E.; Burne, T.H. Vitamin D in synaptic plasticity, cognitive function, and neuropsychiatric illness. Trends Neurosci. 2019, 42, 293–306. [Google Scholar] [CrossRef]
- Bivona, G.; Agnello, L.; Bellia, C.; Iacolino, G.; Scazzone, C.; Lo Sasso, B.; Ciaccio, M. Non-skeletal activities of vitamin D: From physiology to brain pathology. Medicina 2019, 55, 341. [Google Scholar] [CrossRef] [PubMed]
- Bosworth, C.R.; Levin, G.; Robinson-Cohen, C.; Hoofnagle, A.N.; Ruzinski, J.; Young, B.; Schwatrz, S.M.; Himmelfarb, J.; Kestenbaum, B.; de Boer, I.H. The serum 24,25-dihydroxyvitamin D concentration, a marker of vitamin D catabolism, is reduced in chronic kidney disease. Kidney Int. 2012, 82, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Cereda, G.; Enrico, P.; Cippolino, V.; Devecchio, G.; Brambilla, P. The role of vitamin D in bipolar disorder: Epidemiology and influence on disease activity. J. Affect. Disord. 2021, 278, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, A.; Maina, G.; Bolognesi, S.; Rosso, G.; Beccarini Crescenzi, B.; Zanobini, F.; Goracci, A.; Facchi, E.; Favaretto, E.; Baldini, I.; et al. Prevalence and correlates of vitamin D deficiency in a sample of 290 inpatients with mental illness. Front. Psychiatry 2019, 10, 167. [Google Scholar] [CrossRef]
- Rihal, V.; Kaur, A.; Singh, T.G.; Abdel-Daim, M.M. Therapeutic and mechanistic intervention of vitamin D in neuropsychiatric disorders. Psychiatry Res. 2022, 317, 114782. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Cui, X.; Eyles, D.W. Vitamin D and the central nervous system: Causative and preventative mechanisms in brain disorders. Nutrients 2022, 14, 4353. [Google Scholar] [CrossRef]
- Goodwill, A.M.; Szoeke, C. A systemic review and meta-analysis of the effect of low vitamin D on cognition. J. Am. Geriatr. Soc. 2017, 65, 2161–2168. [Google Scholar] [CrossRef]
- Pavlovic, A.; Abel, K.; Barlow, C.E.; Farrell, S.W.; Weiner, M.; DeFina, L.F. The association between serum vitamin d level and cognitive function in older adults: Cooper Center Longitudinal Study. Prev. Med. 2018, 113, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.L.C.; Lutsey, P.L.; Alonso, A.; Gottesman, R.F.; Sharrett, A.R.; Carson, K.A.; Gross, M.; Post, W.S.; Knopman, D.S.; Mosley, T.H.; et al. Vitamin D and cognitive function and dementia risk in a biracial cohort: The ARIC Brain MRI Study. Eur. J. Neurol. 2014, 21, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Maddock, J.; Zhou, A.; Cavadino, A.; Kuzma, E.; Bao, Y.; Smart, M.C.; Saum, K.-U.; Schöttker, B.; Engmann, J.; Kjaergaard, M.; et al. Vitamin D and cognitive function: A Mendelian randomisation study. Sci. Rep. 2017, 7, 13230. [Google Scholar] [CrossRef] [PubMed]
- Farrell, C.; Soldo, J.; Williams, P.; Herrmann, M. 25-Hydroxyvitamin D testing: Challenging the performance of current automated immunoassays. Clin. Chem. Lab. Med. 2012, 50, 1953–1963. [Google Scholar] [CrossRef] [PubMed]
- Zelzer, S.; Meinitzer, A.; Enko, D.; Simstich, S.; Le Goff, C.; Cavalier, E.; Herrmann, M.; Goessler, W. Simultaneous determination of 24, 25-and 25, 26-dihydroxyvitamin D3 in serum samples with liquid-chromatography mass spectrometry—A useful tool for the assessment of vitamin D metabolism. J. Chromatogr. B 2020, 1158, 122394. [Google Scholar] [CrossRef]
- Alonso, N.; Zelzer, S.; Eibinger, G.; Herrmann, M. Vitamin D metabolites: Analytical challenges and clinical relevance. Calcif. Tissue Int. 2023, 112, 158–177. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Talaei, S.A.; Djazayeri, A.; Salami, M. Vitamin D supplementation restores suppressed synaptic plasticity in Alzheimer’s disease. Nutr. Neurosci. 2014, 17, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, S.D.; Ruthazer, E.S.; Kennedy, T.E. Guiding synaptic plasticity: Novel roles for netrin-1 in synaptic plasticity and memory formation in the adult brain. J. Physiol. 2021, 599, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Reininghaus, E.Z.; McIntyre, R.S.; Reininghaus, B.; Geisler, S.; Bengesser, S.A.; Lackner, N.; Hecht, K.; Birner, A.; Kattnig, F.; Unterweger, R.; et al. Tryptophan breakdown is increased in euthymic overweight individuals with bipolar disorder: A preliminary report. Bipolar Disord. 2014, 16, 432–440. [Google Scholar] [CrossRef]
- Wittchen, H.U.; Wunderlich, U.; Gruschwitz, S.; Zaudig, M. SCID: Clinical Interview for DSM-IV (German Version); Hogrefe: Göttingen, Germany, 1997. [Google Scholar]
- Hamilton, M. Development of a rating scale for primary depressive illness. Br. J. Soc. Clin. Psychol. 1967, 6, 278–296. [Google Scholar] [CrossRef] [PubMed]
- Young, R.C.; Biggs, J.T.; Ziegler, V.E.; Meyer, D.A. A rating scale for mania: Reliability, validity and sensitivity. Br. J. Psychiatry 1978, 133, 429–435. [Google Scholar] [CrossRef]
- Ross, A.C.; Taylor, C.L.; Yaktine, A.L.; Del Valle, H.B. (Eds.) Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Herrmann, M.; Zelzer, S.; Cavalier, E.; Kleber, M.; Drexler-Herlmber, C.; Schlenke, P.; Curcic, P.; Keppel, M.H.; Enko, D.; Scharnagl, H.; et al. Functional assessment of vitamin D status by a novel metabolic approach: The low vitamin D profile concept. Clin. Chem. 2023; revision under review. [Google Scholar]
- Reitan, R.M. Trail Making Test: Manual for Administration, Scoring and Interpretation; Indiana University Medical Center: Indianapolis, IN, USA, 1958. [Google Scholar]
- Bäumler, G. Farbe-Wort-Interferenztest (FWIT) Nach J. R. Stroop; Handanweisung; Hogrefe: Göttingen, Germany, 1985. [Google Scholar]
- Niemann, H.; Sturm, W.; Thöne-Otto, A.I.T.; Willmes, K. CVLT California Verbal Learning Test. German Adaptation. Manual; Pearson Assessment: Frankfurt, Germany, 2008. [Google Scholar]
- Lehrl, S. Mehrfachwahl-Wortschatz-Intelligenztest MWT-B; Spitta: Balingen, Germany, 2005. [Google Scholar]
- Zelzer, S.; Hofer, E.; Meinitzer, A.; Fritz-Petrin, E.; Simstich, S.; Goessler, W.; Schmidt, R.; Herrmann, M. Association of vitamin D metabolites with cognitive function and brain atrophy in elderly individuals—The austrian stroke prevention study. Aging 2021, 13, 9455–9467. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, S.; Teng, Z.; Tan, Y.; Xu, X.; Yang, M.; Zhao, Z.; Liu, J.; Tang, H.; Xiang, H.; et al. Association between abnormal glycolipid level and cognitive dysfunction in drug-naïve patients with bipolar disorder. J. Affect. Disord. 2022, 297, 477–485. [Google Scholar] [CrossRef]
- Barbosa, I.G.; de Almeida Ferreira, R.; Rocha, N.P.; Mol, G.C.; Leite, F.D.M.C.; Bauer, I.E.; Teixeira, A.L. Predictors of cognitive performance in bipolar disorder: The role of educational degree and inflammatory markers. J. Psychiatr. Res. 2018, 106, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Jorde, R.; Sneve, M.; Figenschau, Y.; Svartberg, J.; Waterloo, K. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: Randomized double blind trial. J. Intern. Med. 2008, 264, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Huiberts, L.M.; Smolders, K.C. Effects of vitamin D on mood and sleep in the healthy population: Interpretations from the serotonergic pathway. Sleep Med. Rev. 2021, 55, 101379. [Google Scholar] [CrossRef]
- Paterson, A.; Parker, G. Lithium and cognition in those with bipolar disorder. Int. Clin. Psychopharmacol. 2017, 32, 57–62. [Google Scholar] [CrossRef]
- Holmes, M.K.; Erickson, K.; Luckenbaugh, D.A.; Drevets, W.C.; Bain, E.E.; Cannon, D.M.; Snow, J.; Sahakian, B.J.; Husseini, K.M.; Zarate, C.A. A comparison of cognitive functioning in medicated and unmedicated subjects with bipolar depression. Bipolar Disord. 2008, 10, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Kinuta, K.; Tanaka, H.; Moriwake, T.; Aya, K.; Kato, S.; Seino, Y. Vitamin D Is an Important Factor in Estrogen Biosynthesis of Both Female and Male Gonads. Endocrinology 2000, 141, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, M.; Boisen, I.M.; Mortensen, L.J.; Lanske, B.; Juul, A.; Blomberg Jensen, M. Reproductive Endocrinology of Vitamin D. Mol. Cell. Endocrinol. 2017, 453, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Bebbington, P.E.; Dunn, G.; Jenkins, R.; Lewis, G.; Brugha, T.; Farrell, M.; Meltzer, H. The Influence of Age and Sex on the Prevalence of Depressive Conditions: Report from the National Survey of Psychiatric Morbidity. Psychol. Med. 1998, 28, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, S.; Barbieri, V.; Di Pierro, A.M.; Rossi, F.; Widmann, T.; Lucchiari, M.; Pusceddu, I.; Pilz, S.; Obermayer-Pietsch, B.; Herrmann, M. LC–MS/MS based 25(OH)D status in a large Southern European outpatient cohort: Gender- and age-specific differences. Eur. J. Nutr. 2019, 58, 2511–2520. [Google Scholar] [CrossRef] [PubMed]
| Patients with BD (n = 86) | Healthy Controls (n = 93) | Differences | |
|---|---|---|---|
| Age (Years), M (±SD) | 45.17 (13.15) | 37.75 (15.33) | U = 2732.00 **, Z = −3.66 |
| Sex (n, %) | χ2 = 10.07 *, φ = 0.24 | ||
| Male Female | 47 (54.7%) 39 (45.3%) | 29 (31.2%) 64 (68.8%) | |
| Premorbid IQ, M (±SD) | 109.51 (15.23) | 112.18 (14.71) | U = 3579.50, Z = −1.22, p = 0.224 |
| YMRS, M (±SD) | 0.93 (1.88) | 0.12 (0.55) | U = 3082.50 **, Z = −4.13 |
| HAMD, M (±SD) | 4.63 (3.45) | 0.27 (0.95) | U = 902.50 **, Z = −9.78 |
| Vitamin D variables, (n, %) | |||
| 25(OH)D, M (±SD) | 56.37 (23.64) nmol/L | 57.29 (23.95) nmol/L | U = 3813.50, Z = −0.54 |
| 24,25(OH)2D3, M (±SD) | 3.59 (2.11) nmol/L | 4.02 (2.51) nmol/L | U = 3608.50, Z = −1.13 |
| VMR, M (±SD) | 6.31 (2.08) % | 6.91 (2.51) % | U = 3465.00, Z = −1.54 |
| 25(OH)D | χ2 = 0.32, φ = 0.04, p = 0.570 | ||
| <50 nmol/L >50 nmol/L | 35 (40.7%) 51 (59.3%) | 34 (36.6%) 59 (63.4%) | |
| 24,25(OH)2D3 | χ2 = 1.39, φ = 0.09, p = 0.239 | ||
| <3 nmol/L >3 nmol/L | 36 (41.9%) 50 (58.1%) | 31 (33.3%) 62 (66.7%) | |
| VMR (n, %) | χ2 = 0.05, φ = 0.02, p = 0.829 | ||
| <4% >4% | 13 (15.1%) 73 (84.9%) | 13 (14.0%) 80 (86.0%) | |
| Cognitive variables, M (±SD) | |||
| TMT-A (s) | 35.21 (11.98) | 26.04 (9.74) | U = 2082.50 **, Z = −5.53 |
| TMT-B (s) | 90.99 (51.14) | 57.12 (20.96) | U = 2036.00 **, Z = −5.67 |
| Stroop color word reading (s) | 33.15 (7.06) | 29.06 (4.63) | U = 2465.00 **, Z = −4.43 |
| Stroop color naming (s) | 51.70 (9.57) | 44.11 (6.92) | U = 2051.00 **, Z = −5.62 |
| Stroop interference (s) | 85.16 (24.33) | 67.67 (14.73) | U = 2054.00 **, Z = −5.62 |
| CVLT trial 1–5 | 49.74 (13.26) | 60.48 (11.80) | U = 2141.00 **, Z = −5.37 |
| CVLT short delay free recall | 10.02 (3.51) | 12.82 (2.96) | U = 2090.00 **, Z = −5.54 |
| CVLT short delay cued recall | 11.03 (3.31) | 13.27 (2.66) | U = 2363.00 **, Z = −4.76 |
| CVLT long delay free recall | 10.58 (3.57) | 13.24 (3.09) | U = 2182.50 **, Z = −5.28 |
| CVLT long delay cued recall | 11.34 (3.29) | 13.46 (2.91) | U = 2390.00 **, Z = −4.68 |
| Attention | Memory | Executive Function | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | 95%CI | β | t | p | B | 95%CI | β | t | p | B | 95%CI | β | t | p | ||
| Model 1 | Age | −0.02 | [−0.04, −0.01] | −0.28 | −2.70 | 0.008 | −0.02 | [−0.04, −0.01] | −0.30 | −2.83 | 0.006 | −0.03 | [−0.04, −0.01] | −0.35 | −3.39 | 0.001 |
| Model 2 | Age | −0.02 | [−0.04, −0.01] | −0.32 | −3.01 | 0.003 | −0.03 | [−0.04, −0.01] | −0.38 | −3.71 | <0.001 | −0.03 | [−0.05, −0.02] | −0.42 | −4.15 | <0.001 |
| Premorbid IQ | 0.01 | [−0.00, 0.02] | 0.16 | 1.49 | 0.140 | 0.02 | [0.01, 0.04] | 0.33 | 3.27 | 0.002 | 0.02 | [0.01, 0.03] | 0.30 | 2.93 | 0.004 | |
| Model 3 | Age | −0.2 | [−0.04, −0.01] | −0.31 | −2.86 | 0.005 | −0.03 | [−0.04, −0.01] | −0.37 | −3.58 | <0.001 | −0.03 | [−0.05, −0.02] | −0.42 | −4.06 | <0.001 |
| Premorbid IQ | 0.01 | [−0.01, 0.02] | 0.14 | 1.34 | 0.185 | 0.02 | [0.01, 0.03] | 0.32 | 3.13 | 0.002 | 0.02 | [0.01, 0.03] | 0.29 | 2.84 | 0.006 | |
| 25(OH)D | −0.26 | [−0.68, 0.16] | −0.13 | −1.22 | 0.226 | −0.19 | [−0.59, 0.22] | −0.09 | −0.92 | 0.359 | −0.09 | [−0.49, 0.32] | −0.04 | −0.42 | 0.674 | |
| Model 4 | Age | −0.02 | [−0.04, −0.01] | −0.31 | −2.91 | 0.005 | −0.03 | [−0.04, −0.01] | −0.37 | −3.56 | <0.001 | −0.03 | [−0.05, −0.02] | −0.42 | −4.11 | <0.001 |
| Premorbid IQ | 0.01 | [−0.01, 0.02] | 0.13 | 1.15 | 0.252 | 0.02 | [0.01, 0.04] | 0.32 | 3.09 | 0.003 | 0.02 | [0.00, 0.03] | 0.27 | 2.65 | 0.010 | |
| 25(OH)D | −0.05 | [−0.57, 0.47] | −0.03 | −0.20 | 0.841 | −0.19 | [−0.69, 0.31] | −0.09 | −0.75 | 0.453 | 0.11 | [−0.38, 0.61] | 0.06 | 0.46 | 0.649 | |
| 24,25(OH)2D3 | −0.35 | [−0.87, 0.17] | −0.17 | −1.35 | 0.181 | 0.01 | [−0.50, 0.50] | 0.01 | 0.020 | 0.985 | −0.34 | [−0.83, 0.15] | −0.17 | −1.37 | 0.176 | |
| Model 5 | Age | −0.02 | [−0.04, −0.01] | −0.31 | −2.89 | 0.005 | −0.03 | [−0.04, −0.01] | −0.37 | −3.54 | <0.001 | −0.03 | [−0.05, −0.02] | −0.42 | −4.10 | <0.001 |
| Premorbid IQ | 0.01 | [−0.01, 0.02] | 0.13 | 1.18 | 0.240 | 0.02 | [0.01, 0.04] | 0.33 | 3.13 | 0.002 | 0.02 | [0.01, 0.03] | 0.28 | 2.70 | 0.008 | |
| 25(OH)D | −0.03 | [−0.56, 0.49] | −0.02 | −0.13 | 0.899 | −0.16 | [−0.67, 0.34] | −0.08 | −0.64 | 0.523 | 0.14 | [−0.35, 0.64] | 0.07 | 0.57 | 0.569 | |
| 24,25(OH)2D3 | −0.29 | [−0.85, 0.27] | −0.14 | −1.02 | 0.313 | 0.10 | [−0.44, 0.64] | 0.05 | 0.36 | 0.722 | −0.24 | [−0.77, 0.30] | −0.12 | −0.89 | 0.375 | |
| VMR | −0.21 | [−0.87, 0.46] | −0.08 | −0.63 | 0.534 | −0.29 | [−0.93, 0.35] | −0.10 | −0.91 | 0.364 | −0.32 | [−0.95, 0.31] | −0.12 | −1.01 | 0.315 | |
| Patients with BD (n = 86) | Healthy Controls (n = 93) | ||
|---|---|---|---|
| Optimal | 49 (57.0%) | 62 (66.7%) | χ2 = 1.78, φ = 0.10, p = 0.182 |
| Non-optimal | 37 (43.0%) | 31 (33.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leser, B.; Dalkner, N.; Tmava-Berisha, A.; Fellendorf, F.T.; Unterrainer, H.-F.; Stross, T.; Maget, A.; Platzer, M.; Bengesser, S.A.; Häussl, A.; et al. The Influence of Vitamin D Status on Cognitive Ability in Patients with Bipolar Disorder and Healthy Controls. Nutrients 2023, 15, 4111. https://doi.org/10.3390/nu15194111
Leser B, Dalkner N, Tmava-Berisha A, Fellendorf FT, Unterrainer H-F, Stross T, Maget A, Platzer M, Bengesser SA, Häussl A, et al. The Influence of Vitamin D Status on Cognitive Ability in Patients with Bipolar Disorder and Healthy Controls. Nutrients. 2023; 15(19):4111. https://doi.org/10.3390/nu15194111
Chicago/Turabian StyleLeser, Bernadette, Nina Dalkner, Adelina Tmava-Berisha, Frederike T. Fellendorf, Human-Friedrich Unterrainer, Tatjana Stross, Alexander Maget, Martina Platzer, Susanne A. Bengesser, Alfred Häussl, and et al. 2023. "The Influence of Vitamin D Status on Cognitive Ability in Patients with Bipolar Disorder and Healthy Controls" Nutrients 15, no. 19: 4111. https://doi.org/10.3390/nu15194111
APA StyleLeser, B., Dalkner, N., Tmava-Berisha, A., Fellendorf, F. T., Unterrainer, H.-F., Stross, T., Maget, A., Platzer, M., Bengesser, S. A., Häussl, A., Zwigl, I., Birner, A., Queissner, R., Stix, K., Wels, L., Schönthaler, E. M. D., Lenger, M., Schwerdtfeger, A. R., Zelzer, S., ... Reininghaus, E. Z. (2023). The Influence of Vitamin D Status on Cognitive Ability in Patients with Bipolar Disorder and Healthy Controls. Nutrients, 15(19), 4111. https://doi.org/10.3390/nu15194111