Association between Dietary Choline Intake and Cardiovascular Diseases: National Health and Nutrition Examination Survey 2011–2016
Abstract
:1. Introduction
2. Materials and Methods
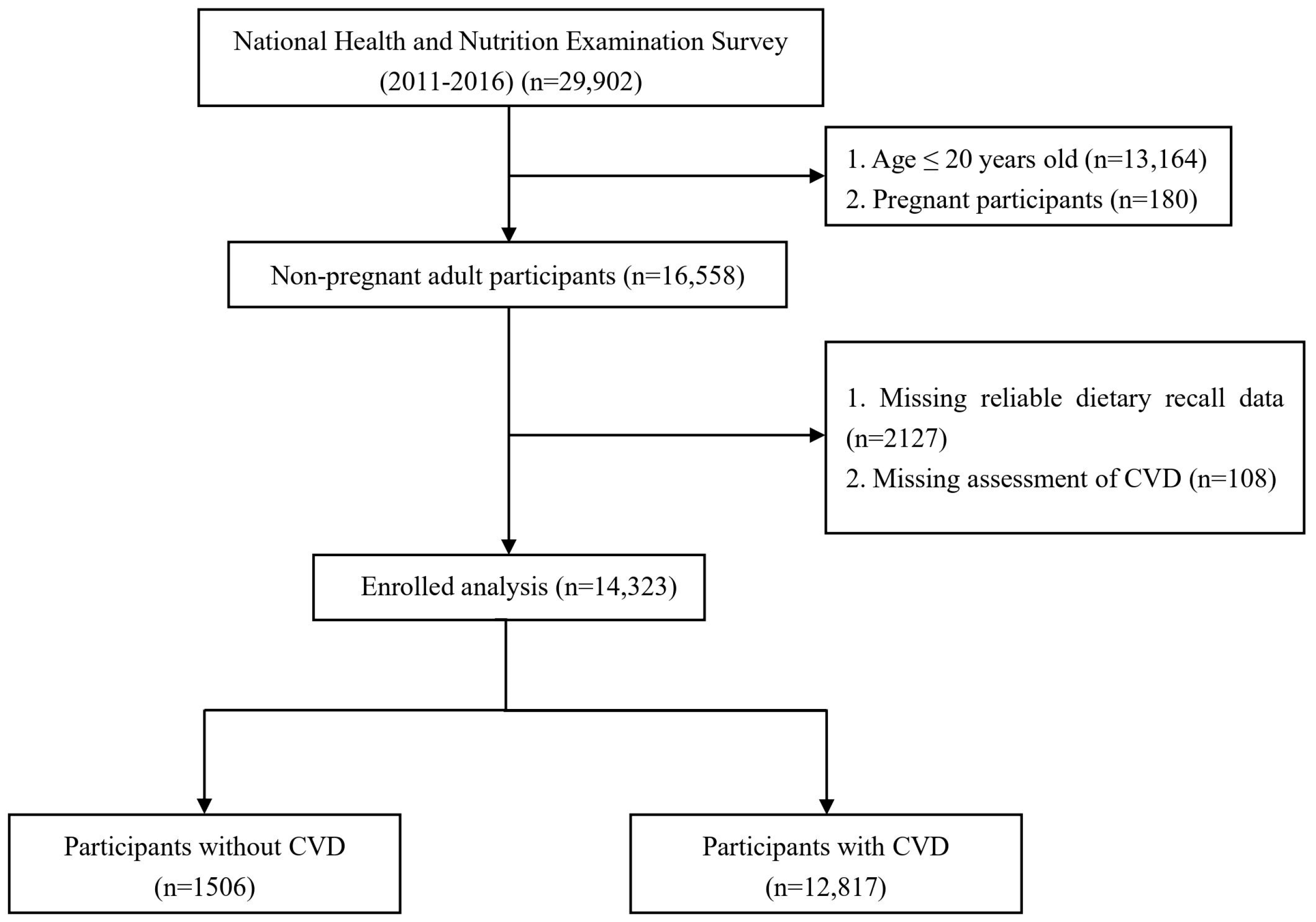
2.1. Study Population
2.2. Assessment of CVD
2.3. Assessment of Dietary Choline Intake
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Participants
3.2. Association between Dietary Choline Intake and CVD
3.3. Subgroup Analysis of Dietary Choline Intake and CVD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kassebaum, N.J.; Arora, M.; Barber, R.M.; Bhutta, Z.A.; Brown, J.; Carter, A.; Casey, D.C.; Charlston, F.J.; Coates, M.M.; Coggeshall, M.; et al. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1603–1658. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Després, J.-P.; Turner, M.B.; Fullerton, H.J.; et al. Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef] [PubMed]
- Bekdash, R.A. Neuroprotective Effects of Choline and Other Methyl Donors. Nutrients 2019, 11, 2995. [Google Scholar] [CrossRef] [PubMed]
- Díez-Ricote, L.; Ruiz-Valderrey, P.; Micó, V.; Blanco, R.; Tomé-Carneiro, J.; Dávalos, A.; Ordovás, J.M.; Daimiel, L. TMAO Upregulates Members of the miR-17/92 Cluster and Impacts Targets Associated with Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 12107. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.; Weeks, T.L.; Hazen, S.L. Gut Microbiota and Cardiovascular Disease. Circ. Res. 2020, 127, 553–570. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Y.; Rimm, E.B.; Hu, F.B.; Albert, C.M.; Rexrode, K.M.; Manson, J.E.; Li, Q. Dietary phosphatidylcholine and risk of all-cause and cardiovascular-specific mortality among US women and men. Am. J. Clin. Nutr. 2016, 104, 173–180. [Google Scholar] [CrossRef]
- Meyer, K.A.; Shea, J.W. Dietary Choline and Betaine and Risk of CVD: A Systematic Review and Meta-Analysis of Prospective Studies. Nutrients 2017, 9, 711. [Google Scholar] [CrossRef]
- Chen, C.; Ye, Y.; Zhang, Y.; Pan, X.F.; Pan, A. Weight change across adulthood in relation to all cause and cause specific mortality: Prospective cohort study. BMJ 2019, 367, 15584. [Google Scholar] [CrossRef]
- Bao, W.; Liu, B.; Rong, S.; Dai, S.Y.; Trasande, L.; Lehmler, H.J. Association Between Bisphenol A Exposure and Risk of All-Cause and Cause-Specific Mortality in US Adults. JAMA Netw. Open 2020, 3, e2011620. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45 (Suppl. S1), S17–S38. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M. ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J.; Greene, T.; Stevens, L.A.; Zhang, Y.L.; Hendriksen, S.; Kusek, J.W.; Van Lente, F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 2006, 145, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Dalmeijer, G.W.; Olthof, M.R.; Verhoef, P.; Bots, M.L.; van der Schouw, Y.T. Prospective study on dietary intakes of folate, betaine, and choline and cardiovascular disease risk in women. Eur. J. Clin. Nutr. 2008, 62, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Bidulescu, A.; Chambless, L.E.; Siega-Riz, A.M.; Zeisel, S.H.; Heiss, G. Usual choline and betaine dietary intake and incident coronary heart disease: The Atherosclerosis Risk in Communities (ARIC) study. BMC Cardiovasc. Disord. 2007, 7, 20. [Google Scholar] [CrossRef]
- Millard, H.R.; Musani, S.K.; Dibaba, D.T.; Talegawkar, S.A.; Taylor, H.A.; Tucker, K.L.; Bidulescu, A. Dietary choline and betaine; associations with subclinical markers of cardiovascular disease risk and incidence of CVD, coronary heart disease and stroke: The Jackson Heart Study. Eur. J. Nutr. 2018, 57, 51–60. [Google Scholar] [CrossRef]
- Golzarand, M.; Mirmiran, P.; Azizi, F. Association between dietary choline and betaine intake and 10.6-year cardiovascular disease in adults. Nutr. J. 2022, 21, 1. [Google Scholar] [CrossRef]
- Díez-Ricote, L.; San-Cristobal, R.; Concejo, M.J.; Martínez-González, M.Á.; Corella, D.; Salas-Salvadó, J.; Goday, A.; Martínez, J.A.; Alonso-Gómez, Á.M.; Wärnberg, J.; et al. One-year longitudinal association between changes in dietary choline or betaine intake and cardiometabolic variables in the PREvención con DIeta MEDiterránea-Plus (PREDIMED-Plus) trial. Am. J. Clin. Nutr. 2022, 116, 1565–1579. [Google Scholar] [CrossRef]
- Hartiala, J.; Bennett, B.J.; Tang, W.H.; Wang, Z.; Stewart, A.F.; Roberts, R.; Allayee, H.; McPherson, R.; Lusis, A.J.; Hazen, S.L.; et al. Comparative genome-wide association studies in mice and humans for trimethylamine N-oxide, a proatherogenic metabolite of choline and L-carnitine. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1307–1313. [Google Scholar] [CrossRef]
- Bidulescu, A.; Chambless, L.E.; Siega-Riz, A.M.; Zeisel, S.H.; Heiss, G. Repeatability and measurement error in the assessment of choline and betaine dietary intake: The Atherosclerosis Risk in Communities (ARIC) study. Nutr. J. 2009, 8, 14. [Google Scholar] [CrossRef]
- Wiedeman, A.M.; Barr, S.I.; Green, T.J.; Xu, Z.; Innis, S.M.; Kitts, D.D. Dietary Choline Intake: Current State of Knowledge Across the Life Cycle. Nutrients 2018, 10, 1513. [Google Scholar] [CrossRef]
- Mazidi, M.; Katsiki, N.; Mikhailidis, D.P.; Banach, M. Dietary choline is positively related to overall and cause-specific mortality: Results from individuals of the National Health and Nutrition Examination Survey and pooling prospective data. Br. J. Nutr. 2019, 122, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Detopoulou, P.; Panagiotakos, D.B.; Antonopoulou, S.; Pitsavos, C.; Stefanadis, C. Dietary choline and betaine intakes in relation to concentrations of inflammatory markers in healthy adults: The ATTICA study. Am. J. Clin. Nutr. 2008, 87, 424–430. [Google Scholar] [CrossRef]
- Purohit, V.; Abdelmalek, M.F.; Barve, S.; Benevenga, N.J.; Halsted, C.H.; Kaplowitz, N.; Kharbanda, K.K.; Liu, Q.-Y.; Lu, S.C.; McClain, C.J.; et al. Role of S-adenosylmethionine, folate, and betaine in the treatment of alcoholic liver disease: Summary of a symposium. Am. J. Clin. Nutr. 2007, 86, 14–24. [Google Scholar] [CrossRef]
- Jones, P.J.H.; Senanayake, V.K.; Pu, S.; Jenkins, D.J.A.; Connelly, P.W.; Lamarche, B.; Couture, P.; Charest, A.; Baril-Gravel, L.; West, S.G.; et al. DHA-enriched high-oleic acid canola oil improves lipid profile and lowers predicted cardiovascular disease risk in the canola oil multicenter randomized controlled trial. Am. J. Clin. Nutr. 2014, 100, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Ganjali, S.; Gotto, A.M.; Ruscica, M., Jr.; Atkin, S.L.; Butler, A.E.; Banach, M.; Sahebkar, A. Monocyte-to-HDL-cholesterol ratio as a prognostic marker in cardiovascular diseases. J. Cell. Physiol. 2018, 233, 9237–9746. [Google Scholar] [CrossRef]
- Poursalehi, D.; Lotfi, K.; Mirzaei, S.; Asadi, A.; Akhlaghi, M.; Saneei, P. Association between methyl donor nutrients and metabolic health status in overweight and obese adolescents. Sci. Rep. 2022, 12, 17045. [Google Scholar] [CrossRef]

| Variables | Total | Quartiles of Dietary Choline Intake | p-Value | |||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| Participants, n | 14,323 | 3566 | 3578 | 3583 | 3596 | |
| Male (%) | 7016 (48.8) | 1226 (32.2) | 1455 (40.1) | 1806 (49.9) | 2529 (70.8) | <0.001 |
| Age (mean ± SD) (years) | 48 ± 17 | 47 ± 17 | 49 ± 17 | 50 ± 16 | 48 ± 16 | <0.001 |
| Race (%) | <0.001 | |||||
| Mexican-American | 1936 (8.6) | 455 (8.6) | 445 (7.8) | 479 (8.2) | 557 (9.7) | |
| Other Hispanic | 1529 (6.0) | 419 (6.9) | 410 (6.3) | 358 (5.4) | 342 (5.4) | |
| Non-Hispanic White | 5598 (65.9) | 1300 (61.9) | 1427 (66.9) | 1456 (68.1) | 1415 (66.2) | |
| Non-Hispanic Black | 3208 (11.1) | 871 (13.0) | 807 (11.3) | 775 (10.2) | 755 (10.2) | |
| Other Race | 2052 (8.4) | 521 (9.7) | 489 (7.7) | 515 (8.1) | 527 (8.5) | |
| Education level (%) | <0.001 | |||||
| Below high school | 3175 (15.0) | 1006 (19.3) | 811 (15.3) | 686 (12.4) | 672 (13.4) | |
| High school | 3117 (20.9) | 825 (23.4) | 735 (20.6) | 752 (19.7) | 805 (20.1) | |
| Above high school | 8031 (64.1) | 1735 (57.2) | 2032 (64.1) | 2145 (67.9) | 2119 (66.5) | |
| Marital status (%) | <0.001 | |||||
| Married | 7327 (54.4) | 1608 (46.5) | 1797 (54.0) | 1991 (59.8) | 1931 (56.4) | |
| Separated | 3141 (19.0) | 938 (22.6) | 837 (19.8) | 738 (18.3) | 628 (15.7) | |
| Never married | 3855 (26.6) | 1020 (30.9) | 944 (26.2) | 854 (21.9) | 1037 (27.8) | |
| BMI (mean ± SD) (kg/m2) | 29.14 ± 6.86 | 29.22 ± 6.86 | 29.06 ± 7.19 | 29.14 ± 6.74 | 29.16 ± 6.67 | 0.9 |
| WBC (mean ± SD) (1000 cells/μL) | 7.29 ± 2.24 | 7.46 ± 2.34 | 7.26 ± 2.25 | 7.20 ± 2.22 | 7.26 ± 2.17 | 0.01 |
| Lymphocyte cells (mean ± SD) (1000 cells/μL) | 2.14 ± 0.99 | 2.21 ± 0.94 | 2.14 ± 0.95 | 2.10 ± 1.15 | 2.11 ± 0.90 | 0.001 |
| Monocyte cells (mean ± SD) (1000 cells/μL) | 0.57 ± 0.21 | 0.56 ± 0.21 | 0.56 ± 0.19 | 0.57 ± 0.21 | 0.59 ± 0.22 | 0.001 |
| Neutrophils cells (mean ± SD) (1000 cells/μL) | 4.33 ± 1.68 | 4.44 ± 1.78 | 4.31 ± 1.68 | 4.28 ± 1.58 | 4.31 ± 1.68 | 0.077 |
| Eosinophils cells (mean ± SD) (1000 cells/μL) | 0.20 ± 0.16 | 0.20 ± 0.16 | 0.20 ± 0.16 | 0.20 ± 0.15 | 0.21 ± 0.16 | 0.282 |
| Basophils cells (mean ± SD) (1000 cells/μL) | 0.05 ± 0.06 | 0.05 ± 0.06 | 0.05 ± 0.06 | 0.05 ± 0.05 | 0.05 ± 0.06 | 0.478 |
| PLT (mean ± SD) (1000 cells/μL) | 237.6 ± 60.2 | 245.9 ± 62.8 | 239.3 ± 59.5 | 236.1 ± 60.8 | 230.0 ± 57.0 | <0.001 |
| RBC (mean ± SD) (million cells/μL) | 4.67 ± 0.48 | 4.59 ± 0.48 | 4.64 ± 0.49 | 4.68 ± 0.47 | 4.76 ± 0.47 | <0.001 |
| Hb (mean ± SD) (g/dL) | 14.15 ± 1.46 | 13.81 ± 1.51 | 14.04 ± 1.42 | 14.20 ± 1.42 | 14.51 ± 1.42 | <0.001 |
| Alb (mean ± SD) (g/dL) | 4.33 ± 0.34 | 4.29 ± 0.35 | 4.33 ± 0.34 | 4.34 ± 0.34 | 4.37 ± 0.33 | <0.001 |
| ALT (mean ± SD) (U/L) | 25.5 ± 22.3 | 23.8 ± 15.9 | 24.9 ± 32.0 | 25.7 ± 18.0 | 27.5 ± 19.4 | <0.001 |
| AST (mean ± SD) (U/L) | 25.8 ± 16.1 | 25.2 ± 16.6 | 25.5 ± 17.7 | 25.7 ± 13.3 | 26.7 ± 16.7 | 0.013 |
| BUN (mean ± SD) (mg/dL) | 13.8 ± 5.5 | 12.8 ± 6.1 | 13.6 ± 5.2 | 14.0 ± 5.3 | 14.7 ± 5.3 | <0.001 |
| Cr (mean ± SD) (mg/dL) | 0.89 ± 0.37 | 0.88 ± 0.55 | 0.87 ± 0.29 | 0.88 ± 0.27 | 0.92 ± 0.31 | <0.001 |
| UA (mean ± SD) (mg/dL) | 5.44 ± 1.40 | 5.27 ± 1.41 | 5.35 ± 1.36 | 5.42 ± 1.42 | 5.68 ± 1.38 | <0.001 |
| HbA1c (mean ± SD) (%) | 5.65 ± 0.96 | 5.67 ± 1.03 | 5.64 ± 0.92 | 5.65 ± 0.93 | 5.66 ± 0.96 | 0.656 |
| Smoking status (%) | <0.001 | |||||
| Never smoker | 8086 (55.5) | 2075 (57.5) | 2157 (58.5) | 2012 (54.6) | 1842 (51.7) | |
| Former smoker | 3391 (25.1) | 687 (19.4) | 779 (23.1) | 949 (29.1) | 976 (28.1) | |
| Current smoker | 2846 (19.4) | 804 (23.1) | 642 (18.4) | 622 (16.3) | 778 (20.2) | |
| Drinking status (%) | <0.001 | |||||
| Non-drinker | 4563 (24.8) | 1470 (33.0) | 1275 (28.3) | 1087 (23.8) | 731 (15.3) | |
| Low-to-moderate drinker | 8479 (63.3) | 1871 (56.9) | 2036 (61.2) | 2173 (64.0) | 2399 (70.3) | |
| Heavy drinker | 1281 (11.8) | 225 (10.1) | 267 (10.5) | 323 (12.1) | 466 (14.4) | |
| eGFR (mean ± SD) (mL/min/1.73 m2) | 99.4 ± 29.7 | 101.1 ± 30.2 | 98.5 ± 27.8 | 99.6 ± 33.6 | 98.7 ± 26.5 | 0.132 |
| Physical activity category (%) | <0.001 | |||||
| Below | 5707 (35.9) | 1623 (40.7) | 1500 (37.9) | 1391 (35.7) | 1193 (29.9) | |
| Meet | 1536 (10.4) | 358 (9.5) | 410 (11.2) | 428 (12.5) | 340 (8.4) | |
| Exceed | 7080 (53.7) | 1585 (49.7) | 1668 (51.0) | 1764 (51.7) | 2063 (61.7) | |
| Hypertension (%) | 5340 (33.6) | 1380 (33.7) | 1352 (34.5) | 1367 (34.5) | 1241 (31.8) | 0.355 |
| DM (%) | 9324 (62.8) | 2478 (68.8) | 2169 (56.4) | 2329 (63.5) | 2348 (63.1) | <0.001 |
| PIR (%) | <0.001 | |||||
| 0–1 | 3291 (16.0) | 1030 (22.2) | 832 (16.1) | 717 (12.9) | 712 (13.4) | |
| 1–3 | 5857 (36.2) | 1517 (39.7) | 1448 (36.6) | 1416 (34.4) | 1476 (34.4) | |
| >3 | 5175 (47.9) | 1019 (38.1) | 1298 (47.3) | 1450 (52.7) | 1408 (52.2) | |
| HR (mean ± SD) (bpm) | 73 ± 12 | 74 ± 12 | 73 ± 12 | 72 ± 12 | 72 ± 12 | <0.001 |
| SBP (mean ± SD) (mmHg) | 123 ± 17 | 123 ± 18 | 122 ± 17 | 122 ± 16 | 123 ± 16 | 0.428 |
| DBP (mean ± SD) (mmHg) | 71 ± 12 | 71 ± 12 | 70 ± 12 | 71 ± 11 | 71 ± 12 | 0.333 |
| Apo B (mean ± SD) (mg/dL) | 91.2 ± 24.7 | 91.7 ± 24.8 | 89.6 ± 24.2 | 91.7 ± 24.2 | 91.8 ± 25.4 | 0.01 |
| HDL-C (mean ± SD) (mg/dL) | 53.7 ± 16.8 | 53.9 ± 18.0 | 54.8 ± 17.1 | 54.1 ± 16.4 | 52.0 ± 15.8 | <0.001 |
| Triglyceride (mean ± SD) (mg/dL) | 187.5 ± 171.5 | 191.1 ± 172.9 | 179.3 ± 174.6 | 190.0 ± 166.1 | 189.9 ± 172.2 | 0.115 |
| LDL-C (mean ± SD) (mg/dL) | 102.6 ± 37.9 | 102.0 ± 38.0 | 101.8 ± 36.7 | 103.4 ± 38.3 | 103.2 ± 38.4 | 0.3 |
| Total cholesterol (mean ± SD) (mg/dL) | 193.7 ± 41.5 | 193.9 ± 41.6 | 192.3 ± 41.5 | 195.4 ± 41.4 | 193.1 ± 41.5 | 0.118 |
| Non-CVD | CVD | p-Value | |
|---|---|---|---|
| n | 12,817 | 1506 | |
| Male (%) | 6190 (48.4) | 826 (53.2) | 0.026 |
| Age (mean ± SD) (years) | 47 ± 16 | 65 ± 14 | <0.001 |
| Race (%) | <0.001 | ||
| Mexican-American | 1806 (8.9) | 130 (5.1) | |
| Other Hispanic | 1384 (6.1) | 145 (4.0) | |
| Non-Hispanic White | 4853 (65.4) | 745 (71.1) | |
| Non-Hispanic Black | 2844 (11.0) | 364 (12.8) | |
| Other Race | 1930 (8.6) | 122 (7.0) | |
| Education level (%) | <0.001 | ||
| Below high school | 2721 (14.3) | 454 (21.6) | |
| High school | 2733 (20.4) | 384 (26.3) | |
| Above high school | 7363 (65.3) | 668 (52.2) | |
| Marital status (%) | <0.001 | ||
| Married | 6589 (54.5) | 738 (53.6) | |
| Separated | 2573 (17.7) | 568 (33.0) | |
| Never married | 3655 (27.8) | 200 (13.4) | |
| Choline intake (mean ± SD) (mg/d) | 318.4 ± 165.5 | 297.2 ± 148.1 | 0.001 |
| BMI (mean ± SD) (kg/m2) | 28.99 ± 6.78 | 30.77 ± 7.47 | <0.001 |
| WBC (mean ± SD) (1000 cells/μL) | 7.26 ± 2.16 | 7.55 ± 2.95 | 0.001 |
| Lymphocyte cells (mean ± SD) (1000 cells/μL) | 2.15 ± 0.79 | 2.05 ± 2.17 | 0.003 |
| Monocyte cells (mean ± SD) (1000 cells/μL) | 0.56 ± 0.20 | 0.63 ± 0.23 | <0.001 |
| Neutrophils cells (mean ± SD) (1000 cells/μL) | 4.31 ± 1.68 | 4.59 ± 1.68 | <0.001 |
| Eosinophils cells (mean ± SD) (1000 cells/μL) | 0.20 ± 0.15 | 0.24 ± 0.22 | <0.001 |
| Basophils cells (mean ± SD) (1000 cells/μL) | 0.05 ± 0.06 | 0.05 ± 0.06 | 0.073 |
| PLT (mean ± SD) (1000 cells/μL) | 239.2 ± 59.3 | 220.3 ± 67.1 | <0.001 |
| RBC (mean ± SD) (million cells/μL) | 4.68 ± 0.47 | 4.55 ± 0.52 | <0.001 |
| Hb (mean ± SD) (g/dL) | 14.18 ± 1.45 | 13.87 ± 1.59 | <0.001 |
| Alb (mean ± SD) (g/dL) | 4.35 ± 0.34 | 4.18 ± 0.35 | <0.001 |
| ALT (mean ± SD) (U/L) | 25.48 ± 18.00 | 26.06 ± 48.10 | 0.686 |
| AST (mean ± SD) (U/L) | 25.66 ± 14.53 | 27.06 ± 27.66 | 0.114 |
| BUN (mean ± SD) (mg/dL) | 13.47 ± 5.00 | 17.32 ± 8.53 | <0.001 |
| Cr (mean ± SD) (mg/dL) | 0.87 ± 0.34 | 1.07 ± 0.56 | <0.001 |
| UA (mean ± SD) (mg/dL) | 5.40 ± 1.38 | 5.81 ± 1.59 | <0.001 |
| HbA1c (mean ± SD) (%) | 5.60 ± 0.90 | 6.19 ± 1.31 | <0.001 |
| Smoking status (%) | <0.001 | ||
| Never smoker | 7477 (57.0) | 609 (39.4) | |
| Former smoker | 2845 (24.0) | 546 (36.7) | |
| Current smoker | 2495 (19.0) | 351 (23.9) | |
| Drinking status (%) | <0.001 | ||
| Non-drinker | 3972 (24.1) | 591 (32.6) | |
| Low-to-moderate drinker | 7682 (63.9) | 797 (57.6) | |
| Heavy drinker | 1163 (12.0) | 118 (9.8) | |
| eGFR (mean ± SD) (ml/min/1.73 m2) | 101.2 ± 29.1 | 80.9 ± 29.2 | <0.001 |
| Physical activity category (%) | <0.001 | ||
| Below | 4839 (34.2) | 868 (53.4) | |
| Meet | 1381 (10.4) | 155 (10.9) | |
| Exceed | 6597 (55.4) | 483 (35.7) | |
| Hypertension (%) | 4203 (29.9) | 1137 (72.2) | <0.001 |
| DM (%) | 8076 (60.9) | 1248 (82.7) | <0.001 |
| PIR (%) | <0.001 | ||
| 0–1 | 2872 (15.6) | 419 (20.2) | |
| 1–3 | 5143 (35.2) | 714 (46.6) | |
| >3 | 4802 (49.3) | 373 (33.2) | |
| HR (mean ± SD) (bpm) | 73 ± 12 | 70 ± 12 | <0.001 |
| SBP (mean ± SD) (mmHg) | 122 ± 17 | 129 ± 20 | <0.001 |
| DBP (mean ± SD) (mmHg) | 71 ± 11 | 67 ± 14 | <0.001 |
| Apo B (mean ± SD) (mg/dL) | 91.5 ± 24.7 | 88.4 ± 24.43 | 0.001 |
| HDL-C (mean ± SD) (mg/dL) | 54.1 ± 16.8 | 50.0 ± 16.1 | <0.001 |
| Triglyceride (mean ± SD) (mg/dL) | 188.2 ± 173.0 | 181.0 ± 154.0 | 0.212 |
| LDL-C (mean ± SD) (mg/dL) | 103.5 ± 37.9 | 93.3 ± 36.5 | <0.001 |
| Total cholesterol (mean ± SD) (mg/dL) | 195.1 ± 41.2 | 179.3 ± 42.1 | <0.001 |
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Choline | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| Q1 | reference | reference | reference | |||
| Q2 | 0.904 (0.727, 1.125) | 0.359 | 0.758 (0.583, 0.985) | 0.039 | 0.863 (0.659, 1.131) | 0.271 |
| Q3 | 0.905 (0.739, 1.109) | 0.329 | 0.733 (0.572, 0.940) | 0.016 | 0.848 (0.654, 1.098) | 0.200 |
| Q4 | 0.728 (0.580, 0.914) | 0.007 | 0.611 (0.467, 0.799) | 0.001 | 0.693 (0.520, 0.923) | 0.014 |
| Subgroup | OR (95%CI) | p-Value |
|---|---|---|
| Overall | ||
| Q1 | Reference | |
| Q2 | 0.863 (0.659, 1.131) | 0.271 |
| Q3 | 0.848 (0.654, 1.098) | 0.200 |
| Q4 | 0.693 (0.520, 0.923) | 0.014 |
| CHF | ||
| Q1 | Reference | |
| Q2 | 0.807 (0.578, 1.127) | 0.197 |
| Q3 | 0.998 (0.668, 1.490) | 0.992 |
| Q4 | 0.743 (0.501, 1.103) | 0.133 |
| CHD | ||
| Q1 | Reference | |
| Q2 | 1.035 (0.733, 1.461) | 0.837 |
| Q3 | 0.874 (0.666, 1.146) | 0.314 |
| Q4 | 0.788 (0.539, 1.151) | 0.207 |
| Stroke | ||
| Q1 | Reference | |
| Q2 | 0.848 (0.609, 1.182) | 0.316 |
| Q3 | 0.858 (0.584, 1.262) | 0.421 |
| Q4 | 0.646 (0.457, 0.913) | 0.016 |
| Subgroup | OR (95%CI) | p-Value |
|---|---|---|
| Overall | ||
| Q1 | Reference | |
| Q2 | 0.863 (0.659, 1.131) | 0.271 |
| Q3 | 0.848 (0.654, 1.098) | 0.200 |
| Q4 | 0.693 (0.520, 0.923) | 0.014 |
| Age < 60 years | ||
| Q1 | Reference | |
| Q2 | 0.914 (0.609, 1.370) | 0.650 |
| Q3 | 0.706 (0.475, 1.048) | 0.081 |
| Q4 | 0.819 (0.558, 1.202) | 0.294 |
| Age ≥ 60 years | ||
| Q1 | Reference | |
| Q2 | 0.871 (0.640, 1.185) | 0.364 |
| Q3 | 0.931 (0.685, 1.265) | 0.634 |
| Q4 | 0.669 (0.479, 0.934) | 0.020 |
| Female | ||
| Q1 | Reference | |
| Q2 | 0.768 (0.583, 1.011) | 0.059 |
| Q3 | 0.838 (0.605, 1.161) | 0.274 |
| Q4 | 0.788 (0.528, 1.176) | 0.232 |
| Male | ||
| Q1 | Reference | |
| Q2 | 1.079 (0.644, 1.808) | 0.764 |
| Q3 | 0.898 (0.617, 1.307) | 0.560 |
| Q4 | 0.722 (0.453, 1.152) | 0.163 |
| BMI < 30 kg/m2 | ||
| Q1 | Reference | |
| Q2 | 0.890 (0.642, 1.234) | 0.469 |
| Q3 | 0.794 (0.626, 1.007) | 0.056 |
| Q4 | 0.680 (0.487, 0.949) | 0.025 |
| BMI ≥ 30 kg/m2 | ||
| Q1 | Reference | |
| Q2 | 0.836 (0.571, 1.226) | 0.344 |
| Q3 | 0.935 (0.587, 1.488) | 0.767 |
| Q4 | 0.742 (0.489, 1.125) | 0.152 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, R.; Yang, M.; Yue, C.; Shi, Y.; Tan, Y.; Zha, L.; Zhang, J.; Chen, S. Association between Dietary Choline Intake and Cardiovascular Diseases: National Health and Nutrition Examination Survey 2011–2016. Nutrients 2023, 15, 4036. https://doi.org/10.3390/nu15184036
Zhou R, Yang M, Yue C, Shi Y, Tan Y, Zha L, Zhang J, Chen S. Association between Dietary Choline Intake and Cardiovascular Diseases: National Health and Nutrition Examination Survey 2011–2016. Nutrients. 2023; 15(18):4036. https://doi.org/10.3390/nu15184036
Chicago/Turabian StyleZhou, Rong, Mei Yang, Chaofu Yue, Yi Shi, Yanan Tan, Lingfeng Zha, Junxia Zhang, and Shaoliang Chen. 2023. "Association between Dietary Choline Intake and Cardiovascular Diseases: National Health and Nutrition Examination Survey 2011–2016" Nutrients 15, no. 18: 4036. https://doi.org/10.3390/nu15184036
APA StyleZhou, R., Yang, M., Yue, C., Shi, Y., Tan, Y., Zha, L., Zhang, J., & Chen, S. (2023). Association between Dietary Choline Intake and Cardiovascular Diseases: National Health and Nutrition Examination Survey 2011–2016. Nutrients, 15(18), 4036. https://doi.org/10.3390/nu15184036







