Isolation, Identification, Activity Evaluation, and Mechanism of Action of Neuroprotective Peptides from Walnuts: A Review
Abstract
:1. Introduction
2. Preparation, Isolation, Purification, and Identification of Walnut Peptides
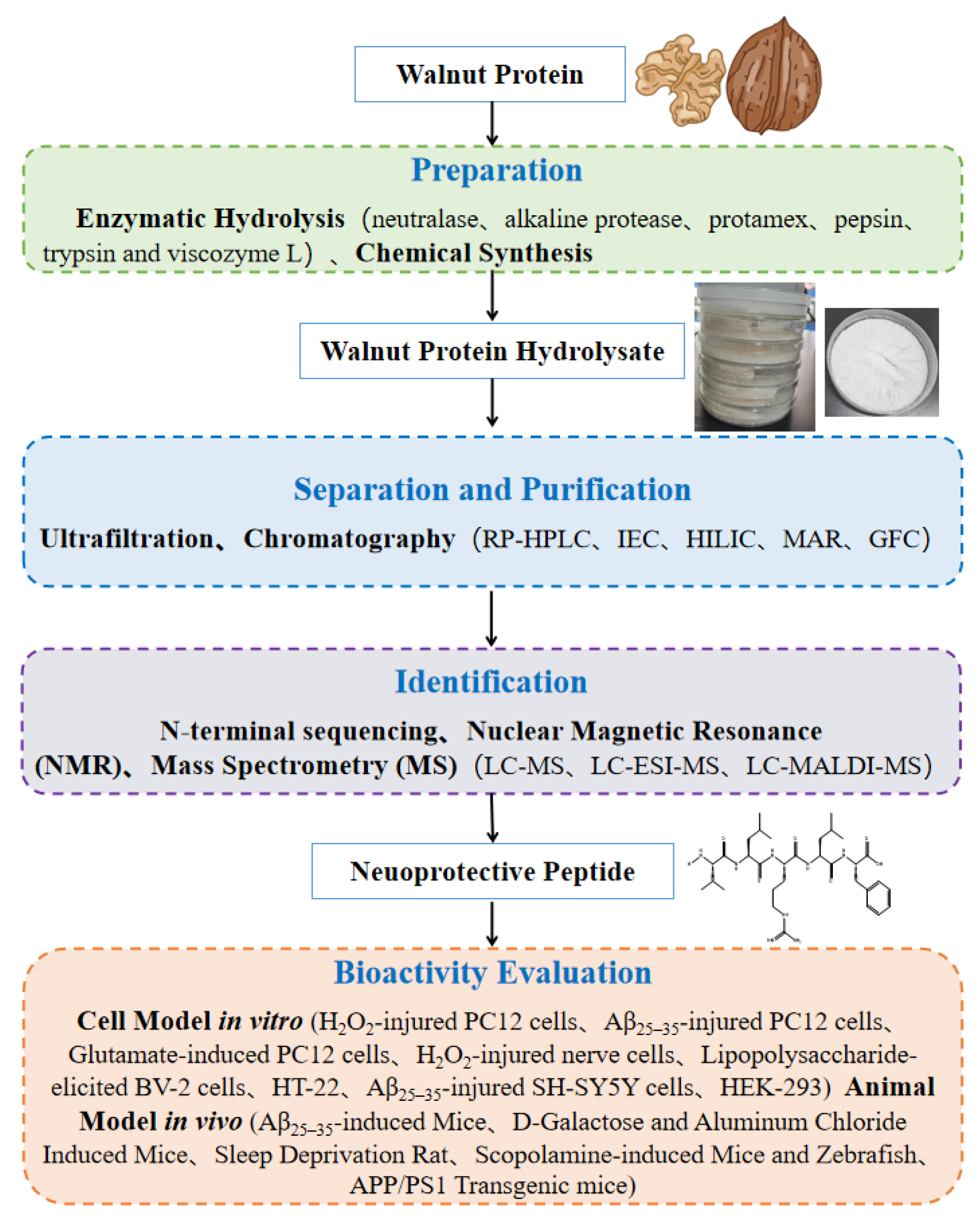
2.1. Preparation of Walnut Peptides
2.2. Separation, Purification and Structural Identification of Walnut Peptides
3. Assessment of Neuroprotective Effects of Walnut Peptides
3.1. In Vitro Assays
3.2. In Vivo Assays
4. Mechanisms of the Neuroprotective Effects of Walnut Peptides
4.1. Antioxidant Effects
4.2. Anti-Inflammatory Effects
4.3. Autophagy Induction
4.4. Regulation of the Cholinergic System
4.5. Improvement of the Gut Microbiota
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abel, T.; Lattal, K.M. Molecular Mechanisms of Memory Acquisition, Consolidation and Retrieval. Curr. Opin. Neurobiol. 2001, 11, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia Prevention, Intervention, and Care: 2020 Report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.; Zubair, H.; Pursell, S.; Shahab, M. Neurodegenerative Diseases: Regenerative Mechanisms and Novel Therapeutic Approaches. Brain Sci. 2018, 8, 177. [Google Scholar] [CrossRef] [PubMed]
- Schauer, E.; Wronski, R.; Patockova, J.; Moessler, H.; Doppler, E.; Hutter-Paier, B.; Windisch, M. Neuroprotection of Cerebrolysin in Tissue Culture Models of Brain Ischemia: Post Lesion Application Indicates a Wide Therapeutic Window. J. Neural Transm. 2006, 113, 855–868. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.S.; Muresanu, D.F.; Nozari, A.; Lafuente, J.V.; Tian, Z.R.; Ozkizilcik, A.; Manzhulo, I.; Mössler, H.; Sharma, A. Nanowired Delivery of Cerebrolysin with Neprilysin and P-Tau Antibodies Induces Superior Neuroprotection in Alzheimer’s Disease. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2019; Volume 245, pp. 145–200. [Google Scholar]
- Hartbauer, M.; Hutter-Paier, B.; Windisch, M. Effects of Cerebrolysin on the Outgrowth and Protection of Processes of Cultured Brain Neurons. J. Neural Transm. 2001, 108, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Perlikowska, R. Whether Short Peptides Are Good Candidates for Future Neuroprotective Therapeutics? Peptides 2021, 140, 170528. [Google Scholar] [CrossRef] [PubMed]
- Cahoon, D.; Shertukde, S.P.; Avendano, E.E.; Tanprasertsuk, J.; Scott, T.M.; Johnson, E.J.; Chung, M.; Nirmala, N. Walnut Intake, Cognitive Outcomes and Risk Factors: A Systematic Review and Meta-Analysis. Ann. Med. 2021, 53, 972–998. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Sun, Y.; Zhang, W.; Huang, X.; Xue, R.; Zhang, Y.; Wang, Y. Walnut Diets Up-Regulate the Decreased Hippocampal Neurogenesis and Age-Related Cognitive Dysfunction in d-Galactose Induced Aged Rats. Food Funct. 2018, 9, 4755–4762. [Google Scholar] [CrossRef]
- Pei, Q.; Liu, Y.; Peng, S. Fatty Acid Profiling in Kernels Coupled with Chemometric Analyses as a Feasible Strategy for the Discrimination of Different Walnuts. Foods 2022, 11, 500. [Google Scholar] [CrossRef]
- Lee, M.J.; Park, S.H.; Han, J.H.; Hong, Y.K.; Hwang, S.; Lee, S.; Kim, D.; Han, S.Y.; Kim, E.S.; Cho, K.S. The Effects of Hempseed Meal Intake and Linoleic Acid on Drosophila Models of Neurodegenerative Diseases and Hypercholesterolemia. Mol. Cells 2011, 31, 337–342. [Google Scholar] [CrossRef]
- Gao, H.; Yan, P.; Zhang, S.; Huang, H.; Huang, F.; Sun, T.; Deng, Q.; Huang, Q.; Chen, S.; Ye, K.; et al. Long-Term Dietary Alpha-Linolenic Acid Supplement Alleviates Cognitive Impairment Correlate with Activating Hippocampal CREB Signaling in Natural Aging Rats. Mol. Neurobiol. 2016, 53, 4772–4786. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhao, Z.; Dai, S.; Che, X.; Liu, W. Identification and Quantification of Bioactive Compounds in Diaphragma juglandis Fructus by UHPLC-Q-Orbitrap HRMS and UHPLC-MS/MS. J. Agric. Food Chem. 2019, 67, 3811–3825. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhou, Y.; Sheng, S.; Li, J.; Wang, G.; Zhang, F. Ellagic Acid Protects Dopamine Neurons via Inhibition of NLRP3 Inflammasome Activation in Microglia. Oxid. Med. Cell. Longev. 2020, 2020, 2963540. [Google Scholar] [CrossRef] [PubMed]
- Aron, P.M.; Kennedy, J.A. Flavan-3-Ols: Nature, Occurrence and Biological Activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Shi, C.; Wang, M.; Zhou, M.; Liang, M.; Zhang, T.; Yuan, E.; Wang, Z.; Yao, M.; Ren, J. Tryptophan Residue Enhances in Vitro Walnut Protein-Derived Peptides Exerting Xanthine Oxidase Inhibition and Antioxidant Activities. J. Funct. Foods 2019, 53, 276–285. [Google Scholar] [CrossRef]
- Wang, C.; Tu, M.; Wu, D.; Chen, H.; Chen, C.; Wang, Z.; Jiang, L. Identification of an ACE-Inhibitory Peptide from Walnut Protein and Its Evaluation of the Inhibitory Mechanism. Int. J. Mol. Sci. 2018, 19, 1156. [Google Scholar] [CrossRef]
- Ma, S.; Huang, D.; Zhai, M.; Yang, L.; Peng, S.; Chen, C.; Feng, X.; Weng, Q.; Zhang, B.; Xu, M. Isolation of a Novel Bio-Peptide from Walnut Residual Protein Inducing Apoptosis and Autophagy on Cancer Cells. BMC Complement. Altern. Med. 2015, 15, 413. [Google Scholar] [CrossRef]
- Wang, Q.; Zhi, T.; Han, P.; Li, S.; Xia, J.; Chen, Z.; Wang, C.; Wu, Y.; Jia, Y.; Ma, A. Potential Anti-Inflammatory Activity of Walnut Protein Derived Peptide Leucine-Proline-Phenylalanine in Lipopolysaccharides-Irritated RAW264.7 Cells. Food Agric. Immunol. 2021, 32, 663–678. [Google Scholar] [CrossRef]
- Wang, J.; Du, K.; Fang, L.; Liu, C.; Min, W.; Liu, J. Evaluation of the Antidiabetic Activity of Hydrolyzed Peptides Derived from Juglans mandshurica Maxim. Fruits in Insulin-Resistant HepG2 Cells and Type 2 Diabetic Mice. J. Food Biochem. 2018, 42, e12518. [Google Scholar]
- Fang, L.; Ren, D.; Cui, L.; Liu, C.; Wang, J.; Liu, W.; Min, W.; Liu, J. Antifatigue, Antioxidant and Immunoregulatory Effects of Peptides Hydrolyzed from Manchurian Walnut (Juglans mandshurica Maxim.) on Mice. Grain Oil Sci. Technol. 2018, 1, 44–52. [Google Scholar] [CrossRef]
- Feng, L.; Wang, X.; Peng, F.; Liao, J.; Nai, Y.; Lei, H.; Li, M.; Xu, H. Walnut Protein Hydrolysates Play a Protective Role on Neurotoxicity Induced by D-Galactose and Aluminum Chloride in Mice. Molecules 2018, 23, 2308. [Google Scholar] [CrossRef] [PubMed]
- Katayama, S.; Nakamura, S. Emerging Roles of Bioactive Peptides on Brain Health Promotion. Int. J. Food Sci. Technol. 2019, 54, 1949–1955. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Duan, Y.; Ma, H. Plant Protein-Derived Antioxidant Peptides: Isolation, Identification, Mechanism of Action and Application in Food Systems: A Review. Trends Food Sci. Technol. 2020, 105, 308–322. [Google Scholar] [CrossRef]
- Li, H.; Gao, J.; Zhao, F.; Liu, X.; Ma, B. Bioactive Peptides from Edible Mushrooms—The Preparation, Mechanisms, Structure—Activity Relationships and Prospects. Foods 2023, 12, 2935. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, H.; Xing, R.; Li, P. Characterization, Preparation, and Purification of Marine Bioactive Peptides. BioMed Res. Int. 2017, 2017, 9746720. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Yu, Q.; Shen, Y.; Chu, Q.; Chen, G.; Fen, S.; Yang, M.; Yuan, L.; McClements, D.J.; Sun, Q. Production, Bioactive Properties, and Potential Applications of Fish Protein Hydrolysates: Developments and Challenges. Trends Food Sci. Technol. 2021, 110, 687–699. [Google Scholar] [CrossRef]
- Kleekayai, T.; Le Gouic, A.V.; Deracinois, B.; Cudennec, B.; FitzGerald, R.J. In Vitro Characterisation of the Antioxidative Properties of Whey Protein Hydrolysates Generated under pH- and Non pH-Controlled Conditions. Foods 2020, 9, 582. [Google Scholar] [CrossRef]
- Jia, L.; Wang, L.; Liu, C.; Liang, Y.; Lin, Q. Bioactive Peptides from Foods: Production, Function, and Application. Food Funct. 2021, 12, 7108–7125. [Google Scholar] [CrossRef]
- Yang, F.; Chen, X.; Huang, M.; Yang, Q.; Cai, X.; Chen, X.; Du, M.; Huang, J.; Wang, S. Molecular Characteristics and Structure–Activity Relationships of Food-Derived Bioactive Peptides. J. Integr. Agric. 2021, 20, 2313–2332. [Google Scholar] [CrossRef]
- Chen, N.; Yang, H.; Sun, Y.; Niu, J.; Liu, S. Purification and Identification of Antioxidant Peptides from Walnut (Juglans regia L.) Protein Hydrolysates. Peptides 2012, 38, 344–349. [Google Scholar] [CrossRef]
- Gu, X.; Hou, Y.-K.; Li, D.; Wang, J.-Z.; Wang, F.-J. Separation, Purification, and Identification of Angiotensin I–Converting Enzyme Inhibitory Peptides from Walnut (Juglans regia L.) Hydrolyzate. Int. J. Food Prop. 2015, 18, 266–276. [Google Scholar] [CrossRef]
- Li, T.; Lin, L.; Li, C.; Zheng, J.; Chen, B.; Shen, Y.; Ren, D. Amelioration of Walnut-Derived Novel Peptides against d-Galactose-Induced Cognitive Impairment by Modulating the Gut Microbiota Composition. Food Funct. 2023, 14, 4228–4241. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Su, G.; Zhang, Q.; Zhao, T.; Liu, Y.; Zheng, L.; Zhao, M. Walnut (Juglans regia) Peptides Reverse Sleep Deprivation-Induced Memory Impairment in Rat via Alleviating Oxidative Stress. J. Agric. Food Chem. 2018, 66, 10617–10627. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Chen, D.; Qu, M.; Yu, L.; Mo, Z. Optimization of Preparation Process of Walnut Peptide and Its Improvement of Memory Function. Sci. Technol. Food Ind. 2021, 42, 135–141. [Google Scholar]
- Wang, S.; Su, G.; Zhang, X.; Song, G.; Zhang, L.; Zheng, L.; Zhao, M. Characterization and Exploration of Potential Neuroprotective Peptides in Walnut (Juglans regia) Protein Hydrolysate against Cholinergic System Damage and Oxidative Stress in Scopolamine-Induced Cognitive and Memory Impairment Mice and Zebrafish. J. Agric. Food Chem. 2021, 69, 2773–2783. [Google Scholar] [CrossRef] [PubMed]
- Piovesana, S.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Montone, C.M.; Zenezini Chiozzi, R.; Laganà, A. Recent Trends and Analytical Challenges in Plant Bioactive Peptide Separation, Identification and Validation. Anal. Bioanal. Chem. 2018, 410, 3425–3444. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; An, Q.; Le, L.; Geng, F.; Jiang, L.; Yan, J.; Xiang, D.; Peng, L.; Zou, L.; Zhao, G.; et al. Prospects of Cereal Protein-Derived Bioactive Peptides: Sources, Bioactivities Diversity, and Production. Crit. Rev. Food Sci. Nutr. 2022, 62, 2855–2871. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, M.; Lin, L.; Wang, J.; Sun-Waterhouse, D.; Dong, Y.; Zhuang, M.; Su, G. Identification of Antioxidative Peptides from Defatted Walnut Meal Hydrolysate with Potential for Improving Learning and Memory. Food Res. Int. 2015, 78, 216–223. [Google Scholar] [CrossRef]
- Feng, L.; Peng, F.; Wang, X.; Li, M.; Lei, H.; Xu, H. Identification and Characterization of Antioxidative Peptides Derived from Simulated In Vitro Gastrointestinal Digestion of Walnut Meal Proteins. Food Res. Int. 2019, 116, 518–526. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Sánchez, A.; Ramos, Y.; Schmidt, A.; Müller, M.; Betancourt, L.; González, L.J.; Vera, R.; Padron, G.; Besada, V. In Silico Analysis of Accurate Proteomics, Complemented by Selective Isolation of Peptides. J. Proteom. 2011, 74, 2071–2082. [Google Scholar] [CrossRef]
- Gagnon, H.; Franck, J.; Wisztorski, M.; Day, R.; Fournier, I.; Salzet, M. TARGETED MASS Spectrometry Imaging: Specific Targeting Mass Spectrometry Imaging Technologies from History to Perspective. Prog. Histochem. Cytochem. 2012, 47, 133–174. [Google Scholar] [CrossRef] [PubMed]
- Girolamo, F.; Lante, I.; Muraca, M.; Putignani, L. The Role of Mass Spectrometry in the “Omics” Era. Curr. Org. Chem. 2013, 17, 2891–2905. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhong, C.; Zeng, G.; Zhang, X.; Xiang, L.; Wan, C.; Yu, Y. Identification and Characterization of a Novel Tetrapeptide from Enzymatic Hydrolysates of Baijiu Byproduct. Food Sci. Hum. Wellness 2022, 11, 1641–1649. [Google Scholar] [CrossRef]
- You, L.; Zhao, M.; Regenstein, J.; Ren, J. Purification and Identification of Antioxidative Peptides from Loach (Misgurnus anguillicaudatus) Protein Hydrolysate by Consecutive Chromatography and Electrospray Ionization-Mass Spectrometry. Food Res. Int. 2010, 43, 1167–1173. [Google Scholar] [CrossRef]
- Gouda, K.; Gowda, L.; Rao, A.; Prakash, V. Angiotensin I-Converting Enzyme Inhibitory Peptide Derived from Glycinin, the 11S Globulin of Soybean (Glycine max). J. Agric. Food Chem. 2006, 54, 4568–4573. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Zhang, L.; Song, W.; Zhang, C.; Hua, Y.; Chen, Y.; Li, X. Separation, Identification and Molecular Binding Mechanism of Dipeptidyl Peptidase IV Inhibitory Peptides Derived from Walnut (Juglans regia L.) Protein. Food Chem. 2021, 347, 129062. [Google Scholar] [CrossRef]
- Sheng, J.; Yang, X.; Chen, J.; Peng, T.; Yin, X.; Liu, W.; Liang, M.; Wan, J.; Yang, X. Antioxidative Effects and Mechanism Study of Bioactive Peptides from Defatted Walnut (Juglans regia L.) Meal Hydrolysate. J. Agric. Food Chem. 2019, 67, 3305–3312. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Guo, Y.; Ma, H. Production, Bioactivities and Bioavailability of Bioactive Peptides Derived from Walnut Origin by-Products: A Review. Crit. Rev. Food Sci. Nutr. 2022, 1–16. [Google Scholar] [CrossRef]
- Li, X.; Guo, M.; Chi, J.; Ma, J. Bioactive Peptides from Walnut Residue Protein. Molecules 2020, 25, 1285. [Google Scholar] [CrossRef]
- Duff, K.; Suleman, F. Transgenic Mouse Models of Alzheimer’s Disease: How Useful Have They Been for Therapeutic Development? Brief. Funct. Genom. Proteom. 2004, 3, 47–59. [Google Scholar] [CrossRef]
- Zhao, T.; Zheng, L.; Zhang, Q.; Wang, S.; Zhao, Q.; Su, G.; Zhao, M. Stability towards the Gastrointestinal Simulated Digestion and Bioactivity of PAYCS and Its Digestive Product PAY with Cognitive Improving Properties. Food Funct. 2019, 10, 2439–2449. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.; Potashkin, J. Shared Dysregulated Pathways Lead to Parkinson’s Disease and Diabetes. Trends Mol. Med. 2013, 19, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun-Waterhouse, D.; Neil Waterhouse, G.I.; Zheng, L.; Su, G.; Zhao, M. Effects of Food-Derived Bioactive Peptides on Cognitive Deficits and Memory Decline in Neurodegenerative Diseases: A Review. Trends Food Sci. Technol. 2021, 116, 712–732. [Google Scholar] [CrossRef]
- Das, K. Assessment of PC12 Cell Differentiation and Neurite Growth: A Comparison of Morphological and Neurochemical Measures. Neurotoxicol. Teratol. 2004, 26, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wang, J.; Lu, H.; Fang, L.; Qin, H.; Liu, C.; Min, W. Neuroprotection by Walnut-Derived Peptides through Autophagy Promotion via Akt/mTOR Signaling Pathway against Oxidative Stress in PC12 Cells. J. Agric. Food Chem. 2020, 68, 3638–3648. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wang, J.; Chen, A.; Liu, J.; Feng, X.; Shao, L. Involvement of PINK1/Parkin-Mediated Mitophagy in ZnO Nanoparticle-Induced Toxicity in BV-2 Cells. Int. J. Nanomed. 2017, 12, 1891–1903. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Qin, H.; Wu, D.; Liu, C.; Fang, L.; Wang, J.; Liu, X.; Min, W. Walnut Peptide WEKPPVSH in Alleviating Oxidative Stress and Inflammation in Lipopolysaccharide-Activated BV-2 Microglia via the Nrf2/HO-1 and NF-ĸB/P38 MAPK Pathways. J. Biosci. Bioeng. 2021, 132, 496–504. [Google Scholar] [CrossRef]
- Morroni, F.; Sita, G.; Djemil, A.; D’Amico, M.; Pruccoli, L.; Cantelli-Forti, G.; Hrelia, P.; Tarozzi, A. Comparison of Adaptive Neuroprotective Mechanisms of Sulforaphane and Its Interconversion Product Erucin in In Vitro and In Vivo Models of Parkinson’s Disease. J. Agric. Food Chem. 2018, 66, 856–865. [Google Scholar] [CrossRef]
- Feng, L.; Wu, Y.; Wang, J.; Han, Y.; Huang, J.; Xu, H. Neuroprotective Effects of a Novel Tetrapeptide SGGY from Walnut against H2O2-Stimulated Oxidative Stress in SH-SY5Y Cells: Possible Involved JNK, P38 and Nrf2 Signaling Pathways. Foods 2023, 12, 1490. [Google Scholar] [CrossRef]
- Sreenivasamurthy, S.; Laul, M.; Zhao, N.; Kim, T.; Zhu, D. Current Progress of Cerebral Organoids for Modeling Alzheimer’s Disease Origins and Mechanisms. Bioeng. Transl. Med. 2023, 8, e10378. [Google Scholar] [CrossRef]
- Koss, E.; Patterson, M.; Oenby, R.; Stuckey, J.; Whitehouse, P. Memory Evaluation in Alzheimer’s Disease Caregivers’ Appraisals and Objective Testing. Arch. Neurol. 1993, 50, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Pallas, M.; Camins, A.; Smith, M.; Perry, G.; Lee, H.; Casadesus, G. From Aging to Alzheimer’s Disease: Unveiling “The Switch” with the Senescence-Accelerated Mouse Model (SAMP8). J. Alzheimer’s Dis. 2008, 15, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Disterhoft, J.; Moyer, J.; Thompson, L. The Calcium Rationale in Aging and Alzheimer’s Disease Evidence from an Animal-Model of Normal Aging. In Calcium Hypothesis of Aging and Dementia; Disterhoft, J., Gispen, W., Traber, J., Khachaturian, Z., Eds.; The New York Academy of Sciences: New York, NY, USA, 1994; Volume 747, pp. 382–406. ISBN 0077-8923. [Google Scholar]
- Dang, Q.; Wu, D.; Li, Y.; Fang, L.; Liu, C.; Wang, X.; Liu, X.; Min, W. Walnut-Derived Peptides Ameliorate d-Galactose-Induced Memory Impairments in a Mouse Model via Inhibition of MMP-9-Mediated Blood–Brain Barrier Disruption. Food Res. Int. 2022, 162, 112029. [Google Scholar] [CrossRef] [PubMed]
- Tamayev, R.; Matsuda, S.; Giliberto, L.; Arancio, O.; D’Adamio, L. APP Heterozygosity Averts Memory Deficit in Knockin Mice Expressing the Danish Dementia BRI2 Mutant. EMBO J. 2011, 30, 2501–2509. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Amakye, W.; Guo, L.; Gong, C.; Zhao, Y.; Yao, M.; Ren, J. Walnut-Derived Peptide PW5 Ameliorates Cognitive Impairments and Alters Gut Microbiota in APP/PS1 Transgenic Mice. Mol. Nutr. Food Res. 2019, 63, 1900326. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, A.; Gupta, S.; Dwivedi, S.; Kumar, D.; Shaikh, M.; Negi, A. Preclinical Models for Alzheimer’s Disease: Past, Present, and Future Approaches. ACS Omega 2022, 7, 47504–47517. [Google Scholar] [CrossRef] [PubMed]
- Geng, M.; Zhao, F.; Lu, H.; Fang, L.; Wang, J.; Liu, C.; Min, W. Insights into the Hippocampus Proteome and Phosphorylation Modification Alterations in C57BL/6 Revealed the Memory Improvement Mechanisms of a Walnut-Derived Peptide. Food Res. Int. 2022, 156, 111311. [Google Scholar] [CrossRef]
- Wong, L.-W.; Tann, J.Y.; Ibanez, C.F.; Sajikumar, S. The P75 Neurotrophin Receptor Is an Essential Mediator of Impairments in Hippocampal-Dependent Associative Plasticity and Memory Induced by Sleep Deprivation. J. Neurosci. 2019, 39, 5452–5465. [Google Scholar] [CrossRef]
- Irwin, M.; Vitiello, M. Implications of Sleep Disturbance and Inflammation for Alzheimer’s Disease Dementia. Lancet Neurol. 2019, 18, 296–306. [Google Scholar] [CrossRef]
- Poovaiah, N.; Davoudi, Z.; Peng, H.; Schlichtmann, B.; Mallapragada, S.; Narasimhan, B.; Wang, Q. Treatment of Neurodegenerative Disorders through the Blood-Brain Barrier Using Nanocarriers. Nanoscale 2018, 10, 16962–16983. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, G. Mitochondrial Dysfunction in Parkinson’s Disease. Transl. Neurodegener. 2016, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Domanskyi, A.; Parlato, R. Oxidative Stress in Neurodegenerative Diseases. Antioxidants 2022, 11, 504. [Google Scholar] [CrossRef] [PubMed]
- Filosto, M.; Scarpelli, M.; Cotelli, M.; Vielmi, V.; Todeschini, A.; Gregorelli, V.; Tonin, P.; Tomelleri, G.; Padovani, A. The Role of Mitochondria in Neurodegenerative Diseases. J. Neurol. 2011, 258, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Martin, L. Neuronal Cell Death in Nervous System Development, Disease, and Injury (Review). Int. J. Mol. Med. 2001, 7, 455–478. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, J.; Skach, W. Protein Folding and Quality Control in the Endoplasmic Reticulum: Recent Lessons from Yeast and Mammalian Cell Systems. Curr. Opin. Cell Biol. 2011, 23, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J. Oxidative Stress in Neurodegeneration: Cause or Consequence? Nat. Med. 2004, 10, S18–S25. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Zhang, L.; Yu, H.; Meng, J.; Sun, Z.; Lu, R. Protective Effect of Whey Protein Hydrolysates on H2O2-Induced PC12 Cells Oxidative Stress via a Mitochondria-Mediated Pathway. Food Chem. 2013, 141, 847–852. [Google Scholar] [CrossRef]
- Hsieh, H.; Yang, C. Role of Redox Signaling in Neuroinflammation and Neurodegenerative Diseases. BioMed Res. Int. 2013, 2013, 484613. [Google Scholar] [CrossRef]
- Jia, S.; Lu, Z.; Gao, Z.; An, J.; Wu, X.; Li, X.; Dai, X.; Zheng, Q.; Sun, Y. Chitosan Oligosaccharides Alleviate Cognitive Deficits in an Amyloid-Beta(1-42)-Induced Rat Model of Alzheimer’s Disease. Int. J. Biol. Macromol. 2016, 83, 416–425. [Google Scholar] [CrossRef]
- Cho, C.; Kim, E.; Kim, J.; Choi, S.; Yang, S.; Cho, S. N-Adamantyl-4-Methylthiazol-2-Amine Suppresses Amyloid Beta-Induced Neuronal Oxidative Damage in Cortical Neurons. Free Radic. Res. 2016, 50, 678–690. [Google Scholar] [CrossRef]
- Sheng, J.; Yang, X.; Liu, Q.; Luo, H.; Yin, X.; Liang, M.; Liu, W.; Lan, X.; Wan, J.; Yang, X. Coadministration with Tea Polyphenols Enhances the Neuroprotective Effect of Defatted Walnut Meal Hydrolysate against Scopolamine-Induced Learning and Memory Deficits in Mice. J. Agric. Food Chem. 2020, 68, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, S.; Wang, Y.; Chen, S.; Yao, W.; Gao, X. Neuroprotective Effects of Silk Fibroin Hydrolysate against Aβ(25-35) Induced Cytotoxicity in SH-SY5Y Cells and Primary Hippocampal Neurons by Regulating ROS Inactivation of PP2A. J. Funct. Foods 2018, 45, 100–109. [Google Scholar] [CrossRef]
- Martinon, F. Signaling by ROS Drives Inflammasome Activation. Eur. J. Immunol. 2010, 40, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Cortés, N.; Andrade, V.; Guzmán-Martínez, L.; Estrella, M.; Maccioni, R. Neuroimmune Tau Mechanisms: Their Role in the Progression of Neuronal Degeneration. Int. J. Mol. Sci. 2018, 19, 956. [Google Scholar] [CrossRef]
- Alvarez, X.A.; Lombardi, V.R.; Fernandez-Novoa, L.; Garcia, M.; Sampedro, C.; Cagiao, A.; Cacabelos, R.; Windisch, M. Cerebrolysin Reduces Microglial Activation In Vivo and In Vitro: A Potential Mechanism of Neuroprotection. J. Neural Transm. Suppl. 2000, 59, 281–292. [Google Scholar] [PubMed]
- Lv, Y.; Wei, K.; Meng, X.; Huang, Y.; Zhang, T.; Li, Z. Separation and Identification of Iron-Chelating Peptides from Defatted Walnut Flake by nanoLC-ESI-MS/MS and de Novo Sequencing. Process Biochem. 2017, 59, 223–228. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, L.; Zhao, T.; Zhang, Q.; Liu, Y.; Sun, B.; Su, G.; Zhao, M. Inhibitory Effects of Walnut (Juglans regia) Peptides on Neuroinflammation and Oxidative Stress in Lipopolysaccharide-Induced Cognitive Impairment Mice. J. Agric. Food Chem. 2020, 68, 2381–2392. [Google Scholar] [CrossRef]
- Liu, C.; Guo, Y.; Zhao, F.; Qin, H.; Lu, H.; Fang, L.; Wang, J.; Min, W. Potential Mechanisms Mediating the Protective Effects of a Peptide from Walnut (Juglans mandshurica Maxim.) against Hydrogen Peroxide Induced Neurotoxicity in PC12 Cells. Food Funct. 2019, 10, 3491–3501. [Google Scholar] [CrossRef]
- Reddy, P.H.; Oliver, D.M. Amyloid Beta and Phosphorylated Tau-Induced Defective Autophagy and Mitophagy in Alzheimer’s Disease. Cells 2019, 8, 488. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Sun, M. Autophagy and Alzheimer’s Disease. Cell. Mol. Neurobiol. 2017, 37, 377–388. [Google Scholar] [CrossRef]
- Perez Ortiz, J.M.; Swerdlow, R.H. Mitochondrial Dysfunction in Alzheimer’s Disease: Role in Pathogenesis and Novel Therapeutic Opportunities. Br. J. Pharmacol. 2019, 176, 3489–3507. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fang, L.; Lu, H.; Liu, C.; Wang, J.; Wu, D.; Min, W. Walnut-Derived Peptide Enhances Mitophagy via JNK-Mediated PINK1 Activation to Reduce Oxidative Stress in HT-22 Cells. J. Agric. Food Chem. 2022, 70, 2630–2642. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Liu, C.; Fang, L.; Lu, H.; Wang, J.; Gao, Y.; Gabbianelli, R.; Min, W. Walnut-Derived Peptide Activates PINK1 via the NRF2/KEAP1/HO-1 Pathway, Promotes Mitophagy, and Alleviates Learning and Memory Impairments in a Mice Model. J. Agric. Food Chem. 2021, 69, 2758–2772. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhou, Y.; Ban, Y.; Mi, J.; He, Y.; Li, X.; Liu, Z.; Wang, K.; Zhu, G.; Liu, W.; et al. Development of Naringenin-O-Alkylamine Derivatives as Multifunctional Agents for the Treatment of Alzheimer’s Disease. J. Enzyme Inhib. Med. Chem. 2022, 37, 792–816. [Google Scholar] [CrossRef] [PubMed]
- Drachman, D.A.; Leavitt, J. Human Memory and the Cholinergic System: A Relationship to Aging? Arch. Neurol. 1974, 30, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Rodriguez, J.J.; Pacheco-Herrero, M.; Thyssen, D.; Murillo-Carretero, M.I.; Berrocoso, E.; Spires-Jones, T.L.; Bacskai, B.J.; Garcia-Alloza, M. Rapid β-Amyloid Deposition and Cognitive Impairment after Cholinergic Denervation in APP/PS1 Mice. J. Neuropathol. Exp. Neurol. 2013, 72, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Mesulam, M.-M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The Cholinergic System in the Pathophysiology and Treatment of Alzheimer’s Disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef]
- Wu, S.; Bekhit, A.; Wu, Q.; Chen, M.; Liao, X.; Wang, J.; Ding, Y. Bioactive Peptides and Gut Microbiota: Candidates for a Novel Strategy for Reduction and Control of Neurodegenerative Diseases. Trends Food Sci. Technol. 2021, 108, 164–176. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Alzheimer’s Disease and Gut Microbiota Modifications: The Long Way between Preclinical Studies and Clinical Evidence. Pharmacol. Res. 2018, 129, 329–336. [Google Scholar] [CrossRef]
- Quigley, E.M.M. Microbiota-Brain-Gut Axis and Neurodegenerative Diseases. Curr. Neurol. Neurosci. Rep. 2017, 17, 94. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Feng, T.; Zhang, J.; Huang, X.; Wang, T.; Xie, Z.; Chu, X.; Yang, J.; Wang, H.; et al. Sodium Oligomannate Therapeutically Remodels Gut Microbiota and Suppresses Gut Bacterial Amino Acids-Shaped Neuroinflammation to Inhibit Alzheimer’s Disease Progression. Cell Res. 2019, 29, 787–803. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Yi, X.; Zhu, Y.; Yu, H.; Huang, S.; Liu, Z.; Zhang, X.; Xia, G.; Shen, X. Tilapia Head Protein Hydrolysate Attenuates Scopolamine-Induced Cognitive Impairment through the Gut-Brain Axis in Mice. Foods 2021, 10, 3129. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zheng, L.; Zhao, T.; Zhang, Q.; Su, G.; Zhao, M. The Neuroprotective Effect of Walnut-Derived Peptides against Glutamate-Induced Damage in PC12 Cells: Mechanism and Bioavailability. Food Sci. Hum. Wellness 2022, 11, 933–942. [Google Scholar] [CrossRef]
- Li, W.; Zhao, T.; Zhang, J.; Xu, J.; Sun-Waterhouse, D.; Zhao, M.; Su, G. Effect of Walnut Protein Hydrolysate on Scopolamine-Induced Learning and Memory Deficits in Mice. J. Food Sci. Technol. 2017, 54, 3102–3110. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Zhao, F.; Liu, C.; Wang, J.; Guo, Y.; Liu, J.; Min, W. Antioxidant Hydrolyzed Peptides from Manchurian Walnut (Juglans mandshurica Maxim.) Attenuate Scopolamine-Induced Memory Impairment in Mice. J. Sci. Food Agric. 2018, 98, 5142–5152. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Bordoni, L.; Min, W.; Gabbianelli, R. Insights into the Hippocampus Proteomics Reveal Epigenetic Properties of Walnut-Derived Peptides in a Low-Grade Neuroinflammation Model. J. Agric. Food Chem. 2023, 71, 8252–8263. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.; Mora, L.; Toldrá, F. Characterisation of the Antioxidant Peptide AEEEYPDL and Its Quantification in Spanish Dry-Cured Ham. Food Chem. 2018, 258, 8–15. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, L.; Luo, D.; Zhao, M. In Vitro Simulated Gastrointestinal Digestion Stability of a Neuroprotective Octapeptide WCPFSRSF and Prediction of Potential Bioactive Peptides in Its Digestive Fragments by Multiple Bioinformatics Tools. J. Agric. Food Chem. 2023, 71, 6987–6998. [Google Scholar] [CrossRef]
- Lafarga, T.; Hayes, M. Bioactive Protein Hydrolysates in the Functional Food Ingredient Industry: Overcoming Current Challenges. Food Rev. Int. 2017, 33, 217–246. [Google Scholar] [CrossRef]
- Tuorila, H.; Cardello, A. Consumer Responses to an Off-Flavor in Juice in the Presence of Specific Health Claims. Food Qual. Prefer. 2002, 13, 561–569. [Google Scholar] [CrossRef]
- Leksrisompong, P.; Gerard, P.; Lopetcharat, K.; Drake, M. Bitter Taste Inhibiting Agents for Whey Protein Hydrolysate and Whey Protein Hydrolysate Beverages. J. Food Sci. 2012, 77, S282–S287. [Google Scholar] [CrossRef]

| Preparation Method | Advantages | Disadvantages |
|---|---|---|
| Natural extraction | Simple operation | Natural raw materials, limited production, purification of natural peptides is difficult. |
| Chemical synthesis | Mainly used for the preparation of short peptides with short development cycle, rapid production, high purity | High cost, by-products, not environmentally friendly. |
| Chemical hydrolysis | The hydrolysis reaction is rapid, thorough, low cost, and low investment | Controlling hydrolytic peptides can be challenging due to the sensitivity of the amino acids, which are easily destroyed. Additionally, the resulting hydrolysate may have a dark appearance, and acid hydrolysis can produce the toxic substance chloropropanol. |
| Enzymatic hydrolysis | Mild conditions, high enzyme specificity, high product purity, and high hydrolysis efficiency | It is difficult to purify the peptide mixture by hydrolysis. |
| Genetic recombination | Mainly used for the preparation of larger peptides | The research and development process is challenging owing to the long development cycle and the immaturity of the related technology, with limited production ability of short peptides. |
| Fermentation | Waste resources can be used, reducing the economic burden | The selection of microorganisms is relatively strict and there are safety problems. |
| Separation and Purification of Peptides | Advantages | Disadvantages |
|---|---|---|
| Ultrafiltration | Peptides with specific molecular weight can be easily intercepted for industrial use | Poor separation ability |
| Reversed-phase high-performance liquid chromatography | Good separation effect and reproducibility | High cost |
| Ion-exchange chromatography | High resolution, large injection volume, acid and alkali resistance, and simple operation are appropriate for industrial scale-up of pure peptide processes | Consumables are expensive, slow, small range, and greatly affected by the environment |
| Gel filtration chromatography | The high resolution is conducive to the purification of small-molecular-weight peptides | Consumables are expensive |
| Hydrophilic interaction chromatography | High specificity | Carriers are expensive |
| Macroporous resin chromatographic column | Fast adsorption speed, gentle desorption conditions, easy regeneration treatment, long service period | The purity of the separation is relatively low |
| Identification of Peptides | Advantages | Disadvantages |
|---|---|---|
| N-terminal sequencing | A traditional method for amino acid sequencing | Not effective for N-terminal blocked polypeptides |
| Nuclear magnetic resonance | Can be used to analyse the composition and amino acid sequence of each component in a quantitative mixture | Can only analyse small peptides with less than 30 amino acids |
| Mass spectrometry | High sensitivity, capability to detect N-terminally blocked peptides | High cost |
| Raw Materials | Preparation | Isolation and Purification | Identification Method | In Vivo and In Vitro Models | Mechanism | Peptide Sequence | References |
|---|---|---|---|---|---|---|---|
| Defatted walnut meal | Hydrolysed at 55 °C, pH8.0 for 12 h by pancreatin (at a substrate to enzyme ratio of 20:1 w/w) | SP-825 macroporous adsorption resin; medium-pressure liquid chromatography | UPLC-ESI-MS/MS | H2O2-injured PC12 cells/D-galactose-induced learning and memory impairments in mice | (1) Antioxidant activity; (2) activating intracellular antioxidant enzymes (SOD and GSH-px) through Keap1 inhibition, inhibiting ROS production, Ca2+ influx, and MMP collapse as well as regulating the expression of apoptosis-related proteins | WSREEQEREE, ADIYTEEAGR | [39,105] |
| Defatted walnut meal | Hydrolysed at 55 °C, pH7.0 for 16 h with an enzyme mixture of pancreatin and viscozyme L (at a protease to substrate ratio of 0.8% w/w) | NA | NA | ORAC and ABTS assay/scopolamine-induced memory deficits in mice | (1) Regulating the cholinergic system (increasing the AChR amount and upregulating the mRNA expression of ChAT); (2) protecting neurons in the central nervous system from free radical damage (scavenging free radicals) | NA | [106] |
| Walnut protein | Hydrolysed by adding two proteases (complex plant hydrolase and pancreatin) at a protease/substrate ratio of 1.0% and 1.0% (w/w) in controlled conditions (pH 7.0, 55 °C for 12 h) | Ultrafiltration membrane (MW < 3 kDa), Sephadex G-15 gel filtration chromatography (2.6 × 70.0 cm2) | UPLC-ESI-QTOF-MS/MS | Memory deficits induced by sleep deprivation in rats/glutamate-induced apoptosis in PC12 cells | (1) Reduction of antioxidant defence (CAT, GSH-px, and SOD) and an increase of MDA content; (2) inhibiting Ca2+ influx and MMP collapse; (3) regulate the expression of apoptosis-related proteins (Bax and Bcl-2) | GGW, VYY, LLPF | [34] |
| Walnut protein | Viscozyme L (protease/substrate 1.0%, w/w) and pancreatin (protease/substrate 1.0%, w/w) at pH 7.0, 55 °C for 12 h | Ultrafiltration membrane (MW < 3 kDa), Sephadex G-15 gel filtration chromatography | UPLC–ESI-QTOF-MS/MS | LPS-activated inflammation in BV-2 cells/LPS-induced learning and memory deficits in mice | (1) Anti-inflammatory; (2) antioxidative properties | LPF, GVYY, APTLW | [89] |
| Walnut protein | Complex plant hydrolase and pancreatin with a 1.0% (w/w) enzyme/substrate ratio and hydrolysed at pH 7.0, 55 °C for 12 h | NA | UPLC-ESI-QTOF-MS/MS | Scopolamine-induced cognitive and memory impairment in mice and zebrafish | (1) Ameliorative effect on cholinergic system damage; (2) reducing oxidative stress | FY, SGFDAE | [36] |
| Defatted walnut meal | Simulated gastrointestinal digestion (pepsin/protein ratio of 1:10 w/w pH2.0, 37 °C, 3 h) and pancreatin (pancreatin/protein ratio of 1:10 W/W, pH7.4, 37 °C, 3 h) sequentially | Ultrafiltration, gel filtration chromatography, and RP-HPLC | UPLC-ESI-QTOF-MS | H2O2-stimulated SH-SY5Y cells/D-galactose and aluminium chloride administration to mice | (1) Alleviated oxidative stress; (2) reversed cholinergic dysfunction; (3) suppressed the release of proinflammatory cytokines in the brains of mice; (4) decline in the phosphorylation of JNK and P38 and the nuclear translation of Nrf2 | TY, SGGY | [22,40,60] |
| Defatted walnut dregs | Dissociated with alkali protease (200 U/mg) over the course of 4 h at a substrate ratio (E/S) of 1:50 (w/w) at 55 °C. | Sephadex G-25 | LC-ESI-MS/MS | HEK-293-E22G cell model of intracellular Aβ42 aggregation/APP/PS1 mouse model | (1) Reducing β-amyloid plaques in the brain; (2) alters the gut microbiota and serum metabolites compositions | PPKNW | [23,67] |
| Defatted walnut meal | Hydrolysed by compound proteases and alkaline proteaseat 50–55 °C with agitation for 18–24 h | Ultracentrifugation (8000 rpm, 20 min) | HPLC-MALDI-TOF-MS, de novo sequencing | H2O2-injured SH-SY5Y cells/scopolamine-induced learning and memory deficits in mice | (1) Hydroxyl radical scavenging; (2) ROS reduction | VEGNLQVLRPR, LAGNPHQQQQN, HNLDTQTESDV, AGNDGFEYVTLK, AELQVVDHLGQTV, EQEEEESTGRMK, QQRQQQGI, WSVWEQELEDR | [48,83] |
| Manchurian walnuts | Fermented for 3 h separately using neutrase (9000 U/g) at pH 7.0 and 52.5 °C and using alcalase (7000 U/g) at pH 8.4 and 55.5 °C | Ultrafiltration (>10 kDa, 3–10 kDa, <3 kDa) | H2O2-induced PC12 cells/ scopolamine-induced in mice | (1) Reduction of oxidative stress; (2) inhibition of neural cell apoptosis; (3) regulation of various neurotransmitters; (4) maintaining hippocampal CA3 pyramidal neurons and upregulation of p-CaMK II levels | Manchurian walnut hydrolysed peptide (<3 kDa) | [107] | |
| Manchurian walnuts | NA | Sephadex G-15, RP-HPLC | HPLC-ESI-Q-TOF-MS/MS | H2O2-induced PC12 cells | (1) Reducing ROS generation and enhancing intracellular antioxidant enzymes (SOD, CAT and GSH-px); (2) suppressed the expression of IKKβ and p65 to inhibit NF-κB pathway activation, attenuating the neurotoxic cascade by overexpression of IL-1β and TNF-α; (3) inhibited apoptosis by suppressing the caspase signal pathway; (4) upregulated the expression of p-CREB and synaptophysin | EVSGPGLSPN | [90] |
| Walnut-derived peptide | Chemical synthesis | NA | NA | H2O2-treated HT-22 cells/scopolamine-induced cognitive-impaired mice | (1) Alleviating oxidative stress; (2) promoted the expression of mitophagy-related proteins and activated the NRF2/KEAP1/HO-1 pathway | TWLPLPR, YVLLPSPK, KVPPLLY | [56] |
| Walnut-derived peptide | Chemical synthesis | NA | NA | LPS-stimulated BV-2 microglia | (1) Reducing ROS generation and enhancing antioxidant enzymes (SOD and CAT) activity; (2) reducing NO generation, attenuating inflammatory factors (TNF-α, IL-1β, IL-6), and decreasing the expression of inflammatory response-related enzymes (iNOS and COX2); (3) activating the Nrf2/HO-1 pathway and inhibiting the NF-κB/p38 MAPK pathway | WEKPPVSH | [58] |
| Walnut-derived peptide | Chemical synthesis | NA | NA | Scopolamine-injured mice | Maintains lysosome homeostasis | EVSGPGLSPN | [69] |
| Walnut-derived peptide | Chemical synthesis | NA | NA | D-galactose-induced mice/Aβ25–35–injured bend.3 cells | Maintains the blood-brain barrier integrity by inhibiting the expression and activity of matrix metalloproteinase 9 | TWLPLPR | [65] |
| Walnut-derived peptide | Enzyme hydrolysis, chemical synthesis | NA | NA | Scopolamine-induced cognitive deficits in mice/LPS-induced THP-1 cells | Decreased the activities of DNA methyltransferases | Walnut hydrolysate proteins (<3 kDa) YVLLPSPK | [108] |
| Walnut protein | Alkaline protease (pH 9.0, 55 °C) hydrolysis | Ultrafiltration | LC-MS/MS | D-galactose-induced cognitive-impaired mice | (1) Inhibiting oxidative stress; (2) inhibiting neuroinflammation; (3) modulating the gut microbiota and serum metabolite compositions. | RLWPF, VLRLF | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Bai, Y.-Y.; Hong, Z.-S.; Xie, J.; Tian, Y. Isolation, Identification, Activity Evaluation, and Mechanism of Action of Neuroprotective Peptides from Walnuts: A Review. Nutrients 2023, 15, 4085. https://doi.org/10.3390/nu15184085
Zhang L, Bai Y-Y, Hong Z-S, Xie J, Tian Y. Isolation, Identification, Activity Evaluation, and Mechanism of Action of Neuroprotective Peptides from Walnuts: A Review. Nutrients. 2023; 15(18):4085. https://doi.org/10.3390/nu15184085
Chicago/Turabian StyleZhang, Li, Yu-Ying Bai, Zi-Shan Hong, Jing Xie, and Yang Tian. 2023. "Isolation, Identification, Activity Evaluation, and Mechanism of Action of Neuroprotective Peptides from Walnuts: A Review" Nutrients 15, no. 18: 4085. https://doi.org/10.3390/nu15184085
APA StyleZhang, L., Bai, Y.-Y., Hong, Z.-S., Xie, J., & Tian, Y. (2023). Isolation, Identification, Activity Evaluation, and Mechanism of Action of Neuroprotective Peptides from Walnuts: A Review. Nutrients, 15(18), 4085. https://doi.org/10.3390/nu15184085




