Hormonal Determinants of Growth and Weight Gain in the Human Fetus and Preterm Infant
Abstract
1. Introduction: The Metabolic Risks of Preterm Birth
- (a)
- Inadequate reserve of skeletal myocytes and pancreatic beta cells, which undergo neogenesis and proliferation primarily in late gestation and early infancy [9,10,11]. A relative reduction in skeletal myocytes in preterm infants reduces muscle insulin sensitivity [12,13,14], limits future energy expenditure [15,16], and contributes to relative adiposity [17], while a reduction in beta cell mass, delayed maturation of glucose transporter 2, and defective processing of proinsulin [10,11,18,19,20,21] attenuate (in those born AGA, not SGA) the insulin secretory response to insulin resistance.
- (b)
- Incomplete maturation of the vascular tree [22] and a deficiency of renal nephrons [23,24,25], a majority of which are formed in late gestation and the early postnatal period. In combination, these may impair endothelial and renal function [26] and predispose to future hypertension [17,27,28,29,30].
- (c)
- Deficiencies of hormones and growth factors that normally emerge or increase in late gestation (see discussion below).
- (d)
- Adaptive hormonal responses to preterm delivery and various intrauterine and perinatal insults including hypoxia, malnutrition, cardiorespiratory and gastrointestinal disorders, neurologic insults, and infectious diseases (see discussion below).
- (e)
- Effects of various therapeutic agents including glucocorticoids, which impede skeletal muscle and bone growth and reduce tissue insulin sensitivity (see discussion below).
2. Methodologic Approach to the Narrative Review
3. Rates of Linear Growth, Weight Gain, and Fat Deposition during Fetal and Early Postnatal Development
4. Roles of Maternal and Placental Hormones, Growth Factors, and Cytokines in Maternal Metabolism and Transplacental Nutrient Delivery
5. Control of Fetal Growth and Weight Gain by Placental and Fetal Hormones, Growth Factors, and Adipocytokines
6. Insulin
7. Insulin-like Growth Factors (IGFs) and IGF Binding Proteins
8. Regulation of Fetal Insulin, IGFs, and IGF Binding Proteins by Nutrients and Hormones
8.1. Macronutrients
8.2. Growth Hormone (GH), Placental Lactogen, and Prolactin
9. Thyroid Hormones
10. Cortisol and the Sex Steroids
11. Leptin, Ghrelin, Adiponectin, and Other Adipocytokines
12. Effects of Maternal Disorders on Fetal Hormones, Growth Factors, and Adipocytokines and Fetal Growth
12.1. Obesity
12.2. Excessive Gestational Weight Gain
12.3. Gestational Diabetes
12.4. Smoking
12.5. Hypertensive Disorders of Pregnancy
12.6. Malnutrition
13. Effects of Maternal Disorders on Placental Nutrient Delivery
14. Effects of Preterm Delivery on Neonatal Hormones, Growth Factors, and Adipocytokines
15. Implications of Preterm Birth for Neonatal Growth, Weight Gain, and Metabolic Function
16. Effects of Nutritional and Pharmacologic Interventions on Growth and Weight Gain in Preterm Infants: Correlation with Hormones, Growth Factors, and Adipocytokines
16.1. Breast Feeding and Breast Milk Hormones
16.2. Glucocorticoid Therapy
17. Summary
18. Gaps in Knowledge
- How do sex differences in fetal and postnatal growth and weight gain influence the risks of future metabolic and cardiovascular disease in infants born preterm?
- Do the lactogenic hormones or growth hormone increase beta cell mass or insulin production in the human fetus? Might other hormones or growth factors serve as fetal beta cell tropins?
- Do macronutrients regulate human fetal IGF production during early and mid-gestation? How is this control exerted? What are the roles of lactogenic hormones in the control of IGF production in the human fetus?
- Do fetal adipocytokines or ghrelin directly modulate human fetal growth, weight gain, or metabolic function?
- What are the effects of maternal pathologies on human fetal hormones, cytokines, and growth factors during the course of intrauterine development? How would this information help us to understand the pathogenesis of growth failure or excess adiposity in affected preterm infants and their potential risks for long-term complications?
- Do hormones, cytokines, and growth factors in breast milk modulate the growth or weight gain of the preterm or term infant? How are these effects mediated?
- How should we optimize nutrition to promote growth and cognitive development in preterm infants while limiting long term cardiovascular and metabolic risks? Does the preferential storage of fat relative to skeletal muscle and bone growth provide a survival advantage for the preterm infant? Might there be an association between neonatal fat storage, energy availability, and long-term cognitive function?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Markopoulou, P.; Papanikolaou, E.; Analytis, A.; Zoumakis, E.; Siahanidou, T. Preterm Birth as a Risk Factor for Metabolic Syndrome and Cardiovascular Disease in Adult Life: A Systematic Review and Meta-Analysis. J. Pediatr. 2019, 210, 69–80.e65. [Google Scholar] [CrossRef]
- Harder, T.; Rodekamp, E.; Schellong, K.; Dudenhausen, J.W.; Plagemann, A. Birth weight and subsequent risk of type 2 diabetes: A meta-analysis. Am. J. Epidemiol. 2007, 165, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Whincup, P.H.; Kaye, S.J.; Owen, C.G.; Huxley, R.; Cook, D.G.; Anazawa, S.; Barrett-Connor, E.; Bhargava, S.K.; Birgisdottir, B.E.; Carlsson, S.; et al. Birth weight and risk of type 2 diabetes: A systematic review. JAMA 2008, 300, 2886–2897. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, E.; Gamborg, M.; Sorensen, T.I.; Baker, J.L. Sex Differences in the Association Between Birth Weight and Adult Type 2 Diabetes. Diabetes 2015, 64, 4220–4225. [Google Scholar] [CrossRef]
- Yoshida-Montezuma, Y.; Stone, E.; Iftikhar, S.; De Rubeis, V.; Andreacchi, A.T.; Keown-Stoneman, C.; Mbuagbaw, L.; Brown, H.K.; de Souza, R.J.; Anderson, L.N. The association between late preterm birth and cardiometabolic conditions across the life course: A systematic review and meta-analysis. Paediatr. Perinat. Epidemiol. 2022, 36, 264–275. [Google Scholar] [CrossRef]
- Hack, M.; Schluchter, M.; Cartar, L.; Rahman, M.; Cuttler, L.; Borawski, E. Growth of very low birth weight infants to age 20 years. Pediatrics 2003, 112, e30–e38. [Google Scholar] [CrossRef] [PubMed]
- Yarmolinsky, J.; Mueller, N.T.; Duncan, B.B.; Chor, D.; Bensenor, I.M.; Griep, R.H.; Appel, L.J.; Barreto, S.M.; Schmidt, M.I. Sex-specific associations of low birth weight with adult-onset diabetes and measures of glucose homeostasis: Brazilian Longitudinal Study of Adult Health. Sci. Rep. 2016, 6, 37032. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C. Sexual dimorphism of body composition. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Nastase, L.; Cretoiu, D.; Stoicescu, S.M. Skeletal Muscle Damage in Intrauterine Growth Restriction. Adv. Exp. Med. Biol. 2018, 1088, 93–106. [Google Scholar] [CrossRef]
- Butler, P.C.; Meier, J.J.; Butler, A.E.; Bhushan, A. The replication of beta cells in normal physiology, in disease and for therapy. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.J.; Butler, A.E.; Saisho, Y.; Monchamp, T.; Galasso, R.; Bhushan, A.; Rizza, R.A.; Butler, P.C. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 2008, 57, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
- Rotteveel, J.; van Weissenbruch, M.M.; Twisk, J.W.; Delemarre-Van de Waal, H.A. Infant and childhood growth patterns, insulin sensitivity, and blood pressure in prematurely born young adults. Pediatrics 2008, 122, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Mathai, S.; Cutfield, W.S.; Derraik, J.G.; Dalziel, S.R.; Harding, J.E.; Robinson, E.; Biggs, J.; Jefferies, C.; Hofman, P.L. Insulin sensitivity and beta-cell function in adults born preterm and their children. Diabetes 2012, 61, 2479–2483. [Google Scholar] [CrossRef]
- Kajantie, E.; Strang-Karlsson, S.; Hovi, P.; Wehkalampi, K.; Lahti, J.; Kaseva, N.; Jarvenpaa, A.L.; Raikkonen, K.; Eriksson, J.G.; Andersson, S. Insulin sensitivity and secretory response in adults born preterm: The Helsinki Study of Very Low Birth Weight Adults. J. Clin. Endocrinol. Metab. 2015, 100, 244–250. [Google Scholar] [CrossRef]
- Sipola-Leppanen, M.; Hovi, P.; Andersson, S.; Wehkalampi, K.; Vaarasmaki, M.; Strang-Karlsson, S.; Jarvenpaa, A.L.; Makitie, O.; Eriksson, J.G.; Kajantie, E. Resting energy expenditure in young adults born preterm--the Helsinki study of very low birth weight adults. PLoS ONE 2011, 6, e17700. [Google Scholar] [CrossRef]
- Kensara, O.A.; Wooton, S.A.; Phillips, D.I.; Patel, M.; Hoffman, D.J.; Jackson, A.A.; Elia, M.; Hertfordshire Study, G. Substrate-energy metabolism and metabolic risk factors for cardiovascular disease in relation to fetal growth and adult body composition. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E365–E371. [Google Scholar] [CrossRef]
- Sipola-Leppanen, M.; Vaarasmaki, M.; Tikanmaki, M.; Matinolli, H.M.; Miettola, S.; Hovi, P.; Wehkalampi, K.; Ruokonen, A.; Sundvall, J.; Pouta, A.; et al. Cardiometabolic risk factors in young adults who were born preterm. Am. J. Epidemiol. 2015, 181, 861–873. [Google Scholar] [CrossRef]
- Bloomfield, F.H. Impact of prematurity for pancreatic islet and beta-cell development. J. Endocrinol. 2018, 238, R161–R171. [Google Scholar] [CrossRef] [PubMed]
- King, R.A.; Smith, R.M.; Dahlenburg, G.W. Long term postnatal development of insulin secretion in early premature infants. Early Hum. Dev. 1986, 13, 285–294. [Google Scholar] [CrossRef]
- Richardson, C.C.; Hussain, K.; Jones, P.M.; Persaud, S.; Lobner, K.; Boehm, A.; Clark, A.; Christie, M.R. Low levels of glucose transporters and K+ATP channels in human pancreatic beta cells early in development. Diabetologia 2007, 50, 1000–1005. [Google Scholar] [CrossRef]
- Mitanchez-Mokhtari, D.; Lahlou, N.; Kieffer, F.; Magny, J.F.; Roger, M.; Voyer, M. Both relative insulin resistance and defective islet beta-cell processing of proinsulin are responsible for transient hyperglycemia in extremely preterm infants. Pediatrics 2004, 113, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Norman, M. Low birth weight and the developing vascular tree: A systematic review. Acta Paediatr. 2008, 97, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Zohdi, V.; Sutherland, M.R.; Lim, K.; Gubhaju, L.; Zimanyi, M.A.; Black, M.J. Low Birth Weight due to Intrauterine Growth Restriction and/or Preterm Birth: Effects on Nephron Number and Long-Term Renal Health. Int. J. Nephrol. 2012, 2012, 136942. [Google Scholar] [CrossRef]
- Chehade, H.; Simeoni, U.; Guignard, J.P.; Boubred, F. Preterm Birth: Long Term Cardiovascular and Renal Consequences. Curr. Pediatr. Rev. 2018, 14, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Dyson, A.; Kent, A.L. The Effect of Preterm Birth on Renal Development and Renal Health Outcome. Neoreviews 2019, 20, e725–e736. [Google Scholar] [CrossRef] [PubMed]
- Engan, B.; Engan, M.; Greve, G.; Vollsaeter, M.; Hufthammer, K.O.; Leirgul, E. Vascular Endothelial Function Assessed by Flow-Mediated Vasodilatation in Young Adults Born Very Preterm or with Extremely Low Birthweight: A Regional Cohort Study. Front. Pediatr. 2021, 9, 734082. [Google Scholar] [CrossRef]
- Cheong, J.L.Y.; Haikerwal, A.; Wark, J.D.; Irving, L.; Garland, S.M.; Patton, G.C.; Cheung, M.M.; Doyle, L.W.; Victorian Infant Collaborative Study Group. Cardiovascular Health Profile at Age 25 Years in Adults Born Extremely Preterm or Extremely Low Birthweight. Hypertension 2020, 76, 1838–1846. [Google Scholar] [CrossRef]
- Hovi, P.; Vohr, B.; Ment, L.R.; Doyle, L.W.; McGarvey, L.; Morrison, K.M.; Evensen, K.A.; van der Pal, S.; Grunau, R.E.; Collaboration, A.A.B.P.I.; et al. Blood Pressure in Young Adults Born at Very Low Birth Weight: Adults Born Preterm International Collaboration. Hypertension 2016, 68, 880–887. [Google Scholar] [CrossRef]
- Paquette, K.; Fernandes, R.O.; Xie, L.F.; Cloutier, A.; Fallaha, C.; Girard-Bock, C.; Mian, M.O.R.; Lukaszewski, M.A.; Masse, B.; El-Jalbout, R.; et al. Kidney Size, Renal Function, Ang (Angiotensin) Peptides, and Blood Pressure in Young Adults Born Preterm. Hypertension 2018, 72, 918–928. [Google Scholar] [CrossRef]
- Bergvall, N.; Iliadou, A.; Johansson, S.; de Faire, U.; Kramer, M.S.; Pawitan, Y.; Pedersen, N.L.; Lichtenstein, P.; Cnattingius, S. Genetic and shared environmental factors do not confound the association between birth weight and hypertension: A study among Swedish twins. Circulation 2007, 115, 2931–2938. [Google Scholar] [CrossRef]
- Soto, N.; Bazaes, R.A.; Pena, V.; Salazar, T.; Avila, A.; Iniguez, G.; Ong, K.K.; Dunger, D.B.; Mericq, M.V. Insulin sensitivity and secretion are related to catch-up growth in small-for-gestational-age infants at age 1 year: Results from a prospective cohort. J. Clin. Endocrinol. Metab. 2003, 88, 3645–3650. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, C.; Urquidi, C.; Pittaluga, E.; Iniguez, G.; Avila, A.; Carrasco, F.; Mericq, V. Differences in body composition and resting energy expenditure in childhood in preterm children born with very low birth weight. Horm. Res. Paediatr. 2013, 79, 347–355. [Google Scholar] [CrossRef]
- Dasinger, J.H.; Alexander, B.T. Gender differences in developmental programming of cardiovascular diseases. Clin. Sci. 2016, 130, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Nugent, J.T.; Lu, Y.; Deng, Y.; Sharifi, M.; Greenberg, J.H. Effect Measure Modification by Birth Weight on the Association Between Overweight or Obesity and Hypertension in Children and Adolescents. JAMA Pediatr. 2023, 177, 735–737. [Google Scholar] [CrossRef]
- Kensara, O.A.; Wootton, S.A.; Phillips, D.I.; Patel, M.; Jackson, A.A.; Elia, M.; Hertfordshire Study, G. Fetal programming of body composition: Relation between birth weight and body composition measured with dual-energy X-ray absorptiometry and anthropometric methods in older Englishmen. Am. J. Clin. Nutr. 2005, 82, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Bann, D.; Wills, A.; Cooper, R.; Hardy, R.; Aihie Sayer, A.; Adams, J.; Kuh, D.; Scientific, N.; Data Collection, T. Birth weight and growth from infancy to late adolescence in relation to fat and lean mass in early old age: Findings from the MRC National Survey of Health and Development. Int. J. Obes. 2014, 38, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ley, S.H.; Tobias, D.K.; Chiuve, S.E.; VanderWeele, T.J.; Rich-Edwards, J.W.; Curhan, G.C.; Willett, W.C.; Manson, J.E.; Hu, F.B.; et al. Birth weight and later life adherence to unhealthy lifestyles in predicting type 2 diabetes: Prospective cohort study. BMJ 2015, 351, h3672. [Google Scholar] [CrossRef]
- Kiserud, T.; Piaggio, G.; Carroli, G.; Widmer, M.; Carvalho, J.; Neerup Jensen, L.; Giordano, D.; Cecatti, J.G.; Abdel Aleem, H.; Talegawkar, S.A.; et al. The World Health Organization Fetal Growth Charts: A Multinational Longitudinal Study of Ultrasound Biometric Measurements and Estimated Fetal Weight. PLoS Med. 2017, 14, e1002220. [Google Scholar] [CrossRef]
- Fowden, A.L.; Szemere, J.; Hughes, P.; Gilmour, R.S.; Forhead, A.J. The effects of cortisol on the growth rate of the sheep fetus during late gestation. J. Endocrinol. 1996, 151, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, E.E.; O’Donnell, A.M.; Nelson, S.E.; Fomon, S.J. Body composition of the reference fetus. Growth 1976, 40, 329–341. [Google Scholar]
- Widdowson, E. Growth and composition of the fetus and newborn. In Biology of Gestation; Assali, N.S., Ed.; Academic Press: New York, NY, USA, 1968; Volume II, pp. 1–49. [Google Scholar]
- Lapillonne, A.; Braillon, P.; Claris, O.; Chatelain, P.G.; Delmas, P.D.; Salle, B.L. Body composition in appropriate and in small for gestational age infants. Acta Paediatr. 1997, 86, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Hamatschek, C.; Yousuf, E.I.; Mollers, L.S.; So, H.Y.; Morrison, K.M.; Fusch, C.; Rochow, N. Fat and Fat-Free Mass of Preterm and Term Infants from Birth to Six Months: A Review of Current Evidence. Nutrients 2020, 12, 288. [Google Scholar] [CrossRef]
- Murphy-Alford, A.J.; Johnson, W.; Nyati, L.H.; Santos, I.S.; Hills, A.P.; Ariff, S.; Wickramasinghe, V.P.; Kuriyan, R.; Lucas, M.N.; Costa, C.S.; et al. Body composition reference charts for infants from birth to 24 months: Multicenter Infant Body Composition Reference Study. Am. J. Clin. Nutr. 2023, 117, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Merklin, R.J. Growth and distribution of human fetal brown fat. Anat. Rec. 1974, 178, 637–645. [Google Scholar] [CrossRef]
- Moragas, A.; Toran, N. Prenatal development of brown adipose tissue in man. A morphometric and biomathematical study. Biol. Neonate 1983, 43, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Newbern, D.; Freemark, M. Placental hormones and the control of maternal metabolism and fetal growth. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Coltart, T.M. Adipose tissue metabolism in pregnancy: The lipolytic effect of human placental lactogen. Br. J. Obstet. Gynaecol. 1978, 85, 43–46. [Google Scholar] [CrossRef]
- Sferruzzi-Perri, A.N.; Sandovici, I.; Constancia, M.; Fowden, A.L. Placental phenotype and the insulin-like growth factors: Resource allocation to fetal growth. J. Physiol. 2017, 595, 5057–5093. [Google Scholar] [CrossRef]
- Sandovici, I.; Georgopoulou, A.; Perez-Garcia, V.; Hufnagel, A.; Lopez-Tello, J.; Lam, B.Y.H.; Schiefer, S.N.; Gaudreau, C.; Santos, F.; Hoelle, K.; et al. The imprinted Igf2-Igf2r axis is critical for matching placental microvasculature expansion to fetal growth. Dev. Cell 2022, 57, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Guadix, P.; Corrales, I.; Vilarino-Garcia, T.; Rodriguez-Chacon, C.; Sanchez-Jimenez, F.; Jimenez-Cortegana, C.; Duenas, J.L.; Sanchez-Margalet, V.; Perez-Perez, A. Expression of nutrient transporters in placentas affected by gestational diabetes: Role of leptin. Front. Endocrinol. 2023, 14, 1172831. [Google Scholar] [CrossRef]
- von Versen-Hoynck, F.; Rajakumar, A.; Parrott, M.S.; Powers, R.W. Leptin affects system A amino acid transport activity in the human placenta: Evidence for STAT3 dependent mechanisms. Placenta 2009, 30, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Duval, F.; Santos, E.D.; Poidatz, D.; Serazin, V.; Gronier, H.; Vialard, F.; Dieudonne, M.N. Adiponectin Inhibits Nutrient Transporters and Promotes Apoptosis in Human Villous Cytotrophoblasts: Involvement in the Control of Fetal Growth. Biol. Reprod. 2016, 94, 111. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, A.; Zeller, M.; Fernandez, A., Jr.; Zalcberg, L.J.; Bartlett, R.J.; Ricordi, C.; Pietropaolo, M.; Eisenbarth, G.S.; Bennett, S.T.; Patel, D.D. The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat. Genet. 1997, 15, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.E.; Abu-Amero, S.N.; Bell, G.; Wakeling, E.L.; Kingsnorth, A.; Stanier, P.; Jauniaux, E.; Bennett, S.T. Evidence that insulin is imprinted in the human yolk sac. Diabetes 2001, 50, 199–203. [Google Scholar] [CrossRef]
- Economides, D.L.; Proudler, A.; Nicolaides, K.H. Plasma insulin in appropriate- and small-for-gestational-age fetuses. Am. J. Obstet. Gynecol. 1989, 160, 1091–1094. [Google Scholar] [CrossRef]
- Salardi, S.; Orsini, L.F.; Cacciari, E.; Righetti, F.; Donati, S.; Mandini, M.; Cicognani, A.; Bovicelli, L. Growth hormone, insulin-like growth factor I, insulin and C-peptide during human fetal life: In-utero study. Clin. Endocrinol. 1991, 34, 187–190. [Google Scholar] [CrossRef]
- Boucher, J.; Softic, S.; El Ouaamari, A.; Krumpoch, M.T.; Kleinridders, A.; Kulkarni, R.N.; O’Neill, B.T.; Kahn, C.R. Differential Roles of Insulin and IGF-1 Receptors in Adipose Tissue Development and Function. Diabetes 2016, 65, 2201–2213. [Google Scholar] [CrossRef] [PubMed]
- Kniss, D.A.; Shubert, P.J.; Zimmerman, P.D.; Landon, M.B.; Gabbe, S.G. Insulinlike growth factors. Their regulation of glucose and amino acid transport in placental trophoblasts isolated from first-trimester chorionic villi. J. Reprod. Med. 1994, 39, 249–256. [Google Scholar]
- Ericsson, A.; Hamark, B.; Powell, T.L.; Jansson, T. Glucose transporter isoform 4 is expressed in the syncytiotrophoblast of first trimester human placenta. Hum. Reprod. 2005, 20, 521–530. [Google Scholar] [CrossRef]
- Jansson, N.; Greenwood, S.L.; Johansson, B.R.; Powell, T.L.; Jansson, T. Leptin stimulates the activity of the system A amino acid transporter in human placental villous fragments. J. Clin. Endocrinol. Metab. 2003, 88, 1205–1211. [Google Scholar] [CrossRef]
- Castillo-Castrejon, M.; Jansson, T.; Powell, T.L. No evidence of attenuation of placental insulin-stimulated Akt phosphorylation and amino acid transport in maternal obesity and gestational diabetes mellitus. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E1037–E1049. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.J.; Crace, C.J.; Strain, A.J.; Milner, R.D. Regulation of amino acid uptake and deoxyribonucleic acid synthesis in isolated human fetal fibroblasts and myoblasts: Effect of human placental lactogen, somatomedin-C, multiplication-stimulating activity, and insulin. J. Clin. Endocrinol. Metab. 1986, 62, 753–760. [Google Scholar] [CrossRef]
- Lee, I.L.; Barr, E.L.M.; Longmore, D.; Barzi, F.; Brown, A.D.H.; Connors, C.; Boyle, J.A.; Kirkwood, M.; Hampton, V.; Lynch, M.; et al. Cord blood metabolic markers are strong mediators of the effect of maternal adiposity on fetal growth in pregnancies across the glucose tolerance spectrum: The PANDORA study. Diabetologia 2020, 63, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Luo, Z.C.; Nuyt, A.M.; Audibert, F.; Wei, S.Q.; Abenhaim, H.A.; Bujold, E.; Julien, P.; Huang, H.; Levy, E.; et al. Large-for-Gestational-Age May Be Associated with Lower Fetal Insulin Sensitivity and beta-Cell Function Linked to Leptin. J. Clin. Endocrinol. Metab. 2018, 103, 3837–3844. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.E.; De Franco, E.; Freathy, R.M.; Fetal, I.; Growth, C.; Flanagan, S.E.; Hattersley, A.T. Monogenic disease analysis establishes that fetal insulin accounts for half of human fetal growth. J. Clin. Investig. 2023, 133, e16430. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.E.; Hattersley, A.T.; Flanagan, S.E.; Freathy, R.M. Two decades since the fetal insulin hypothesis: What have we learned from genetics? Diabetologia 2021, 64, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Hammami, M.; Walters, J.C.; Hockman, E.M.; Koo, W.W. Disproportionate alterations in body composition of large for gestational age neonates. J. Pediatr. 2001, 138, 817–821. [Google Scholar] [CrossRef]
- Durnwald, C.; Huston-Presley, L.; Amini, S.; Catalano, P. Evaluation of body composition of large-for-gestational-age infants of women with gestational diabetes mellitus compared with women with normal glucose tolerance levels. Am. J. Obstet. Gynecol. 2004, 191, 804–808. [Google Scholar] [CrossRef]
- Goya, L.; de la Puente, A.; Ramos, S.; Martin, M.A.; Escriva, F.; Pascual-Leone, A.M. Regulation of insulin-like growth factor-I and -II by glucose in primary cultures of fetal rat hepatocytes. J. Biol. Chem. 1999, 274, 24633–24640. [Google Scholar] [CrossRef] [PubMed]
- Han, V.K.; Lund, P.K.; Lee, D.C.; D’Ercole, A.J. Expression of somatomedin/insulin-like growth factor messenger ribonucleic acids in the human fetus: Identification, characterization, and tissue distribution. J. Clin. Endocrinol. Metab. 1988, 66, 422–429. [Google Scholar] [CrossRef]
- Han, V.K.; D’Ercole, A.J.; Lund, P.K. Cellular localization of somatomedin (insulin-like growth factor) messenger RNA in the human fetus. Science 1987, 236, 193–197. [Google Scholar] [CrossRef]
- Strain, A.J.; Hill, D.J.; Swenne, I.; Milner, R.D. Regulation of DNA synthesis in human fetal hepatocytes by placental lactogen, growth hormone, and insulin-like growth factor I/somatomedin-C. J. Cell Physiol. 1987, 132, 33–40. [Google Scholar] [CrossRef]
- Smith, P.J.; Wise, L.S.; Berkowitz, R.; Wan, C.; Rubin, C.S. Insulin-like growth factor-I is an essential regulator of the differentiation of 3T3-L1 adipocytes. J. Biol. Chem. 1988, 263, 9402–9408. [Google Scholar] [CrossRef]
- Boney, C.M.; Moats-Staats, B.M.; Stiles, A.D.; D’Ercole, A.J. Expression of insulin-like growth factor-I (IGF-I) and IGF-binding proteins during adipogenesis. Endocrinology 1994, 135, 1863–1868. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M.; Zhang, H.; Schulz, T.J.; Huang, T.L.; Espinoza, D.O.; Kristiansen, K.; Unterman, T.G.; Tseng, Y.H. Insulin/IGF-I regulation of necdin and brown adipocyte differentiation via CREB- and FoxO1-associated pathways. Endocrinology 2011, 152, 3680–3689. [Google Scholar] [CrossRef] [PubMed]
- Miki, H.; Yamauchi, T.; Suzuki, R.; Komeda, K.; Tsuchida, A.; Kubota, N.; Terauchi, Y.; Kamon, J.; Kaburagi, Y.; Matsui, J.; et al. Essential role of insulin receptor substrate 1 (IRS-1) and IRS-2 in adipocyte differentiation. Mol. Cell. Biol. 2001, 21, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.N.; Holzenberger, M.; Shih, D.Q.; Ozcan, U.; Stoffel, M.; Magnuson, M.A.; Kahn, C.R. beta-cell-specific deletion of the Igf1 receptor leads to hyperinsulinemia and glucose intolerance but does not alter beta-cell mass. Nat. Genet. 2002, 31, 111–115. [Google Scholar] [CrossRef]
- Ueki, K.; Okada, T.; Hu, J.; Liew, C.W.; Assmann, A.; Dahlgren, G.M.; Peters, J.L.; Shackman, J.G.; Zhang, M.; Artner, I.; et al. Total insulin and IGF-I resistance in pancreatic beta cells causes overt diabetes. Nat. Genet. 2006, 38, 583–588. [Google Scholar] [CrossRef]
- Voutilainen, R.; Miller, W.L. Developmental and hormonal regulation of mRNAs for insulin-like growth factor II and steroidogenic enzymes in human fetal adrenals and gonads. DNA 1988, 7, 9–15. [Google Scholar] [CrossRef]
- Birnbacher, R.; Amann, G.; Breitschopf, H.; Lassmann, H.; Suchanek, G.; Heinz-Erian, P. Cellular localization of insulin-like growth factor II mRNA in the human fetus and the placenta: Detection with a digoxigenin-labeled cRNA probe and immunocytochemistry. Pediatr. Res. 1998, 43, 614–620. [Google Scholar] [CrossRef]
- Conover, C.A.; Rosenfeld, R.G.; Hintz, R.L. Insulin-like growth factor II binding and action in human fetal fibroblasts. J. Cell Physiol. 1987, 133, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Selenou, C.; Brioude, F.; Giabicani, E.; Sobrier, M.L.; Netchine, I. IGF2: Development, Genetic and Epigenetic Abnormalities. Cells 2022, 11, 1886. [Google Scholar] [CrossRef]
- Calderari, S.; Gangnerau, M.N.; Thibault, M.; Meile, M.J.; Kassis, N.; Alvarez, C.; Portha, B.; Serradas, P. Defective IGF2 and IGF1R protein production in embryonic pancreas precedes beta cell mass anomaly in the Goto-Kakizaki rat model of type 2 diabetes. Diabetologia 2007, 50, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- de Miguel-Santos, L.; Fernandez-Millan, E.; Angeles Martin, M.; Escriva, F.; Alvarez, C. Maternal undernutrition increases pancreatic IGF-2 and partially suppresses the physiological wave of beta-cell apoptosis during the neonatal period. J. Mol. Endocrinol. 2010, 44, 25–36. [Google Scholar] [CrossRef]
- Vaughan, O.R.; Rosario, F.J.; Powell, T.L.; Jansson, T. Regulation of Placental Amino Acid Transport and Fetal Growth. Prog. Mol. Biol. Transl. Sci. 2017, 145, 217–251. [Google Scholar] [CrossRef]
- Lassarre, C.; Hardouin, S.; Daffos, F.; Forestier, F.; Frankenne, F.; Binoux, M. Serum insulin-like growth factors and insulin-like growth factor binding proteins in the human fetus. Relationships with growth in normal subjects and in subjects with intrauterine growth retardation. Pediatr. Res. 1991, 29, 219–225. [Google Scholar] [CrossRef]
- Langford, K.; Nicolaides, K.; Miell, J.P. Maternal and fetal insulin-like growth factors and their binding proteins in the second and third trimesters of human pregnancy. Hum. Reprod. 1998, 13, 1389–1393. [Google Scholar] [CrossRef]
- Haj-Ahmad, L.M.; Mahmoud, M.M.; Sweis, N.W.G.; Bsisu, I.; Alghrabli, A.M.; Ibrahim, A.M.; Zayed, A.A. Serum IGF-1 to IGFBP-3 Molar Ratio: A Promising Diagnostic Tool for Growth Hormone Deficiency in Children. J. Clin. Endocrinol. Metab. 2023, 108, 986–994. [Google Scholar] [CrossRef]
- Martin-Rivada, A.; Guerra-Cantera, S.; Campillo-Calatayud, A.; Andres-Esteban, E.M.; Sanchez Holgado, M.; Martos-Moreno, G.A.; Pozo, J.; Guemes, M.; Soriano-Guillen, L.; Pellicer, A.; et al. Pappalysins and Stanniocalcins and Their Relationship with the Peripheral IGF Axis in Newborns and During Development. J. Clin. Endocrinol. Metab. 2022, 107, 2912–2924. [Google Scholar] [CrossRef]
- Hellstrom, A.; Sigurdsson, J.; Lofqvist, C.; Hellgren, G.; Kistner, A. The IGF system and longitudinal growth in preterm infants in relation to gestational age, birth weight and gender. Growth Horm. IGF Res. 2020, 51, 46–57. [Google Scholar] [CrossRef]
- Cianfarani, S.; Germani, D.; Rossi, L.; Argiro, G.; Boemi, S.; Lemon, M.; Holly, J.M.; Branca, F. IGF-I and IGF-binding protein-1 are related to cortisol in human cord blood. Eur. J. Endocrinol. 1998, 138, 524–529. [Google Scholar] [CrossRef]
- Leger, J.; Oury, J.F.; Noel, M.; Baron, S.; Benali, K.; Blot, P.; Czernichow, P. Growth factors and intrauterine growth retardation. I. Serum growth hormone, insulin-like growth factor (IGF)-I, IGF-II, and IGF binding protein 3 levels in normally grown and growth-retarded human fetuses during the second half of gestation. Pediatr. Res. 1996, 40, 94–100. [Google Scholar] [CrossRef]
- Verhaeghe, J.; Van Bree, R.; Van Herck, E.; Laureys, J.; Bouillon, R.; Van Assche, F.A. C-peptide, insulin-like growth factors I and II, and insulin-like growth factor binding protein-1 in umbilical cord serum: Correlations with birth weight. Am. J. Obstet. Gynecol. 1993, 169, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Tzschoppe, A.; Riedel, C.; von Kries, R.; Struwe, E.; Rascher, W.; Dorr, H.G.; Beckmann, M.W.; Schild, R.L.; Goecke, T.W.; Flyvbjerg, A.; et al. Differential effects of low birthweight and intrauterine growth restriction on umbilical cord blood insulin-like growth factor concentrations. Clin. Endocrinol. 2015, 83, 739–745. [Google Scholar] [CrossRef]
- Yalinbas, E.E.; Binay, C.; Simsek, E.; Aksit, M.A. The Role of Umbilical Cord Blood Concentration of IGF-I, IGF-II, Leptin, Adiponectin, Ghrelin, Resistin, and Visfatin in Fetal Growth. Am. J. Perinatol. 2019, 36, 600–608. [Google Scholar] [CrossRef]
- Hawkes, C.P.; Murray, D.M.; Kenny, L.C.; Kiely, M.; O’B Hourihane, J.; Irvine, A.D.; Wu, Z.; Argon, Y.; Reitz, R.E.; McPhaul, M.J.; et al. Correlation of Insulin-Like Growth Factor-I and -II Concentrations at Birth Measured by Mass Spectrometry and Growth from Birth to Two Months. Horm. Res. Paediatr. 2018, 89, 122–131. [Google Scholar] [CrossRef]
- Giudice, L.C.; de Zegher, F.; Gargosky, S.E.; Dsupin, B.A.; de las Fuentes, L.; Crystal, R.A.; Hintz, R.L.; Rosenfeld, R.G. Insulin-like growth factors and their binding proteins in the term and preterm human fetus and neonate with normal and extremes of intrauterine growth. J. Clin. Endocrinol. Metab. 1995, 80, 1548–1555. [Google Scholar] [CrossRef]
- He, H.; Zhu, W.T.; Nuyt, A.M.; Marc, I.; Julien, P.; Huang, R.; Dubois, L.; Wei, S.Q.; Zhang, J.; Levy, E.; et al. Cord Blood IGF-I, Proinsulin, Leptin, HMW Adiponectin, and Ghrelin in Short or Skinny Small-for-Gestational-Age Infants. J. Clin. Endocrinol. Metab. 2021, 106, e3049–e3057. [Google Scholar] [CrossRef] [PubMed]
- Walenkamp, M.J.; Wit, J.M. Genetic disorders in the growth hormone-insulin-like growth factor-I axis. Horm. Res. 2006, 66, 221–230. [Google Scholar] [CrossRef]
- Walenkamp, M.J.; Wit, J.M. Single gene mutations causing SGA. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 433–446. [Google Scholar] [CrossRef]
- Savage, M.O.; Hwa, V.; David, A.; Rosenfeld, R.G.; Metherell, L.A. Genetic Defects in the Growth Hormone-IGF-I Axis Causing Growth Hormone Insensitivity and Impaired Linear Growth. Front. Endocrinol. 2011, 2, 95. [Google Scholar] [CrossRef] [PubMed]
- Smerieri, A.; Petraroli, M.; Ziveri, M.A.; Volta, C.; Bernasconi, S.; Street, M.E. Effects of cord serum insulin, IGF-II, IGFBP-2, IL-6 and cortisol concentrations on human birth weight and length: Pilot study. PLoS ONE 2011, 6, e29562. [Google Scholar] [CrossRef]
- Street, M.E.; Seghini, P.; Fieni, S.; Ziveri, M.A.; Volta, C.; Martorana, D.; Viani, I.; Gramellini, D.; Bernasconi, S. Changes in interleukin-6 and IGF system and their relationships in placenta and cord blood in newborns with fetal growth restriction compared with controls. Eur. J. Endocrinol. 2006, 155, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Pham, N.V.; Nguyen, M.T.; Hu, J.F.; Vu, T.H.; Hoffman, A.R. Dissociation of IGF2 and H19 imprinting in human brain. Brain Res. 1998, 810, 1–8. [Google Scholar] [CrossRef]
- Begemann, M.; Zirn, B.; Santen, G.; Wirthgen, E.; Soellner, L.; Buttel, H.M.; Schweizer, R.; van Workum, W.; Binder, G.; Eggermann, T. Paternally Inherited IGF2 Mutation and Growth Restriction. N. Engl. J. Med. 2015, 373, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Masunaga, Y.; Inoue, T.; Yamoto, K.; Fujisawa, Y.; Sato, Y.; Kawashima-Sonoyama, Y.; Morisada, N.; Iijima, K.; Ohata, Y.; Namba, N.; et al. IGF2 Mutations. J. Clin. Endocrinol. Metab. 2020, 105, 116–125. [Google Scholar] [CrossRef]
- Bartz, S.; Mody, A.; Hornik, C.; Bain, J.; Muehlbauer, M.; Kiyimba, T.; Kiboneka, E.; Stevens, R.; Bartlett, J.; St Peter, J.V.; et al. Severe acute malnutrition in childhood: Hormonal and metabolic status at presentation, response to treatment, and predictors of mortality. J. Clin. Endocrinol. Metab. 2014, 99, 2128–2137. [Google Scholar] [CrossRef]
- Clemmons, D.R.; Underwood, L.E. Nutritional regulation of IGF-I and IGF binding proteins. Annu. Rev. Nutr. 1991, 11, 393–412. [Google Scholar] [CrossRef]
- Thissen, J.P.; Ketelslegers, J.M.; Underwood, L.E. Nutritional regulation of the insulin-like growth factors. Endocr. Rev. 1994, 15, 80–101. [Google Scholar] [CrossRef]
- Estivariz, C.F.; Ziegler, T.R. Nutrition and the insulin-like growth factor system. Endocrine 1997, 7, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Guasti, L.; Silvennoinen, S.; Bulstrode, N.W.; Ferretti, P.; Sankilampi, U.; Dunkel, L. Elevated FGF21 leads to attenuated postnatal linear growth in preterm infants through GH resistance in chondrocytes. J. Clin. Endocrinol. Metab. 2014, 99, E2198–E2206. [Google Scholar] [CrossRef]
- Larque, E.; Ruiz-Palacios, M.; Koletzko, B. Placental regulation of fetal nutrient supply. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 292–297. [Google Scholar] [CrossRef]
- Illsley, N.P.; Baumann, M.U. Human placental glucose transport in fetoplacental growth and metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165359. [Google Scholar] [CrossRef]
- Duttaroy, A.K.; Basak, S. Maternal Fatty Acid Metabolism in Pregnancy and Its Consequences in the Feto-Placental Development. Front. Physiol. 2021, 12, 787848. [Google Scholar] [CrossRef]
- Osborn, B.H.; Fowlkes, J.; Han, V.K.; Freemark, M. Nutritional regulation of insulin-like growth factor-binding protein gene expression in the ovine fetus and pregnant ewe. Endocrinology 1992, 131, 1743–1750. [Google Scholar] [CrossRef]
- Boehmer, B.H.; Baker, P.R.; Brown, L.D.; Wesolowski, S.R.; Rozance, P.J. Leucine acutely potentiates glucose-stimulated insulin secretion in fetal sheep. J. Endocrinol. 2020, 247, 115–126. [Google Scholar] [CrossRef]
- Roth, S.; Abernathy, M.P.; Lee, W.H.; Pratt, L.; Denne, S.; Golichowski, A.; Pescovitz, O.H. Insulin-like growth factors I and II peptide and messenger RNA levels in macrosomic infants of diabetic pregnancies. J. Soc. Gynecol. Investig. 1996, 3, 78–84. [Google Scholar] [CrossRef]
- Lindsay, R.S.; Westgate, J.A.; Beattie, J.; Pattison, N.S.; Gamble, G.; Mildenhall, L.F.; Breier, B.H.; Johnstone, F.D. Inverse changes in fetal insulin-like growth factor (IGF)-1 and IGF binding protein-1 in association with higher birth weight in maternal diabetes. Clin. Endocrinol. 2007, 66, 322–328. [Google Scholar] [CrossRef]
- Davenport, M.L.; D’Ercole, A.J.; Underwood, L.E. Effect of maternal fasting on fetal growth, serum insulin-like growth factors (IGFs), and tissue IGF messenger ribonucleic acids. Endocrinology 1990, 126, 2062–2067. [Google Scholar] [CrossRef]
- Oliver, M.H.; Harding, J.E.; Breier, B.H.; Evans, P.C.; Gluckman, P.D. Glucose but not a mixed amino acid infusion regulates plasma insulin-like growth factor-I concentrations in fetal sheep. Pediatr. Res. 1993, 34, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Gaylord, T.D.; Bowsher, R.R.; Hlaing, M.; Moorehead, H.; Liechty, E.A. Nutritional regulation of circulating insulin-like growth factors (IGFs) and their binding proteins in the ovine fetus. Endocr. J. 1997, 44, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.S.; Bialek, P.; Anzo, M.; Khosravi, J.; Yee, S.P.; Han, V.K. Elevated circulating insulin-like growth factor binding protein-1 is sufficient to cause fetal growth restriction. Endocrinology 2006, 147, 1175–1186. [Google Scholar] [CrossRef]
- Li, J.; Owens, J.A.; Owens, P.C.; Saunders, J.C.; Fowden, A.L.; Gilmour, R.S. The ontogeny of hepatic growth hormone receptor and insulin-like growth factor I gene expression in the sheep fetus during late gestation: Developmental regulation by cortisol. Endocrinology 1996, 137, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Goodyer, C.G.; Figueiredo, R.M.; Krackovitch, S.; De Souza Li, L.; Manalo, J.A.; Zogopoulos, G. Characterization of the growth hormone receptor in human dermal fibroblasts and liver during development. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E1213–E1220. [Google Scholar] [CrossRef]
- Kenth, G.; Mergelas, J.A.; Goodyer, C.G. Developmental changes in the human GH receptor and its signal transduction pathways. J. Endocrinol. 2008, 198, 71–82. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leger, J.; Noel, M.; Limal, J.M.; Czernichow, P. Growth factors and intrauterine growth retardation. II. Serum growth hormone, insulin-like growth factor (IGF) I, and IGF-binding protein 3 levels in children with intrauterine growth retardation compared with normal control subjects: Prospective study from birth to two years of age. Study Group of IUGR. Pediatr. Res. 1996, 40, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Arosio, M.; Cortelazzi, D.; Persani, L.; Palmieri, E.; Casati, G.; Baggiani, A.M.; Gambino, G.; Beck-Peccoz, P. Circulating levels of growth hormone, insulin-like growth factor-I and prolactin in normal, growth retarded and anencephalic human fetuses. J. Endocrinol. Investig. 1995, 18, 346–353. [Google Scholar] [CrossRef]
- Binder, G.; Weidenkeller, M.; Blumenstock, G.; Langkamp, M.; Weber, K.; Franz, A.R. Rational approach to the diagnosis of severe growth hormone deficiency in the newborn. J. Clin. Endocrinol. Metab. 2010, 95, 2219–2226. [Google Scholar] [CrossRef] [PubMed]
- Silbergeld, A.; Lazar, L.; Erster, B.; Keret, R.; Tepper, R.; Laron, Z. Serum growth hormone binding protein activity in healthy neonates, children and young adults: Correlation with age, height and weight. Clin. Endocrinol. 1989, 31, 295–303. [Google Scholar] [CrossRef]
- Massa, G.; de Zegher, F.; Vanderschueren-Lodeweyckx, M. Serum growth hormone-binding proteins in the human fetus and infant. Pediatr. Res. 1992, 32, 69–72. [Google Scholar] [CrossRef][Green Version]
- Low, L.C.; Tam, S.Y.; Kwan, E.Y.; Tsang, A.M.; Karlberg, J. Onset of significant GH dependence of serum IGF-I and IGF-binding protein 3 concentrations in early life. Pediatr. Res. 2001, 50, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Hwa, V. Human growth disorders associated with impaired GH action: Defects in STAT5B and JAK2. Mol. Cell Endocrinol. 2021, 519, 111063. [Google Scholar] [CrossRef]
- Pena-Almazan, S.; Buchlis, J.; Miller, S.; Shine, B.; MacGillivray, M. Linear growth characteristics of congenitally GH-deficient infants from birth to one year of age. J. Clin. Endocrinol. Metab. 2001, 86, 5691–5694. [Google Scholar] [CrossRef][Green Version]
- Thorpe-Beeston, J.G.; Snijders, R.J.; Felton, C.V.; Nicolaides, K.H. Serum prolactin concentration in normal and small for gestational age fetuses. Br. J. Obstet. Gynaecol. 1992, 99, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Perlman, M.; Schenker, J.; Glassman, M.; Ben-david, M. Prolonged hyperprolactinemia in preterm infants. J. Clin. Endocrinol. Metab. 1978, 47, 894–897. [Google Scholar] [CrossRef] [PubMed]
- Fleenor, D.; Arumugam, R.; Freemark, M. Growth hormone and prolactin receptors in adipogenesis: STAT-5 activation, suppressors of cytokine signaling, and regulation of insulin-like growth factor I. Horm. Res. 2006, 66, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.J.; Crace, C.J.; Milner, R.D. Incorporation of [3H]thymidine by isolated fetal myoblasts and fibroblasts in response to human placental lactogen (HPL): Possible mediation of HPL action by release of immunoreactive SM-C. J. Cell Physiol. 1985, 125, 337–344. [Google Scholar] [CrossRef]
- Schoknecht, P.A.; McGuire, M.A.; Cohick, W.S.; Currie, W.B.; Bell, A.W. Effect of chronic infusion of placental lactogen on ovine fetal growth in late gestation. Domest. Anim. Endocrinol. 1996, 13, 519–528. [Google Scholar] [CrossRef]
- Houghton, D.J.; Shackleton, P.; Obiekwe, B.C.; Chard, T. Relationship of maternal and fetal levels of human placental lactogen to the weight and sex of the fetus. Placenta 1984, 5, 455–458. [Google Scholar] [CrossRef]
- Knopp, R.H.; Bergelin, R.O.; Wahl, P.W.; Walden, C.E. Relationships of infant birth size to maternal lipoproteins, apoproteins, fuels, hormones, clinical chemistries, and body weight at 36 weeks gestation. Diabetes 1985, 34 (Suppl. S2), 71–77. [Google Scholar] [CrossRef]
- Hill, D.J.; Freemark, M.; Strain, A.J.; Handwerger, S.; Milner, R.D. Placental lactogen and growth hormone receptors in human fetal tissues: Relationship to fetal plasma human placental lactogen concentrations and fetal growth. J. Clin. Endocrinol. Metab. 1988, 66, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.M.; Goetzmann, L.N.; Cantlon, J.D.; Jeckel, K.M.; Winger, Q.A.; Anthony, R.V. Development of ovine chorionic somatomammotropin hormone-deficient pregnancies. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R837–R846. [Google Scholar] [CrossRef] [PubMed]
- Hord, T.K.; Tanner, A.R.; Kennedy, V.C.; Lynch, C.S.; Winger, Q.A.; Rozance, P.J.; Anthony, R.V. Impact of Chorionic Somatomammotropin In Vivo RNA Interference Phenotype on Uteroplacental Expression of the IGF Axis. Life 2023, 13, 1261. [Google Scholar] [CrossRef]
- Mittal, P.; Espinoza, J.; Hassan, S.; Kusanovic, J.P.; Edwin, S.S.; Nien, J.K.; Gotsch, F.; Than, N.G.; Erez, O.; Mazaki-Tovi, S.; et al. Placental growth hormone is increased in the maternal and fetal serum of patients with preeclampsia. J. Matern. Fetal Neonatal Med. 2007, 20, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Vickers, M.H.; Stanley, J.L.; Baker, P.N.; Perry, J.K. Human Placental Growth Hormone Variant in Pathological Pregnancies. Endocrinology 2018, 159, 2186–2198. [Google Scholar] [CrossRef] [PubMed]
- Pepe, G.J.; Lynch, T.J.; Davies, W.A.; Albrecht, E.D. Regulation of baboon fetal pituitary prolactin expression by estrogen. Biol. Reprod. 2009, 80, 1189–1195. [Google Scholar] [CrossRef]
- Nanbu-Wakao, R.; Fujitani, Y.; Masuho, Y.; Muramatu, M.; Wakao, H. Prolactin enhances CCAAT enhancer-binding protein-beta (C/EBP beta) and peroxisome proliferator-activated receptor gamma (PPAR gamma) messenger RNA expression and stimulates adipogenic conversion of NIH-3T3 cells. Mol. Endocrinol. 2000, 14, 307–316. [Google Scholar] [CrossRef][Green Version]
- Viengchareun, S.; Servel, N.; Feve, B.; Freemark, M.; Lombes, M.; Binart, N. Prolactin receptor signaling is essential for perinatal brown adipocyte function: A role for insulin-like growth factor-2. PLoS ONE 2008, 3, e1535. [Google Scholar] [CrossRef]
- Lopez-Vicchi, F.; De Winne, C.; Ornstein, A.M.; Sorianello, E.; Toneatto, J.; Becu-Villalobos, D. Severe Hyperprolactinemia Promotes Brown Adipose Tissue Whitening and Aggravates High Fat Diet Induced Metabolic Imbalance. Front. Endocrinol. 2022, 13, 883092. [Google Scholar] [CrossRef]
- Le, J.A.; Wilson, H.M.; Shehu, A.; Devi, Y.S.; Aguilar, T.; Gibori, G. Prolactin activation of the long form of its cognate receptor causes increased visceral fat and obesity in males as shown in transgenic mice expressing only this receptor subtype. Horm. Metab. Res. 2011, 43, 931–937. [Google Scholar] [CrossRef]
- Colao, A.; Sarno, A.D.; Cappabianca, P.; Briganti, F.; Pivonello, R.; Somma, C.D.; Faggiano, A.; Biondi, B.; Lombardi, G. Gender differences in the prevalence, clinical features and response to cabergoline in hyperprolactinemia. Eur. J. Endocrinol. 2003, 148, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Salenave, S.; Ancelle, D.; Bahougne, T.; Raverot, G.; Kamenicky, P.; Bouligand, J.; Guiochon-Mantel, A.; Linglart, A.; Souchon, P.F.; Nicolino, M.; et al. Macroprolactinomas in children and adolescents: Factors associated with the response to treatment in 77 patients. J. Clin. Endocrinol. Metab. 2015, 100, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Al Sabie, F.; Tariq, Z.; Erickson, D.; Donegan, D. Association between Prolactinoma and Body Mass Index. Endocr. Pract. 2021, 27, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Freemark, M.; Fleenor, D.; Driscoll, P.; Binart, N.; Kelly, P. Body weight and fat deposition in prolactin receptor-deficient mice. Endocrinology 2001, 142, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Flint, D.J.; Binart, N.; Boumard, S.; Kopchick, J.J.; Kelly, P. Developmental aspects of adipose tissue in GH receptor and prolactin receptor gene disrupted mice: Site-specific effects upon proliferation, differentiation and hormone sensitivity. J. Endocrinol. 2006, 191, 101–111. [Google Scholar] [CrossRef]
- Fleenor, D.; Oden, J.; Kelly, P.A.; Mohan, S.; Alliouachene, S.; Pende, M.; Wentz, S.; Kerr, J.; Freemark, M. Roles of the lactogens and somatogens in perinatal and postnatal metabolism and growth: Studies of a novel mouse model combining lactogen resistance and growth hormone deficiency. Endocrinology 2005, 146, 103–112. [Google Scholar] [CrossRef][Green Version]
- Nielsen, J.H. Effects of growth hormone, prolactin, and placental lactogen on insulin content and release, and deoxyribonucleic acid synthesis in cultured pancreatic islets. Endocrinology 1982, 110, 600–606. [Google Scholar] [CrossRef]
- Brelje, T.C.; Allaire, P.; Hegre, O.; Sorenson, R.L. Effect of prolactin versus growth hormone on islet function and the importance of using homologous mammosomatotropic hormones. Endocrinology 1989, 125, 2392–2399. [Google Scholar] [CrossRef]
- Brelje, T.C.; Scharp, D.W.; Lacy, P.E.; Ogren, L.; Talamantes, F.; Robertson, M.; Friesen, H.G.; Sorenson, R.L. Effect of homologous placental lactogens, prolactins, and growth hormones on islet B-cell division and insulin secretion in rat, mouse, and human islets: Implication for placental lactogen regulation of islet function during pregnancy. Endocrinology 1993, 132, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Terra, L.F.; Garay-Malpartida, M.H.; Wailemann, R.A.; Sogayar, M.C.; Labriola, L. Recombinant human prolactin promotes human beta cell survival via inhibition of extrinsic and intrinsic apoptosis pathways. Diabetologia 2011, 54, 1388–1397. [Google Scholar] [CrossRef]
- Banerjee, R.R.; Cyphert, H.A.; Walker, E.M.; Chakravarthy, H.; Peiris, H.; Gu, X.; Liu, Y.; Conrad, E.; Goodrich, L.; Stein, R.W.; et al. Gestational Diabetes Mellitus From Inactivation of Prolactin Receptor and MafB in Islet beta-Cells. Diabetes 2016, 65, 2331–2341. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Hernandez, G.; Adan-Castro, E.; Diaz-Lezama, N.; Ruiz-Herrera, X.; Martinez de la Escalera, G.; Macotela, Y.; Clapp, C. Global Deletion of the Prolactin Receptor Aggravates Streptozotocin-Induced Diabetes in Mice. Front. Endocrinol. 2021, 12, 619696. [Google Scholar] [CrossRef]
- Huang, C. Wild-type offspring of heterozygous prolactin receptor-null female mice have maladaptive beta-cell responses during pregnancy. J. Physiol. 2013, 591, 1325–1338. [Google Scholar] [CrossRef]
- Freemark, M.; Driscoll, P.; Maaskant, R.; Petryk, A.; Kelly, P.A. Ontogenesis of prolactin receptors in the human fetus in early gestation. Implications for tissue differentiation and development. J. Clin. Investig. 1997, 99, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Kleinberger, J.W.; Takane, K.K.; Salim, F.; Fiaschi-Taesch, N.; Pappas, K.; Parsons, R.; Jiang, J.; Zhang, Y.; Liu, H.; et al. Augmented Stat5 Signaling Bypasses Multiple Impediments to Lactogen-Mediated Proliferation in Human beta-Cells. Diabetes 2015, 64, 3784–3797. [Google Scholar] [CrossRef] [PubMed]
- Moldrup, A.; Petersen, E.D.; Nielsen, J.H. Effects of sex and pregnancy hormones on growth hormone and prolactin receptor gene expression in insulin-producing cells. Endocrinology 1993, 133, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Swenne, I.; Hill, D.J.; Strain, A.J.; Milner, R.D. Growth hormone regulation of somatomedin C/insulin-like growth factor I production and DNA replication in fetal rat islets in tissue culture. Diabetes 1987, 36, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.J.; Riley, S.C.; Bassett, N.S.; Waters, M.J. Localization of the growth hormone receptor, identified by immunocytochemistry, in second trimester human fetal tissues and in placenta throughout gestation. J. Clin. Endocrinol. Metab. 1992, 75, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Kim, Y.G.; Kim, K.; Osonoi, S.; Wang, S.; Saunders, D.C.; Wang, J.; Yang, K.; Kim, H.; Lee, J.; et al. Serotonin Regulates Adult beta-Cell Mass by Stimulating Perinatal beta-Cell Proliferation. Diabetes 2020, 69, 205–214. [Google Scholar] [CrossRef]
- Varvarigou, A.; Vagenakis, A.G.; Makri, M.; Beratis, N.G. Growth hormone, insulin-like growth factor-I and prolactin in small for gestational age neonates. Biol. Neonate 1994, 65, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Diaz, A.; Villar, J.; Matorras-Weinig, R.; Valenzuela-Ruiz, P. Intrauterine growth retardation at term: Association between anthropometric and endocrine parameters. Acta Obstet. Gynecol. Scand. 1996, 75, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Galsgaard, E.D.; Nielsen, J.H.; Moldrup, A. Regulation of prolactin receptor (PRLR) gene expression in insulin-producing cells. Prolactin and growth hormone activate one of the rat prlr gene promoters via STAT5a and STAT5b. J. Biol. Chem. 1999, 274, 18686–18692. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, A.; Marino, R.; Ribas, A.; Rossi, J.; Ciaccio, M.; Oleastro, M.; Ornani, A.; Paz, R.; Rivarola, M.A.; Zelazko, M.; et al. Characterization of immunodeficiency in a patient with growth hormone insensitivity secondary to a novel STAT5b gene mutation. Pediatrics 2006, 118, e1584–e1592. [Google Scholar] [CrossRef] [PubMed]
- Hwa, V.; Little, B.; Adiyaman, P.; Kofoed, E.M.; Pratt, K.L.; Ocal, G.; Berberoglu, M.; Rosenfeld, R.G. Severe growth hormone insensitivity resulting from total absence of signal transducer and activator of transcription 5b. J. Clin. Endocrinol. Metab. 2005, 90, 4260–4266. [Google Scholar] [CrossRef] [PubMed]
- Forhead, A.J.; Fowden, A.L. Thyroid hormones in fetal growth and prepartum maturation. J. Endocrinol. 2014, 221, R87–R103. [Google Scholar] [CrossRef] [PubMed]
- Vulsma, T.; Gons, M.H.; de Vijlder, J.J. Maternal-fetal transfer of thyroxine in congenital hypothyroidism due to a total organification defect or thyroid agenesis. N. Engl. J. Med. 1989, 321, 13–16. [Google Scholar] [CrossRef]
- Loubiere, L.S.; Vasilopoulou, E.; Bulmer, J.N.; Taylor, P.M.; Stieger, B.; Verrey, F.; McCabe, C.J.; Franklyn, J.A.; Kilby, M.D.; Chan, S.Y. Expression of thyroid hormone transporters in the human placenta and changes associated with intrauterine growth restriction. Placenta 2010, 31, 295–304. [Google Scholar] [CrossRef]
- Koopdonk-Kool, J.M.; de Vijlder, J.J.; Veenboer, G.J.; Ris-Stalpers, C.; Kok, J.H.; Vulsma, T.; Boer, K.; Visser, T.J. Type II and type III deiodinase activity in human placenta as a function of gestational age. J. Clin. Endocrinol. Metab. 1996, 81, 2154–2158. [Google Scholar] [CrossRef]
- Santini, F.; Chiovato, L.; Ghirri, P.; Lapi, P.; Mammoli, C.; Montanelli, L.; Scartabelli, G.; Ceccarini, G.; Coccoli, L.; Chopra, I.J.; et al. Serum iodothyronines in the human fetus and the newborn: Evidence for an important role of placenta in fetal thyroid hormone homeostasis. J. Clin. Endocrinol. Metab. 1999, 84, 493–498. [Google Scholar] [CrossRef]
- Stanley, E.L.; Hume, R.; Visser, T.J.; Coughtrie, M.W. Differential expression of sulfotransferase enzymes involved in thyroid hormone metabolism during human placental development. J. Clin. Endocrinol. Metab. 2001, 86, 5944–5955. [Google Scholar] [CrossRef]
- van Wassenaer, A.G.; Kok, J.H.; Endert, E.; Vulsma, T.; de Vijlder, J.J. Thyroxine administration to infants of less than 30 weeks’ gestational age does not increase plasma triiodothyronine concentrations. Acta Endocrinol. 1993, 129, 139–146. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thorpe-Beeston, J.G.; Nicolaides, K.H.; Felton, C.V.; Butler, J.; McGregor, A.M. Maturation of the secretion of thyroid hormone and thyroid-stimulating hormone in the fetus. N. Engl. J. Med. 1991, 324, 532–536. [Google Scholar] [CrossRef]
- Radunovic, N.; Dumez, Y.; Nastic, D.; Mandelbrot, L.; Dommergues, M. Thyroid function in fetus and mother during the second half of normal pregnancy. Biol. Neonate 1991, 59, 139–148. [Google Scholar] [CrossRef]
- Fisher, D.A. Thyroid system immaturities in very low birth weight premature infants. Semin. Perinatol. 2008, 32, 387–397. [Google Scholar] [CrossRef]
- Forhead, A.J.; Curtis, K.; Kaptein, E.; Visser, T.J.; Fowden, A.L. Developmental control of iodothyronine deiodinases by cortisol in the ovine fetus and placenta near term. Endocrinology 2006, 147, 5988–5994. [Google Scholar] [CrossRef]
- Macek Jilkova, Z.; Pavelka, S.; Flachs, P.; Hensler, M.; Kus, V.; Kopecky, J. Modulation of type I iodothyronine 5’-deiodinase activity in white adipose tissue by nutrition: Possible involvement of leptin. Physiol. Res. 2010, 59, 561–569. [Google Scholar] [CrossRef]
- Boelen, A.; van Beeren, M.; Vos, X.; Surovtseva, O.; Belegri, E.; Saaltink, D.J.; Vreugdenhil, E.; Kalsbeek, A.; Kwakkel, J.; Fliers, E. Leptin administration restores the fasting-induced increase of hepatic type 3 deiodinase expression in mice. Thyroid 2012, 22, 192–199. [Google Scholar] [CrossRef]
- Lanham, S.A.; Fowden, A.L.; Roberts, C.; Cooper, C.; Oreffo, R.O.; Forhead, A.J. Effects of hypothyroidism on the structure and mechanical properties of bone in the ovine fetus. J. Endocrinol. 2011, 210, 189–198. [Google Scholar] [CrossRef]
- Richards, G.E.; Morrow, D.A.; Thominet, J.L.; Silverman, B.L.; Gluckman, P.D. The effect of thyroidectomy on growth hormone regulation in the ovine fetus. J. Dev. Physiol. 1993, 19, 165–169. [Google Scholar]
- Shields, B.M.; Knight, B.A.; Hill, A.; Hattersley, A.T.; Vaidya, B. Fetal thyroid hormone level at birth is associated with fetal growth. J. Clin. Endocrinol. Metab. 2011, 96, E934–E938. [Google Scholar] [CrossRef]
- Polak, M.; Legac, I.; Vuillard, E.; Guibourdenche, J.; Castanet, M.; Luton, D. Congenital hyperthyroidism: The fetus as a patient. Horm. Res. 2006, 65, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.H.; Reviczky, A.; Padbury, J.F. Thyroid hormones augment catecholamine-stimulated brown adipose tissue thermogenesis in the ovine fetus. Endocrinology 1984, 114, 1065–1069. [Google Scholar] [CrossRef]
- Harris, S.E.; De Blasio, M.J.; Zhao, X.; Ma, M.; Davies, K.; Wooding, F.B.P.; Hamilton, R.S.; Blache, D.; Meredith, D.; Murray, A.J.; et al. Thyroid Deficiency Before Birth Alters the Adipose Transcriptome to Promote Overgrowth of White Adipose Tissue and Impair Thermogenic Capacity. Thyroid 2020, 30, 794–805. [Google Scholar] [CrossRef]
- Camm, E.J.; Inzani, I.; De Blasio, M.J.; Davies, K.L.; Lloyd, I.R.; Wooding, F.B.P.; Blache, D.; Fowden, A.L.; Forhead, A.J. Thyroid Hormone Deficiency Suppresses Fetal Pituitary-Adrenal Function Near Term: Implications for the Control of Fetal Maturation and Parturition. Thyroid 2021, 31, 861–869. [Google Scholar] [CrossRef]
- Kajantie, E.; Dunkel, L.; Turpeinen, U.; Stenman, U.H.; Wood, P.J.; Nuutila, M.; Andersson, S. Placental 11 beta-hydroxysteroid dehydrogenase-2 and fetal cortisol/cortisone shuttle in small preterm infants. J. Clin. Endocrinol. Metab. 2003, 88, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Bagnoli, F.; Mori, A.; Fommei, C.; Coriolani, G.; Badii, S.; Tomasini, B. ACTH and cortisol cord plasma concentrations in preterm and term infants. J. Perinatol. 2013, 33, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Fowden, A.L.; Forhead, A.J. Glucocorticoids as regulatory signals during intrauterine development. Exp. Physiol. 2015, 100, 1477–1487. [Google Scholar] [CrossRef]
- Seckl, J.R. Prenatal glucocorticoids and long-term programming. Eur. J. Endocrinol. 2004, 151 (Suppl. S3), U49–U62. [Google Scholar] [CrossRef]
- Verkauskiene, R.; Beltrand, J.; Claris, O.; Chevenne, D.; Deghmoun, S.; Dorgeret, S.; Alison, M.; Gaucherand, P.; Sibony, O.; Levy-Marchal, C. Impact of fetal growth restriction on body composition and hormonal status at birth in infants of small and appropriate weight for gestational age. Eur. J. Endocrinol. 2007, 157, 605–612. [Google Scholar] [CrossRef]
- Aoki, M.; Urakami, T.; Nagano, N.; Aoki, R.; Morioka, I. Association of Plasma Cortisol Levels with Gestational Age and Anthropometric Values at Birth in Preterm Infants. Int. J. Environ. Res. Public Health 2022, 19, 11448. [Google Scholar] [CrossRef]
- Dave-Sharma, S.; Wilson, R.C.; Harbison, M.D.; Newfield, R.; Azar, M.R.; Krozowski, Z.S.; Funder, J.W.; Shackleton, C.H.; Bradlow, H.L.; Wei, J.Q.; et al. Examination of genotype and phenotype relationships in 14 patients with apparent mineralocorticoid excess. J. Clin. Endocrinol. Metab. 1998, 83, 2244–2254. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, E.C.; Seckl, J.R.; Holmes, M.C.; Wyrwoll, C.S. Foetal and placental 11beta-HSD2: A hub for developmental programming. Acta Physiol. 2014, 210, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Bose, H.S.; Sugawara, T.; Strauss, J.F., 3rd; Miller, W.L.; International Congenital Lipoid Adrenal Hyperplasia Consortium. The pathophysiology and genetics of congenital lipoid adrenal hyperplasia. N. Engl. J. Med. 1996, 335, 1870–1878. [Google Scholar] [CrossRef]
- de Zegher, F.; Devlieger, H.; Eeckels, R. Fetal growth: Boys before girls. Horm. Res. 1999, 51, 258–259. [Google Scholar] [CrossRef] [PubMed]
- de Zegher, F.; Francois, I.; Boehmer, A.L.; Saggese, G.; Muller, J.; Hiort, O.; Sultan, C.; Clayton, P.; Brauner, R.; Cacciari, E.; et al. Androgens and fetal growth. Horm. Res. 1998, 50, 243–244. [Google Scholar] [CrossRef]
- Miles, H.L.; Gidlof, S.; Nordenstrom, A.; Ong, K.K.; Hughes, I.A. The role of androgens in fetal growth: Observational study in two genetic models of disordered androgen signalling. Arch. Dis. Child Fetal Neonatal Ed. 2010, 95, F435–F438. [Google Scholar] [CrossRef]
- Poyrazoglu, S.; Darendeliler, F.; Ahmed, S.F.; Hughes, I.; Bryce, J.; Jiang, J.; Rodie, M.; Hiort, O.; Hannema, S.E.; Bertelloni, S.; et al. Birth Weight in Different Etiologies of Disorders of Sex Development. J. Clin. Endocrinol. Metab. 2017, 102, 1044–1050. [Google Scholar] [CrossRef][Green Version]
- Morishima, A.; Grumbach, M.M.; Simpson, E.R.; Fisher, C.; Qin, K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J. Clin. Endocrinol. Metab. 1995, 80, 3689–3698. [Google Scholar] [CrossRef]
- Smith, E.P.; Boyd, J.; Frank, G.R.; Takahashi, H.; Cohen, R.M.; Specker, B.; Williams, T.C.; Lubahn, D.B.; Korach, K.S. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N. Engl. J. Med. 1994, 331, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Yu, W.; Fu, L.; Li, F.; Jing, J.; Cui, X.; Wang, S.; Cao, Q.; Xue, B.; Shi, H. Postnatal leptin surge is critical for the transient induction of the developmental beige adipocytes in mice. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E453–E461. [Google Scholar] [CrossRef]
- Yuen, B.S.; Owens, P.C.; Muhlhausler, B.S.; Roberts, C.T.; Symonds, M.E.; Keisler, D.H.; McFarlane, J.R.; Kauter, K.G.; Evens, Y.; McMillen, I.C. Leptin alters the structural and functional characteristics of adipose tissue before birth. FASEB J. 2003, 17, 1102–1104. [Google Scholar] [CrossRef] [PubMed]
- Henson, M.C.; Swan, K.F.; O’Neil, J.S. Expression of placental leptin and leptin receptor transcripts in early pregnancy and at term. Obstet. Gynecol. 1998, 92, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Henson, M.C.; Castracane, V.D.; O’Neil, J.S.; Gimpel, T.; Swan, K.F.; Green, A.E.; Shi, W. Serum leptin concentrations and expression of leptin transcripts in placental trophoblast with advancing baboon pregnancy. J. Clin. Endocrinol. Metab. 1999, 84, 2543–2549. [Google Scholar] [CrossRef] [PubMed]
- Lepercq, J.; Challier, J.C.; Guerre-Millo, M.; Cauzac, M.; Vidal, H.; Hauguel-de Mouzon, S. Prenatal leptin production: Evidence that fetal adipose tissue produces leptin. J. Clin. Endocrinol. Metab. 2001, 86, 2409–2413. [Google Scholar] [CrossRef]
- Cetin, I.; Morpurgo, P.S.; Radaelli, T.; Taricco, E.; Cortelazzi, D.; Bellotti, M.; Pardi, G.; Beck-Peccoz, P. Fetal plasma leptin concentrations: Relationship with different intrauterine growth patterns from 19 weeks to term. Pediatr. Res. 2000, 48, 646–651. [Google Scholar] [CrossRef]
- Geary, M.; Herschkovitz, R.; Pringle, P.J.; Rodeck, C.H.; Hindmarsh, P.C. Ontogeny of serum leptin concentrations in the human. Clin. Endocrinol. 1999, 51, 189–192. [Google Scholar] [CrossRef]
- Schubring, C.; Siebler, T.; Kratzsch, J.; Englaro, P.; Blum, W.F.; Triep, K.; Kiess, W. Leptin serum concentrations in healthy neonates within the first week of life: Relation to insulin and growth hormone levels, skinfold thickness, body mass index and weight. Clin. Endocrinol. 1999, 51, 199–204. [Google Scholar] [CrossRef]
- Pighetti, M.; Tommaselli, G.A.; D’Elia, A.; Di Carlo, C.; Mariano, A.; Di Carlo, A.; Nappi, C. Maternal serum and umbilical cord blood leptin concentrations with fetal growth restriction. Obstet. Gynecol. 2003, 102, 535–543. [Google Scholar] [CrossRef]
- Simpson, J.; Smith, A.D.; Fraser, A.; Sattar, N.; Lindsay, R.S.; Ring, S.M.; Tilling, K.; Davey Smith, G.; Lawlor, D.A.; Nelson, S.M. Programming of Adiposity in Childhood and Adolescence: Associations with Birth Weight and Cord Blood Adipokines. J. Clin. Endocrinol. Metab. 2017, 102, 499–506. [Google Scholar] [CrossRef]
- Kajantie, E.; Hytinantti, T.; Hovi, P.; Andersson, S. Cord plasma adiponectin: A 20-fold rise between 24 weeks gestation and term. J. Clin. Endocrinol. Metab. 2004, 89, 4031–4036. [Google Scholar] [CrossRef]
- Kitamura, S.; Yokota, I.; Hosoda, H.; Kotani, Y.; Matsuda, J.; Naito, E.; Ito, M.; Kangawa, K.; Kuroda, Y. Ghrelin concentration in cord and neonatal blood: Relation to fetal growth and energy balance. J. Clin. Endocrinol. Metab. 2003, 88, 5473–5477. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Li, B.; Xu, X.; Liu, S.; Li, Z.; Li, M.; Wang, D. Umbilical Cord Blood Adiponectin, Leptin, Insulin, and Ghrelin in Premature Infants and Their Association with Birth Outcomes. Front. Endocrinol. 2021, 12, 738964. [Google Scholar] [CrossRef] [PubMed]
- Gat-Yablonski, G.; Phillip, M. Leptin and regulation of linear growth. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Beghini, M.; Brandt, S.; Korber, I.; Kohlsdorf, K.; Vollbach, H.; Lennerz, B.; Denzer, C.; Shalitin, S.; Santini, F.; Blum, W.F.; et al. Serum IGF1 and linear growth in children with congenital leptin deficiency before and after leptin substitution. Int. J. Obes. 2021, 45, 1448–1456. [Google Scholar] [CrossRef]
- Farooqi, I.S.; Wangensteen, T.; Collins, S.; Kimber, W.; Matarese, G.; Keogh, J.M.; Lank, E.; Bottomley, B.; Lopez-Fernandez, J.; Ferraz-Amaro, I.; et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N. Engl. J. Med. 2007, 356, 237–247. [Google Scholar] [CrossRef]
- Poher, A.L.; Tschop, M.H.; Muller, T.D. Ghrelin regulation of glucose metabolism. Peptides 2018, 100, 236–242. [Google Scholar] [CrossRef]
- Tong, J.; Prigeon, R.L.; Davis, H.W.; Bidlingmaier, M.; Kahn, S.E.; Cummings, D.E.; Tschop, M.H.; D’Alessio, D. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes 2010, 59, 2145–2151. [Google Scholar] [CrossRef] [PubMed]
- Gualillo, O.; Caminos, J.; Blanco, M.; Garcia-Caballero, T.; Kojima, M.; Kangawa, K.; Dieguez, C.; Casanueva, F. Ghrelin, a novel placental-derived hormone. Endocrinology 2001, 142, 788–794. [Google Scholar] [CrossRef]
- Cortelazzi, D.; Cappiello, V.; Morpurgo, P.S.; Ronzoni, S.; Nobile De Santis, M.S.; Cetin, I.; Beck-Peccoz, P.; Spada, A. Circulating levels of ghrelin in human fetuses. Eur. J. Endocrinol. 2003, 149, 111–116. [Google Scholar] [CrossRef]
- Bellone, S.; Prodam, F.; Savastio, S.; Avanzo, D.; Pagani, A.; Trovato, L.; Walker, G.E.; Genoni, G.; Bona, G. Acylated/unacylated ghrelin ratio in cord blood: Correlation with anthropometric and metabolic parameters and pediatric lifespan comparison. Eur. J. Endocrinol. 2012, 166, 115–120. [Google Scholar] [CrossRef]
- Farquhar, J.; Heiman, M.; Wong, A.C.; Wach, R.; Chessex, P.; Chanoine, J.P. Elevated umbilical cord ghrelin concentrations in small for gestational age neonates. J. Clin. Endocrinol. Metab. 2003, 88, 4324–4327. [Google Scholar] [CrossRef] [PubMed]
- Yokota, I.; Kitamura, S.; Hosoda, H.; Kotani, Y.; Kangawa, K. Concentration of the n-octanoylated active form of ghrelin in fetal and neonatal circulation. Endocr. J. 2005, 52, 271–276. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Warchol, M.; Wojciechowska, M.; Kupsz, J.; Sot-Szewczyk, M.H.; Michalak, M.; Kolodziejski, P.; Pruszynska-Oszmalek, E.; Krauss, H. Association of cord blood ghrelin, leptin and insulin concentrations in term newborns with anthropometric parameters at birth. J. Pediatr. Endocrinol. Metab. 2018, 31, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Wortley, K.E.; del Rincon, J.P.; Murray, J.D.; Garcia, K.; Iida, K.; Thorner, M.O.; Sleeman, M.W. Absence of ghrelin protects against early-onset obesity. J. Clin. Investig. 2005, 115, 3573–3578. [Google Scholar] [CrossRef]
- Pantel, J.; Legendre, M.; Cabrol, S.; Hilal, L.; Hajaji, Y.; Morisset, S.; Nivot, S.; Vie-Luton, M.P.; Grouselle, D.; de Kerdanet, M.; et al. Loss of constitutive activity of the growth hormone secretagogue receptor in familial short stature. J. Clin. Investig. 2006, 116, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Pinar, H.; Basu, S.; Hotmire, K.; Laffineuse, L.; Presley, L.; Carpenter, M.; Catalano, P.M.; Hauguel-de Mouzon, S. High molecular mass multimer complexes and vascular expression contribute to high adiponectin in the fetus. J. Clin. Endocrinol. Metab. 2008, 93, 2885–2890. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, S.; Bulfamante, G.; Cortelazzi, D.; Barresi, V.; Cetin, I.; Mantovani, G.; Bondioni, S.; Beck-Peccoz, P.; Spada, A. Adiponectin expression in human fetal tissues during mid- and late gestation. J. Clin. Endocrinol. Metab. 2005, 90, 2397–2402. [Google Scholar] [CrossRef]
- Makker, K.; Zhang, M.; Wang, G.; Hong, X.; Aziz, K.; Brady, T.M.; Wang, X. Longitudinal Trajectory and Early Life Determinant of Childhood Adipokines: Findings From a Racially Diverse Birth Cohort. J. Clin. Endocrinol. Metab. 2023, 108, 1747–1757. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Saget, S.; Lu, C.; Hay, W.W., Jr.; Karsenty, G.; Shao, J. Adiponectin Promotes Maternal beta-Cell Expansion through Placental Lactogen Expression. Diabetes 2021, 70, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Lekva, T.; Roland, M.C.P.; Michelsen, A.E.; Friis, C.M.; Aukrust, P.; Bollerslev, J.; Henriksen, T.; Ueland, T. Large Reduction in Adiponectin During Pregnancy Is Associated with Large-for-Gestational-Age Newborns. J. Clin. Endocrinol. Metab. 2017, 102, 2552–2559. [Google Scholar] [CrossRef]
- Balachandiran, M.; Bobby, Z.; Dorairajan, G.; Gladwin, V.; Vinayagam, V.; Packirisamy, R.M. Decreased maternal serum adiponectin and increased insulin-like growth factor-1 levels along with increased placental glucose transporter-1 expression in gestational diabetes mellitus: Possible role in fetal overgrowth. Placenta 2021, 104, 71–80. [Google Scholar] [CrossRef]
- Sivan, E.; Mazaki-Tovi, S.; Pariente, C.; Efraty, Y.; Schiff, E.; Hemi, R.; Kanety, H. Adiponectin in human cord blood: Relation to fetal birth weight and gender. J. Clin. Endocrinol. Metab. 2003, 88, 5656–5660. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, R.S.; Walker, J.D.; Havel, P.J.; Hamilton, B.A.; Calder, A.A.; Johnstone, F.D.; Scottish Multicentre Study of Diabetes, P. Adiponectin is present in cord blood but is unrelated to birth weight. Diabetes Care 2003, 26, 2244–2249. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Wattez, J.S.; Lee, S.; Guo, Z.; Schaack, J.; Hay, W.W., Jr.; Zita, M.M.; Parast, M.; Shao, J. Knockout maternal adiponectin increases fetal growth in mice: Potential role for trophoblast IGFBP-1. Diabetologia 2016, 59, 2417–2425. [Google Scholar] [CrossRef] [PubMed]
- Mayeur, S.; Wattez, J.S.; Lukaszewski, M.A.; Lecoutre, S.; Butruille, L.; Drougard, A.; Eberle, D.; Bastide, B.; Laborie, C.; Storme, L.; et al. Apelin Controls Fetal and Neonatal Glucose Homeostasis and Is Altered by Maternal Undernutrition. Diabetes 2016, 65, 554–560. [Google Scholar] [CrossRef]
- Vaughan, O.R.; Powell, T.L.; Jansson, T. Apelin is a novel regulator of human trophoblast amino acid transport. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E810–E816. [Google Scholar] [CrossRef]
- Hanssens, S.; Marousez, L.; Pecheux, O.; Besengez, C.; Storme, L.; Deruelle, P.; Eberle, D.; Lesage, J. Maternal obesity reduces apelin level in cord blood without altering the placental apelin/elabela-APJ system. Placenta 2022, 128, 112–115. [Google Scholar] [CrossRef]
- Jiang, S.; Teague, A.M.; Tryggestad, J.B.; Lyons, T.J.; Chernausek, S.D. Fetal circulating human resistin increases in diabetes during pregnancy and impairs placental mitochondrial biogenesis. Mol. Med. 2020, 26, 76. [Google Scholar] [CrossRef]
- Martos-Moreno, G.A.; Barrios, V.; Saenz de Pipaon, M.; Pozo, J.; Dorronsoro, I.; Martinez-Biarge, M.; Quero, J.; Argente, J. Influence of prematurity and growth restriction on the adipokine profile, IGF1, and ghrelin levels in cord blood: Relationship with glucose metabolism. Eur. J. Endocrinol. 2009, 161, 381–389. [Google Scholar] [CrossRef]
- Zamojska, J.; Niewiadomska-Jarosik, K.; Wosiak, A.; Gruca, M.; Smolewska, E. Serum Adipocytokines Profile in Children Born Small and Appropriate for Gestational Age-A Comparative Study. Nutrients 2023, 15, 868. [Google Scholar] [CrossRef]
- Chang, Y.H.; Chang, D.M.; Lin, K.C.; Shin, S.J.; Lee, Y.J. Visfatin in overweight/obesity, type 2 diabetes mellitus, insulin resistance, metabolic syndrome and cardiovascular diseases: A meta-analysis and systemic review. Diabetes Metab. Res. Rev. 2011, 27, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Ooi, S.Q.; Chan, R.M.; Poh, L.K.; Loke, K.Y.; Heng, C.K.; Chan, Y.H.; Gan, S.U.; Lee, K.O.; Lee, Y.S. Visfatin and its genetic variants are associated with obesity-related morbidities and cardiometabolic risk in severely obese children. Pediatr. Obes. 2014, 9, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, L.; Sebastiani, G.; Lopez-Bermejo, A.; Diaz, M.; Gomez-Roig, M.D.; de Zegher, F. Gender specificity of body adiposity and circulating adiponectin, visfatin, insulin, and insulin growth factor-I at term birth: Relation to prenatal growth. J. Clin. Endocrinol. Metab. 2008, 93, 2774–2778. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Wang, S.; Yang, M.N.; Dong, Y.; He, H.; Fang, F.; Huang, R.; Yu, X.G.; Zhang, G.H.; Zhao, X.; et al. Fetuin-A in Infants Born Small- or Large-for-Gestational-Age. Front. Endocrinol. 2020, 11, 567955. [Google Scholar] [CrossRef]
- Driscoll, A.K.; Gregory, E.C.W. Increases in Prepregnancy Obesity: United States, 2016-2019. NCHS Data Brief 2020, 392, 1–8. [Google Scholar]
- Strauss, A.; Rochow, N.; Kunze, M.; Hesse, V.; Dudenhausen, J.W.; Voigt, M. Obesity in pregnant women: A 20-year analysis of the German experience. Eur. J. Clin. Nutr. 2021, 75, 1757–1763. [Google Scholar] [CrossRef]
- Zhang, C.; Hediger, M.L.; Albert, P.S.; Grewal, J.; Sciscione, A.; Grobman, W.A.; Wing, D.A.; Newman, R.B.; Wapner, R.; D’Alton, M.E.; et al. Association of Maternal Obesity with Longitudinal Ultrasonographic Measures of Fetal Growth: Findings From the NICHD Fetal Growth Studies-Singletons. JAMA Pediatr. 2018, 172, 24–31. [Google Scholar] [CrossRef]
- Catalano, P.M.; Presley, L.; Minium, J.; Hauguel-de Mouzon, S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 2009, 32, 1076–1080. [Google Scholar] [CrossRef]
- Brynhildsen, J.; Sydsjo, G.; Blomberg, M.; Claesson, I.M.; Theodorsson, E.; Nystrom, F.; Sydsjo, A.; Josefsson, A. Leptin and adiponectin in cord blood from children of normal weight, overweight and obese mothers. Acta Paediatr. 2013, 102, 620–624. [Google Scholar] [CrossRef]
- Allbrand, M.; Aman, J.; Lodefalk, M. Placental ghrelin and leptin expression and cord blood ghrelin, adiponectin, leptin, and C-peptide levels in severe maternal obesity. J. Matern. Fetal Neonatal Med. 2018, 31, 2839–2846. [Google Scholar] [CrossRef]
- Makker, K.; Zhang, M.; Wang, G.; Hong, X.; Aziz, K.B.; Wang, X. Maternal and fetal factors affecting cord plasma leptin and adiponectin levels and their ratio in preterm and term newborns: New insight on fetal origins of metabolic dysfunction. Precis. Nutr. 2022, 1, e00013. [Google Scholar]
- Stirrat, L.I.; Just, G.; Homer, N.Z.M.; Andrew, R.; Norman, J.E.; Reynolds, R.M. Glucocorticoids are lower at delivery in maternal, but not cord blood of obese pregnancies. Sci. Rep. 2017, 7, 10263. [Google Scholar] [CrossRef]
- Carlsen, E.M.; Renault, K.M.; Jensen, R.B.; Norgaard, K.; Jensen, J.E.; Nilas, L.; Cortes, D.; Michaelsen, K.F.; Pryds, O. The Association between Newborn Regional Body Composition and Cord Blood Concentrations of C-Peptide and Insulin-Like Growth Factor I. PLoS ONE 2015, 10, e0121350. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, Z.M.; Qiu, Q.; Gruslin, A.; Adamo, K.B. Characterization of the insulin-like growth factor axis in term pregnancies complicated by maternal obesity. Hum. Reprod. 2012, 27, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Voerman, E.; Amiano, P.; Barros, H.; Beilin, L.J.; Bergstrom, A.; Charles, M.A.; Chatzi, L.; Chevrier, C.; Chrousos, G.P.; et al. Impact of maternal body mass index and gestational weight gain on pregnancy complications: An individual participant data meta-analysis of European, North American and Australian cohorts. BJOG 2019, 126, 984–995. [Google Scholar] [CrossRef] [PubMed]
- QuickStats: Gestational Weight Gain* Among Women with Full-Term, Singleton Births, Compared with Recommendations-48 States and the District of Columbia, 2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 1121. [CrossRef]
- Rifas-Shiman, S.L.; Fleisch, A.; Hivert, M.F.; Mantzoros, C.; Gillman, M.W.; Oken, E. First and second trimester gestational weight gains are most strongly associated with cord blood levels of hormones at delivery important for glycemic control and somatic growth. Metabolism 2017, 69, 112–119. [Google Scholar] [CrossRef]
- Logan, C.A.; Bornemann, R.; Koenig, W.; Reister, F.; Walter, V.; Fantuzzi, G.; Weyermann, M.; Brenner, H.; Genuneit, J.; Rothenbacher, D. Gestational Weight Gain and Fetal-Maternal Adiponectin, Leptin, and CRP: Results of two birth cohorts studies. Sci. Rep. 2017, 7, 41847. [Google Scholar] [CrossRef]
- Patro-Malysza, J.; Trojnar, M.; Skorzynska-Dziduszko, K.E.; Kimber-Trojnar, Z.; Darmochwal-Kolarz, D.; Czuba, M.; Leszczynska-Gorzelak, B. Leptin and Ghrelin in Excessive Gestational Weight Gain-Association between Mothers and Offspring. Int. J. Mol. Sci. 2019, 20, 2398. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: A Global Perspective. Curr. Diab. Rep. 2016, 16, 7. [Google Scholar] [CrossRef]
- Mirabelli, M.; Chiefari, E.; Tocci, V.; Greco, E.; Foti, D.; Brunetti, A. Gestational diabetes: Implications for fetal growth, intervention timing, and treatment options. Curr. Opin. Pharmacol. 2021, 60, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sovio, U.; Murphy, H.R.; Smith, G.C. Accelerated Fetal Growth Prior to Diagnosis of Gestational Diabetes Mellitus: A Prospective Cohort Study of Nulliparous Women. Diabetes Care 2016, 39, 982–987. [Google Scholar] [CrossRef] [PubMed]
- de Santis, M.S.; Taricco, E.; Radaelli, T.; Spada, E.; Rigano, S.; Ferrazzi, E.; Milani, S.; Cetin, I. Growth of fetal lean mass and fetal fat mass in gestational diabetes. Ultrasound. Obstet. Gynecol. 2010, 36, 328–337. [Google Scholar] [CrossRef]
- Sletner, L.; Jenum, A.K.; Yajnik, C.S.; Morkrid, K.; Nakstad, B.; Rognerud-Jensen, O.H.; Birkeland, K.I.; Vangen, S. Fetal growth trajectories in pregnancies of European and South Asian mothers with and without gestational diabetes, a population-based cohort study. PLoS ONE 2017, 12, e0172946. [Google Scholar] [CrossRef] [PubMed]
- Brand, J.S.; West, J.; Tuffnell, D.; Bird, P.K.; Wright, J.; Tilling, K.; Lawlor, D.A. Gestational diabetes and ultrasound-assessed fetal growth in South Asian and White European women: Findings from a prospective pregnancy cohort. BMC Med. 2018, 16, 203. [Google Scholar] [CrossRef]
- Li, M.; Hinkle, S.N.; Grantz, K.L.; Kim, S.; Grewal, J.; Grobman, W.A.; Skupski, D.W.; Newman, R.B.; Chien, E.K.; Sciscione, A.; et al. Glycaemic status during pregnancy and longitudinal measures of fetal growth in a multi-racial US population: A prospective cohort study. Lancet Diabetes Endocrinol. 2020, 8, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Group, H.S.C.R.; Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef]
- Lawlor, D.A.; West, J.; Fairley, L.; Nelson, S.M.; Bhopal, R.S.; Tuffnell, D.; Freeman, D.J.; Wright, J.; Whitelaw, D.C.; Sattar, N. Pregnancy glycaemia and cord-blood levels of insulin and leptin in Pakistani and white British mother-offspring pairs: Findings from a prospective pregnancy cohort. Diabetologia 2014, 57, 2492–2500. [Google Scholar] [CrossRef]
- Vasilakos, L.K.; Steinbrekera, B.; Santillan, D.A.; Santillan, M.K.; Brandt, D.S.; Dagle, D.; Roghair, R.D. Umbilical Cord Blood Leptin and IL-6 in the Presence of Maternal Diabetes or Chorioamnionitis. Front. Endocrinol. 2022, 13, 836541. [Google Scholar] [CrossRef]
- Ott, R.; Stupin, J.H.; Loui, A.; Eilers, E.; Melchior, K.; Rancourt, R.C.; Schellong, K.; Ziska, T.; Dudenhausen, J.W.; Henrich, W.; et al. Maternal overweight is not an independent risk factor for increased birth weight, leptin and insulin in newborns of gestational diabetic women: Observations from the prospective ‘EaCH’ cohort study. BMC Pregnancy Childbirth 2018, 18, 250. [Google Scholar] [CrossRef] [PubMed]
- Karakulak, M.; Saygili, U.; Temur, M.; Yilmaz, O.; Ozun Ozbay, P.; Calan, M.; Cosar, H. Comparison of umbilical cord ghrelin concentrations in full-term pregnant women with or without gestational diabetes. Endocr. Res. 2017, 42, 79–85. [Google Scholar] [CrossRef]
- Patel, N.; Hellmuth, C.; Uhl, O.; Godfrey, K.; Briley, A.; Welsh, P.; Pasupathy, D.; Seed, P.T.; Koletzko, B.; Poston, L.; et al. Cord Metabolic Profiles in Obese Pregnant Women: Insights Into Offspring Growth and Body Composition. J. Clin. Endocrinol. Metab. 2018, 103, 346–355. [Google Scholar] [CrossRef]
- Gomez-Diaz, R.A.; Gomez-Medina, M.P.; Ramirez-Soriano, E.; Lopez-Robles, L.; Aguilar-Salinas, C.A.; Saucedo, R.; Zarate, A.; Valladares-Salgado, A.; Wacher, N.H. Lower Plasma Ghrelin Levels are Found in Women with Diabetes-Complicated Pregnancies. J. Clin. Res. Pediatr. Endocrinol. 2016, 8, 425–431. [Google Scholar] [CrossRef]
- Kanai, Y.; Kamoda, T.; Saito, M.; Fujiyama, S.; Nishimura, K.; Iwabuchi, A.; Miyazono, Y.; Hamada, H.; Sumazaki, R. Cord blood insulin-like growth factor (IGF)-1, IGF-binding proteins and adiponectin, and birth size in offspring of women with mild gestational diabetes. Early Hum. Dev. 2016, 93, 39–42. [Google Scholar] [CrossRef]
- Oken, E.; Morton-Eggleston, E.; Rifas-Shiman, S.L.; Switkowski, K.M.; Hivert, M.F.; Fleisch, A.F.; Mantzoros, C.; Gillman, M.W. Sex-Specific Associations of Maternal Gestational Glycemia with Hormones in Umbilical Cord Blood at Delivery. Am. J. Perinatol. 2016, 33, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Herbstman, J.; Apelberg, B.J.; Witter, F.R.; Panny, S.; Goldman, L.R. Maternal, infant, and delivery factors associated with neonatal thyroid hormone status. Thyroid 2008, 18, 67–76. [Google Scholar] [CrossRef]
- Liu, K.; Wu, H.Y.; Xu, Y.H. Study on the relationship between the expression of IGF-1 in umbilical cord blood and abnormal glucose metabolism during pregnancy. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 647–651. [Google Scholar] [PubMed]
- Hall, K.; Hansson, U.; Lundin, G.; Luthman, M.; Persson, B.; Povoa, G.; Stangenberg, M.; Ofverholm, U. Serum levels of somatomedins and somatomedin-binding protein in pregnant women with type I or gestational diabetes and their infants. J. Clin. Endocrinol. Metab. 1986, 63, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Yan-Jun, L.; Tsushima, T.; Minei, S.; Sanaka, M.; Nagashima, T.; Yanagisawa, K.; Omori, Y. Insulin-like growth factors (IGFs) and IGF-binding proteins (IGFBP-1, -2 and -3) in diabetic pregnancy: Relationship to macrosomia. Endocr. J. 1996, 43, 221–231. [Google Scholar] [CrossRef]
- Jaddoe, V.W.; Verburg, B.O.; de Ridder, M.A.; Hofman, A.; Mackenbach, J.P.; Moll, H.A.; Steegers, E.A.; Witteman, J.C. Maternal smoking and fetal growth characteristics in different periods of pregnancy: The generation R study. Am. J. Epidemiol. 2007, 165, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.; Alramadhan, S.; Iniguez, C.; Duijts, L.; Jaddoe, V.W.; Den Dekker, H.T.; Crozier, S.; Godfrey, K.M.; Hindmarsh, P.; Vik, T.; et al. A systematic review of maternal smoking during pregnancy and fetal measurements with meta-analysis. PLoS ONE 2017, 12, e0170946. [Google Scholar] [CrossRef]
- Fernandez-Rodriguez, B.; Gomez, A.R.; Jimenez Moreno, B.S.; de Alba, C.; Galindo, A.; Villalain, C.; Pallas, C.; Herraiz, I. Smoking influence on early and late fetal growth. J. Perinat. Med. 2022, 50, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Pringle, P.J.; Geary, M.P.; Rodeck, C.H.; Kingdom, J.C.; Kayamba-Kay’s, S.; Hindmarsh, P.C. The influence of cigarette smoking on antenatal growth, birth size, and the insulin-like growth factor axis. J. Clin. Endocrinol. Metab. 2005, 90, 2556–2562. [Google Scholar] [CrossRef]
- Fleisch, A.F.; Rifas-Shiman, S.L.; Rokoff, L.B.; Hivert, M.F.; Mantzoros, C.S.; Oken, E. Associations of maternal prenatal smoking with umbilical cord blood hormones: The Project Viva cohort. Metabolism 2017, 72, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Bouhours-Nouet, N.; Boux de Casson, F.; Rouleau, S.; Douay, O.; Mathieu, E.; Bouderlique, C.; Gillard, P.; Limal, J.M.; Descamps, P.; Coutant, R. Maternal and cord blood ghrelin in the pregnancies of smoking mothers: Possible markers of nutrient availability for the fetus. Horm. Res. 2006, 66, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Luo, Z.C.; Dejemli, A.; Delvin, E.; Zhang, J. Maternal Smoking and Metabolic Health Biomarkers in Newborns. PLoS ONE 2015, 10, e0143660. [Google Scholar] [CrossRef] [PubMed]
- Chelchowska, M.; Gajewska, J.; Maciejewski, T.M.; Mazur, J.; Oltarzewski, M.; Ambroszkiewicz, J. Associations between Maternal and Fetal Levels of Total Adiponectin, High Molecular Weight Adiponectin, Selected Somatomedins, and Birth Weight of Infants of Smoking and Non-Smoking Mothers. Int. J. Environ. Res. Public Health 2020, 17, 4781. [Google Scholar] [CrossRef]
- Ingvarsson, R.F.; Bjarnason, A.O.; Dagbjartsson, A.; Hardardottir, H.; Haraldsson, A.; Thorkelsson, T. The effects of smoking in pregnancy on factors influencing fetal growth. Acta Paediatr. 2007, 96, 383–386. [Google Scholar] [CrossRef]
- Proctor, L.K.; Kfouri, J.; Hiersch, L.; Aviram, A.; Zaltz, A.; Kingdom, J.; Barrett, J.; Melamed, N. Association between hypertensive disorders and fetal growth restriction in twin compared with singleton gestations. Am. J. Obstet. Gynecol. 2019, 221, 251.e251–251.e258. [Google Scholar] [CrossRef]
- Magalhaes, E.; Meio, M.; Peixoto-Filho, F.M.; Gonzalez, S.; da Costa, A.C.C.; Moreira, M.E.L. Pregnancy-induced hypertension, preterm birth, and cord blood adipokine levels. Eur. J. Pediatr. 2020, 179, 1239–1246. [Google Scholar] [CrossRef]
- Mateus, J.; Newman, R.B.; Zhang, C.; Pugh, S.J.; Grewal, J.; Kim, S.; Grobman, W.A.; Owen, J.; Sciscione, A.C.; Wapner, R.J.; et al. Fetal growth patterns in pregnancy-associated hypertensive disorders: NICHD Fetal Growth Studies. Am. J. Obstet. Gynecol. 2019, 221, 635.e631–635.e616. [Google Scholar] [CrossRef] [PubMed]
- Bakker, R.; Steegers, E.A.; Hofman, A.; Jaddoe, V.W. Blood pressure in different gestational trimesters, fetal growth, and the risk of adverse birth outcomes: The generation R study. Am. J. Epidemiol. 2011, 174, 797–806. [Google Scholar] [CrossRef]
- Aydin, S.; Guzel, S.P.; Kumru, S.; Aydin, S.; Akin, O.; Kavak, E.; Sahin, I.; Bozkurt, M.; Halifeoglu, I. Serum leptin and ghrelin concentrations of maternal serum, arterial and venous cord blood in healthy and preeclamptic pregnant women. J. Physiol. Biochem. 2008, 64, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Kharb, S.; Nanda, S. Patterns of Biomarkers in Cord Blood During Pregnancy and Preeclampsia. Curr. Hypertens. Rev. 2017, 13, 57–64. [Google Scholar] [CrossRef]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Bhowmik, B.; Siddique, T.; Majumder, A.; Mdala, I.; Hossain, I.A.; Hassan, Z.; Jahan, I.; Moreira, N.; Alim, A.; Basit, A.; et al. Maternal BMI and nutritional status in early pregnancy and its impact on neonatal outcomes at birth in Bangladesh. BMC Pregnancy Childbirth 2019, 19, 413. [Google Scholar] [CrossRef] [PubMed]
- Wiley, A.S.; Lubree, H.G.; Joshi, S.M.; Bhat, D.S.; Ramdas, L.V.; Rao, A.S.; Thuse, N.V.; Deshpande, V.U.; Yajnik, C.S. Cord IGF-I concentrations in Indian newborns: Associations with neonatal body composition and maternal determinants. Pediatr. Obes. 2016, 11, 151–157. [Google Scholar] [CrossRef]
- Mahajan, S.; Aalinkeel, R.; Shah, P.; Singh, S.; Gupta, N.; Kochupillai, N. Nutritional anaemia dysregulates endocrine control of fetal growth. Br. J. Nutr. 2008, 100, 408–417. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kelly, A.C.; Powell, T.L.; Jansson, T. Placental function in maternal obesity. Clin. Sci. 2020, 134, 961–984. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, L.M.; Ananth, C.V.; Prasad, V.; Nath, C.; Vintzileos, A.M.; New Jersey Placental Abruption Study Investigators. The influence of maternal cigarette smoking on placental pathology in pregnancies complicated by abruption. Am. J. Obstet. Gynecol. 2007, 197, 275.e1–275.e5. [Google Scholar] [CrossRef] [PubMed]
- Walker, N.; Filis, P.; O’Shaughnessy, P.J.; Bellingham, M.; Fowler, P.A. Nutrient transporter expression in both the placenta and fetal liver are affected by maternal smoking. Placenta 2019, 78, 10–17. [Google Scholar] [CrossRef]
- Coutant, R.; Boux de Casson, F.; Douay, O.; Mathieu, E.; Rouleau, S.; Beringue, F.; Gillard, P.; Limal, J.M.; Descamps, P. Relationships between placental GH concentration and maternal smoking, newborn gender, and maternal leptin: Possible implications for birth weight. J. Clin. Endocrinol. Metab. 2001, 86, 4854–4859. [Google Scholar] [CrossRef]
- Dimitriadis, E.; Rolnik, D.L.; Zhou, W.; Estrada-Gutierrez, G.; Koga, K.; Francisco, R.P.V.; Whitehead, C.; Hyett, J.; da Silva Costa, F.; Nicolaides, K.; et al. Pre-eclampsia. Nat. Rev. Dis. Primers. 2023, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Steinbrekera, B.; Colaizy, T.T.; Vasilakos, L.K.; Johnson, K.J.; Santillan, D.A.; Haskell, S.E.; Roghair, R.D. Origins of neonatal leptin deficiency in preterm infants. Pediatr. Res. 2019, 85, 1016–1023. [Google Scholar] [CrossRef]
- Williams, F.L.; Simpson, J.; Delahunty, C.; Ogston, S.A.; Bongers-Schokking, J.J.; Murphy, N.; van Toor, H.; Wu, S.Y.; Visser, T.J.; Hume, R.; et al. Developmental trends in cord and postpartum serum thyroid hormones in preterm infants. J. Clin. Endocrinol. Metab. 2004, 89, 5314–5320. [Google Scholar] [CrossRef] [PubMed]
- Chmielewska, A.; Farooqi, A.; Domellof, M.; Ohlund, I. Lean Tissue Deficit in Preterm Infants Persists up to 4 Months of Age: Results from a Swedish Longitudinal Study. Neonatology 2020, 117, 80–87. [Google Scholar] [CrossRef]
- Casirati, A.; Somaschini, A.; Perrone, M.; Vandoni, G.; Sebastiani, F.; Montagna, E.; Somaschini, M.; Caccialanza, R. Preterm birth and metabolic implications on later life: A narrative review focused on body composition. Front. Nutr. 2022, 9, 978271. [Google Scholar] [CrossRef] [PubMed]
- Kajantie, E.; Dunkel, L.; Rutanen, E.M.; Seppala, M.; Koistinen, R.; Sarnesto, A.; Andersson, S. IGF-I, IGF binding protein (IGFBP)-3, phosphoisoforms of IGFBP-1, and postnatal growth in very low birth weight infants. J. Clin. Endocrinol. Metab. 2002, 87, 2171–2179. [Google Scholar] [CrossRef]
- Conover, C.A.; Divertie, G.D.; Lee, P.D. Cortisol increases plasma insulin-like growth factor binding protein-1 in humans. Acta Endocrinol. 1993, 128, 140–143. [Google Scholar] [CrossRef][Green Version]
- Menuelle, P.; Babajko, S.; Plas, C. Insulin-like growth factor (IGF) binding proteins modulate the glucocorticoid-dependent biological effects of IGF-II in cultured fetal rat hepatocytes. Endocrinology 1999, 140, 2232–2240. [Google Scholar] [CrossRef][Green Version]
- Mistry, J.N.; Silvennoinen, S.; Zaman, F.; Savendahl, L.; Mariniello, K.; Hall, C.; Howard, S.R.; Dunkel, L.; Sankilampi, U.; Guasti, L. The crosstalk between FGF21 and GH leads to weakened GH receptor signaling and IGF1 expression and is associated with growth failure in very preterm infants. Front. Endocrinol. 2023, 14, 1105602. [Google Scholar] [CrossRef]
- Daliot, J.; Laron-Kenet, T.; Wattad, M.; Ben-Dor, A.; Lilos, P.; Laron, Z. The relationship between serum levels of prolactin and growth hormone in the early postnatal period. Pediatr. Res. 2017, 82, 796–800. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shimizu, T.; Kitamura, T.; Yoshikawa, N.; Suganuma, H.; Hisata, K.; Tanaka, K.; Shinohara, K.; Yamashiro, Y. Plasma levels of active ghrelin until 8 weeks after birth in preterm infants: Relationship with anthropometric and biochemical measures. Arch. Dis. Child Fetal Neonatal Ed. 2007, 92, F291–F292. [Google Scholar] [CrossRef] [PubMed]
- Bellone, S.; Baldelli, R.; Radetti, G.; Rapa, A.; Vivenza, D.; Petri, A.; Savastio, S.; Zaffaroni, M.; Broglio, F.; Ghigo, E.; et al. Ghrelin secretion in preterm neonates progressively increases and is refractory to the inhibitory effect of food intake. J. Clin. Endocrinol. Metab. 2006, 91, 1929–1933. [Google Scholar] [CrossRef] [PubMed]
- Chaoimh, C.N.; Murray, D.M.; Kenny, L.C.; Irvine, A.D.; Hourihane, J.O.; Kiely, M. Cord blood leptin and gains in body weight and fat mass during infancy. Eur. J. Endocrinol. 2016, 175, 403–410. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ong, K.K.; Ahmed, M.L.; Sherriff, A.; Woods, K.A.; Watts, A.; Golding, J.; Dunger, D.B. Cord blood leptin is associated with size at birth and predicts infancy weight gain in humans. ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. J. Clin. Endocrinol. Metab. 1999, 84, 1145–1148. [Google Scholar] [CrossRef]
- Kaar, J.L.; Brinton, J.T.; Crume, T.; Hamman, R.F.; Glueck, D.H.; Dabelea, D. Leptin levels at birth and infant growth: The EPOCH study. J. Dev. Orig. Health Dis. 2014, 5, 214–218. [Google Scholar] [CrossRef]
- Ertl, T.; Funke, S.; Sarkany, I.; Szabo, I.; Rascher, W.; Blum, W.F.; Sulyok, E. Postnatal changes of leptin levels in full-term and preterm neonates: Their relation to intrauterine growth, gender and testosterone. Biol. Neonate 1999, 75, 167–176. [Google Scholar] [CrossRef]
- Harigaya, A.; Onigata, K.; Nako, Y.; Nagashima, K.; Morikawa, A. Role of serum leptin in the regulation of weight gain in early infancy. Biol. Neonate 1999, 75, 234–238. [Google Scholar] [CrossRef]
- Volberg, V.; Heggeseth, B.; Harley, K.; Huen, K.; Yousefi, P.; Dave, V.; Tyler, K.; Vedar, M.; Eskenazi, B.; Holland, N. Adiponectin and leptin trajectories in Mexican-American children from birth to 9 years of age. PLoS ONE 2013, 8, e77964. [Google Scholar] [CrossRef]
- Hansen-Pupp, I.; Hellgren, G.; Hard, A.L.; Smith, L.; Hellstrom, A.; Lofqvist, C. Early Surge in Circulatory Adiponectin Is Associated with Improved Growth at Near Term in Very Preterm Infants. J. Clin. Endocrinol. Metab. 2015, 100, 2380–2387. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.Y.; Chen, X.Y.; Han, T.; Jin, Y.; Chen, T.T.; Wang, Z.H.; Zhao, Z.Y.; Zhu, Z.W. Associations between cord blood metabolic factors and early-childhood growth and overweight and obesity. Front. Endocrinol. 2023, 14, 1164747. [Google Scholar] [CrossRef]
- Wang, G.; Johnson, S.; Gong, Y.; Polk, S.; Divall, S.; Radovick, S.; Moon, M.; Paige, D.; Hong, X.; Caruso, D.; et al. Weight Gain in Infancy and Overweight or Obesity in Childhood across the Gestational Spectrum: A Prospective Birth Cohort Study. Sci. Rep. 2016, 6, 29867. [Google Scholar] [CrossRef]
- Meek, J.Y.; Noble, L.; Section on Breastfeeding. Policy Statement: Breastfeeding and the Use of Human Milk. Pediatrics 2022, 150, e2022057988. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.G.; Stellwagen, L.M.; Noble, L.; Kim, J.H.; Poindexter, B.B.; Puopolo, K.M.; Section on Breastfeeding, Committee on Nutrition, Committee on Fetus And Newborn. Promoting Human Milk and Breastfeeding for the Very Low Birth Weight Infant. Pediatrics 2021, 148, e2021054272. [Google Scholar] [CrossRef]
- Committee on Nutrition, Section on Breastfeeding, Committee on Fetus and Newborn. Donor Human Milk for the High-Risk Infant: Preparation, Safety, and Usage Options in the United States. Pediatrics 2017, 139, e20163440. [Google Scholar] [CrossRef]
- Engstrom, E.; Niklasson, A.; Wikland, K.A.; Ewald, U.; Hellstrom, A. The role of maternal factors, postnatal nutrition, weight gain, and gender in regulation of serum IGF-I among preterm infants. Pediatr. Res. 2005, 57, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.J.; Underwood, L.E.; Keyes, L.; Clemmons, D.R. Use of insulin-like growth factor I (IGF-I) and IGF-binding protein measurements to monitor feeding of premature infants. J. Clin. Endocrinol. Metab. 1997, 82, 3982–3988. [Google Scholar] [CrossRef]
- Yumani, D.F.J.; Calor, A.K.; van Weissenbruch, M.M. The Course of IGF-1 Levels and Nutrient Intake in Extremely and Very Preterm Infants During Hospitalisation. Nutrients 2020, 12, 675. [Google Scholar] [CrossRef]
- Belfort, M.B.; Edwards, E.M.; Greenberg, L.T.; Parker, M.G.; Ehret, D.Y.; Horbar, J.D. Diet, weight gain, and head growth in hospitalized US very preterm infants: A 10-year observational study. Am. J. Clin. Nutr. 2019, 109, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Socha, P.; Grote, V.; Gruszfeld, D.; Janas, R.; Demmelmair, H.; Closa-Monasterolo, R.; Subias, J.E.; Scaglioni, S.; Verduci, E.; Dain, E.; et al. Milk protein intake, the metabolic-endocrine response, and growth in infancy: Data from a randomized clinical trial. Am. J. Clin. Nutr. 2011, 94, 1776S–1784S. [Google Scholar] [CrossRef]
- Mehta, R.; Petrova, A. Urinary levels of energy metabolism hormones in association with the proportional intake of maternal milk and weight gain in very preterm neonates. J. Neonatal. Perinatal. Med. 2022, 15, 599–607. [Google Scholar] [CrossRef] [PubMed]
- York, D.J.; Smazal, A.L.; Robinson, D.T.; De Plaen, I.G. Human Milk Growth Factors and Their Role in NEC Prevention: A Narrative Review. Nutrients. 2021, 13, 3751. [Google Scholar] [CrossRef] [PubMed]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Chatmethakul, T.; Schmelzel, M.L.; Johnson, K.J.; Walker, J.R.; Santillan, D.A.; Colaizy, T.T.; Roghair, R.D. Postnatal Leptin Levels Correlate with Breast Milk Leptin Content in Infants Born before 32 Weeks Gestation. Nutrients 2022, 14, 5224. [Google Scholar] [CrossRef] [PubMed]
- Galante, L.; Reynolds, C.M.; Milan, A.M.; Alexander, T.; Bloomfield, F.H.; Jiang, Y.; Asadi, S.; Muelbert, M.; Cameron-Smith, D.; Pundir, S.; et al. Metabolic Hormone Profiles in Breast Milk From Mothers of Moderate-Late Preterm Infants Are Associated with Growth From Birth to 4 Months in a Sex-Specific Manner. Front. Nutr. 2021, 8, 641227. [Google Scholar] [CrossRef]
- Joung, K.E.; Martin, C.R.; Cherkerzian, S.; Kellogg, M.; Belfort, M.B. Human Milk Hormone Intake in the First Month of Life and Physical Growth Outcomes in Preterm Infants. J. Clin. Endocrinol. Metab. 2021, 106, 1793–1803. [Google Scholar] [CrossRef]
- Ramiro-Cortijo, D.; Singh, P.; Herranz Carrillo, G.; Gila-Diaz, A.; Martin-Cabrejas, M.A.; Martin, C.R.; Arribas, S.M. Association of maternal body composition and diet on breast milk hormones and neonatal growth during the first month of lactation. Front. Endocrinol. 2023, 14, 1090499. [Google Scholar] [CrossRef]
- Casabiell, X.; Piñeiro, V.; Tomé, M.A.; Peinó, R.; Diéguez, C.; Casanueva, F.F. Presence of leptin in colostrum and/or breast milk from lactating mothers: A potential role in the regulation of neonatal food intake. J. Clin. Endocrinol. Metab. 1997, 82, 4270–4273. [Google Scholar] [CrossRef]
- Sánchez, J.; Oliver, P.; Miralles, O.; Ceresi, E.; Picó, C.; Palou, A. Leptin orally supplied to neonate rats is directly uptaken by the immature stomach and may regulate short-term feeding. Endocrinology. 2005, 146, 2575–2582. [Google Scholar] [CrossRef]
- Xu, R.J.; Wang, T. Gastrointestinal absorption of insulin-like growth factor-I in neonatal pigs. J. Pediatr. Gastroenterol. Nutr. 1996, 23, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Burrin, D.G. Is milk-borne insulin-like growth factor-I essential for neonatal development? J. Nutr. 1997, 127 (Suppl. S5), 975S–979S. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, N.S.; Grosvenor, C.E. Transfer of milk prolactin to the plasma of neonatal rats by intestinal absorption. J. Endocrinol. 1978, 79, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Drozdowski, L.; Thomson, A.B. Intestinal hormones and growth factors: Effects on the small intestine. World J. Gastroenterol. 2009, 15, 385–406. [Google Scholar] [CrossRef]
- Alavi, K.; Schwartz, M.Z.; Prasad, R.; O’Connor, D.; Funanage, V. Leptin: A new growth factor for the small intestine. J. Pediatr. Surg. 2002, 37, 327–330. [Google Scholar] [CrossRef]
- Wolinski, J.; Biernat, M.; Guilloteau, P.; Westrom, B.R.; Zabielski, R. Exogenous leptin controls the development of the small intestine in neonatal piglets. J. Endocrinol. 2003, 177, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Corpeleijn, W.E.; van Vliet, I.; de Gast-Bakker, D.A.; van der Schoor, S.R.; Alles, M.S.; Hoijer, M.; Tibboel, D.; van Goudoever, J.B. Effect of enteral IGF-1 supplementation on feeding tolerance, growth, and gut permeability in enterally fed premature neonates. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 184–190. [Google Scholar] [CrossRef]
- Stark, A.; Smith, P.B.; Hornik, C.P.; Zimmerman, K.O.; Hornik, C.D.; Pradeep, S.; Clark, R.H.; Benjamin, D.K., Jr.; Laughon, M.; Greenberg, R.G. Medication Use in the Neonatal Intensive Care Unit and Changes from 2010 to 2018. J. Pediatr. 2022, 240, 66–71.e64. [Google Scholar] [CrossRef]
- Doyle, L.W.; Ehrenkranz, R.A.; Halliday, H.L. Early (<8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst. Rev. 2014, 10, CD001146. [Google Scholar] [CrossRef]
- Yeh, T.F.; Lin, Y.J.; Lin, H.C.; Huang, C.C.; Hsieh, W.S.; Lin, C.H.; Tsai, C.H. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N. Engl. J. Med. 2004, 350, 1304–1313. [Google Scholar] [CrossRef]
- Van Goudoever, J.B.; Wattimena, J.D.; Carnielli, V.P.; Sulkers, E.J.; Degenhart, H.J.; Sauer, P.J. Effect of dexamethasone on protein metabolism in infants with bronchopulmonary dysplasia. J. Pediatr. 1994, 124, 112–118. [Google Scholar] [CrossRef]
- Leitch, C.A.; Ahlrichs, J.; Karn, C.; Denne, S.C. Energy expenditure and energy intake during dexamethasone therapy for chronic lung disease. Pediatr. Res. 1999, 46, 109–113. [Google Scholar] [CrossRef]
- LeFlore, J.L.; Engle, W.D. Growth and neurodevelopment in extremely low-birth-weight neonates exposed to postnatal steroid therapy. Am. J. Perinatol. 2011, 28, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.C.; Lam, C.W.; Lee, C.H.; Fok, T.F.; Chan, I.H.; Ma, K.C.; Wong, E. Changes in serum leptin concentration after corticosteroid treatment in preterm infants. Acta Paediatr. 2002, 91, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Skinner, A.M.; Battin, M.; Solimano, A.; Daaboul, J.; Kitson, H.F. Growth and growth factors in premature infants receiving dexamethasone for bronchopulmonary dysplasia. Am. J. Perinatol. 1997, 14, 539–546. [Google Scholar] [CrossRef]
- Huysman, M.W.; Hokken-Koelega, A.C.; Hop, W.C.; Sauer, P.J. Effect of dexamethasone treatment on serum GH, IGF-I, and the binding proteins IGFBP-1 and -3 in ventilated very preterm infants. Pediatr. Res. 2003, 54, 37–43. [Google Scholar] [CrossRef]
- Yeh, T.F.; Lin, Y.J.; Huang, C.C.; Chen, Y.J.; Lin, C.H.; Lin, H.C.; Hsieh, W.S.; Lien, Y.J. Early dexamethasone therapy in preterm infants: A follow-up study. Pediatrics 1998, 101, E7. [Google Scholar] [CrossRef]
- Rijken, M.; Wit, J.M.; Le Cessie, S.; Veen, S.; Leiden Follow-Up Project on Prematurity. The effect of perinatal risk factors on growth in very preterm infants at 2 years of age: The Leiden Follow-Up Project on Prematurity. Early Hum. Dev. 2007, 83, 527–534. [Google Scholar] [CrossRef]
- Wang, D.; Vandermeulen, J.; Atkinson, S.A. Early life factors predict abnormal growth and bone accretion at prepuberty in former premature infants with/without neonatal dexamethasone exposure. Pediatr. Res. 2007, 61, 111–116. [Google Scholar] [CrossRef]
- Watterberg, K.L.; Shaffer, M.L.; Mishefske, M.J.; Leach, C.L.; Mammel, M.C.; Couser, R.J.; Abbasi, S.; Cole, C.H.; Aucott, S.W.; Thilo, E.H.; et al. Growth and neurodevelopmental outcomes after early low-dose hydrocortisone treatment in extremely low birth weight infants. Pediatrics 2007, 120, 40–48. [Google Scholar] [CrossRef]
- Watterberg, K.L.; Walsh, M.C.; Li, L.; Chawla, S.; D’Angio, C.T.; Goldberg, R.N.; Hintz, S.R.; Laughon, M.M.; Yoder, B.A.; Kennedy, K.A.; et al. Hydrocortisone to Improve Survival without Bronchopulmonary Dysplasia. N. Engl. J. Med. 2022, 386, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Peltoniemi, O.M.; Lano, A.; Puosi, R.; Yliherva, A.; Bonsante, F.; Kari, M.A.; Hallman, M.; Neonatal Hydrocortisone Working Group. Trial of early neonatal hydrocortisone: Two-year follow-up. Neonatology 2009, 95, 240–247. [Google Scholar] [CrossRef]
- Peltoniemi, O.M.; Lano, A.; Yliherva, A.; Kari, M.A.; Hallman, M.; Neonatal Hydrocortisone Working Group. Randomised trial of early neonatal hydrocortisone demonstrates potential undesired effects on neurodevelopment at preschool age. Acta Paediatr. 2016, 105, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Onland, W.; Cools, F.; Kroon, A.; Rademaker, K.; Merkus, M.P.; Dijk, P.H.; van Straaten, H.L.; Te Pas, A.B.; Mohns, T.; Bruneel, E.; et al. Effect of Hydrocortisone Therapy Initiated 7 to 14 Days After Birth on Mortality or Bronchopulmonary Dysplasia among Very Preterm Infants Receiving Mechanical Ventilation: A Randomized Clinical Trial. JAMA 2019, 321, 354–363. [Google Scholar] [CrossRef]
- Baud, O.; Maury, L.; Lebail, F.; Ramful, D.; El Moussawi, F.; Nicaise, C.; Zupan-Simunek, V.; Coursol, A.; Beuchee, A.; Bolot, P.; et al. Effect of early low-dose hydrocortisone on survival without bronchopulmonary dysplasia in extremely preterm infants (PREMILOC): A double-blind, placebo-controlled, multicentre, randomised trial. Lancet 2016, 387, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Tijsseling, D.; Ter Wolbeek, M.; Derks, J.B.; de Vries, W.B.; Heijnen, C.J.; van Bel, F.; Mulder, E.J.H. Neonatal corticosteroid therapy affects growth patterns in early infancy. PLoS ONE 2018, 13, e0192162. [Google Scholar] [CrossRef]
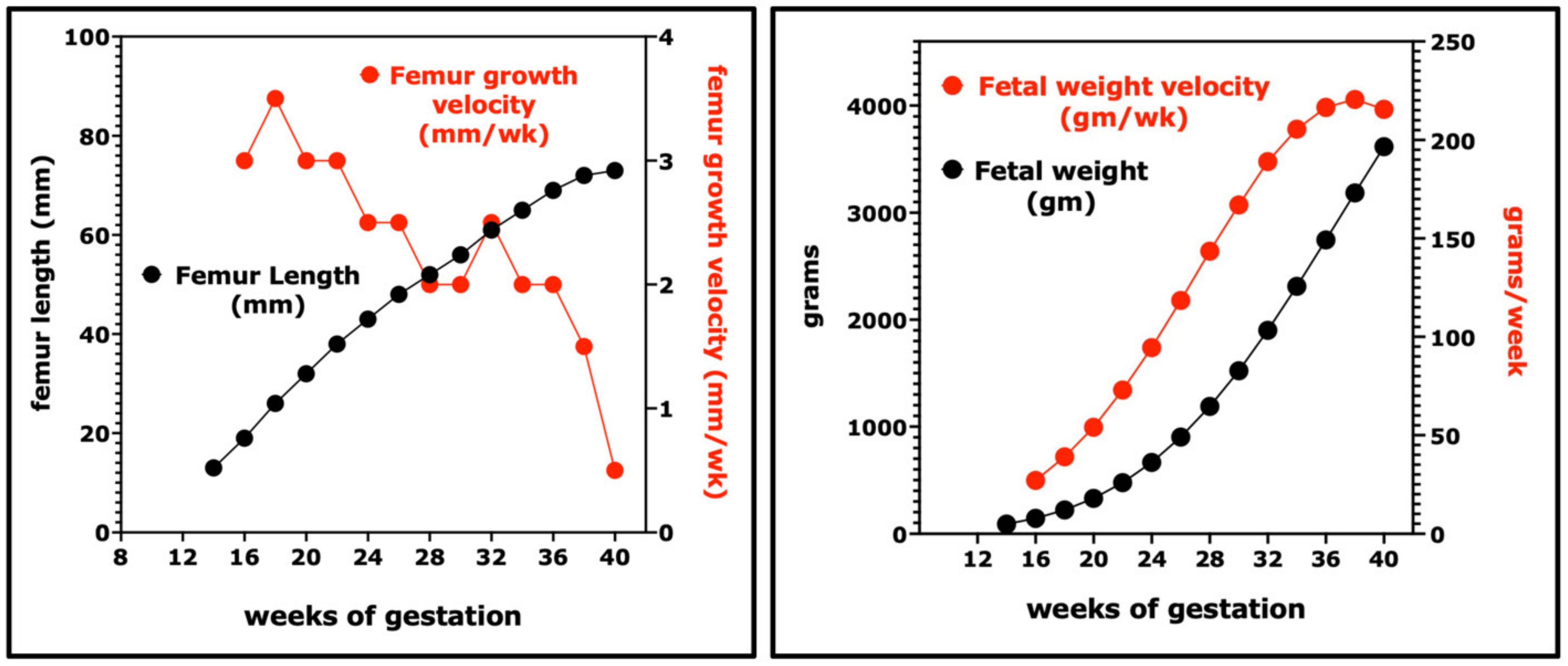
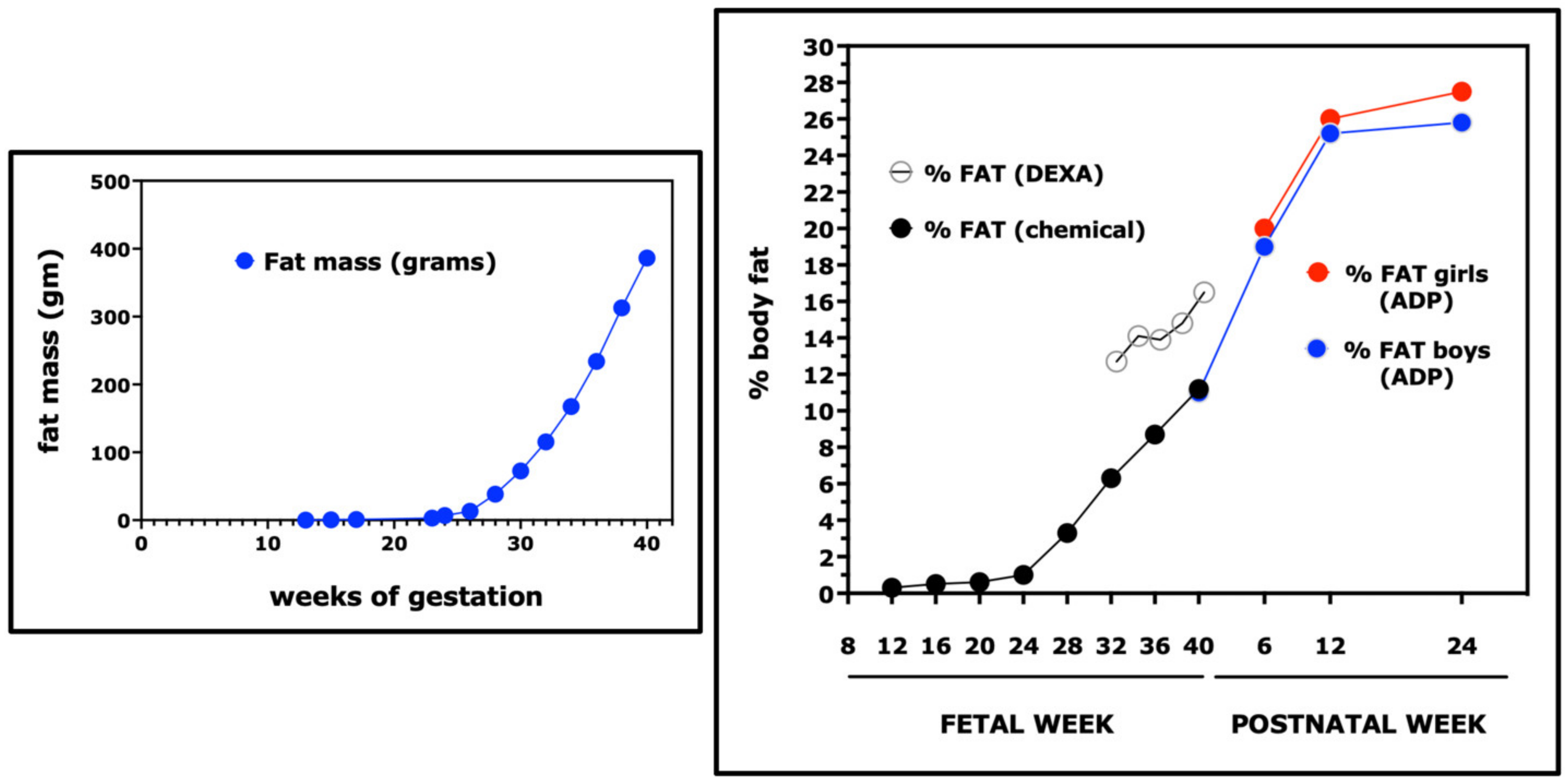
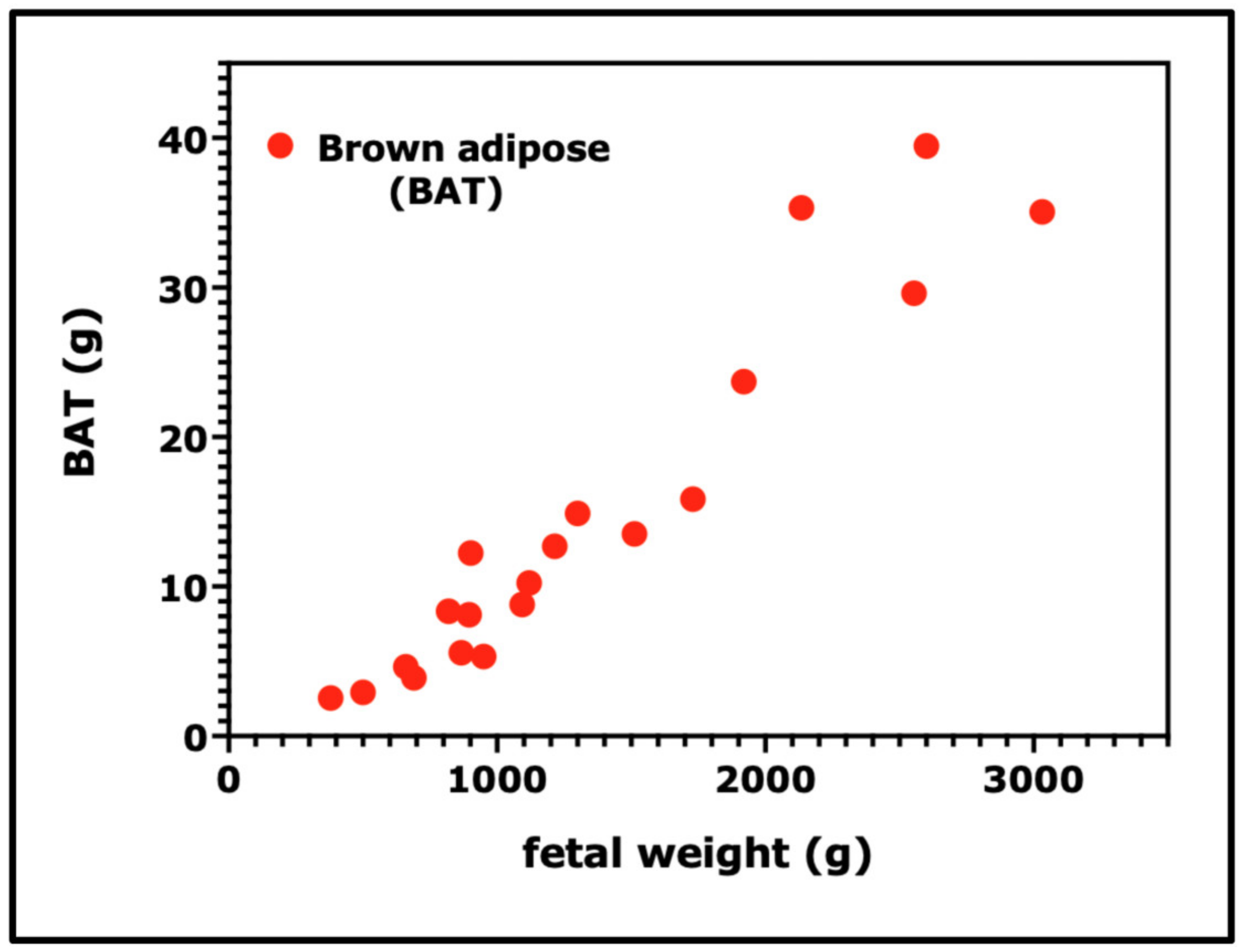

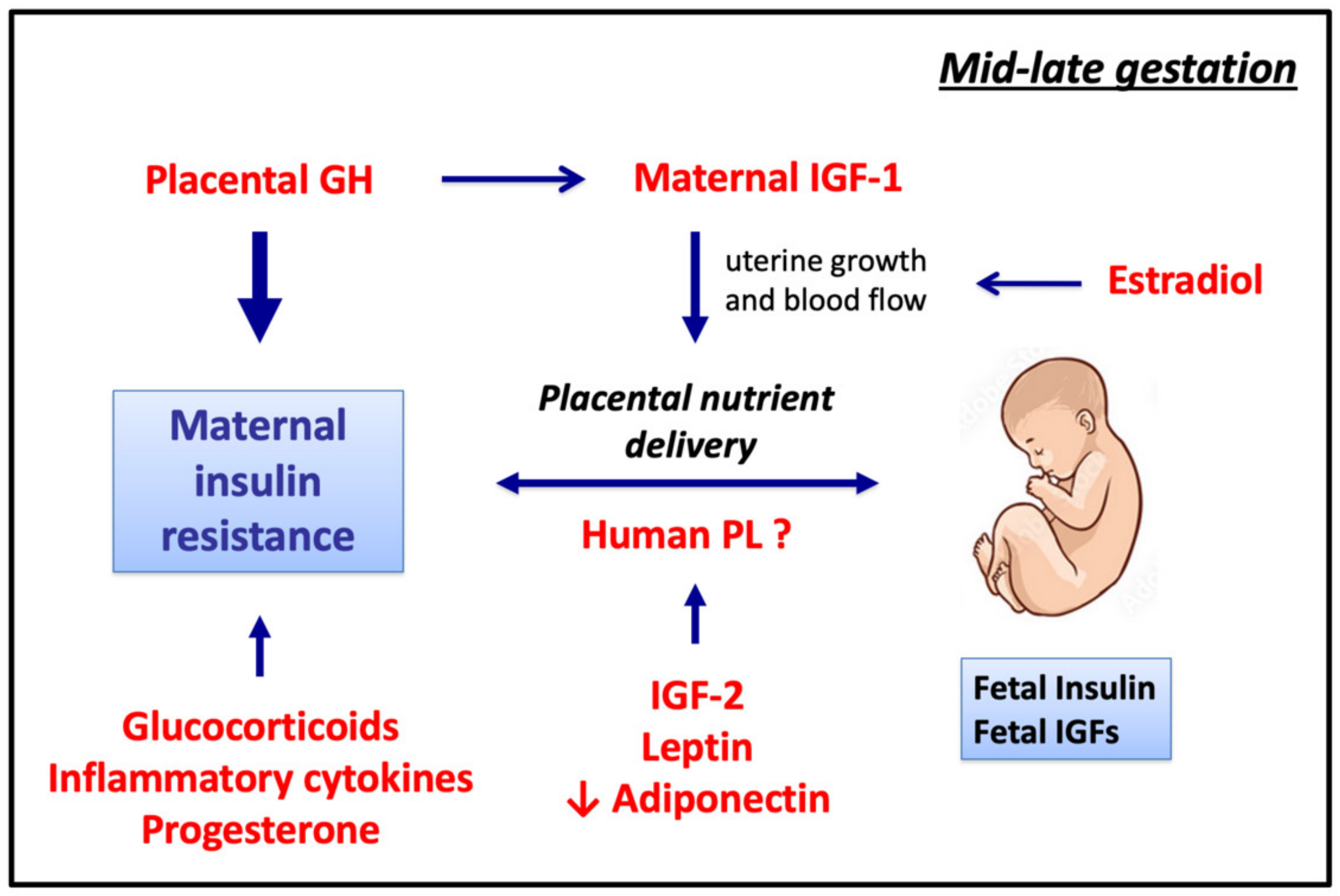


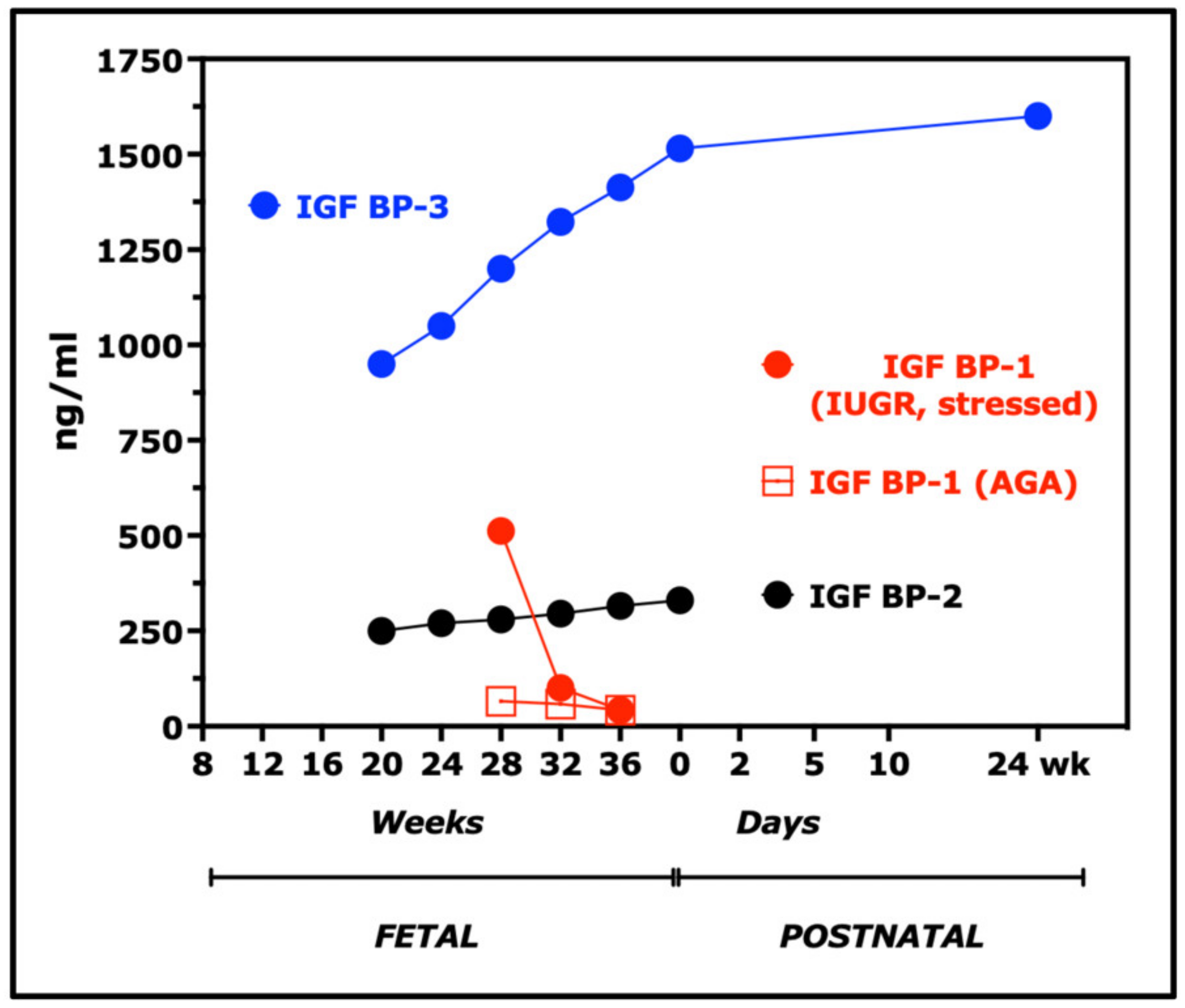

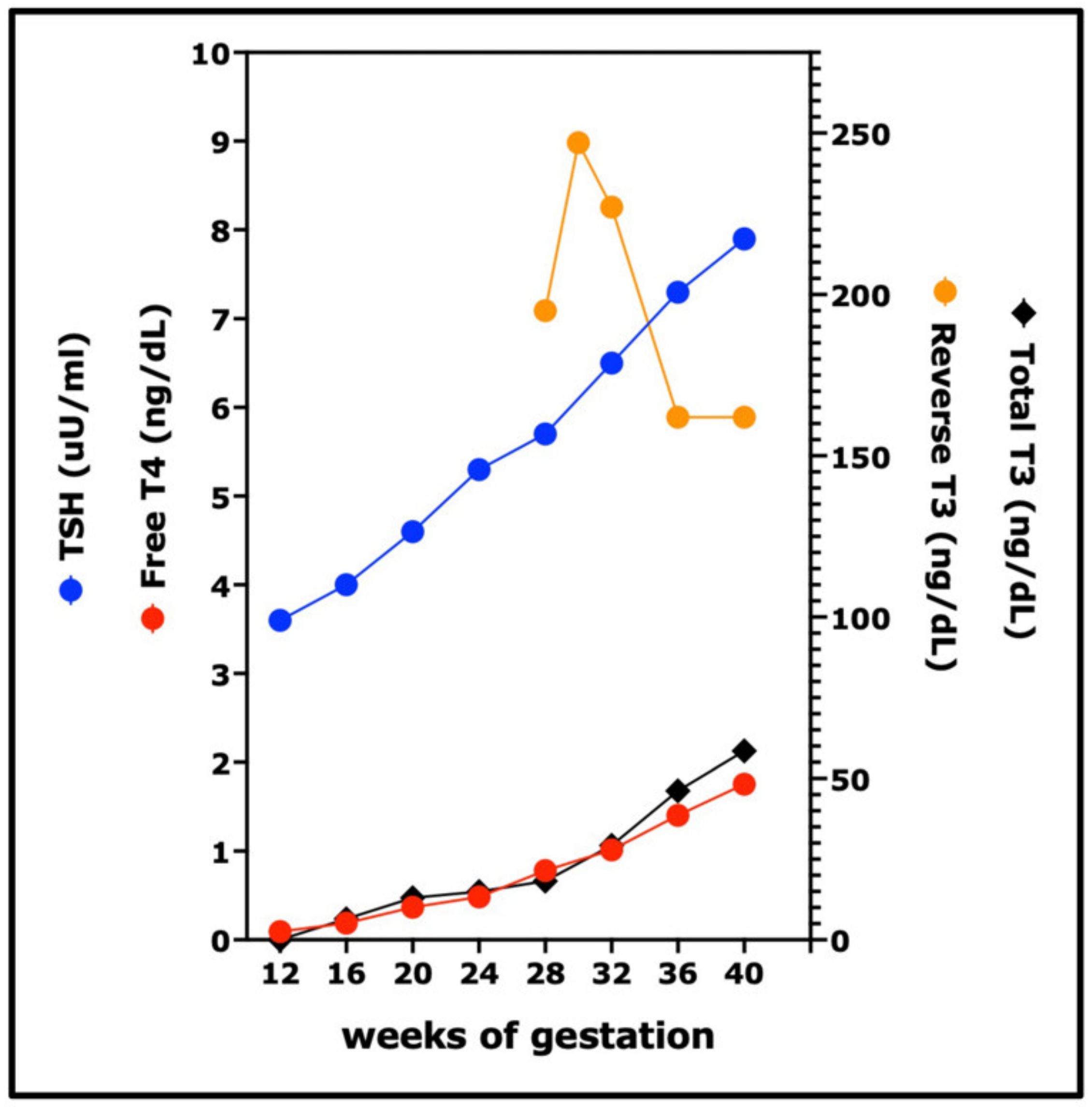
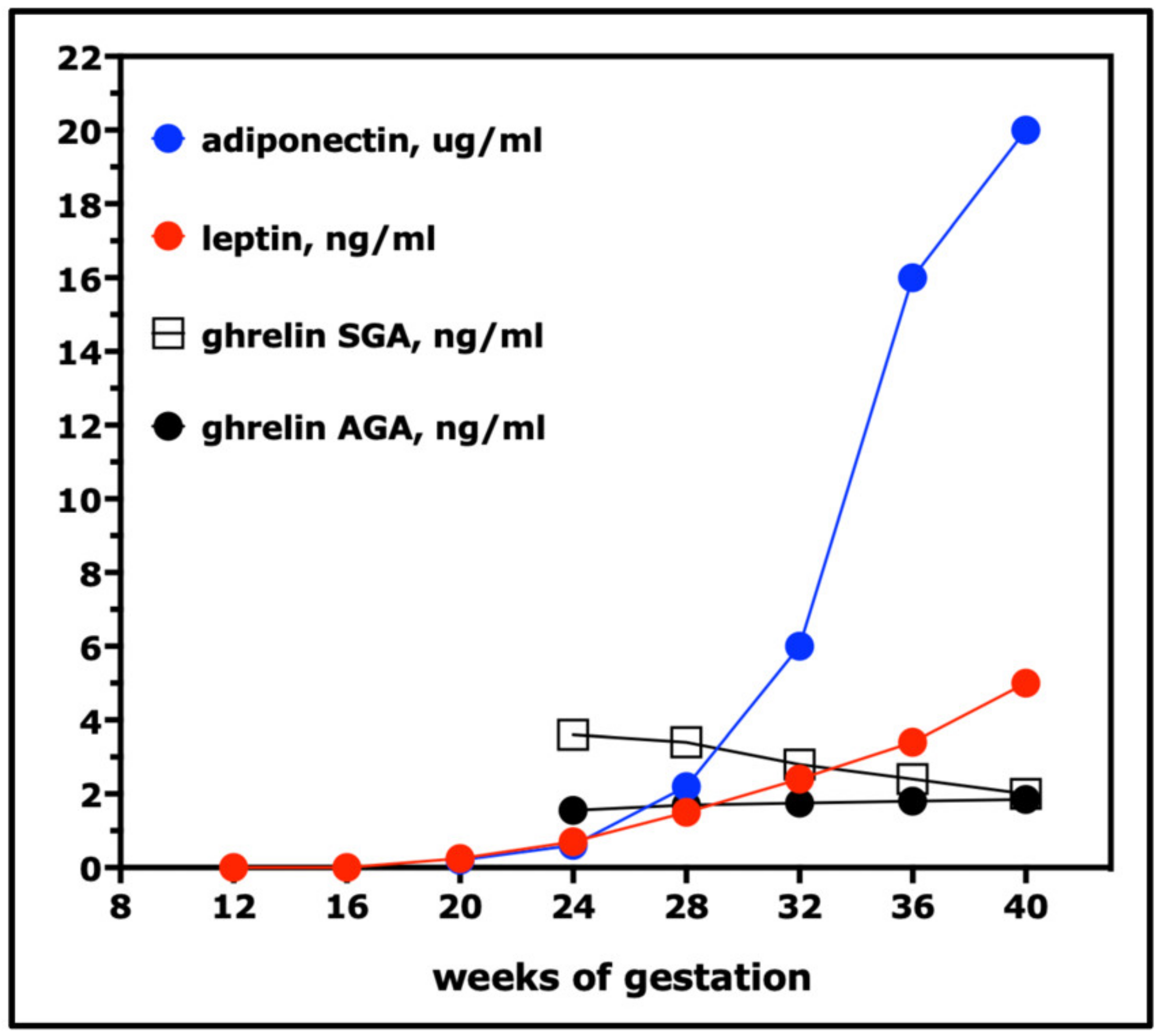
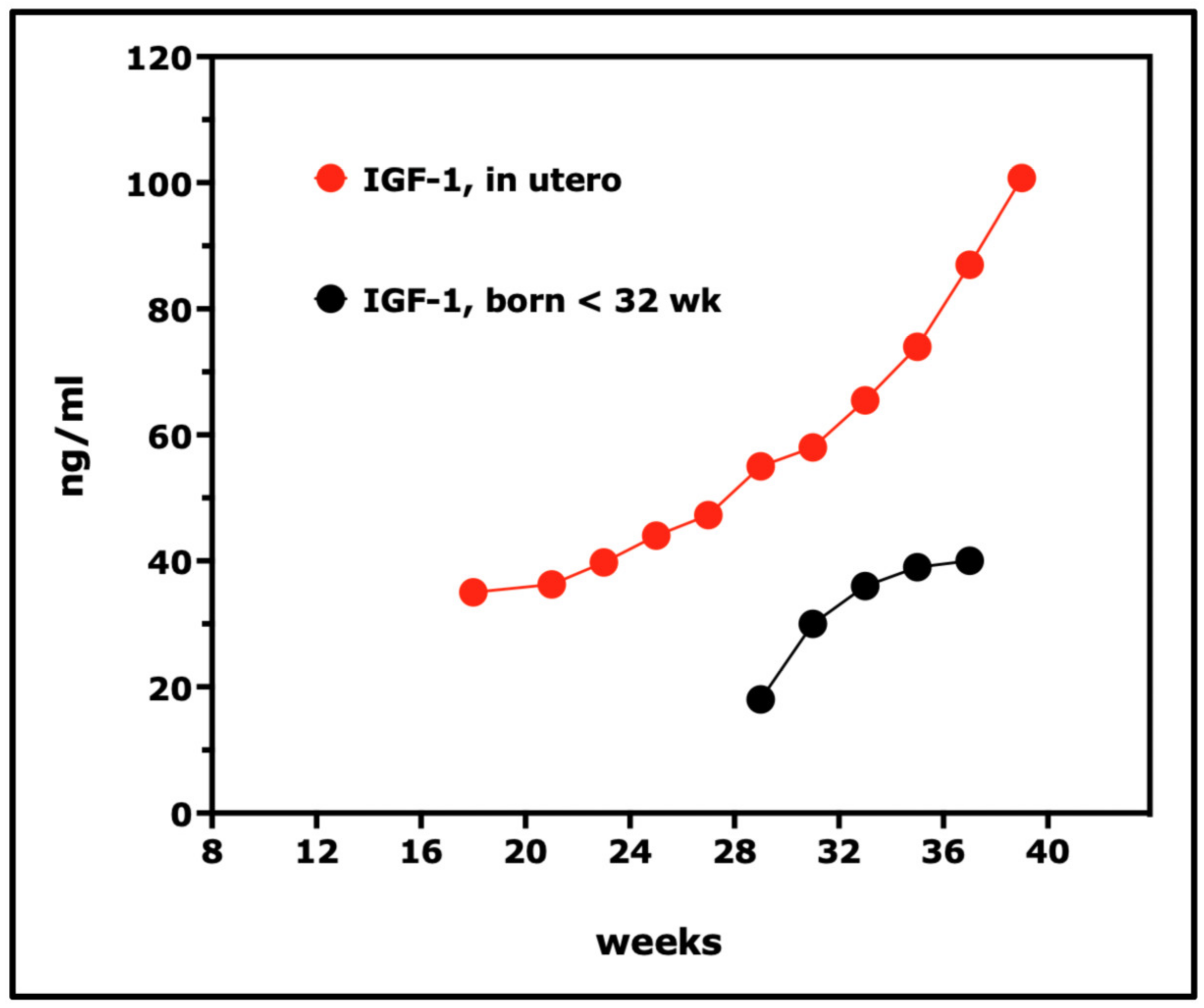
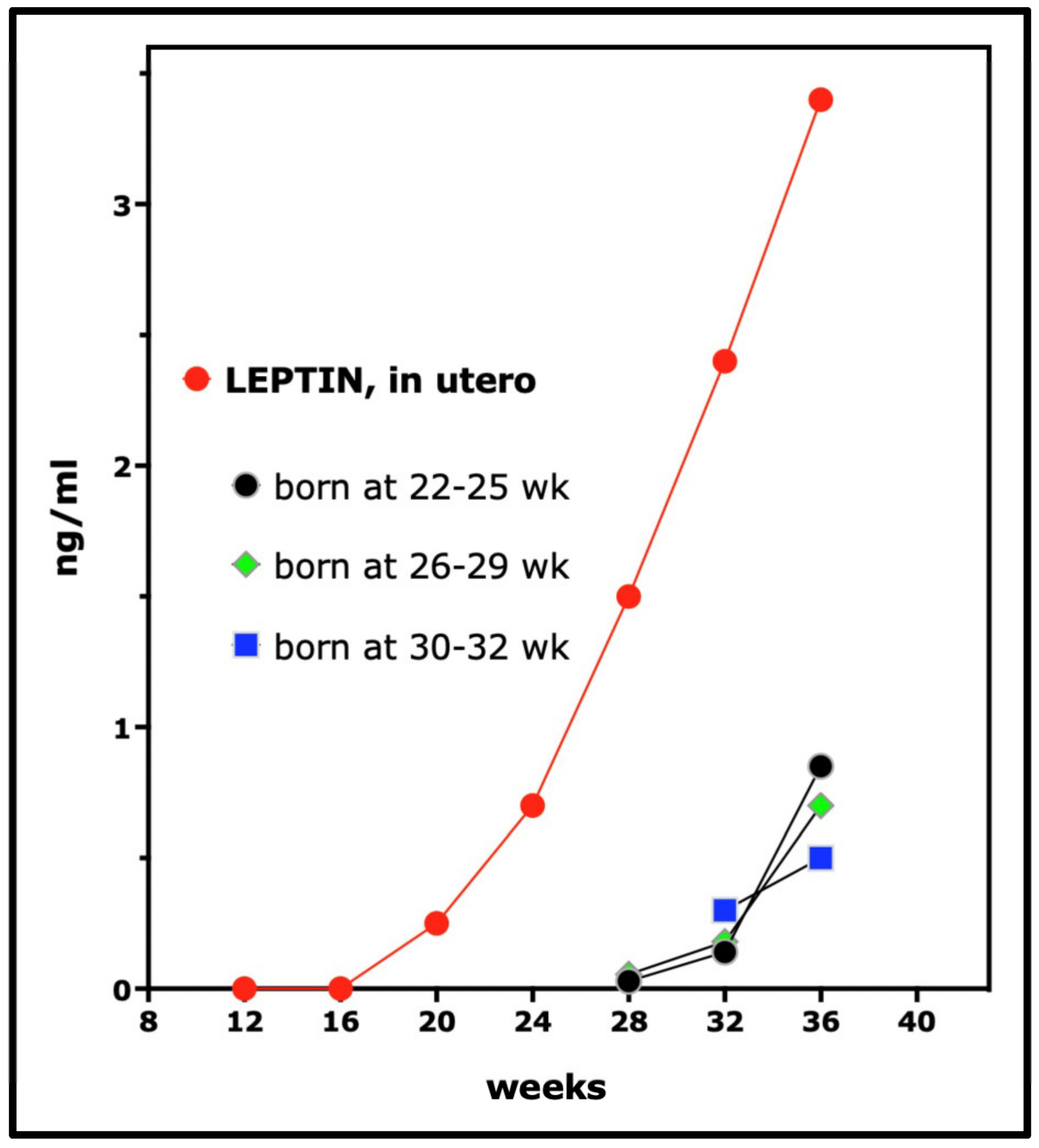
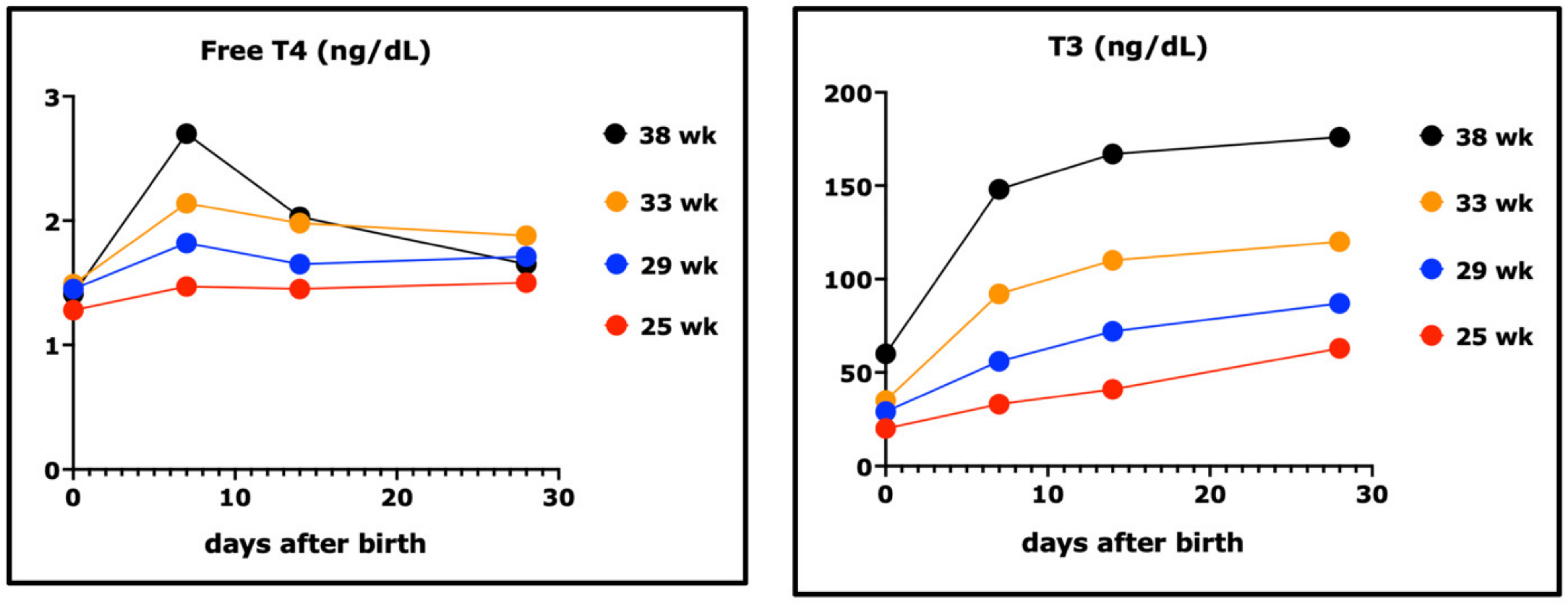
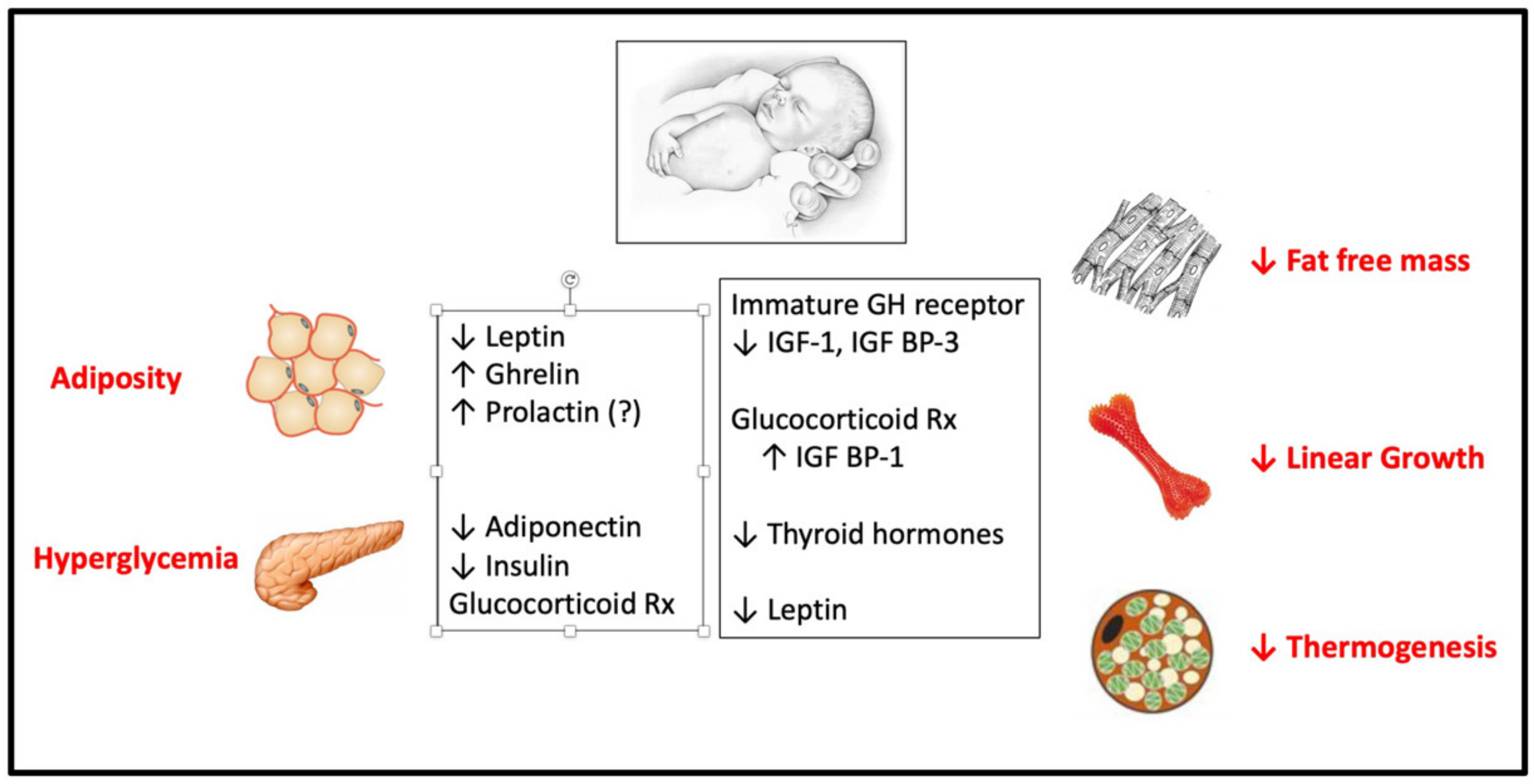
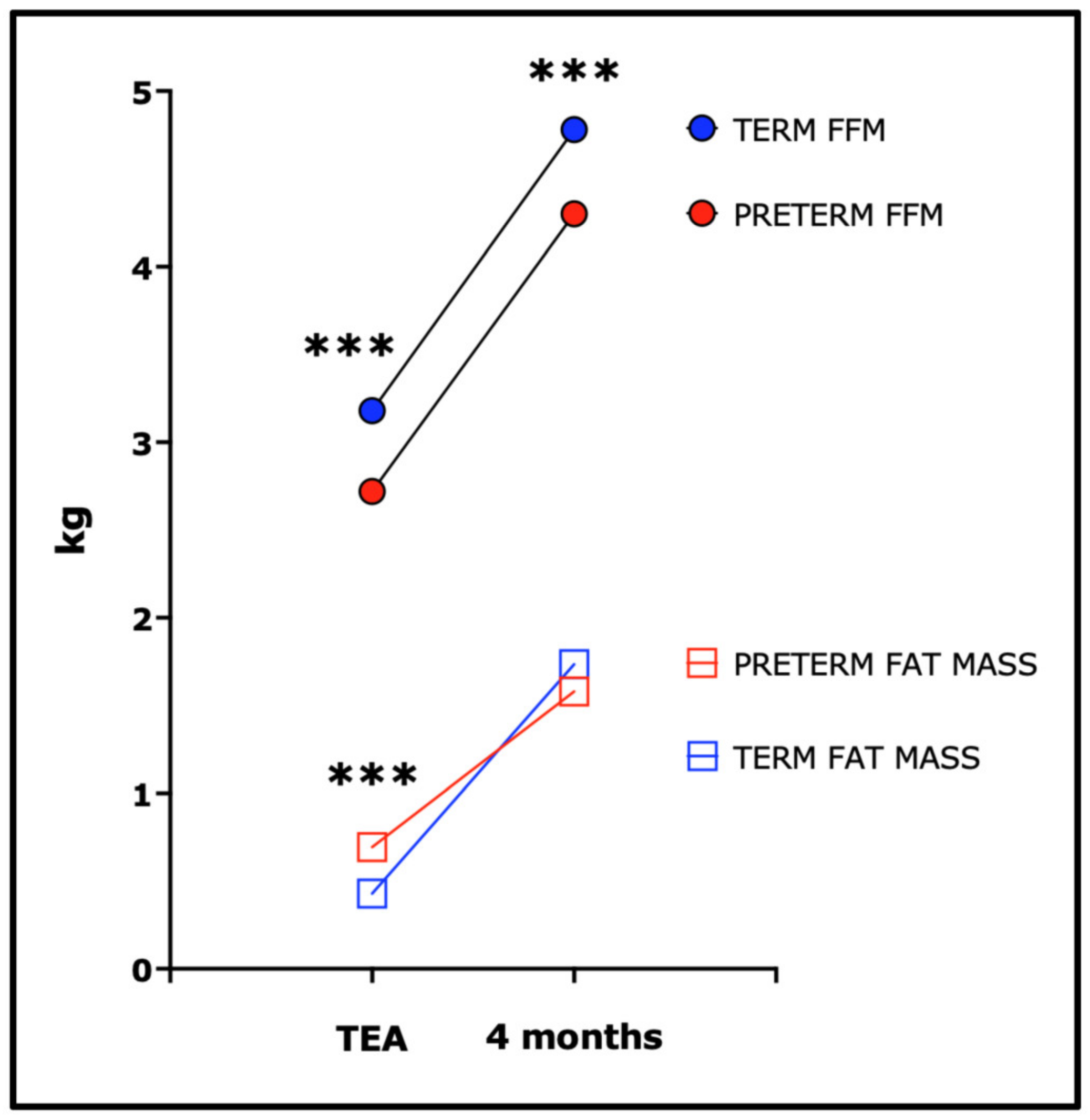
| Fetal Hormone/Growth Factor/Cytokine (or Cognate Receptor) | Source | Proposed Biological Actions in the Fetus | Correlation of Fetal Level with Birth Length | Correlation of Fetal Level with Birth Weight/Fat Mass | Effect of Fetal Deficiency/Deletion or Excess (Human) on Fetal Weight or Length |
|---|---|---|---|---|---|
| Insulin | Yolk sac and fetal thymus in early development (? contribution to fetal plasma levels); then, pancreatic beta cells. | Exerts adipogenic, lipogenic, and glycogenic effects in fetal tissues and the induction of placental glucose transport and amino acid uptake. Stimulates amino acid uptake (but not DNA synthesis) in human fetal myoblasts and fibroblasts. | Weakly correlated with birth length. Low in SGA/IUGR. | Strongly correlated with birth weight and extremity and abdominal fat. | Marked decrease in birth weight, lesser decrease in birth length in pancreatic agenesis or defects in beta cell development or insulin production. Macrosomia in infants of diabetic mothers associated with fetal hyperinsulinemia. |
| IGF-1 | Connective tissues and mesenchymal cells in diverse fetal tissues including perichondrium. Placental villous cytotrophoblast, extravillous trophoblast, and microvillous membrane. | Exerts mitogenic, anti-apoptotic, and adipogenic effects in fetal tissues. Induces placental trophoblast migration and invasion, and the differentiation of cytotrophoblasts into syncytiotrophoblasts; stimulates placental glucose transport and amino acid uptake. | Correlated with birth length and fat-free mass. Low in SGA/IUGR. | Strongly correlated with birth weight and fat mass. Increased in children born LGA. Low birth weight associated with low IGF-1 and high IGFBP-1 and IGFBP-2. | Severe IUGR in patients with mutations in IGF-1 or IGF-1 receptor. |
| IGF-2 | Fetal endothelial cells, chondroblasts, hepatocytes, myocytes, adrenocortical cells, and epithelial cells of the developing kidney and choroid plexus. Placental chorionic plate, chorionic mesoderm, cytotrophoblast, and extravillous trophoblast. | Promotes trophoblast migration and invasion, placental growth and maturation, and nutrient transport. Exerts mitogenic, anti-apoptotic, and adipogenic effects in fetal tissues and promotes fetal chondogenesis and myogenesis. | Weakly corelated with birth length and fat-free mass. Low in SGA/IUGR. | Variably correlated with birth weight. Commonly low in SGA/IUGR; variably elevated in fetal macrosomia. | Severe IUGR in patients with paternally inherited IGF-2 missense mutations. Intrauterine growth restriction and placental hypoplasia in Russell–Silver syndrome associated with a loss of paternal expression of IGF-2; conversely, fetal overgrowth and placentomegaly in Beckwith–Wiedemann syndrome associated with bi-allelic IGF-2 (over) expression caused by a loss of maternal imprinting. |
| Pituitary GH (GH-N) | Fetal pituitary | Effects on fetal IGF-1 blunted because of immaturity of hepatic GH receptor. Decline in levels after birth may facilitate insulin-dependent lipogenesis and fat deposition. | No significant correlation | None or inverse | Variable and mild decrease in birth length (mean −0.7–1.0 SD), relative adiposity (−0.3–0.4 SD) in hypopituitarism, GH deficiency, or mutations in GH receptor. |
| Placental GH (GH-V) | Placenta | Stimulates a rise in maternal IGF-1 after mid-gestation and may promote trophoblast invasion. Induces maternal insulin resistance in mid–late gestation. Little or no GH-V in the fetal circulation. | N/A | N/A. Low maternal levels in IUGR | Variable reduction in birth weight in GH-V gene deletions (in association with HPL deletions). |
| Human Placental Lactogen (hPL) | Placenta | Stimulates amino acid uptake, DNA synthesis, and IGF-1 secretion in human fetal myoblasts and fibroblasts and DNA synthesis and IGF-1 secretion in human fetal hepatocytes. Effects on human fetal or maternal pancreatic beta cell mass and insulin production unclear. | Fetal hPL correlates positively with fetal IGF-1 and IGF-2 in late gestation. Birth weight and length correlate positively with maternal but not cord blood hPL. | Fetal hPL correlates positively with fetal IGF-1 and IGF-2 in late gestation. Birth weight and length correlate positively with maternal but not cord blood hPL. | Variable reduction in birth weight in HPL gene deletions (some in association with GH-V deletions). |
| Prolactin | Fetal pituitary | Stimulates white and brown adipogenesis in rodent cell lines. May promote postnatal “whitening” of brown adipose tissue. Stimulates pancreatic beta cell replication in postnatal rodents but effects on human fetal or maternal pancreatic beta cell mass and insulin production unclear. | ? | Variably high in SGA infants | Decreased beta cell mass and brown adipose depots in neonatal PRLR-knockout mice. Combined defects in PRLR signaling and GH production in the mouse reduce weight on postnatal day 7. |
| Thyroid hormone | Maternal and fetal thyroid | Increases BAT development and enzymes for thermogenesis in the neonatal period. Promotes fetal bone growth and bone maturation. | Weak association of cord free T4 (not free T3) with birth length. | Weak association of cord free T4 (not free T3) with birth weight. | Variable increase in birth weight and delayed bone maturation in severe primary congenital hypothyroidism; low birth weight in fetal hyperthyroidism. |
| Cortisol | Fetal adrenal | Promotes the maturation of respiratory and gastrointestinal functions and liver glycogenesis in late gestation. Modulates thyroid deiodinase activity. May facilitate expression of the hepatic GH receptor in late gestation and the early postnatal period. Inhibits linear growth at supraphysiologic concentrations. | Glucocorticoid excess (endogenous or exogenous) reduces IGF-1 and increases IGFBP-1 and inhibits linear growth. | Cord blood cortisol variably increased in IUGR; birth weight correlates with placental 11 beta-HSD2 activity. | Birth weight reduced markedly in patients with mutations in 11 beta-HSD2. Normal birth weight in congenital lipoid adrenal hyperplasia (mutation of Steroidogenic Acute Regulatory Protein). |
| Sex steroids | Placenta, fetal adrenal and gonads. | ? effects on fetal bone growth and maturation. | Birth length slightly greater in boys than girls. | Birth weight slightly greater in boys than girls. | Variable slight decrease in birth weight in males with complete androgen resistance; no effects of androgen synthetic defects, ovarian hypogonadism, or mutations in aromatase or estrogen receptor. Birth weight normal in states of androgen excess (congenital adrenal hyperplasia) in females or males. |
| Leptin | Placenta and fetal adipose | Increases sympathetic nervous system activity and fetal BAT development. Exerts growth-promoting effects in postnatal rodents. | Weak or no association with birth length. | Strongly correlated with birth weight and neonatal fat mass; low in intrauterine growth restriction. | Normal birth weight and length in patients with mutations in leptin or leptin receptor. |
| Ghrelin | Placenta and fetal tissues including the pancreas. | Stimulates food intake, fat storage and growth hormone secretion in the postnatal period. | Inversely related to birth length (limited data). | Inversely related to birth weight z score. | Normal birth weight and length in a child with a mutation that abolishes constitutive ghrelin receptor activity. Birth weight normal in ghrelin knockout mice. |
| Adiponectin | Fetal vascular endothelial cells in skin, kidney, cortex, skeletal and smooth muscle. Low-level expression in fetal adipose. | Inhibits placental amino acid glucose transport and stimulates placental IGFBP-1 expression. Increases the expression of placental lactogen in human placental explants and cells. | ? | Rises markedly between 24 weeks and term and correlates variably with birth weight and adiposity. | Deletion in pregnant mice reduces placental IGFBP-1 expression and increases birth weight. |
| Maternal Condition | Second Trimester | Third Trimester | Fullterm Birth |
|---|---|---|---|
| Obesity | ↑ femur and humerus lengths (~21 weeks) | ↑ femur and humerus lengths ↑ EFW (~30 weeks) ↑ AC (~32 weeks) | ↑ length ↑ weight ↑ risk of LGA |
| Gestational diabetes | Possible period of ↓ EFW and AC (~14–20 weeks) ↑ femur length (~24 weeks) ↑ AC (~24 weeks) | Rapid growth ↑ femur length ↑ AC ↑ subscapular thickness ↑ fat and lean mass of the upper arm and thigh (~28 weeks) | Larger and heavier than peers with: ↑ total tissue mass ↑ percentage fat mass ↑ risk of LGA |
| Smoking | ↓ femur length (~18–24 weeks) ↓ HC (~25 weeks) | ↓ femur length ↓ HC ↓ AC ↓ EFW | ↑ ponderal index ↓ length > ↓ HC > ↓ weight ↑ risk of SGA |
| Severe preeclampsia | ↓ EFW (~22 weeks) ↓ AC (~23 weeks) | ↓ AC ↓ EFW | Likely to be delivered pre-term ↓ weight > ↓ ponderal index > ↓ length ↑ risk SGA and asymmetric SGA |
| Cord Blood Hormone Levels | |||||||
|---|---|---|---|---|---|---|---|
| Maternal Condition | IGF-1 | IGFBP-3 | Leptin | Adiponectin | Ghrelin | Insulin/C-Peptide | T4/fT4 |
| Obesity | ↔ | - | ↑ | ↔ | ↔ | ↑ | - |
| Excessive gestational weight gain | ↔ | ↑ | ↑ | ↔ | ↑ (? Males only) | ↑ | - |
| Gestational diabetes | ? (? Sex-dependent) | - | ↑ | ? | ? | ↑ | ↔ |
| Smoking | ↓ | ? | ↔ | ? | ↑ | ↔ | - |
| Hypertensive Disorders of Pregnancy | ↓ | - | ? | ↑ | ? | - | ↓ |
| Malnutrition | ↑ ? | - | - | - | - | - | ↓ ? |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Page, L.; Younge, N.; Freemark, M. Hormonal Determinants of Growth and Weight Gain in the Human Fetus and Preterm Infant. Nutrients 2023, 15, 4041. https://doi.org/10.3390/nu15184041
Page L, Younge N, Freemark M. Hormonal Determinants of Growth and Weight Gain in the Human Fetus and Preterm Infant. Nutrients. 2023; 15(18):4041. https://doi.org/10.3390/nu15184041
Chicago/Turabian StylePage, Laura, Noelle Younge, and Michael Freemark. 2023. "Hormonal Determinants of Growth and Weight Gain in the Human Fetus and Preterm Infant" Nutrients 15, no. 18: 4041. https://doi.org/10.3390/nu15184041
APA StylePage, L., Younge, N., & Freemark, M. (2023). Hormonal Determinants of Growth and Weight Gain in the Human Fetus and Preterm Infant. Nutrients, 15(18), 4041. https://doi.org/10.3390/nu15184041





