Abstract
Fortified balanced energy–protein (BEP) supplementation is a promising intervention for improving maternal health, birth outcomes and infant growth in low- and middle-income countries. This nested biospecimen sub-study aimed to evaluate the physiological effect of multi-micronutrient-fortified BEP supplementation on pregnant and lactating women and their infants. Pregnant women (15–40 years) received either fortified BEP and iron–folic acid (IFA) (intervention) or IFA only (control) throughout pregnancy. The same women were concurrently randomized to receive either a fortified BEP supplement during the first 6 months postpartum in combination with IFA for the first 6 weeks (i.e., intervention) or the postnatal standard of care, which comprised IFA alone for 6 weeks postpartum (i.e., control). Biological specimens were collected at different timepoints. Multi-omics profiles will be characterized to assess the mediating effect of BEP supplementation on the different trial arms and its effect on maternal health, as well as birth and infant growth outcomes. The mediating effect of the exposome in the relationship between BEP supplementation and maternal health, birth outcomes and infant growth were characterized via biomonitoring markers of air pollution, mycotoxins and environmental contaminants. The results will provide holistic insight into the granular physiological effects of prenatal and postnatal BEP supplementation.
1. Background
In many low- and middle-income countries (LMICs), pregnant women struggle to meet the nutritional requirements for sustaining healthy fetal development [1] (UNICEF-WHO, 2019); left unmet, these crucial needs can result in miscarriage [2] (Benammar et al., 2012) and stillbirth [3] (McClure et al., 2009). Born alive, these infants are often of low birth weight (LBW), small for gestational age (SGA) and/or preterm, and they experience severe health and developmental disadvantages, resulting in undue costs to society [4] (Almond et al., 2004).
In 2016, to reduce the risk of stillbirths and SGA births, the WHO published antenatal care guidelines recommending balanced energy–protein (BEP) supplementation during pregnancy for all pregnant women in the context of high prevalenceof population-level undernutrition [5] (WHO, 2016). This recommendation, however, was based on evidence of “moderate certainty” and suggests that BEP supplementation “probably” reduces the rates of stillbirth and SGA birth [5,6] (Ota et al., 2012; WHO, 2016). In an LMIC, any costly antenatal intervention (like BEP supplementation) supported by evidence of “moderate certainty” is unlikely to be prioritized, at least until more robust research and evidence-based data can demonstrate a return on investment. As a result, the Bill & Melinda Gates Foundation (BMGF) built and funded a large-scale multi-centric consortium of seven intervention studies aimed at evaluating the effectiveness of BEP supplementation during pregnancy and lactation [7] (Gernand et al., 2023).
The protocol of the MISAME-III main trial was published previously [8] (Vanslambrouck et al., 2021). The study was a community-based, non-blinded, individually randomized 2 × 2 factorial randomized controlled trial (RCT) involving directly observed daily supplement intake (Figure 1). The primary outcomes were the prevalence of SGA at birth (<10th percentile of the newborns size standards of the International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st) [9] (Villar et al., 2013)) and the length-for-age z-score (LAZ) at 6 months of age (calculated using the WHO’s 2006 growth reference at 6 months of age [10] (de Onis and Branca, 2016)). These results have been published [11,12,13] (de Kok et al., 2022; Argaw, de Kok, et al., 2023; Argaw, Toe, et al., 2023).
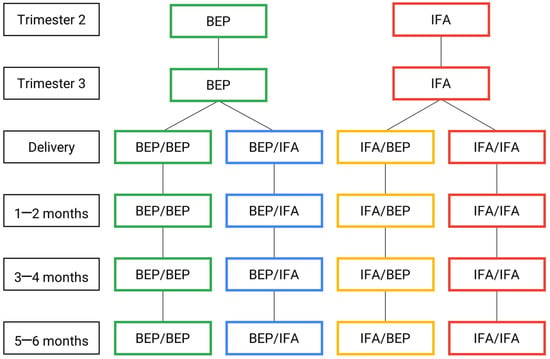
Figure 1.
MISAME-III efficacy trial timeline: intervention and control arms. BEP, micronutrient-fortified balanced energy–protein; IFA, iron–folic acid.
The secondary and exploratory biological outcomes of the prenatal BEP intervention were maternal and newborn body compositions and newborn relative telomere length (TL), with mitochondrial DNA content (mtDNAc) a non-declared outcome that was considered relevant for the trial at the time at which the samples were analyzed. The former has been published [12] (Argaw, Toe, et al., 2023), and the latter is under review [14] (Hanley-Cook et al., no date).
Anthropometry serves as a valuable metric, enabling the comparison of an individual child to a growth reference derived from a healthy population [15] (Perumal, Bassani and Roth, 2018). However, these are relatively crude metrics, and biomarkers are required to fully characterize the physiological changes hypothesized to result from maternal perinatal BEP supplementation [12,13] (de Kok et al., 2022; Argaw et al., 2023).
Thus, this dedicated biospecimen sub-study (BioSpé), which was nested within the larger MISAME-III trial, aimed to evaluate the physiological effects of multi-micronutrient-fortified BEP supplementation on 309 pregnant and lactating women (PLW) and their infants (Table 1).

Table 1.
List of biospecimens collected for the BioSpé study.
Due to a dearth of evidence on maternal and infant physiology during maternal BEP supplementation, we adopt an unbiased, hypothesis-generating approach aimed to uncover biological pathways and discover novel biomarkers for assessing maternal health (gestational weight gain and anemia [16] (Hanley-Cook, Toe, et al., 2022)) as well as infant development and growth (Figure 2).
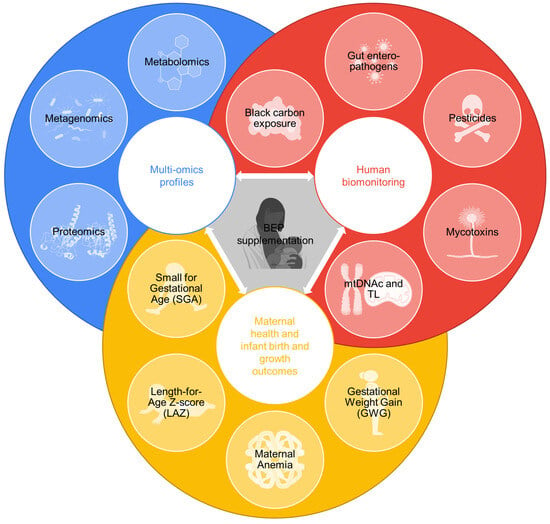
Figure 2.
Data produced in the biospecimen sub-study (BioSpé) of MISAME-III. These data will be leveraged to assess the physiological effects of micronutrient-fortified balanced energy–protein (BEP) supplementation on maternal health, infant birth and infant growth outcomes through an analysis of multi-omics profiles, the human biomonitoring of contaminants and measurements of relative telomere length (TL) and mitochondrial DNA content (mtDNAc).
2. Methods: Study Design, Biospecimen Collection and Rationale
2.1. Study Setting, Participants and Enrolment Procedures
The MISAME-III trial was implemented in 6 rural health-center catchment areas in Burkina Faso. The usual diet during pregnancy is predominantly maize-based, with the addition of leafy vegetables [17] (de Kok, Argaw, et al., 2021). The transmission of malaria is continual with seasonal variations [18] (Hanley-Cook, Argaw, et al., 2022).
PLW aged between 15 and 40 years who lived in the study villages were identified through a census conducted in the research area (n = 10,165). Community support staff visited all eligible participants at their residences every five weeks to identify early pregnancy by screening for self-reported amenorrhea. Women who were suspected of being pregnant were guided to the health center for a urine pregnancy test, and pregnancies were confirmed via ultrasounds. The participants excluded from the study included those who planned to leave the study area during pregnancy or deliver outside the study area and individuals who had a peanut allergy because BEP is an energy-dense peanut paste.
After written informed consent was provided, the participants were randomly assigned to the prenatal intervention arms receiving either the fortified BEP supplements and iron–folic acid (IFA) tablets (i.e., intervention) or the IFA tablets alone (i.e., control), which is the standard of care during pregnancy. The same participants were concurrently randomized to one of two of the postnatal intervention arms, either receiving fortified BEP supplementation during the first 6 months postpartum in combination with IFA for the first 6 weeks (i.e., intervention), or IFA alone for 6 weeks postpartum (i.e., control). Therefore, the participants were randomized into one of the 4 study groups: (1) both pre- and postnatal BEP and IFA supplementation (BEP/BEP); (2) prenatal BEP supplementation and postnatal IFA supplementation only (BEP/IFA); (3) prenatal IFA supplementation and postnatal BEP and IFA supplementation (IFA/BEP); or (4) both pre- and postnatal IFA supplementation (IFA/IFA).
Trained village-based project workers visited the pregnant participants to observe their intake of the BEP supplement and IFA tablets. When the participants were absent from home, the BEP and IFA were provided in advance (thus counting as non-observed intakes). The participants were encouraged to attend scheduled antenatal care (ANC) visits every seven weeks per national policy [19] (Ministère de la Santé—Burkina Faso, 2010).
2.2. Study Supplements
A formative study was conducted to establish a preferred and suitable supplement according to the Burkinabè population [20] (Jones et al., 2021). The chosen supplement was an energy-dense peanut paste fortified with multiple micronutrients. Table 2 [21] (Bill & Melinda Gates Foundation, 2017) provides the nutritional composition of the BEP.

Table 2.
Nutritional values of the BEP supplement for pregnant and lactating women 1.
The PLW in the intervention group received a daily BEP supplement and an IFA tablet (Sidhaant Life Sciences, Delhi, India) containing 65 mg of iron (in the form of FeH2O5S) and 400 μg of folic acid (in the form of C19H19N7O6; the tolerable upper intake level from fortified food or supplements, not including folate from food, is 1000 μg/d [22] (Allen, Carriquiry and Murphy, 2020), whereas those in the control group received a daily IFA tablet only, in accordance with the standard of care in Burkina Faso. Following Burkinabè guidelines, during ANC visits, all participants received malaria prophylaxis (three oral doses of sulfoxide–pyrimethamine).
2.3. Biospecimen Collection
2.3.1. Whole Blood (Mother–Infant Dyads)
A total of 60 µL (2 × 10 µL and 2 × 20 µL) of whole blood was collected via capillary sampling, using a volumetric absorptive microsampling (VAMS) device. The VAMS technology wicks a small, fixed volume of biofluid, which is beneficial for newborns and anemic participants, and poses fewer challenges in the handling, storage and transportation of samples [23] (Vidal et al., 2021). Both 10 µL Mitra Clamshell (2-sampler) and 20 µL Mitra Clamshell (2-Sampler) devices (item numbers: 10,109 and 20,109), namely MitraTM, were obtained from Neoteryx (Torrance, CA, USA).
Samples were collected via the VAMS device at the following timepoints: trimester 2 (19–24 weeks of gestation), trimester 3 (30–34 weeks of gestation) and 5–6 months (147–175 days) postpartum in the PLW participants. In infants, 60 µL of whole blood was collected via the VAMS device at birth and 1–2 months (28–56 days of life), 3–4 months (84–112 days of life) and 5–6 months (140–168 days) postnatally.
The VAMS technology wicks a small, fixed volume of biofluid, which is beneficial for newborns and anemic participants, and poses fewer challenges in the handling, storage and transportation of samples [23] (Vidal et al., 2021). Untargeted metabolomics and mycotoxin analyses were performed on 10 µL and 20 µL VAMS devices, respectively. The VAMS devices (MitraTM) were obtained from Neoteryx (Torrance, CA, USA). To preserve the integrity of the metabolites for the metabolomics analyses, the PLW were asked to not eat breakfast on the morning of the sample collection via the VAMs device. The samples collected via the VAMS device were stored in Mitra Autoracks (96-Sampler, item number: 108) inside a storage solution, using a 96-well plate packed with desiccant bags (item number: AC-SS02); both items were obtained from Neoteryx (Torrance, CA, USA) for long-term storage at −80 °C.
2.3.2. Plasma (Mothers)
A total of 500 µL of whole blood was collected via capillary sampling, using plastic BD microtainer whole-blood tubes which were spray-coated with K2 potassium salt of ethylene diamine tetra acetic acid (EDTA) (BD, Franklin Lakes, NJ, USA). Following centrifugation using a microcentrifuge (VWR International, Leuven, Belgium), 100 µL of plasma was aliquoted into sterile cryotubes (Biosigma, Cona, VE, Italy) via single-channel pipettes (Thermo Fisher Scientific, Merelbeke, Belgium) before it was flash-frozen in 12 L liquid nitrogen storage vessels (Cryopal, Air Liquide, Paris, France) and transferred to a −80 °C freezer. In the PLW, the plasma samples were collected in trimester 2 (19–24 weeks of gestation) and trimester 3 (30–34 weeks of gestation) and 1–2 (28–56 days) and 5–6 months (140–168 days) postnatally.
Plasma was collected as it encompasses a broad spectrum of proteins often utilized as biomarkers to detect the biological pathways influenced by supplementation [24,25] (Weissinger et al., 2006; Chakrabarti et al., 2020).
2.3.3. Cord Blood
Within 30 min of birth, arterial umbilical cord blood was collected in 4 mL BD Vacutainer® plastic whole-blood tubes which were spray-coated with K2 potassium salt of EDTA (BD, Franklin Lakes, NJ, USA). These tubes were gently inverted at least 10 times to thoroughly mix the blood with the anticoagulant. Using micropipettes (Thermo Fisher Scientific, Merelbeke, Belgium), the blood samples were aliquoted into sterile cryotubes and flash-frozen before they were transferred to a −80 °C freezer.
Umbilical cord blood demonstrates lower biovariability and excellent DNA yield and quality [26] (Lin et al., 2019). Consequently, the impact of the BEP and environmental contaminants on newborn relative TL and mtDNAc and the presence of black carbon (BC) particles will be analyzed using the whole arterial blood collected from the umbilical cord.
2.3.4. Urine (Mothers)
First-morning-void urine samples were collected from the participants in sterile 60 mL polypropylene containers (Corning Gosselin SAS, Borre, France) and aliquoted into 5 mL sterile cryotubes (VWR International, Leuven, Belgium), using 1 mL Pasteur pipettes (Deltalab, Heusden-Zolder, Belgium), before they were flash-frozen and stored at −80 °C. The first morning voids were collected to standardize urine collection between participants. The urine samples were collected in trimester 2 (19–24 weeks of gestation) and trimester 3 (30–34 weeks of gestation).
Urine is the most appropriate biological matrix for measuring acute, nonpersistent chemical exposures with rapid half-lives [27,28] (Barr et al., 2005; Esteban and Castaño, 2009); thus, analyses of the prenatal concentrations of pesticides, insecticides and herbicides will be performed on these samples.
2.3.5. Breast Milk (Mothers)
Using an electric breast pump (Medela, Baar, Switzerland), a total of 7.2 mL of breast milk was aliquoted into 4 × 2 mL sterile cryotubes. This sample was drawn from a full expression of the breast adjacent to the breast last used to feed the infant, and the samples were gently inverted to homogenize fore- and hindmilk. Milk samples were collected at the following timepoints: 14–21 days after delivery and 1–2 months (28–56 days of life) and 3–4 months (84–112 days of life) postpartum.
In addition to macronutrients and micronutrients, breast milk also provides numerous bioactive components, including antibacterial peptides, antibodies, cells and microbes [29,30] (Walker and Iyengar, 2014; Ma et al., 2020). These components have an influence on the growth of the newborn and on the development of organs and systems [31,32,33,34,35,36] (Bardanzellu, Fanos and Reali, 2017; Bardanzellu et al., 2018, 2019; Congiu et al., 2019; Bardanzellu, Peroni and Fanos, 2020; Bardanzellu, Reali, et al., 2020). The milk proteome is composed of proteins and endogenous peptides [37] (Dallas et al., 2013). Proteomic studies have reported almost 3000 proteins in human milk [38] (Van Herwijnen et al., 2016).
In this study, breast milk will be analyzed to assess the multi-omics profiles between different arms of the trial [39,40] (Kisuse et al., 2018; Nguyen et al., 2021).
2.3.6. Feces (Mother–Infant Dyads)
Maternal fecal samples (8 g) were collected in a fecal pot and then aliquoted into sterile cryotubes (Biosigma, Cona, VE, Italy), flash-frozen and transferred to a −80 °C freezer. Infant feces (8 g) were collected using a 38 × 50 cm sterile protection sheet (Kimberley-Clark, Irving, TX, USA) which is used like a diaper and wrapped around the newborn, before they were transferred to a OMNIgene•GUT OM-200 collection kit and then sterile cryotubes for storage at −80 °C. The collected feces were assessed for consistency based on the visual Bristol scale. For liquid feces, thorough homogenization was performed using a plastic spoon so that the solid and liquid components were mixed well.
Fecal samples were collected from the PLW in trimester 2 (19–24 weeks) and trimester 3 (30–34 weeks) and 1–2 (28–56 days) and 5–6 months (147–175 days) postpartum, and fecal samples were collected from the infants 1–2 months (28–56 days of life), 3–4 months (84–112 days of life) and 5–6 months (140–168 days) postnatally. Since feces are non-invasive, biologically rich matrixes containing host, microbe and dietary proteins [41] (Gonzalez, Zhang and Elias, 2017), these samples will be analyzed to assess the gut microbiota profiles of the participants and markers of inflammation.
2.4. Rationale for the Analysis of Biospecimens with Related Bio-Measurements
We will apply integrated multi-omics approaches to comprehensively characterize the metabolome, microbiome and proteome of the whole blood, plasma, breast milk and feces during pregnancy and the period of exclusive breastfeeding in both mothers and infants. A summary of the analyses and the laboratories used are shown in Table 3.

Table 3.
Multi-omics profiles investigated in the BioSpé Study (excluding breast milk analyses—see Table 4).
The multi-omics approaches applied will compare postnatal maternal and infant data among and between supplementation groups to identify any differences in the compositions associated with the pre- and postnatal supplementation of BEP. In addition, these analyses will aim to identify maternal and infant features are associated with infant phenotypes of adverse birth outcomes (i.e., LBW, neonatal mortality, SGA, preterm birth, stunting, underweight, wasting and underweight), as well as those correlated with continuous metrics of birth anthropometry (i.e., birth length and weight and chest, head and mid-upper arm circumferences) and with continuous metrics of growth (i.e., infant length and weight and chest, head and mid-upper arm circumference) throughout the period of postnatal follow-up (from birth to 6 months of age).
This section describes selected analyses and the rationale and specific analytical techniques that will be employed.
2.4.1. Metabolomics
Metabolomics identifies and characterizes changes in the metabolites in a biofluid (e.g., blood). Previously, a targeted metabolomics approach was used to measure essential amino acids and other metabolites in 313 Malawian children. The results reported that sixty-two percent of Malawian children with stunting had lower serum concentrations of all essential amino acids in contrast with non-stunted children, as well as lower serum concentrations of conditionally essential amino acids, non-essential amino acids and six sphingolipids and variations in the concentrations of glycerophospholipids [53] (Semba et al., 2016). Likewise, a study by Hemp et al. (2019) [54] found that the breast milk of mothers with stunted infants, in comparison to milk from mothers with a body mass index higher than 18.5, was lower in 6 amino acids/biogenic amines [54] (Hampel et al., 2019).
In the present study, we will apply untargeted metabolomics approaches to analyze maternal and infant blood, as well as targeted and untargeted metabolomics approaches to analyze breast milk. The untargeted metabolomics analyses will be conducted via rapid liquid chromatography–mass spectrometry (rLC-MS), using a previously developed method [42] (Villar et al., 2022), and targeted metabolomics analyses will be conducted using LC-MS-MS and FIA-MS/MS [52] (Langsdorf et al., 2023).
2.4.2. Metagenomics
Early life is a period during which the infant gut microbiome is established, ultimately influencing health and disease later in life [55] (Tanaka and Nakayama, 2017). An important factor affecting the development and composition of the infant gut microbiome is nutrition [56,57,58] (Marques et al., 2010; Bäckhed et al., 2015; Gritz and Bhandari, 2015). Human milk has been demonstrated to induce differences in the composition of the microbiota [58,59] (Marques et al., 2010; Gomez-Llorente et al., 2013). It is established that an inadequate maturation of the gut microbiome can lead to the development of child malnutrition, both moderate and severe [60] (Vray et al., 2018).
To understand this, inStrain will be used to profile fecal metagenomes recovered by shotgun sequencing maternal and infant samples [10] (Olm et al., 2021), and KofamKOALA will be used to obtain functional annotations [44] (Aramaki et al., 2020). Similarly, we will assess differences in the microbiome compositions of milk samples. Within the framework of the International Milk Composition Consortium, the milk microbiome will be analyzed via 16S rRNA sequencing. An additional comparison will be performed to determine the associations of the microbiome with maternal health, adverse birth outcomes and infant growth.
2.4.3. Proteomics
The field of proteomics is a high-throughput approach employed to identify the full spectrum of proteins in an organism, tissue, cells or bodily fluid. This approach investigates the functional states of proteins, including protein–protein interactions and posttranslational modifications [61] (Adeola et al., 2017). Navarro et al. (2015) [62] noted that several pathways differed between interventions with glucosamine and chondroitin supplementation and a placebo (Navarro et al., 2015) [62].
In the present study, the untargeted quantification of proteins and peptides in prenatal maternal plasma and breast milk samples will be conducted according to a standardized liquid chromatography–tandem mass spectrometry (LC-MS/MS) workflow [45] (Mc Ardle et al., 2022).
2.4.4. Breast Milk Characterization
Numerous components from breast milk influence the infant microbiota by enhancing the growth of specific bacteria or limiting the growth of others [63] (Boudry et al., 2021). Human milk oligosaccharides (HMOs) interact with gut microbiota by supporting the growth of beneficial bacterial and providing anti-pathogenic effects [64] (Zhang et al., 2021). Another example is lactoferrin, a non-heme iron-binding protein that plays an important role in iron absorption and protection against bacteria [65] (Demmelmair et al., 2017). The impact of maternal supplementation on the interplay of macronutrients, micronutrients and bioactive compounds in human milk is not yet fully understood, highlighting the need for further investigation.
Breast milk samples were distributed to and will be analyzed at multiple laboratories for macronutrients, micronutrients, oligosaccharides, growth factors, immunoglobulins, cytokines, metabolites and microbes. The analyses to be performed on the breast milk and the laboratories employed are summarized in Table 4.

Table 4.
Summary of the analyses performed on breast milk.
2.5. Human Biomonitoring
Dietary and environmental contaminants, specifically those common in rural, low-income settings (e.g., smoke pollution from cooking, mycotoxins, herbicides, insecticides and pesticides) play important mediating roles and must be considered. The totality of these exposures is the “exposome” [66,67] (Wild, 2005; Miller and Jones, 2014), the study of which is an emerging field with great potential to advance human health research. A biomonitoring analysis will provide insight to determine if the aforementioned exposures act as effect modifiers in the relationship between the provision of BEP and maternal health, birth outcomes and infant growth, as well as any association between the exposure and the relative TL and mtDNAc. A summary of the analyses and the laboratories used are shown in Table 5.

Table 5.
Environmental contaminants investigated in the BioSpé study.
2.5.1. Telomere Length and Mitochondrial DNA
Telomeres protect DNA coding sequences from degradation and prevent the aberrant fusion of chromosomes [81] (Blackburn, Epel and Lin, 2015). In somatic cells, telomeres shorten after each cell division due to the incomplete replication of DNA molecules and maintenance mechanisms that are unable to prevent telomere attrition [82] (Wang et al., 2021). Previous research suggests that short telomeres are associated with cardiovascular disease and mortality [83,84] (Haycock et al., 2014; Wang et al., 2018). In Ghana, prenatal supplementation had no impact on TLs at 4–6 years of age when compared to IFA. However, in Greece, adults receiving a daily combination of vitamin supplements for 6 to 12 months had longer TLs compared to the control group [85] (Tsoukalas et al., 2019).
Mitochondria play a critical role in the production of energy through aerobic respiration, resulting in the formation of adenosine triphosphate [86] (Roger, Muñoz-Gómez and Kamikawa, 2017). The mtDNA is theoretically more susceptible to damage to oxidative stress as it is situated close to the sites of oxidative phosphorylation (e.g., reactive oxygen species). Additionally, mtDNA lacks protection from the histones present in nuclear DNA [87,88] (Tait and Green, 2013; Copeland and Longley, 2014). Mitochondrial dysfunction during the neonatal period and infancy has been related to heart arrhythmia and poor weight gain [89,90] (Gibson et al., 2008; Kohda et al., 2016), whereas in adulthood, mitochondrial dysfunction has been implicated in Alzheimer’s disease and cancer [91] (Druzhyna, Wilson and LeDoux, 2008). In Indonesia, one trial assessed the impact of prenatal MMN supplementation on the mtDNA in venous blood of pregnant women and reported lower post-supplementation mtDNA compared to IFA, indicating improved mitochondrial efficiency [92] (Priliani et al., 2019).
In the scope of this study, the efficacy of a prenatal BEP supplement and an IFA tablet on newborn relative TL and mtDNAc were compared to the efficacy of IFA alone, and differences in relative TL and mtDNAc across adverse birth outcomes (i.e., SGA, LBW or preterm births) were measured via a real-time PCR method, using whole arterial blood collected from the umbilical cord [68,69,70,71,72] (Cawthon, 2002, 2009; Janssen et al., 2012; Martens et al., 2016, 2020) The results of the effect of the fortified BEP supplementation on the newborn genome were reported in a separate manuscript [14] (Hanley-Cook et al., no date).
2.5.2. Air and Smoke Pollution
In Ouagadougou, the capital of Burkina Faso, 60% of households use biomass-based fuels as their primary cooking fuel [93] (Sana et al., 2019). In 2002, it was estimated that 21,500 deaths in Burkina Faso were attributed to domestic air pollution [94] (WHO, 2007). Exposure to particulate matter air pollution, such as BC, during early life has been linked to adverse pregnancy outcomes, including a LBW [95] (Pedersen et al., 2013), increased cardiovascular morbidity and mortality [96,97] (Brook et al., 2010; Nawrot et al., 2011). In 469 mother–newborn pairs, in utero exposure to particulate matter during the third trimester of pregnancy was linked to a lower placental iodine load, an element that is important for fetal brain development and growth [98] (Neven et al., 2021).
In the present study, using confocal microscopy, the level of BC will be assessed in in whole arterial blood collected from the umbilical cord [73,74] (Saenen et al., 2017; Bové et al., 2019).
2.5.3. Mycotoxins
Mycotoxins are secondary fungal metabolites found on food and feed that can disturb gut microbial homeostasis, metabolism and the integrity of the intestinal barrier [1,3,6,8,10,13,15,17,19,20,26,29,39,40,43,45,46,49,50,51,52,53,55,62,66,70,71,72,74,78,80,86,88,90,92,93,95,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113] (Hussein and Brasel, 2001; Vidal et al., 2018). In LMICs, mycotoxins pose health risks due to their high abundance and acute intrinsic toxicity [114] (Yacine Ware et al., 2017). Children are vulnerable due to their lower body mass, higher metabolic rate and developing detoxification system [107] (Peraica, Richter and Rašić, 2014). A study in Ethiopia reported a high occurrence of long-tern maternal aflatoxin exposure and an associated risk of poorer fetal growth trajectories [111] (Tesfamariam et al., 2022). In Burkina Faso, limited biological and toxicological food contamination data are available [101] (Kpoda et al., 2022), and regulations or legislation concerning mycotoxins are often not implemented [115,116] (FAO, 2003; Warth et al., 2012).
Maternal and infant whole blood, extracted via VAMS, will be analyzed for mycotoxins using an adapted LC-MS/MS methodology [23] (Vidal et al., 2021).
2.5.4. Environmental Contaminants
Burkina Faso’s economy relies heavily on the agricultural sector, which provides employment for most of the population and generates nearly half of the gross domestic product, yet disease and animal pests cause significant damage to crops. To address this, plant protection products are used to eradicate pests [106] (Ouedraogo et al., 2011), leading to high levels of exposure among the Burkinabè population [117,118,119] (Lehmann et al., 2017, 2018; Son et al., 2018). A study by [101] Kpoda et al. (2022) reported that more than 58% of the food samples collected from Burkinabè markets (i.e., cereals, oilseeds, vegetables and dried fish) contained at least one pesticide. Researchers postulate that exposure to these pesticides increases the chances of miscarriage and other adverse birth outcomes [101] (Kpoda et al., 2022). Previous studies in India and Egypt reported that interventional strategies reduced pesticide-induced oxidative effects [104,109] (Saad-Hussein et al., 2020; Medithi et al., 2022).
Analyses of environmental contaminants (i.e., herbicides, insecticides and pesticides) in maternal urine will be conducted using LC-MS/MS and LC–high resolution mass spectrometry (LC-HRMS) [75,76] (Gys et al., 2020; Caballero-Casero et al., 2021).
2.5.5. Gut Enteropathogens
The TaqMan array card (TAC) is a 384-well platform that uses primers and probes specific to the targets pre-allocated on the card, allowing for the simultaneous detection of up to 48 targets in one specimen [102,120] (Diaz et al., 2013; Liu et al., 2013). Additional advantages of the TAC assay include its ease of use and excellent reproducibility [121,122] (Weinberg et al., 2013; Diaz et al., 2019). TAC is an ideal platform for multi-pathogen detection in low-resource settings [103,105,121] (Diaz et al., 2019; Moore et al., 2019; Marks et al., 2021). In Nepal, Tanzania and Bangladesh, a TAC analysis detected multiple pathogens with high sensitivity and enhanced the understanding of mixed infections detected in one matrix [78] (Liu et al., 2014).
In this study, a TAC analysis will be performed on maternal and infant fecal samples to assess the pathogen burden, as previously described [77,78] (Liu et al., 2014; Deboer et al., 2018).
2.5.6. Fecal Inflammatory Markers
Calprotectin
Calprotectin is a calcium-binding protein belonging to the S100 series [123,124] (Yui, Nakatani and Mikami, 2003; Jukic et al., 2021) that accounts for 30–60% of the protein content of neutrophils [125] (Dale et al., 1985). Calprotectin interferes in physiological behaviors such as cell differentiation, immune regulation and inflammation [123] (Jukic et al., 2021). The release of calprotectin in the gastrointestinal tract lumen and its excretion in feces are consequences of an inflammatory process that prompts the migration of neutrophils into the gastrointestinal tissue. Calprotectin is therefore a robust and noninvasive marker for intestinal inflammation, whether acute or chronic [126] (Summerton et al., 2002).
Am enzyme-linked immunosorbent assay (ELISA) will be applied to maternal and infant fecal samples to detect calprotectin since it has demonstrated acceptable intra- and inter batch precision, good recovery and dilution linearity across different concentrations [114] (Whitehead et al., 2013).
Short-Chain Fatty Acids
Short-chain fatty acids (SCFAs) are formed in the colon via the fermentation of proteins and non-digestible carbohydrates [127] (Cummings and Macfarlane, 1997). SCFAs are sources of energy for the epithelium and regulate differentiation and proliferation [128,129] (Louis and Flint, 2009; Blad, Tang and Offermanns, 2012). Butyric acid, an essential SCFA, protects against inflammatory bowel disease [130] (Berries et al., 2018), colorectal cancer [53,131] (Scheppach, Bartram and Richter, 1995; Gomes et al., 2018) and cardiovascular disease [86] (Richards et al., 2016). Butyrate regulates the epithelial barrier by organizing proteins, thus reducing the permeability of bacteria [132] (Cani et al., 2008). A study examining dietary effects on gut microbiota found that Burkinabè infants had increased bacterial diversity and higher levels of SCFAs in their feces compared to European children [133] (De Filippo et al., 2010).
A quantitative analysis of SCFAs will be conducted in maternal and infant fecal samples by means of capillary gas chromatography (GC) coupled with a flame ionization detector (FID) [80] (Toe et al., 2020).
3. Metadata
For all participants, questionnaires and lifestyle and clinical data were collected. In addition, the participants’ dietary intakes were assessed using weekly food-group-diversity questionnaires. Complete details on the metadata collection were described previously [113] (Vanslambrouck et al., 2021).
4. Data Quality Control
The MISAME-III field data were collected using SurveySolutions v.21.5 on tablets and synchronized to a cloud-based server weekly. Furthermore, generic validation codes were programmed to limit the entry of implausible values and to improve data quality. Bi-weekly data quality checks were conducted, and missing or inconsistent data were sent back to the field for revision. To ensure the quality of the ultrasound images and the estimations of gestational age (GA), an external gynecologist regularly evaluated 10% of the examinations using a quality checklist and scoring sheet. The trained project workers collected daily data on BEP and IFA compliance in both prenatal study arms via smartphone-assisted personal interviewing programmed in CSPro v.7.3.1. Six supervisors performed monthly lot quality assurance sampling (LQAS) schemes of each home visitor’s work on a random day [8] (Valadez et al., 1996).
5. Ethical Considerations
The protocol of this study was approved by the ethics committee of Ghent University Hospital in Belgium (B670201734334) and the ethics committee of the Institut de Recherche en Sciences de la Santé in Burkina Faso (50-2020/CEIRES). An independent Data and Safety Monitoring Board (DSMB), comprising an endocrinologist, two pediatricians, a gynecologist and an ethicist of both Belgian and Burkinabè nationalities, was established prior to the start of the efficacy trial. The DSMB managed remote safety reviews for adverse and serious events at 9 and 20 months after the initiation of enrolment. The MISAME-III trial was registered on ClinicalTrials.gov (identifier: NCT03533712).
6. Strengths and Limitations
The BioSpé sub-study of the MISAME-III project is unique in that compliance to BEP and IFA supplementation was verified by a community-based network of home visitors, leading to high levels of observed adherence. Moreover, quantitative 24 h dietary recalls were conducted to assess whether the daily energy and micronutrient requirements were met by integrating the BEP supplement with the participants’ regular diets, as well as to eliminate the possibility of any dietary substitution [100] (de Kok, Moore, et al., 2021). Furthermore, by collecting diverse biological specimens from mother–infant dyads at various timepoints, we were able to obtain comprehensive -omics data and conduct biomonitoring on the contaminants present in these samples. In conjunction with lifestyle data, this will assist in evaluating the physiological effect of maternal perinatal BEP supplementation. Additionally, it will facilitate the understanding of the intermediary role of environmental contaminants in the relationship between BEP supplementation, maternal health, birth outcomes and infant growth in Burkina Faso.
An additional strength of this study lies in the comprehensive characterization of multi-omics profiles, including exogenous and endogenous exposures. Additionally, the prospective, longitudinal collection of biospecimens minimizes the possibility of measurement errors in the analysis. Our research group’s diverse expertise across numerous disciplines enables a holistic approach to assessing exposures and biological responses. Moreover, to avoid interlaboratory variability and ensure consistency, all laboratory experimentation for each -omics and exposure analysis will be conducted in the same laboratory.
A notable limitation of this study is its sample size, which is insufficient to thoroughly investigate rare diseases or extreme values for continuous traits unless combined with data from other cohorts. Also, the study is monocentric, and the study population is largely homogenous (i.e., rural and African); therefore, its generalizability to the larger population, and other geographical regions and urban settings, is limited.
In conclusion, the BioSpé study will help generate evidence-based health prevention and intervention strategies that improve maternal health, enhance birth outcomes, promote healthy infant growth and address related health issues such as metabolic disorders and accelerated biological aging. In doing so, the results will help us understand mechanistic pathways that will provide valuable insights to inform policy decisions in public health.
Author Contributions
Author Conceptualization: Y.B.-M., L.O., M.D.B., G.T.H.-C., L.H., L.C.T., P.K., C.L., S.D.S. and T.D.-C.; data curation: Y.B.-M., A.A., L.O., L.D. and T.D.-C.; funding acquisition: L.H., C.L., P.K., M.D.B. and T.D.-C.; investigation: Y.B.-M., L.O., L.C.T. and T.D.-C.; project administration: Y.B.-M., L.O., M.D.B., M.O., A.C., R.G., C.L., P.K., S.D.S. and T.D.-C.; resources: Y.B.-M., L.O., M.O., L.C.T. and T.D.-C.; software: Y.B.-M., A.A., G.T.H.-C., B.d.K., L.D., K.T. and T.D.-C.; supervision: C.L., M.D.B. and T.D.-C.; writing—original draft: Y.B.-M. and T.D.-C.; writing—review and editing: Y.B.-M., L.O., M.D.B., A.A., G.T.H.-C., L.D., K.T., L.H., L.C.T., C.L., S.D.S. and T.D.-C. All authors have read and agreed to the published version of the manuscript.
Funding
The work is supported by the Bill & Melinda Gates Foundation (OPP1175213). The MDB is supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement No 946192, HUMYCO). The funders had no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Commissie voor Medische Ethiek (CME) of Ghent University Hospital (protocol code: B670201734334 and date of 10/08/2020) and the Comité d’Éthique Institutionnel de la Recherche En Sciences de la Santé (CEIRES) of the Institut de Recherche en Sciences de la Santé (IRSS) (protocol code 50-2020/CEIRES and date of 22/10/2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Given the personal nature of the data, data will be made available through a data-sharing agreement. Please contact carl.lachat@ugent.be for any queries. Supporting study documents, including the study protocol and questionnaires, are publicly available on the study’s website: https://misame3.ugent.be (accessed on 18 September 2023).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
ANC, antenatal care; BC, black carbon; BEP, balanced energy–protein; BioSpé, biospecimen sub-study; BMGF, Bill & Melinda Gates Foundation; DSMB, Data and Safety Monitoring Board; EDTA, ethylene diamine tetra acetic acid; ELISA, enzyme-linked immunosorbent assay; FIA-MS/MS, flow injection analysis–tandem mass spectrometry; FID, flame ionization detector; GA, gestational age; GC, gas chromatography; GWG, gestational weight gain; HMO, human milk oligosaccharides; IFA, iron–folic acid; LAZ, length-for-age z-score; LBW, low birth weight; LC-MS/MS, liquid chromatography–tandem mass spectrometry; LC-HRMS, liquid chromatography–high resolution mass spectrometry; LMIC, low- or middle-income country; LQAS, lot quality assurance sampling; MISAME, micronutriments pour la santé de la mère et de l’enfant; mtDNAc, mitochondrial DNA content; PLW, pregnant and lactating women; rLC-MS, rapid liquid chromatography–tandem mass spectrometry; RCT, randomized controlled trial; qPCR, quantitative polymerase chain reaction; SCFA, short-chain fatty acids; SGA, small-for-gestational age; TAC, TaqMan array card; TL, telomere length; VAMS, volumetric absorptive microsampling.
References
- UNICEF-WHO. Low Birthweight Estimates: Levels and Trends 2000–2015; World Health Organization: Geneva, Switzerland, 2019; Volume 4, pp. 3–9. Available online: https://www.unicef.org/reports/UNICEF-WHO-low-birthweight-estimates-2019 (accessed on 16 January 2023).
- Benammar, A.; Sermondade, N.; Faure, C.; Dupont, C.; Cedrin-Durnerin, I.; Sifer, C.; Hercberg, S.; Levy, R. Nutrition et fausses couches spontanées: Une revue de la littérature. Gynécologie Obs. Fertil. 2012, 40, 162–169. [Google Scholar] [CrossRef] [PubMed]
- McClure, E.M.; Saleem, S.; Pasha, O.; Goldenberg, R.L. Stillbirth in developing countries: A review of causes, risk factors and prevention strategies. J. Matern.-Fetal Neonatal Med. 2009, 22, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Almond, D.; Chay, K.; Lee, D. The Costs of Low Birth Weight. Q. J. Econ. 2004, 120, 1031–1083. [Google Scholar] [CrossRef]
- WHO. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. In WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience; World Health Organization: Geneva, Switzerland, 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK409108/ (accessed on 25 January 2022).
- Ota, E.; Tobe-Gai, R.; Mori, R.; Farrar, D. Antenatal dietary advice and supplementation to increase energy and protein intake. Cochrane Database Syst. Rev. 2012, 9, CD000032. [Google Scholar] [CrossRef]
- Gernand, A.D.; Gallagher, K.; Bhandari, N.; Kolsteren, P.; Lee, A.C.; Shafiq, Y.; Taneja, S.; Tielsch, J.M.; Abate, F.W.; Baye, E.; et al. Harmonization of maternal balanced energy-protein supplementation studies for individual participant data (IPD) meta-analyses—Finding and creating similarities in variables and data collection. BMC Pregnancy Childbirth 2023, 23, 107. [Google Scholar] [CrossRef]
- Vanslambrouck, K.; de Kok, B.; Toe, L.C.; De Cock, N.; Ouedraogo, M.; Dailey-Chwalibóg, T.; Hanley-Cook, G.; Ganaba, R.; Lachat, C.; Huybregts, L.; et al. Effect of balanced energy-protein supplementation during pregnancy and lactation on birth outcomes and infant growth in rural Burkina Faso: Study protocol for a randomised controlled trial. BMJ Open 2021, 11, e038393. [Google Scholar] [CrossRef]
- Villar, J.; Altman, D.G.; Purwar, M.; Noble, J.A.; Knight, H.E.; Ruyan, P.; Ismail, L.C.; Barros, F.C.; Lambert, A.; Papageorghiou, A.T.; et al. The objectives, design and implementation of the INTERGROWTH-21st Project. BJOG Int. J. Obstet. Gynaecol. 2013, 120 (Suppl. 2), 9–26. [Google Scholar] [CrossRef]
- De Onis, M.; Branca, F. Childhood stunting: A global perspective. Matern. Child Nutr. 2016, 12 (Suppl. 1), 12–26. [Google Scholar] [CrossRef]
- Argaw, A.; Toe, L.C.; Hanley-Cook, G.; Dailey-Chwalibóg, T.; de Kok, B.; Ouédraogo, L.; Compaoré, A.; Ouédraogo, M.; Sawadogo, A.; Ganaba, R.; et al. Effect of prenatal micronutrient-fortified balanced energy-protein supplementation on maternal and newborn body composition: A sub-study from the MISAME-III randomized controlled efficacy trial in rural Burkina Faso. PLoS Med. 2023, 20, e1004242. [Google Scholar] [CrossRef]
- Argaw, A.; de Kok, B.; Toe, L.C.; Hanley-Cook, G.; Dailey-Chwalibóg, T.; Ouédraogo, M.; Compaoré, A.; Vanslambrouck, K.; Ganaba, R.; Kolsteren, P.; et al. Fortified balanced energy–protein supplementation during pregnancy and lactation and infant growth in rural Burkina Faso: A 2 × 2 factorial individually randomized controlled trial. PLoS Med. 2023, 20, e1004186. [Google Scholar] [CrossRef]
- De Kok, B.; Toe, L.C.; Hanley-Cook, G.; Argaw, A.; Ouédraogo, M.; Compaoré, A.; Vanslambrouck, K.; Dailey-Chwalibóg, T.; Ganaba, R.; Kolsteren, P.; et al. Prenatal fortified balanced energy-Protein supplementation and birth outcomes in rural Burkina Faso: A randomized controlled efficacy trial. PLoS Med. 2022, 19, e1004002. [Google Scholar] [CrossRef]
- Hanley-Cook, G.T.; Bastos-Moreira, Y.; Martens, D.S.; Dailey-Chwalibóg, T.; Toe, L.C.; de Kok, B.; Ouédraogo, L.; Argaw, A.; Tesfamariam, K.; Kolsteren, P.; et al. Prenatal multiple micronutrient-fortified balanced energy-protein supplementation and newborn telomere length and mitochondrial DNA content: A randomized controlled efficacy trial in rural Burkina Faso. Manuscript submitted for publication.
- Perumal, N.; Bassani, D.G.; Roth, D.E. Use and Misuse of Stunting as a Measure of Child Health. J. Nutr. 2018, 148, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Hanley-Cook, G.; Toe, L.C.; Tesfamariam, K.; de Kok, B.; Argaw, A.; Compaoré, A.; Ouédraogo, M.; Dailey-Chwalibóg, T.; Kolsteren, P.; Lachat, C.; et al. Fortified Balanced Energy-Protein Supplementation, Maternal Anemia, and Gestational Weight Gain: A Randomized Controlled Efficacy Trial among Pregnant Women in Rural Burkina Faso. J. Nutr. 2022, 152, 2277–2286. [Google Scholar] [CrossRef] [PubMed]
- De Kok, B.; Argaw, A.; Hanley-Cook, G.; Toe, L.C.; Ouédraogo, M.; Dailey-Chwalibóg, T.; Diop, L.; Becquey, E.; Kolsteren, P.; Lachat, C.; et al. Fortified Balanced Energy-Protein Supplements Increase Nutrient Adequacy without Displacing Food Intake in Pregnant Women in Rural Burkina Faso. J. Nutr. 2021, 151, 3831–3840. [Google Scholar] [CrossRef]
- Hanley-Cook, G.T.; Argaw, A.; de Kok, B.; Toe, L.C.; Dailey-Chwalibóg, T.; Ouédraogo, M.; Kolsteren, P.; Huybregts, L.; Lachat, C. Seasonality and Day-to-Day Variability of Dietary Diversity: Longitudinal Study of Pregnant Women Enrolled in a Randomized Controlled Efficacy Trial in Rural Burkina Faso. J. Nutr. 2022, 152, 2145–2154. [Google Scholar] [CrossRef]
- Ministère de la Santé—Burkina Faso. Politique et Normes en Matière de Santé de la Reproduction; Ministère de la Santé: Ouagadougou, Burkina Faso, 2010. Available online: https://www.prb.org/wp-content/uploads/2018/05/Politiques-et-Normes-en-Matière-de-Santeé-de-la-Reproduction-au-Burkina-Faso-2010.pdf (accessed on 9 July 2023).
- Jones, L.; de Kok, B.; Moore, K.; de Pee, S.; Bedford, J.; Vanslambrouck, K.; Toe, L.C.; Lachat, C.; De Cock, N.; Ouédraogo, M.; et al. Acceptability of 12 fortified balanced energy protein supplements—Insights from Burkina Faso. Matern. Child Nutr. 2021, 17, e13067. [Google Scholar] [CrossRef]
- Bill & Melinda Gates Foundation. Framework and Specifications for the Nutritional Composition of a Food Supplement for Pregnant and Lactating Women (PLW) In Undernourished and Low Income Settings; Gates Open Research: Seattle, WA, USA, 2017; pp. 1–23. [Google Scholar] [CrossRef]
- Allen, L.H.; Carriquiry, A.L.; Murphy, S.P. Perspective: Proposed Harmonized Nutrient Reference Values for Populations. Adv. Nutr. 2020, 11, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.; Belova, L.; Stove, C.; De Boevre, M.; De Saeger, S. Volumetric Absorptive Microsampling as an Alternative Tool for Biomonitoring of Multi-Mycotoxin Exposure in Resource-Limited Areas. Toxins 2021, 13, 345. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Eiden, M.; Morin-Rivron, D.; Christinat, N.; Monteiro, J.P.; Kaput, J.; Masoodi, M. Impact of multi-micronutrient supplementation on lipidemia of children and adolescents. Clin. Nutr. 2020, 39, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Weissinger, E.M.; Nguyen-Khoa, T.; Fumeron, C.; Saltiel, C.; Walden, M.; Kaiser, T.; Mischak, H.; Drüeke, T.B.; Lacour, B.; Massy, Z.A. Effects of oral vitamin C supplementation in hemodialysis patients: A proteomic assessment. Proteomics 2006, 6, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Smith, D.L.; Esteves, K.; Drury, S. Telomere length measurement by qPCR—Summary of critical factors and recommendations for assay design. Psychoneuroendocrinology 2019, 99, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Barr, D.B.; Wilder, L.C.; Caudill, S.P.; Gonzalez, A.J.; Needham, L.L.; Pirkle, J.L. Urinary creatinine concentrations in the U.S. population: Implications for urinary biologic monitoring measurements. Environ. Health Perspect. 2005, 113, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Esteban, M.; Castaño, A. Non-invasive matrices in human biomonitoring: A review. Environ. Int. 2009, 35, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, Z.; Zhang, W.; Zhang, C.; Zhang, Y.; Mei, H.; Zhuo, N.; Wang, H.; Wang, L.; Wu, D. Comparison of gut microbiota in exclusively breast-fed and formula-fed babies: A study of 91 term infants. Sci. Rep. 2020, 10, 15792. [Google Scholar] [CrossRef]
- Walker, W.A.; Iyengar, R.S. Breast milk, microbiota, and intestinal immune homeostasis. Pediatr. Res. 2015, 77, 220–228. [Google Scholar] [CrossRef]
- Bardanzellu, F.; Fanos, V.; Reali, A. “Omics” in Human Colostrum and Mature Milk: Looking to Old Data with New Eyes. Nutrients 2017, 9, 843. [Google Scholar] [CrossRef]
- Bardanzellu, F.; Fanos, V.; Strigini, F.A.L.; Artini, P.G.; Peroni, D.G. Human Breast Milk: Exploring the Linking Ring Among Emerging Components. Front. Pediatr. 2018, 6, 215. [Google Scholar] [CrossRef]
- Bardanzellu, F.; Peila, C.; Fanos, V.; Coscia, A. Clinical insights gained through metabolomic analysis of human breast milk. Expert Rev. Proteom. 2019, 16, 909–932. [Google Scholar] [CrossRef]
- Bardanzellu, F.; Puddu, M.; Peroni, D.G.; Fanos, V. The Human Breast Milk Metabolome in Overweight and Obese Mothers. Front. Immunol. 2020, 11, 1533. [Google Scholar] [CrossRef]
- Bardanzellu, F.; Reali, A.; Marcialis, M.A.; Fanos, V. Across the “Sweetest” Properties of Human Breast Milk: Focus on Oligosaccharides. Curr. Pediatr. Rev. 2021, 16, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Congiu, M.; Reali, A.; Deidda, F.; Dessì, A.; Bardanzellu, F.; Fanos, V. Breast Milk for Preterm Multiples: More Proteins, Less Lactose. Twin Res. Hum. Genet. 2019, 22, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Dallas, D.C.; Guerrero, A.; Khaldi, N.; Castillo, P.A.; Martin, W.F.; Smilowitz, J.T.; Bevins, C.L.; Barile, D.; German, J.B.; Lebrilla, C.B. Extensive in vivo Human Milk Peptidomics Reveals Specific Proteolysis Yielding Protective Antimicrobial Peptides. J. Proteome Res. 2013, 12, 2295–2304. [Google Scholar] [CrossRef] [PubMed]
- van Herwijnen, M.J.; Zonneveld, M.I.; Goerdayal, S.; Hoen, E.N.N.; Garssen, J.; Stahl, B.; Altelaar, A.M.; Redegeld, F.A.; Wauben, M.H. Comprehensive Proteomic Analysis of Human Milk-derived Extracellular Vesicles Unveils a Novel Functional Proteome Distinct from Other Milk Components. Mol. Cell. Proteom. 2016, 15, 3412–3423. [Google Scholar] [CrossRef]
- Kisuse, J.; La-Ongkham, O.; Nakphaichit, M.; Therdtatha, P.; Momoda, R.; Tanaka, M.; Fukuda, S.; Popluechai, S.; Kespechara, K.; Sonomoto, K.; et al. Urban Diets Linked to Gut Microbiome and Metabolome Alterations in Children: A Comparative Cross-Sectional Study in Thailand. Front. Microbiol. 2018, 9, 1345. [Google Scholar] [CrossRef]
- Nguyen, Q.P.; Karagas, M.R.; Madan, J.C.; Dade, E.; Palys, T.J.; Morrison, H.G.; Pathmasiri, W.W.; McRitche, S.; Sumner, S.J.; Frost, H.R.; et al. Associations between the gut microbiome and metabolome in early life. BMC Microbiol. 2021, 21, 238. [Google Scholar] [CrossRef]
- Gonzalez, C.G.; Zhang, L.; Elias, J.E. From mystery to mechanism: Can proteomics build systems-level understanding of our gut microbes? Expert Rev. Proteom. 2017, 14, 473–476. [Google Scholar] [CrossRef]
- Villar, J.; Ochieng, R.; Gunier, R.B.; Papageorghiou, A.T.; Rauch, S.; McGready, R.; Gauglitz, J.M.; Barros, F.C.; Vatish, M.; Fernandes, M.; et al. Association between fetal abdominal growth trajectories, maternal metabolite signatures early in pregnancy, and childhood growth and adiposity: Prospective observational multinational INTERBIO-21st fetal study. Lancet Diabetes Endocrinol. 2022, 10, 710–719. [Google Scholar] [CrossRef]
- Olm, M.R.; Crits-Christoph, A.; Bouma-Gregson, K.; Firek, B.A.; Morowitz, M.J.; Banfield, J.F. inStrain profiles population microdiversity from metagenomic data and sensitively detects shared microbial strains. Nat. Biotechnol. 2021, 39, 727–736. [Google Scholar] [CrossRef]
- Aramaki, T.; Blanc-Mathieu, R.; Endo, H.; Ohkubo, K.; Kanehisa, M.; Goto, S.; Ogata, H. KofamKOALA: KEGG Ortholog assignment based on profile HMM and adaptive score threshold. Bioinformatics 2019, 36, 2251–2252. [Google Scholar] [CrossRef]
- Mc Ardle, A.; Binek, A.; Moradian, A.; Orgel, B.C.; Rivas, A.; Washington, K.E.; Phebus, C.; Manalo, D.-M.; Go, J.; Venkatraman, V.; et al. Standardized Workflow for Precise Mid- and High-Throughput Proteomics of Blood Biofluids. Clin. Chem. 2021, 68, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Smilowitz, J.T.; Gho, D.S.; Mirmiran, M.; German, J.B.; Underwood, M.A. Rapid measurement of human milk macronutrients in the neonatal intensive care unit: Accuracy and precision of fourier transform mid-infrared spectroscopy. J. Hum. Lact. 2014, 30, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Hampel, D.; Dror, D.K.; Allen, L.H. Micronutrients in Human Milk: Analytical Methods. Adv. Nutr. 2018, 9 (Suppl. 1), 313S–331S. [Google Scholar] [CrossRef] [PubMed]
- Hampel, D.; York, E.R.; Allen, L.H. Ultra-performance liquid chromatography tandem mass-spectrometry (UPLC-MS/MS) for the rapid, simultaneous analysis of thiamin, riboflavin, flavin adenine dinucleotide, nicotinamide and pyridoxal in human milk. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 903, 7–13. [Google Scholar] [CrossRef]
- Kellman, B.P.; Richelle, A.; Yang, J.-Y.; Chapla, D.; Chiang, A.W.T.; Najera, J.A.; Liang, C.; Fürst, A.; Bao, B.; Koga, N.; et al. Elucidating Human Milk Oligosaccharide biosynthetic genes through network-based multi-omics integration. Nat. Commun. 2022, 13, 2455. [Google Scholar] [CrossRef]
- Ju, H.; Lai, G.; Yan, F. Electrochemiluminescent immunosensing. In Immunosensing for Detection of Protein Biomarkers; Elsevier: Amsterdam, The Netherlands, 2017; pp. 171–206. [Google Scholar] [CrossRef]
- Ramani, S.; Stewart, C.J.; Laucirica, D.R.; Ajami, N.J.; Robertson, B.; Autran, C.A.; Shinge, D.; Rani, S.; Anandan, S.; Hu, L.; et al. Human milk oligosaccharides, milk microbiome and infant gut microbiome modulate neonatal rotavirus infection. Nat. Commun. 2018, 9, 5010. [Google Scholar] [CrossRef]
- Langsdorf, M.; Ömer, G.; Dearth, S.; Röhring, C.; Pham, T.H.; Dallmann, G.; Koal, T. Standardized Quantitative Metabolomics Using the Biocrates MxP® Quant 500 XL Kit across Mass Spectrometer Platforms. 2023. Available online: https://biocrates.com/wp-content/uploads/2023/06/Application-note-Quant500-XL-across-MS-platforms.pdf (accessed on 10 September 2023).
- Semba, R.D.; Shardell, M.; Ashour, F.A.S.; Moaddel, R.; Trehan, I.; Maleta, K.M.; Ordiz, M.I.; Kraemer, K.; Khadeer, M.A.; Ferrucci, L.; et al. Child Stunting is Associated with Low Circulating Essential Amino Acids. EBioMedicine 2016, 6, 246–252. [Google Scholar] [CrossRef]
- Hampel, D.; Shahab-Ferdows, S.; Hossain, M.; Islam, M.M.; Ahmed, T.; Allen, L.H. Validation and Application of Biocrates AbsoluteIDQ® p180 Targeted Metabolomics Kit Using Human Milk. Nutrients 2019, 11, 1733. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakayama, J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol. Int. Off. J. Jpn. Soc. Allergol. 2017, 66, 515–522. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Gritz, E.C.; Ebhandari, V. The Human Neonatal Gut Microbiome: A Brief Review. Front. Pediatr. 2015, 3, 17. [Google Scholar] [CrossRef]
- Marques, T.M.; Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Ryan, C.A.; Stanton, C. Programming infant gut microbiota: Influence of dietary and environmental factors. Curr. Opin. Biotechnol. 2010, 21, 149–156. [Google Scholar] [CrossRef]
- Gomez-Llorente, C.; Plaza-Diaz, J.; Aguilera, M.; Muñoz-Quezada, S.; Bermudez-Brito, M.; Peso-Echarri, P.; Martinez-Silla, R.; Vasallo-Morillas, M.I.; Campaña-Martin, L.; Vives-Piñera, I.; et al. Three Main Factors Define Changes in Fecal Microbiota Associated With Feeding Modality in Infants. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 461–466. [Google Scholar] [CrossRef]
- Vray, M.; Hedible, B.G.; Adam, P.; Tondeur, L.; Manirazika, A.; Randremanana, R.; Mainassara, H.; Briend, A.; Artaud, C.; von Platen, C.; et al. A multicenter, randomized controlled comparison of three renutrition strategies for the management of moderate acute malnutrition among children aged from 6 to 24 months (the MALINEA project). Trials 2018, 19, 666. [Google Scholar] [CrossRef]
- Adeola, H.A.; Blackburn, J.M.; Rebbeck, T.R.; Zerbini, L.F. Emerging proteomics biomarkers and prostate cancer burden in Africa. Oncotarget 2017, 8, 37991–38007. [Google Scholar] [CrossRef]
- Navarro, S.L.; White, E.; Kantor, E.D.; Zhang, Y.; Rho, J.; Song, X.; Milne, G.L.; Lampe, P.D.; Lampe, J.W. Randomized Trial of Glucosamine and Chondroitin Supplementation on Inflammation and Oxidative Stress Biomarkers and Plasma Proteomics Profiles in Healthy Humans. PLoS ONE 2015, 10, e0117534. [Google Scholar] [CrossRef]
- Boudry, G.; Charton, E.; Le Huerou-Luron, I.; Ferret-Bernard, S.; Le Gall, S.; Even, S.; Blat, S. The Relationship Between Breast Milk Components and the Infant Gut Microbiota. Front. Nutr. 2021, 8, 629740. [Google Scholar] [CrossRef]
- Zhang, S.; Li, T.; Xie, J.; Zhang, D.; Pi, C.; Zhou, L.; Yang, W. Gold standard for nutrition: A review of human milk oligosaccharide and its effects on infant gut microbiota. Microb. Cell Factories 2021, 20, 108. [Google Scholar] [CrossRef]
- Demmelmair, H.; Prell, C.; Timby, N.; Lönnerdal, B. Benefits of Lactoferrin, Osteopontin and Milk Fat Globule Membranes for Infants. Nutrients 2017, 9, 817. [Google Scholar] [CrossRef]
- Miller, G.W.; Jones, D.P. The nature of nurture: Refining the definition of the exposome. Toxicol. Sci. Off. J. Soc. Toxicol. 2014, 137, 1–2. [Google Scholar] [CrossRef]
- Wild, C.P. Complementing the genome with an “exposome”: The outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1847–1850. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, R.M. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009, 37, e21. [Google Scholar] [CrossRef] [PubMed]
- Janssen, B.G.; Munters, E.; Pieters, N.; Smeets, K.; Cox, B.; Cuypers, A.; Fierens, F.; Penders, J.; Vangronsveld, J.; Gyselaers, W.; et al. Placental mitochondrial DNA content and particulate air pollution during in utero life. Environ. Health Perspect. 2012, 120, 1346–1352. [Google Scholar] [CrossRef]
- Martens, D.S.; Plusquin, M.; Gyselaers, W.; De Vivo, I.; Nawrot, T.S. Maternal pre-pregnancy body mass index and newborn telomere length. BMC Med. 2016, 14, 148. [Google Scholar] [CrossRef]
- Martens, D.S.; Janssen, B.G.; Bijnens, E.M.; Clemente, D.B.P.; Vineis, P.; Plusquin, M.; Nawrot, T.S. Association of Parental Socioeconomic Status and Newborn Telomere Length. JAMA Netw. Open 2020, 3, e204057. [Google Scholar] [CrossRef]
- Bové, H.; Bongaerts, E.; Slenders, E.; Bijnens, E.M.; Saenen, N.D.; Gyselaers, W.; Van Eyken, P.; Plusquin, M.; Roeffaers, M.B.J.; Ameloot, M.; et al. Ambient black carbon particles reach the fetal side of human placenta. Nat. Commun. 2019, 10, 3866. [Google Scholar] [CrossRef]
- Saenen, N.D.; Bové, H.; Steuwe, C.; Roeffaers, M.B.J.; Provost, E.B.; Lefebvre, W.; Vanpoucke, C.; Ameloot, M.; Nawrot, T.S. Children’s urinary environmental carbon load: A novel marker reflecting residential ambient air pollution exposure? Am. J. Respir. Crit. Care Med. 2017, 196, 873–881. [Google Scholar] [CrossRef]
- Caballero-Casero, N.; Castro, G.; Bastiaensen, M.; Gys, C.; van Larebeke, N.; Schoeters, G.; Covaci, A. Identification of chemicals of emerging concern in urine of Flemish adolescents using a new suspect screening workflow for LC-QTOF-MS. Chemosphere 2021, 280, 130683. [Google Scholar] [CrossRef]
- Gys, C.; Bamai, Y.A.; Araki, A.; Bastiaensen, M.; Caballero-Casero, N.; Kishi, R.; Covaci, A. Biomonitoring and temporal trends of bisphenols exposure in Japanese school children. Environ. Res. 2020, 191, 110172. [Google Scholar] [CrossRef]
- DeBoer, M.D.; Platts-Mills, J.A.; Scharf, R.J.; McDermid, J.M.; Wanjuhi, A.W.; Gratz, J.; Svensen, E.; Swann, J.R.; Donowitz, J.R.; Jatosh, S.; et al. Early Life Interventions for Childhood Growth and Development in Tanzania (ELICIT): A protocol for a randomised factorial, double-blind, placebo-controlled trial of azithromycin, nitazoxanide and nicotinamide. BMJ Open 2018, 8, e021817. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kabir, F.; Manneh, J.; Lertsethtakarn, P.; Begum, S.; Gratz, J.; Becker, S.M.; Operario, D.J.; Taniuchi, M.; Janaki, L.; et al. Development and assessment of molecular diagnostic tests for 15 enteropathogens causing childhood diarrhoea: A multicentre study. Lancet Infect. Dis. 2014, 14, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, S.J.; French, J.; Brookes, M.J.; Ford, C.; Gama, R. Between-assay variability of faecal calprotectin enzyme-linked immunosorbent assay kits. Ann. Clin. Biochem. 2013, 50, 53–61. [Google Scholar] [CrossRef]
- Toe, L.C.; Kerckhof, F.-M.; De Bodt, J.; Morel, F.B.; Ouedraogo, J.-B.; Kolsteren, P.; Van de Wiele, T. A prebiotic-enhanced lipid-based nutrient supplement (LNSp) increases Bifidobacterium relative abundance and enhances short-chain fatty acid production in simulated colonic microbiota from undernourished infants. FEMS Microbiol. Ecol. 2020, 96, fiaa105. [Google Scholar] [CrossRef]
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Nawrot, T.S.; Van Der Stukken, C.; Tylus, D.; Sleurs, H.; Peusens, M.; Alfano, R.; Langie, S.A.; Plusquin, M.; Martens, D.S. Different epigenetic signatures of newborn telomere length and telomere attrition rate in early life. Aging 2021, 13, 14630–14650. [Google Scholar] [CrossRef] [PubMed]
- Haycock, P.C.; Heydon, E.E.; Kaptoge, S.; Butterworth, A.S.; Thompson, A.; Willeit, P. Leucocyte telomere length and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2014, 349, g4227. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhan, Y.; Pedersen, N.L.; Fang, F.; Hägg, S. Telomere Length and All-Cause Mortality: A Meta-analysis. Ageing Res. Rev. 2018, 48, 11–20. [Google Scholar] [CrossRef]
- Tsoukalas, D.; Fragkiadaki, P.; Docea, A.O.; Alegakis, A.K.; Sarandi, E.; Vakonaki, E.; Salataj, E.; Kouvidi, E.; Nikitovic, D.; Kovatsi, L.; et al. Association of nutraceutical supplements with longer telomere length. Int. J. Mol. Med. 2019, 44, 218–226. [Google Scholar] [CrossRef]
- Roger, A.J.; Muñoz-Gómez, S.A.; Kamikawa, R. The Origin and Diversification of Mitochondria. Curr. Biol. CB 2017, 27, R1177–R1192. [Google Scholar] [CrossRef]
- Copeland, W.C.; Longley, M.J. Mitochondrial genome maintenance in health and disease. DNA Repair 2014, 19, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.W.G.; Green, D.R. Mitochondrial Regulation of Cell Death. Cold Spring Harb. Perspect. Biol. 2013, 5, a008706. [Google Scholar] [CrossRef] [PubMed]
- Gibson, K.; Halliday, J.L.; Kirby, D.M.; Yaplito-Lee, J.; Thorburn, D.R.; Boneh, A. Mitochondrial oxidative phosphorylation disorders presenting in neonates: Clinical manifestations and enzy-matic and molecular diagnoses. Pediatrics 2008, 122, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Kohda, M.; Tokuzawa, Y.; Kishita, Y.; Nyuzuki, H.; Moriyama, Y.; Mizuno, Y.; Hirata, T.; Yatsuka, Y.; Yamashita-Sugahara, Y.; Nakachi, Y.; et al. A Comprehensive Genomic Analysis Reveals the Genetic Landscape of Mitochondrial Respiratory Chain Complex Deficiencies. PLoS Genet. 2016, 12, e1005679. [Google Scholar] [CrossRef]
- Druzhyna, N.M.; Wilson, G.L.; LeDoux, S.P. Mitochondrial DNA repair in aging and disease. Mech. Ageing Dev. 2008, 129, 383–390. [Google Scholar] [CrossRef]
- Priliani, L.; Prado, E.L.; Restuadi, R.; Waturangi, D.E.; Shankar, A.H.; Malik, S.G. Maternal Multiple Micronutrient Supplementation Stabilizes Mitochondrial DNA Copy Number in Pregnant Women in Lombok, Indonesia. J. Nutr. 2019, 149, 1309–1316. [Google Scholar] [CrossRef]
- Sana, A.; Meda, N.; Badoum, G.; Kafando, B.; Bouland, C. Primary Cooking Fuel Choice and Respiratory Health Outcomes among Women in Charge of Household Cooking in Ouagadougou, Burkina Faso: Cross-Sectional Study. Int. J. Environ. Res. Public Health 2019, 16, 1040. [Google Scholar] [CrossRef]
- WHO. National Burden of Disease Estimates; World Health Organization: Geneva, Switzerland, 2007; pp. 1–8. Available online: http://www.who.int/indoorair/publications/fuelforlife/ (accessed on 18 January 2023).
- Pedersen, M.; Giorgis-Allemand, L.; Bernard, C.; Aguilera, I.; Andersen, A.-M.N.; Ballester, F.; Beelen, R.M.J.; Chatzi, L.; Cirach, M.; Danileviciute, A.; et al. Ambient air pollution and low birthweight: A European cohort study (ESCAPE). Lancet Respir. Med. 2013, 1, 695–704. [Google Scholar] [CrossRef]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A.; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate Matter Air Pollution and Cardiovascular Disease: An update to the scientific statement from the american heart association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef]
- Nawrot, T.S.; Perez, L.; Künzli, N.; Munters, E.; Nemery, B. Public health importance of triggers of myocardial infarction: A comparative risk assessment. Lancet 2011, 377, 732–740. [Google Scholar] [CrossRef]
- Neven, K.Y.; Wang, C.; Janssen, B.G.; Roels, H.A.; Vanpoucke, C.; Ruttens, A.; Nawrot, T.S. Ambient air pollution exposure during the late gestational period is linked with lower placental iodine load in a Belgian birth cohort. Environ. Int. 2021, 147, 106334. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.S.; Brasel, J.M. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology 2001, 167, 101–134. [Google Scholar] [CrossRef] [PubMed]
- De Kok, B.; Moore, K.; Jones, L.; Vanslambrouck, K.; Toe, L.C.; Ouédraogo, M.; Ganaba, R.; de Pee, S.; Bedford, J.; Lachat, C.; et al. Home consumption of two fortified balanced energy protein supplements by pregnant women in Burkina Faso. Matern. Child Nutr. 2021, 17, e13134. [Google Scholar] [CrossRef] [PubMed]
- Kpoda, D.S.; Bandé, M.; Compaoré, A.M.; Bazié, R.B.S.; Meda, R.N.; Somda, S.; Meda, D.S.; Kpoda, H.B.N.; Somé, S.A.; Sakana, L.; et al. Nutritional, Microbiological, and Toxicological Quality Assessment of Foods Sold in Urban and Suburban Markets in Burkina Faso. Health Secur. 2022, 20, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gratz, J.; Amour, C.; Kibiki, G.; Becker, S.; Janaki, L.; Verweij, J.J.; Taniuchi, M.; Sobuz, S.U.; Haque, R.; et al. A laboratory-developed TaqMan Array Card for simultaneous detection of 19 enteropathogens. J. Clin. Microbiol. 2013, 51, 472–480. [Google Scholar] [CrossRef]
- Marks, F.; Liu, J.; Soura, A.B.; Gasmelseed, N.; Operario, D.J.; Grundy, B.; Wieser, J.; Gratz, J.; Meyer, C.G.; Im, J.; et al. Pathogens That Cause Acute Febrile Illness Among Children and Adolescents in Burkina Faso, Madagascar, and Sudan. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 73, 1338–1345. [Google Scholar] [CrossRef]
- Medithi, S.; Kasa, Y.D.; Kankipati, V.R.; Kodali, V.; Jee, B.; Jonnalagadda, P.R. Impact of Micronutrient Supplementation on Pesticide Residual, Acetylcholinesterase Activity, and Oxidative Stress Among Farm Children Exposed to Pesticides. Front. Public Health 2022, 10, 872125. [Google Scholar] [CrossRef]
- Moore, C.C.; Jacob, S.T.; Banura, P.; Zhang, J.; Stroup, S.; Boulware, D.R.; Scheld, W.M.; Houpt, E.R.; Liu, J. Etiology of Sepsis in Uganda Using a Quantitative Polymerase Chain Reaction-based TaqMan Array Card. Clin. Infect. Dis. 2018, 68, 266–272. [Google Scholar] [CrossRef]
- Ouedraogo, M.; Toe, A.M.; Ouedraogo, T.; Guissou, P.I. Pesticides in Burkina Faso: Overview of the Situation in a Sahelian African Country. In Pesticides in the Modern World—Pesticides Use and Management; InTech: Houston, TX, USA, 2011. [Google Scholar] [CrossRef]
- Peraica, M.; Richter, D.; Rašić, D. Mycotoxicoses in children. Arh. Za Hig. Rada I Toksikol. 2014, 65, 347–363. [Google Scholar] [CrossRef]
- Richards, L.; Li, M.; van Esch, B.; Garssen, J.; Folkerts, G. The effects of short-chain fatty acids on the cardiovascular system. PharmaNutrition 2016, 4, 68–111. [Google Scholar] [CrossRef]
- Saad-Hussein, A.; Ibrahim, K.S.; Abdalla, M.S.; El-Mezayen, H.; Osman, N.F. Effects of zinc supplementation on oxidant/antioxidant and lipids status of pesticides sprayers. J. Complement. Integr. Med. 2020, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Scheppach, W.; Bartram, H.P.; Richter, F. Role of short-chain fatty acids in the prevention of colorectal cancer. Eur. J. Cancer 1995, 31, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Tesfamariam, K.; Argaw, A.; Hanley-Cook, G.; Gebreyesuse, S.H.; Kolsteren, P.; Belachew, T.; Van de Velde, M.; De Saeger, S.; De Boevre, M.; Lachat, C. Chronic maternal aflatoxin exposure is associated with poor fetal growth trajectories: A prospective cohort study in rural Ethiopia. 2022, unpublished work [Preprint]. [Google Scholar]
- Valadez, J.J.; Brown, L.D.; Vargas, W.V.; Morley, D. Using lot quality assurance sampling to assess measurements for growth monitoring in a developing country’s primary health care system. Int. J. Epidemiol. 1996, 25, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.; Mengelers, M.; Yang, S.; De Saeger, S.; De Boevre, M. Mycotoxin Biomarkers of Exposure: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1127–1155. [Google Scholar] [CrossRef] [PubMed]
- Ware, L.Y.; Durand, N.; Nikiema, P.A.; Alter, P.; Fontana, A.; Montet, D.; Barro, N. Occurrence of mycotoxins in commercial infant formulas locally produced in Ouagadougou (Burkina Faso). Food Control 2017, 73, 518–523. [Google Scholar] [CrossRef]
- FAO. Worldwide Regulations for Mycotoxins in Food and Feed in 2003; FAO: Roma, Italy, 2003; Available online: https://www.fao.org/3/y5499e/y5499e0d.htm (accessed on 1 July 2022).
- Warth, B.; Parich, A.; Atehnkeng, J.; Bandyopadhyay, R.; Schuhmacher, R.; Sulyok, M.; Krska, R. Quantitation of Mycotoxins in Food and Feed from Burkina Faso and Mozambique Using a Modern LC-MS/MS Multitoxin Method. J. Agric. Food Chem. 2012, 60, 9352–9363. [Google Scholar] [CrossRef]
- Lehmann, E.; Oltramare, C.; Dibié, J.-J.N.; Konaté, Y.; de Alencastro, L.F. Assessment of human exposure to pesticides by hair analysis: The case of vegetable-producing areas in Burkina Faso. Environ. Int. 2018, 111, 317–331. [Google Scholar] [CrossRef]
- Lehmann, E.; Turrero, N.; Kolia, M.; Konaté, Y.; de Alencastro, L.F. Dietary risk assessment of pesticides from vegetables and drinking water in gardening areas in Burkina Faso. Sci. Total Environ. 2017, 601–602, 1208–1216. [Google Scholar] [CrossRef]
- Son, D.; Zerbo, F.K.B.; Bonzi, S.; Schiffers, B.; Somda, I.; Schiffers, B.; Legreve, A. Assessment of Tomato (Solanum lycopersicum L.) Producers’ Exposure Level to Pesticides, in Kouka and Toussiana (Burkina Faso). Int. J. Environ. Res. Public Health 2018, 15, 204. [Google Scholar] [CrossRef]
- Diaz, M.H.; Waller, J.L.; Napoliello, R.A.; Islam, S.; Wolff, B.J.; Burken, D.J.; Holden, R.L.; Srinivasan, V.; Arvay, M.; McGee, L.; et al. Optimization of Multiple Pathogen Detection Using the TaqMan Array Card: Application for a Population-Based Study of Neonatal Infection. PLoS ONE 2013, 8, e66183. [Google Scholar] [CrossRef]
- Diaz, M.H.; Waller, J.L.; Theodore, M.J.; Patel, N.; Wolff, B.J.; Benitez, A.J.; Morris, T.; Raghunathan, P.L.; Breiman, R.F.; Whitney, C.G.; et al. Development and Implementation of Multiplex TaqMan Array Cards for Specimen Testing at Child Health and Mortality Prevention Surveillance Site Laboratories. Clin. Infect. Dis. 2019, 69 (Suppl. 4), S311–S321. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, G.A.; Schnabel, K.C.; Erdman, D.D.; Prill, M.M.; Iwane, M.K.; Shelley, L.M.; Whitaker, B.L.; Szilagyi, P.G.; Hall, C.B. Field evaluation of TaqMan Array Card (TAC) for the simultaneous detection of multiple respiratory viruses in children with acute respiratory infection. J. Clin. Virol. 2013, 57, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Jukic, A.; Bakiri, L.; Wagner, E.F.; Tilg, H.; Adolph, T.E. Calprotectin: From biomarker to biological function. Gut 2021, 70, 1978–1988. [Google Scholar] [CrossRef] [PubMed]
- Yui, S.; Nakatani, Y.; Mikami, M. Calprotectin (S100A8/S100A9), an Inflammatory Protein Complex from Neutrophils with a Broad Apoptosis-Inducing Activity. Biol. Pharm. Bull. 2003, 26, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Dale, I.; Brandtzaeg, P.; Fagerhol, M.K.; Scott, H. Distribution of a New Myelomonocytic Antigen (L1) in Human Peripheral Blood Leukocytes: Immunofluorescence and Immunoperoxidase Staining Features in Comparison with Lysozyme and Lactoferrin. Am. J. Clin. Pathol. 1985, 84, 24–34. [Google Scholar] [CrossRef]
- Summerton, C.B.; Longlands, M.G.; Wiener, K.; Shreeve, D.R. Faecal calprotectin: A marker of inflammation throughout the intestinal tract. Eur. J. Gastroenterol. Hepatol. 2002, 14, 841–845. [Google Scholar] [CrossRef]
- Cummings, J.; Macfarlane, G. Collaborative JPEN-Clinical Nutrition Scientific Publications Role of intestinal bacteria in nutrient metabolism. J. Parenter. Enter. Nutr. 1997, 21, 357–365. [Google Scholar] [CrossRef]
- Blad, C.C.; Tang, C.; Offermanns, S. G protein-coupled receptors for energy metabolites as new therapeutic targets. Nat. Rev. Drug Discov. 2012, 11, 603–619. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef]
- Berries, G.; Mnf, W.; Kang, Y.; Yang, G.; Zhang, S.; Ross, C.F.; Zhu, M.-J. Goji Berry Modulates Gut Microbiota and Alleviates Colitis in IL-10-Deficient Mice. Mol. Nutr. Food Res. 2018, 62, e1800535. [Google Scholar] [CrossRef]
- Gomes, S.D.; Oliveira, C.S.; Azevedo-Silva, J.; Casanova, M.R.; Barreto, J.; Pereira, H.; Chaves, S.R.; Rodrigues, L.R.; Casal, M.; Côrte-Real, M.; et al. The Role of Diet Related Short-Chain Fatty Acids in Colorectal Cancer Metabolism and Survival: Prevention and Therapeutic Implications. Curr. Med. Chem. 2020, 27, 4087–4108. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet-Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).