Investigating the Combined Effects of Mechanical Stress and Nutrition on Muscle Hypertrophic Signals Using Contractile 3D-Engineered Muscle (3D-EM)
Abstract
:1. Introduction
2. Materials and Methods
2.1. C2C12 Cell Culture
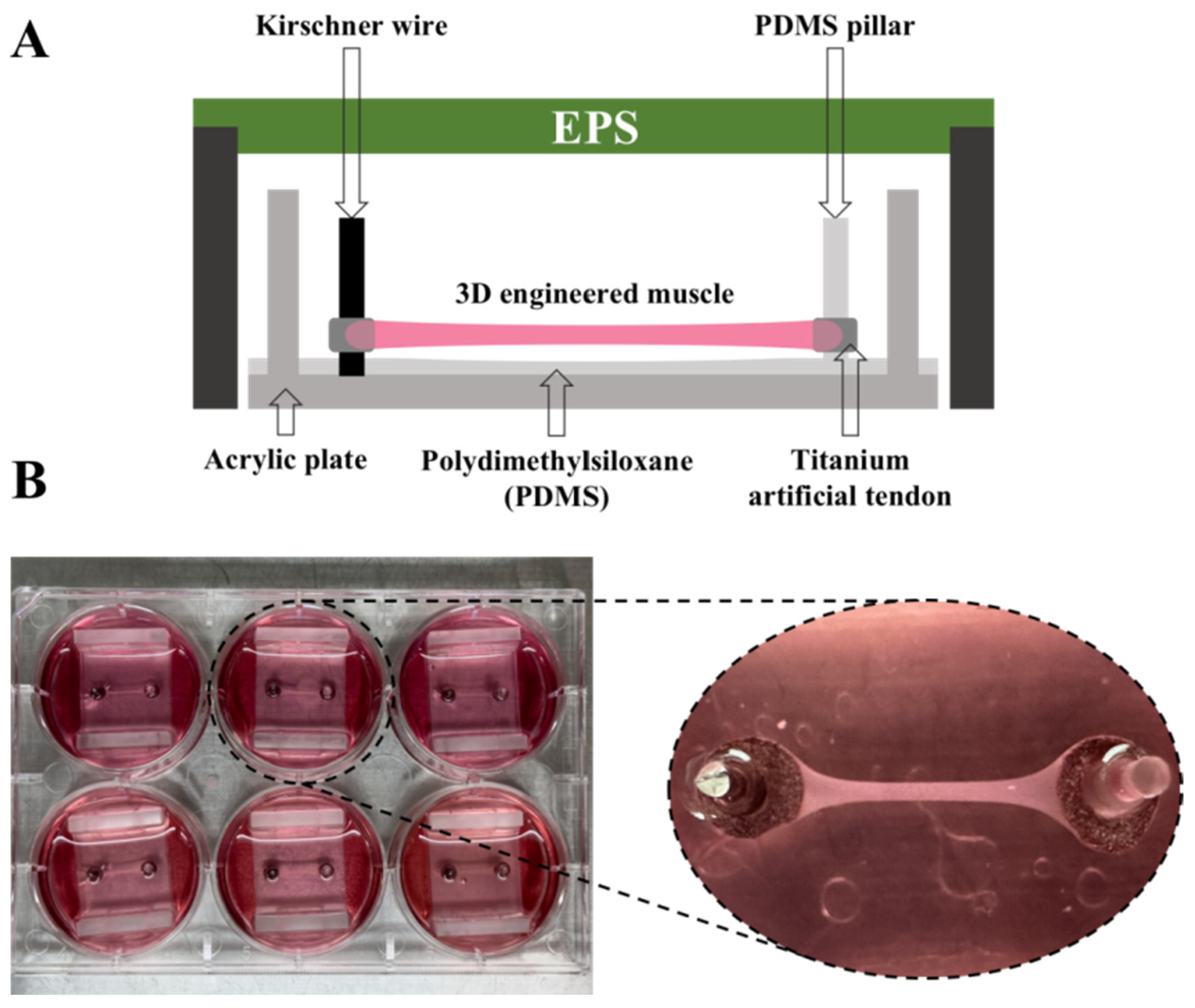
2.2. The Manufacture of 3D-EM
2.3. Experimental Design
2.4. Electrical Pulse Stimulation (EPS)
2.5. Western Blot Analysis
2.6. Statistical Analysis
3. Results
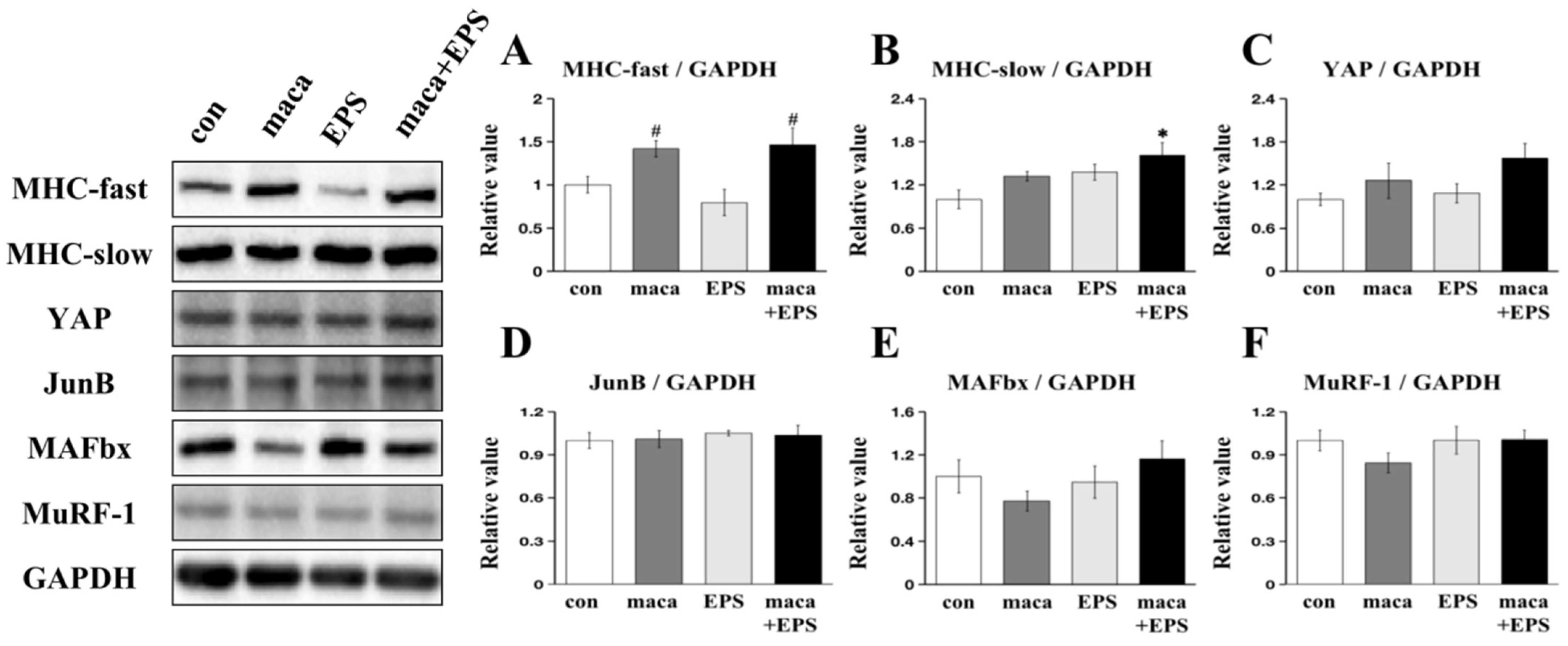
3.1. The Effect of Combining Exercise (i.e., EPS) and Nutrition (i.e., Maca) on the Expression of Proteins Associated with Muscle Growth in 3D-EM
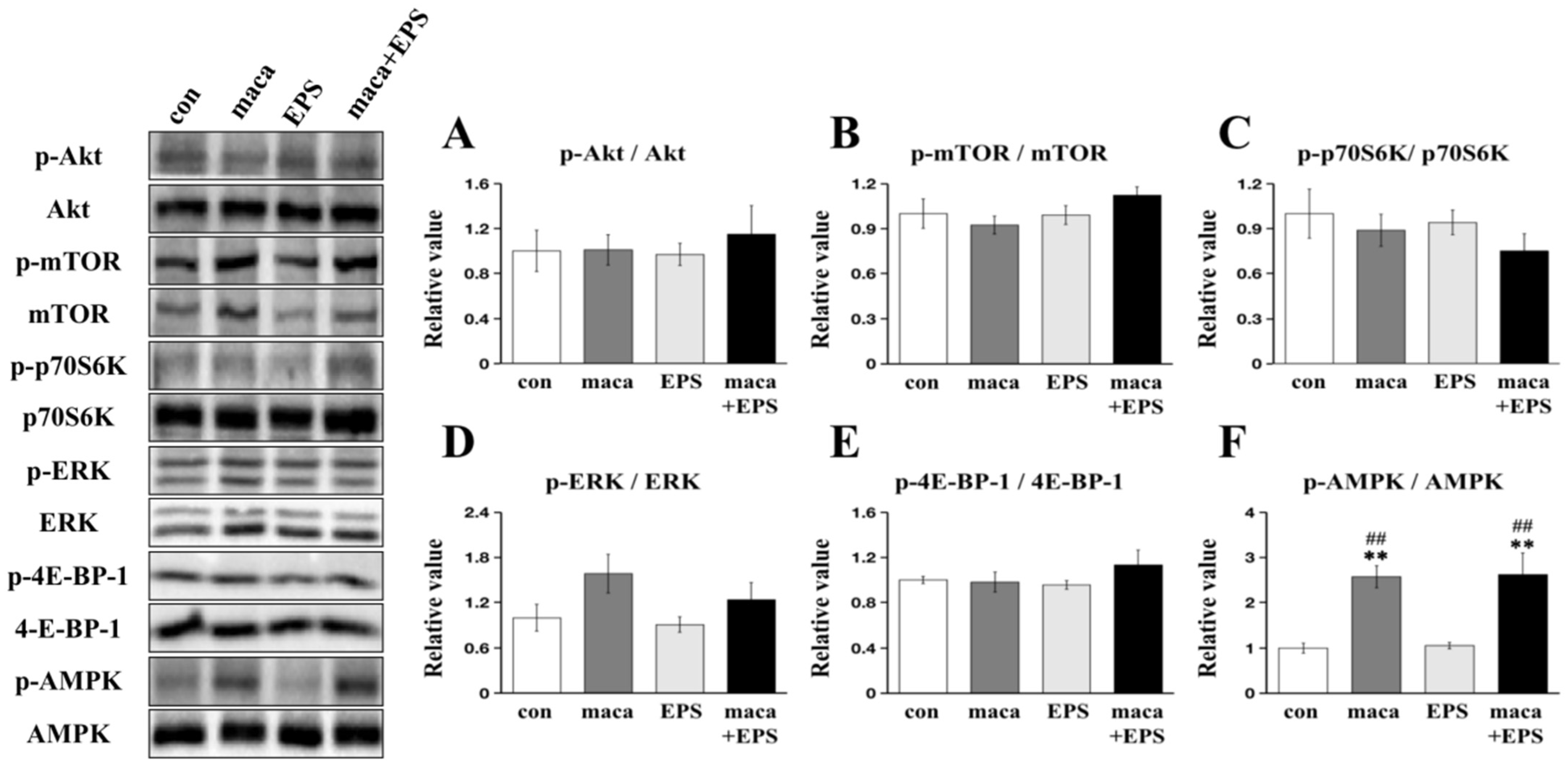
3.2. Changes in Intracellular Signaling Related to Muscle Protein Synthesis and Metabolism
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European Consensus on Definition and Diagnosis. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Sieber, C.C. Malnutrition and Sarcopenia. Aging Clin. Exp. Res. 2019, 31, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Lynch, G.S.; Schertzer, J.D.; Ryall, J.G. Therapeutic Approaches for Muscle Wasting Disorders. Pharmacol. Ther. 2007, 113, 461–487. [Google Scholar] [CrossRef] [PubMed]
- Little, J.P.; Phillips, S.M. Resistance Exercise and Nutrition to Counteract Muscle Wasting. Appl. Physiol. Nutr. Metab. 2009, 34, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.-B.; Lee, H.-S.; Hong, J.S.; Kim, D.H.; Moon, J.M.; Park, Y. Effects of Tannase-Converted Green Tea Extract on Skeletal Muscle Development. BMC Complement Med. Ther. 2020, 20, 47. [Google Scholar] [CrossRef] [PubMed]
- Saud Gany, S.L.; Chin, K.-Y.; Tan, J.K.; Aminuddin, A.; Makpol, S. Curcumin as a Therapeutic Agent for Sarcopenia. Nutrients 2023, 15, 2526. [Google Scholar] [CrossRef] [PubMed]
- Toniolo, L.; Concato, M.; Giacomello, E. Resveratrol, a Multitasking Molecule That Improves Skeletal Muscle Health. Nutrients 2023, 15, 3413. [Google Scholar] [CrossRef]
- Peres, N.d.S.L.; Bortoluzzi, L.C.P.; Marques, L.L.M.; Formigoni, M.; Fuchs, R.H.B.; Droval, A.A.; Cardoso, F.A.R. Medicinal Effects of Peruvian Maca (Lepidium meyenii): A Review. Food Funct. 2020, 11, 83–92. [Google Scholar] [CrossRef]
- Fu, P.; Luo, S.; Liu, Z.; Furuhara, K.; Tsuji, T.; Higashida, H.; Yokoyama, S.; Zhong, J.; Tsuji, C. Oral Supplementation with Maca Improves Social Recognition Deficits in the Valproic Acid Animal Model of Autism Spectrum Disorder. Brain. Sci. 2023, 13, 316. [Google Scholar] [CrossRef]
- Liu, T.; Peng, Z.; Lai, W.; Shao, Y.; Gao, Q.; He, M.; Zhou, W.; Guo, L.; Kang, J.; Jin, X.; et al. The Efficient Synthesis and Anti-Fatigue Activity Evaluation of Macamides: The Unique Bioactive Compounds in Maca. Molecules 2023, 28, 3943. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Park, M.; Kim, B.; Kang, S. Effect of Black Maca Supplementation on Inflammatory Markers and Physical Fitness in Male Elite Athletes. Nutrients 2023, 15, 1618. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Shi, H.; Wang, M.; Xu, Y. Macamide B Suppresses Lung Cancer Progression Potentially via the ATM Signaling Pathway. Oncol. Lett. 2023, 25, 115. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Jin, W.; Dong, X.; Ao, M.; Liu, H.; Yu, L. Safety Evaluation and Protective Effects of Ethanolic Extract from Maca (Lepidium meyenii Walp.) against Corticosterone and H2O2 Induced Neurotoxicity. Regul. Toxicol. Pharmacol. 2020, 111, 104570. [Google Scholar] [CrossRef] [PubMed]
- Orhan, C.; Gencoglu, H.; Tuzcu, M.; Sahin, N.; Ojalvo, S.P.; Sylla, S.; Komorowski, J.R.; Sahin, K. Maca Could Improve Endurance Capacity Possibly by Increasing Mitochondrial Biogenesis Pathways and Antioxidant Response in Exercised Rats. J. Food Biochem. 2022, 46, e14159. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Chang, Y.H. Physicochemical and Antioxidant Properties of Methanol Extract from Maca (Lepidium meyenii Walp.) Leaves and Roots. Food Sci. Technol. 2019, 39, 278–286. [Google Scholar] [CrossRef]
- Takamura, Y.; Nomura, M.; Uchiyama, A.; Fujita, S. Effects of Aerobic Exercise Combined with Panaxatriol Derived from Ginseng on Insulin Resistance and Skeletal Muscle Mass in Type 2 Diabetic Mice. J. Nutr. Sci. Vitaminol. 2017, 63, 339–348. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Fan, L. The Nutritional Composition of Maca in Hypocotyls (Lepidium meyenii Walp.) Cultivated in Different Regions of China. J. Food Qual. 2017, 2017, e3749627. [Google Scholar] [CrossRef]
- Tan, B.; Yin, Y.; Liu, Z.; Li, X.; Xu, H.; Kong, X.; Huang, R.; Tang, W.; Shinzato, I.; Smith, S.B.; et al. Dietary L-Arginine Supplementation Increases Muscle Gain and Reduces Body Fat Mass in Growing-Finishing Pigs. Amino Acids 2009, 37, 169–175. [Google Scholar] [CrossRef]
- Lim, C.H.; Gil, J.H.; Quan, H.; Viet, D.H.; Kim, C.K. Effect of 8-week Leucine Supplementation and Resistance Exercise Training on Muscle Hypertrophy and Satellite Cell Activation in Rats. Physiol. Rep. 2018, 6, e13725. [Google Scholar] [CrossRef]
- Pasiakos, S.M.; McClung, J.P. Supplemental Dietary Leucine and the Skeletal Muscle Anabolic Response to Essential Amino Acids. Nutr. Rev. 2011, 69, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.; Yoshikawa, M.; Sugimoto, T.; Tomoo, K.; Okada, Y.; Hashimoto, T. Effects of Maca on Muscle Hypertrophy in C2C12 Skeletal Muscle Cells. Int. J. Mol. Sci. 2022, 23, 6825. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.; Saleem, A.; Tarnopolsky, M.A. The Potential of Endurance Exercise-Derived Exosomes to Treat Metabolic Diseases. Nat. Rev. Endocrinol. 2016, 12, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, N.; Görgens, S.W.; Thoresen, G.H.; Aas, V.; Eckel, J.; Eckardt, K. Electrical Pulse Stimulation of Cultured Skeletal Muscle Cells as a Model for in Vitro Exercise—Possibilities and Limitations. Acta Physiol. 2017, 220, 310–331. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Davis, J.; Bersch, I.; Goldberg, G.; Gorgey, A.S. Electrical Stimulation and Denervated Muscles after Spinal Cord Injury. Neural. Regen. Res. 2020, 15, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Tarum, J.; Folkesson, M.; Atherton, P.J.; Kadi, F. Electrical Pulse Stimulation: An in Vitro Exercise Model for the Induction of Human Skeletal Muscle Cell Hypertrophy. A Proof-of-Concept Study. Exp. Physiol. 2017, 102, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Khodabukus, A.; Madden, L.; Prabhu, N.K.; Koves, T.R.; Jackman, C.P.; Muoio, D.M.; Bursac, N. Electrical Stimulation Increases Hypertrophy and Metabolic Flux in Tissue-Engineered Human Skeletal Muscle. Biomaterials 2019, 198, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Churchward-Venne, T.A.; Burd, N.A.; Phillips, S.M. Nutritional Regulation of Muscle Protein Synthesis with Resistance Exercise: Strategies to Enhance Anabolism. Nutr. Metab. 2012, 9, 40. [Google Scholar] [CrossRef]
- Ogasawara, R.; Sato, K.; Higashida, K.; Nakazato, K.; Fujita, S. Ursolic Acid Stimulates mTORC1 Signaling after Resistance Exercise in Rat Skeletal Muscle. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E760–E765. [Google Scholar] [CrossRef]
- Miyatake, S.; Hino, K.; Natsui, Y.; Ebisu, G.; Fujita, S. Protein Supplementation Enhances the Effects of Intermittent Loading on Skeletal Muscles by Activating the mTORC1 Signaling Pathway in a Rat Model of Disuse Atrophy. Nutrients 2020, 12, 2729. [Google Scholar] [CrossRef]
- Wang, H.; Brown, P.C.; Chow, E.C.Y.; Ewart, L.; Ferguson, S.S.; Fitzpatrick, S.; Freedman, B.S.; Guo, G.L.; Hedrich, W.; Heyward, S.; et al. 3D Cell Culture Models: Drug Pharmacokinetics, Safety Assessment, and Regulatory Consideration. Clin. Transl. Sci. 2021, 14, 1659–1680. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.S.T.; Passey, S.; Greensmith, L.; Mudera, V.; Lewis, M.P. Characterization and Optimization of a Simple, Repeatable System for the Long Term in Vitro Culture of Aligned Myotubes in 3D. J. Cell. Biochem. 2012, 113, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The Third Dimension Bridges the Gap between Cell Culture and Live Tissue. Nat. Rev. Mol. Cell. Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Ito, A.; Sato, M.; Kawabe, Y.; Kamihira, M. Improved Contractile Force Generation of Tissue-Engineered Skeletal Muscle Constructs by IGF-I and Bcl-2 Gene Transfer with Electrical Pulse Stimulation. Regen. Ther. 2016, 3, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Takagi, S.; Kamon, T.; Yamasaki, K.; Fujisato, T. Development and Evaluation of a Removable Tissue-Engineered Muscle with Artificial Tendons. J. Biosci. Bioeng. 2017, 123, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Nakamura, T.; Yokoyama, S.; Fujisato, T.; Konishi, S.; Hashimoto, T. Investigation of Brain Function-Related Myokine Secretion by Using Contractile 3D-Engineered Muscle. Int. J. Mol. Sci. 2022, 23, 5723. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Okada, Y.; Yamanaka, A.; Ono, N.; Uryu, K.; Maru, I. The Effect of Eleutherococcus Senticosus on Metabolism-Associated Protein Expression in 3T3-L1 and C2C12 Cells. Phys. Act. Nutr. 2020, 24, 13–18. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Hosokawa, M.; Miyashita, K.; Fujita, T.; Nishino, H.; Hashimoto, T. Fucoxanthinol Attenuates Oxidative Stress-Induced Atrophy and Loss in Myotubes and Reduces the Triacylglycerol Content in Mature Adipocytes. Mol. Biol. Rep. 2020, 47, 2703–2711. [Google Scholar] [CrossRef]
- Sakushima, K.; Yoshikawa, M.; Osaki, T.; Miyamoto, N.; Hashimoto, T. Moderate Hypoxia Promotes Skeletal Muscle Cell Growth and Hypertrophy in C2C12 Cells. Biochem. Biophys. Res. Commun. 2020, 525, 921–927. [Google Scholar] [CrossRef]
- Nakamura, T.; Takagi, S.; Okuzaki, D.; Matsui, S.; Fujisato, T. Hypoxia Transactivates Cholecystokinin Gene Expression in 3D-Engineered Muscle. J. Biosc. Bioeng. 2021, 132, 64–70. [Google Scholar] [CrossRef]
- Harada, M.; Nakamura, T.; Yokoyama, S. Design and Construction of A Continuous Quantitative Force Measurement Microdevice for Artificial Skeletal Muscle. In Proceedings of the Mechanical Engineering Congress, Aichi, Japan, 13–16 September 2020. [Google Scholar] [CrossRef]
- Nikolić, N.; Skaret Bakke, S.; Tranheim Kase, E.; Rudberg, I.; Flo Halle, I.; Rustan, A.C.; Thoresen, G.H.; Aas, V. Electrical Pulse Stimulation of Cultured Human Skeletal Muscle Cells as an In Vitro Model of Exercise. PLoS ONE 2012, 7, e33203. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Imai, S.; Yoshikawa, M.; Fujisato, T.; Hashimoto, T.; Nakamura, T. Mechanical Unloading of 3D-Engineered Muscle Leads to Muscle Atrophy by Suppressing Protein Synthesis. J. Appl. Physiol. 2022, 132, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Lena, A.; Anker, M.S.; Springer, J. Muscle Wasting and Sarcopenia in Heart Failure—The Current State of Science. Int. J. Mol. Sci. 2020, 21, 6549. [Google Scholar] [CrossRef] [PubMed]
- Noor, H.; Reid, J.; Slee, A. Resistance Exercise and Nutritional Interventions for Augmenting Sarcopenia Outcomes in Chronic Kidney Disease: A Narrative Review. J. Cachexia Sarcopenia Muscle 2021, 12, 1621–1640. [Google Scholar] [CrossRef] [PubMed]
- Vahlberg, B.; Cederholm, T.; Lindmark, B.; Zetterberg, L.; Hellström, K. Short-Term and Long-Term Effects of a Progressive Resistance and Balance Exercise Program in Individuals with Chronic Stroke: A Randomized Controlled Trial. Disabil. Rehabil. 2017, 39, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Grimm, D. EPA Plan to End Animal Testing Splits Scientists. Science 2019, 365, 1231. [Google Scholar] [CrossRef] [PubMed]
- Badillo-Mata, J.A.; Camacho-Villegas, T.A.; Lugo-Fabres, P.H. 3D Cell Culture as Tools to Characterize Rheumatoid Arthritis Signaling and Development of New Treatments. Cells 2022, 11, 3410. [Google Scholar] [CrossRef]
- Del Valle, J.S.; Chuva de Sousa Lopes, S.M. Bioengineered 3D Ovarian Models as Paramount Technology for Female Health Management and Reproduction. Bioengineering 2023, 10, 832. [Google Scholar] [CrossRef]
- Khodabukus, A.; Baar, K. Streptomycin Decreases the Functional Shift to a Slow Phenotype Induced by Electrical Stimulation in Engineered Muscle. Tissue Eng. Part A 2015, 21, 1003–1012. [Google Scholar] [CrossRef]
- Morley, J.E.; Baumgartner, R.N.; Roubenoff, R.; Mayer, J.; Nair, K.S. Sarcopenia. J. Lab. Clin. Med. 2001, 137, 231–243. [Google Scholar] [CrossRef]
- Friedmann-Bette, B.; Profit, F.; Gwechenberger, T.; Weiberg, N.; Parstorfer, M.; Weber, M.-A.; Streich, N.; Barié, A. Strength Training Effects on Muscular Regeneration after ACL Reconstruction. Med. Sci. Sports Exerc. 2018, 50, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Ferdousi, F.; Fukumitsu, S.; Isoda, H. Maslinic Acid Attenuates Denervation-Induced Loss of Skeletal Muscle Mass and Strength. Nutrients 2021, 13, 2950. [Google Scholar] [CrossRef]
- Kim, M.; Sung, B.; Kang, Y.J.; Kim, D.H.; Lee, Y.; Hwang, S.Y.; Yoon, J.-H.; Yoo, M.-A.; Kim, C.M.; Chung, H.Y.; et al. The Combination of Ursolic Acid and Leucine Potentiates the Differentiation of C2C12 Murine Myoblasts through the mTOR Signaling Pathway. Int. J. Mol. Med. 2015, 35, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Chen, J.; Xu, J.; Cao, J.; Wang, Y.; Thomas, S.S.; Hu, Z. Suppression of Muscle Wasting by the Plant-derived Compound Ursolic Acid in a Model of Chronic Kidney Disease. J. Cachexia Sarcopenia Muscle 2017, 8, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Kang, Y.S.; Noh, E.B.; Seo, B.W.; Seo, D.Y.; Park, G.D.; Kim, S.H. Concurrent Treatment with Ursolic Acid and Low-Intensity Treadmill Exercise Improves Muscle Atrophy and Related Outcomes in Rats. Korean J. Physiol. Pharmacol. 2018, 22, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Khodabukus, A.; Baehr, L.M.; Bodine, S.C.; Baar, K. Role of Contraction Duration in Inducing Fast-to-Slow Contractile and Metabolic Protein and Functional Changes in Engineered Muscle. J. Cell. Physiol. 2015, 230, 2489–2497. [Google Scholar] [CrossRef] [PubMed]
- Raffaello, A.; Milan, G.; Masiero, E.; Carnio, S.; Lee, D.; Lanfranchi, G.; Goldberg, A.L.; Sandri, M. JunB Transcription Factor Maintains Skeletal Muscle Mass and Promotes Hypertrophy. J. Cell Biol. 2010, 191, 101–113. [Google Scholar] [CrossRef]
- Goodman, C.A.; Dietz, J.M.; Jacobs, B.L.; McNally, R.M.; You, J.-S.; Hornberger, T.A. Yes-Associated Protein Is up-Regulated by Mechanical Overload and Is Sufficient to Induce Skeletal Muscle Hypertrophy. FEBS. Lett. 2015, 589, 1491–1497. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C.; Akimoto, T.; Blaauw, B. Molecular Mechanisms of Skeletal Muscle Hypertrophy. J. Neuromuscul. Dis. 2021, 8, 169–183. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, C.; Hou, J.; Su, P.; Yang, Y.; Xia, B.; Zhao, X.; He, R.; Wang, L.; Cao, C.; et al. Red Ginseng Extract Improves Skeletal Muscle Energy Metabolism and Mitochondrial Function in Chronic Fatigue Mice. Front. Pharmacol. 2022, 13, 1077249. [Google Scholar] [CrossRef]
- Thomson, D.M. The Role of AMPK in the Regulation of Skeletal Muscle Size, Hypertrophy, and Regeneration. Int. J. Mol. Sci. 2018, 19, 3125. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Hosokawa, M.; Miyashita, K.; Nishino, H.; Hashimoto, T. Effects of Fucoxanthin on the Inhibition of Dexamethasone-Induced Skeletal Muscle Loss in Mice. Nutrients 2021, 13, 1079. [Google Scholar] [CrossRef]
- Plotkin, D.L.; Roberts, M.D.; Haun, C.T.; Schoenfeld, B.J. Muscle Fiber Type Transitions with Exercise Training: Shifting Perspectives. Sports 2021, 9, 127. [Google Scholar] [CrossRef]
- Nintou, E.; Karligiotou, E.; Vliora, M.; Ioannou, L.G.; Flouris, A.D. Characteristics of the Protocols Used in Electrical Pulse Stimulation of Cultured Cells for Mimicking In Vivo Exercise: A Systematic Review, Meta-Analysis, and Meta-Regression. Int. J. Mol. Sci. 2022, 23, 13446. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, D.; Sugimoto, T.; Matsumura, T.; Yokoyama, S.; Fujisato, T.; Nakamura, T.; Hashimoto, T. Investigating the Combined Effects of Mechanical Stress and Nutrition on Muscle Hypertrophic Signals Using Contractile 3D-Engineered Muscle (3D-EM). Nutrients 2023, 15, 4083. https://doi.org/10.3390/nu15184083
Yi D, Sugimoto T, Matsumura T, Yokoyama S, Fujisato T, Nakamura T, Hashimoto T. Investigating the Combined Effects of Mechanical Stress and Nutrition on Muscle Hypertrophic Signals Using Contractile 3D-Engineered Muscle (3D-EM). Nutrients. 2023; 15(18):4083. https://doi.org/10.3390/nu15184083
Chicago/Turabian StyleYi, Dong, Takeshi Sugimoto, Teppei Matsumura, Sho Yokoyama, Toshia Fujisato, Tomohiro Nakamura, and Takeshi Hashimoto. 2023. "Investigating the Combined Effects of Mechanical Stress and Nutrition on Muscle Hypertrophic Signals Using Contractile 3D-Engineered Muscle (3D-EM)" Nutrients 15, no. 18: 4083. https://doi.org/10.3390/nu15184083
APA StyleYi, D., Sugimoto, T., Matsumura, T., Yokoyama, S., Fujisato, T., Nakamura, T., & Hashimoto, T. (2023). Investigating the Combined Effects of Mechanical Stress and Nutrition on Muscle Hypertrophic Signals Using Contractile 3D-Engineered Muscle (3D-EM). Nutrients, 15(18), 4083. https://doi.org/10.3390/nu15184083





