Seasonal and Regional Differences in Eating Times in a Representative Sample of the Brazilian Population
Abstract
:1. Introduction
2. Methods
2.1. National Household Budget Survey (POF-IBGE/2008–2009)
2.2. Sample Characteristics
2.3. Chrononutritional Variables
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Helm, B.; Visser, M.E.; Schwartz, W.; Kronfeld-Schor, N.; Gerkema, M.; Piersma, T.; Bloch, G. Two Sides of a Coin: Ecological and Chronobiological Perspectives of Timing in the Wild. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160246. [Google Scholar] [CrossRef] [PubMed]
- Altizer, S.; Dobson, A.; Hosseini, P.; Hudson, P.; Pascual, M.; Rohani, P. Seasonality and the Dynamics of Infectious Diseases. Ecol. Lett. 2006, 9, 467–484. [Google Scholar] [CrossRef]
- Dos Santos Barroso, G.; Sichieri, R.; Salles-Costa, R. Relationship of Socio-Economic Factors and Parental Eating Habits with Children’s Food Intake in a Population-Based Study in a Metropolitan Area of Brazil. PublicHealth Nutr. 2014, 17, 156–161. [Google Scholar] [CrossRef]
- de Paula Barbosa Medina, L.; de Azevedo Barros, M.B.; da Silva Sousa, N.F.; Bastos, T.F.; Lima, M.G.; Szwarcwald, C.L. Social Inequalities in the Food Consumption Profile of the Brazilian Population: National Health Survey, 2013. Rev. Bras. Epidemiol. 2019, 22, E190011. [Google Scholar] [CrossRef]
- Stelmach-Mardas, M.; Kleiser, C.; Uzhova, I.; Peñalvo, J.L.; Torre, L.; Palys, W.; Lojko, D.; Nimptsch, K.; Suwalska, A.; Linseisen, J.; et al. Seasonality of Food Groups and Total Energy Intake: A Systematic Review and Meta-Analysis. Eur. J. Clin. Nutr. 2016, 70, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.J. Lifestyle and Circadian Health: Where the Challenges Lie? Nutr. Metab. Insights 2019, 12, 1178638819869024. [Google Scholar] [CrossRef]
- Rossato, S.L.; Olinto, M.T.A.; Henn, R.L.; Moreira, L.B.; Camey, S.A.; Anjos, L.A.; Wahrlich, V.; Waissmann, W.; Fuchs, F.D.; Fuchs, S.C. Seasonal Variation in Food Intake and the Interaction Effects of Sex and Age among Adults in Southern Brazil. Eur. J. Clin. Nutr. 2015, 69, 1015–1022. [Google Scholar] [CrossRef]
- Capita, R.; Alonso-Calleja, C. Differences in Reported Winter and Summer Dietary Intakes in Young Adults in Spain. Int. J. Food Sci. Nutr. 2005, 56, 431–443. [Google Scholar] [CrossRef]
- Shahar, D.R.; Yerushalmi, N.; Lubin, F.; Froom, P.; Shahar, A.; Kristal-Boneh, E. Seasonal Variations in Dietary Intake Affect the Consistency of Dietary Assessment. Eur. J. Epidemiol. 2001, 17, 129–133. [Google Scholar] [CrossRef]
- Spence, C. Explaining Seasonal Patterns of Food Consumption. Int. J. Gastron. Food Sci. 2021, 24, 100332. [Google Scholar] [CrossRef]
- Levy-Costa, R.B.; Sichieri, R.; dos Santos Pontes, N.; Monteiro, C.A. Household Food Availability in Brazil: Distribution and Trends (1974–2003). Rev. Saude Publica 2005, 39, 530–540. [Google Scholar] [CrossRef]
- Rossato, S.L.; Olinto, M.T.A.; Henn, R.L.; dos Anjos, L.A.; Bressan, A.W.; Wahrlich, V. Seasonal Effect on Nutrient Intake in Adults Living in Southern Brazil. Cad. Saude Publica 2010, 26, 2177–2187. [Google Scholar] [CrossRef] [PubMed]
- Instituto Brasileiro de Geografi a e Estatística—IBGE. Pesquisa de Orçamentos Familiares 2008–2009: Despesas, Rendimentos e Condições de Vida; IBGE—Coordenação de Trabalho e Rendimento: Rio de Janeiro, Brazil, 2010; ISBN 9788524041310. [Google Scholar]
- Instituto Brasileiro de Geografi a e Estatística—IBGE. Pesquisa de Orçamentos Familiares 2008–2009: Análise do Consumo Alimentar Pessoal No Brasil; IBGE—Coordenação de Trabalho e Rendimento: Rio de Janeiro, Brazil, 2011; ISBN 9788524041983. [Google Scholar]
- Rodrigues, R.M.; Souza, A.D.M.; Bezerra, I.N.; Pereira, R.A.; Yokoo, E.M.; Sichieri, R. Most Consumed Foods in Brazil: Evolution between 2008–2009 and 2017–2018. Rev. Saude Publica 2021, 55, 4s. [Google Scholar] [CrossRef] [PubMed]
- Hut, R.A.; Paolucci, S.; Dor, R.; Kyriacou, C.P.; Daan, S. Latitudinal Clines: An Evolutionary View on Biological Rhythms †,‡. Proc. R. Soc. B Biol. Sci. 2013, 280, 20130433. [Google Scholar] [CrossRef] [PubMed]
- Leocadio-Miguel, M.A.; Louzada, F.M.M.; Duarte, L.L.; Areas, R.P.; Alam, M.; Freire, M.V.; Fontenele-Araujo, J.; Menna-Barreto, L.; Pedrazzoli, M.; Araujo, J.F.; et al. Latitudinal Cline of Chronotype. Sci. Rep. 2017, 7, 5437. [Google Scholar] [CrossRef]
- van der Merwe, C.; Münch, M.; Kruger, R. Chronotype Differences in Body Composition, Dietary Intake and Eating Behavior Outcomes: A Scoping Systematic Review. Adv. Nutr. 2022, 13, 2357–2405. [Google Scholar] [CrossRef]
- Teixeira, G.P.; Guimarães, K.C.; Soares, A.G.N.S.; Marqueze, E.C.; Moreno, C.R.C.; Mota, M.C.; Crispim, C.A. Role of Chronotype in Dietary Intake, Meal Timing, and Obesity: A Systematic Review. Nutr. Rev. 2022, 81, 75–90. [Google Scholar] [CrossRef]
- Bernstein, S.; Zambell, K.; Amar, M.J.; Arango, C.; Kelley, R.C.; Miszewski, S.G.; Tryon, S.; Courville, A.B. Dietary Intake Patterns Are Consistent Across Seasons in a Cohort of Healthy Adults in a Metropolitan Population. J. Acad. Nutr. Diet. 2016, 116, 38–45. [Google Scholar] [CrossRef]
- Costa, A.F.; Yokoo, E.M.; dos Anjos, L.A.; Wahrlich, V.; Olinto, M.T.A.; Henn, R.L.; Waissmann, W. Seasonal Variation of Food Intake of Adults from Niterói, Rio de Janeiro, Brazil. Rev. Bras. Epidemiol. 2013, 16, 513–524. [Google Scholar] [CrossRef]
- Aparecida Crispim, C.; Carliana Mota, M. New Perspectives on Chrononutrition. Biol. Rhythm Res. 2018, 50, 63–77. [Google Scholar] [CrossRef]
- Do Vale Cardoso Lopes, T.; Borba, M.E.; do Vale Cardoso Lopes, R.; Fisberg, R.M.; Paim, S.L.; Teodoro, V.V.; Zimberg, I.Z.; Crispim, C.A. Eating Late Negatively Affects Sleep Pattern and Apnea Severity in Individuals with Sleep Apnea. J. Clin. Sleep Med. 2019, 15, 383–392. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Mikic, A.; Pietrolungo, C.E. Effects of Diet on Sleep Quality. Adv. Nutr. 2016, 7, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Crispim, C.A.; Zimberg, I.Z.; Gomes Dos Reis, B.; Diniz, R.M.; Tufik, S.; Túlio De Mello, M. Relationship between Food Intake and Sleep Pattern in Healthy Individuals. J. Clin. Sleep Med. 2011, 7, 659–664. [Google Scholar] [CrossRef] [PubMed]
- María Martín-Olalla, J. Latitudinal Trends in Human Primary Activities: Characterizing the Winter Day as a Synchronizer. Sci. Rep. 2018, 8, 5350. [Google Scholar] [CrossRef]
- Hut, R.A.; Beersma, D.G.M. Evolution of Time-Keeping Mechanisms: Early Emergence and Adaptation to Photoperiod. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2141–2154. [Google Scholar] [CrossRef]
- Manoogian, E.N.C.; Chaix, A.; Panda, S. When to Eat: The Importance of Eating Patterns in Health and Disease. J. Biol. Rhythm 2019, 34, 579–581. [Google Scholar] [CrossRef]
- Quante, M.; Wang, R.; Weng, J.; Kaplan, E.R.; Rueschman, M.; Taveras, E.M.; Rifas-Shiman, S.L.; Gillman, M.W.; Redline, S. Seasonal and Weather Variation of Sleep and Physical Activity in 12–14-Year-Old Children. Behav. Sleep Med. 2017, 17, 398–410. [Google Scholar] [CrossRef]
- Cepeda, M.; Koolhaas, C.M.; van Rooij, F.J.A.; Tiemeier, H.; Guxens, M.; Franco, O.H.; Schoufour, J.D. Seasonality of Physical Activity, Sedentary Behavior, and Sleep in a Middle-Aged and Elderly Population: The Rotterdam Study. Maturitas 2018, 110, 41–50. [Google Scholar] [CrossRef]
- Ferguson, T.; Curtis, R.; Fraysse, F.; Lagiseti, R.; Northcott, C.; Virgara, R.; Watson, A.; Maher, C.A. Annual, Seasonal, Cultural and Vacation Patterns in Sleep, Sedentary Behaviour and Physical Activity: A Systematic Review and Meta-Analysis. BMC Public Health 2021, 21, 1384. [Google Scholar] [CrossRef]
- Rubio-Sastre, P.; Gómez-Abellán, P.; Martinez-Nicolas, A.; Ordovás, J.M.; Madrid, J.A.; Garaulet, M. Evening Physical Activity Alters Wrist Temperature Circadian Rhythmicity. J. Biol. Med. Rhythm. Res. 2014, 31, 276–282. [Google Scholar] [CrossRef]
- Thomas, J.M.; Kern, P.A.; Bush, H.M.; McQuerry, K.J.; Black, W.S.; Clasey, J.L.; Pendergast, J.S. Circadian Rhythm Phase Shifts Caused by Timed Exercise Vary with Chronotype. JCI Insight 2020, 5, e134270. [Google Scholar] [CrossRef]
- Mota, M.C.; Silva, C.M.; Balieiro, L.C.T.; Gonçalves, B.F.; Fahmy, W.M.; Crispim, C.A. Association between Social Jetlag Food Consumption and Meal Times in Patients with Obesity-Related Chronic Diseases. PLoS ONE 2019, 14, e0212126. [Google Scholar] [CrossRef]
- McHill, A.W.; Phillips, A.J.K.; Czeisler, C.A.; Keating, L.; Yee, K.; Barger, L.K.; Garaulet, M.; Scheer, F.A.J.L.; Klerman, E.B. Later Circadian Timing of Food Intake Is Associated with Increased Body Fat. Am. J. Clin. Nutr. 2017, 106, 1213–1219. [Google Scholar] [CrossRef]
- Stothard, E.R.; McHill, A.W.; Depner, C.M.; Birks, B.R.; Moehlman, T.M.; Ritchie, H.K.; Guzzetti, J.R.; Chinoy, E.D.; LeBourgeois, M.K.; Axelsson, J.; et al. Circadian Entrainment to the Natural Light-Dark Cycle across Seasons and the Weekend. Curr. Biol. 2017, 27, 508–513. [Google Scholar] [CrossRef]
- Marti-Soler, H.; Guessous, I.; Gaspoz, J.M.; Metcalf, P.; Deschamps, V.; Castetbon, K.; Malyutina, S.; Bobak, M.; Ruidavets, J.B.; Bongard, V.; et al. Seasonality of Nutrient Intake—An Analysis Including over 44,000 Participants in 4 Countries. Clin. Nutr. ESPEN 2017, 21, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Panda, S. The Arrival of Circadian Medicine. Nat. Rev. Endocrinol. 2019, 15, 67–69. [Google Scholar] [CrossRef]
- Longo, V.D.; Panda, S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A.; Dobson, A. Analysing Seasonal Health Data (Statistics for Biology and Health), 1st ed.; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 9783642107474. [Google Scholar]
| Brazil | North (n = 3003) | Northeast (n = 7504) | Midwest (n = 2963) | Southeast (n = 4545) | South (n = 2607) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Spring-Summer | Fall-Winter | Spring-Summer | Fall-Winter | Spring-Summer | Fall-Winter | Spring-Summer | Fall-Winter | Spring-Summer | Fall-Winter | ||
| Sociodemographic Variables # | % | % | % | % | % | % | % | % | % | % | % |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |
| Sex | |||||||||||
| Male | 49.8 | 26.5 | 25.4 | 23.2 | 26.3 | 21.7 | 28.5 | 23.6 | 26.3 | 24.7 | 24.3 |
| (49.0–50.6) | (23.7–29.6) | (22.5–28.5) | (21.3–25.2) | (24.4–28.3) | (19.1–24.5) | (25.5–31.7) | (21.3–26.1) | (24.1–28.8) | (22.1–27.5) | (21.7–27.1) | |
| Female | 50.2 | 23.9 | 24.2 | 23.9 | 26.5 | 20.9 | 28.9 | 23.5 | 26.5 | 25.3 | 25.6 |
| (49.4–50.9) | (21.4–26.6) | (21.5–27.1) | (22.1–25.9) | (24.7–28.5) | (18.5–23.5) | (26.1–32.0) | (21.2–26.0) | (24.2–28.9) | (22.7–28.2) | (22.9–28.5) | |
| Age | |||||||||||
| 18–25 | 21.8 | 13.8 | 11.8 | 11 | 12.5 | 8.2 * | 15.1 * | 10.2 | 10.6 | 9.8 | 10.1 |
| (20.9–22.8) | (11.8–16.1) | (10.0–13.9) | (9.8–12.3) | (11.3–13.9) | (6.8–9.9) | (12.6–18.1) | (8.5–12.1) | (9.2–12.2) | (8.3–11.7) | (8.4–12.1) | |
| 26–35 | 27.3 | 14.8 | 14 | 14 | 15.6 | 12.8 * | 15.8 * | 12.1 | 14 | 12.3 | 12.4 |
| (26.2–28.4) | (12.6–17.4) | (11.9–16.5) | (12.5–15.7) | (13.8–17.6) | (10.8–15.1) | (13.6–18.3) | (10.4–13.9) | (12.2–16.0) | (10.4–14.6) | (10.4–14.7) | |
| 36–45 | 24.7 | 10.8 | 13.1 | 10.9 | 12.4 | 10.7 * | 13.4 * | 11.8 | 13.5 | 11.9 | 14.6 |
| (23.7–25.7) | (8.9–13.0) | (11.1–15.3) | (9.8–12.1) | (11.1–13.7) | (8.9–12.8) | (11.6–15.5) | (10.3–13.5) | (11.8–15.4) | (10.0–14.2) | (12.4–17.1) | |
| 45–59 | 26.2 | 11 | 10.7 | 11.3 | 12.4 | 10.9 * | 13.1 * | 13.1 | 14.8 | 15.9 | 12.9 |
| (25.1–27.3) | (9.2–13.2) | (8.9–12.7) | (10.1–12.6) | (11.1–13.7) | (9.1–13.0) | (11.1–15.3) | (11.4–15.1) | (13.0–16.8) | (13.5–18.6) | (11.1–15.0) | |
| Years of education | |||||||||||
| 0–10 | 56.9 | 30.9 | 29.8 | 32.3 | 34.5 | 22.8 | 31.6 | 25.3 | 26.5 | 26.2 | 27.5 |
| (55.3–58.5) | (27.0–35.2) | (26.2–33.6) | (29.7–35.1) | (31.8–37.3) | (19.7–26.1) | (28.0–35.4) | (22.5–28.4) | (23.7–29.6) | (23.0–29.6) | (24.3–30.9) | |
| >11 | 43.1 | 19.5 | 19.8 | 14.8 | 18.3 | 19.8 | 25.8 | 21.8 | 26.3 | 23.9 | 22.5 |
| (41.5–44.7) | (16.7–22.5) | (17.0–23.0) | (13.2–16.6) | (15.9–21.1) | (17.0–22.9) | (22.0–30.1) | (19.1–24.8) | (23.4–29.6) | (20.7–27.4) | (19.6–25.7) | |
| Race | |||||||||||
| White | 49 | 10.8 | 10.5 | 13.2 | 13.6 | 19.2 | 23.2 | 27.4 | 30.5 | 39.3 | 39.5 |
| (47.4–50.7) | (9.0–13.1) | (8.7–12.8) | (11.7–14.8) | (12.2–15.1) | (16.3–22.5) | (20.0–26.8) | (24.4–30.6) | (27.3–33.9) | (35.0–43.7) | (35.2–44.0) | |
| Black/Brown | 49.7 | 38.5 | 37.3 | 33.5 | 38.6 | 23 | 33.4 | 19.3 | 21.7 | 10.2 | 9.7 |
| (47.4–50.7) | (34.1–43.2) | (33.0–41.7) | (30.7–36.3) | (35.7–41.6) | (20.0–26.2) | (29.7–37.4) | (16.9–22.0) | (19.1–24.5) | (8.3–12.5) | (7.8–12.0) | |
| Asian/Indigenous | 1 | 0.9 | 1.6 | 0.4 | 0.5 | 0.2 | 0.6 | 0.3 | 0.5 | 0.5 | 0.5 |
| (0.7–1.2) | (0.4–2.0) | (1.0–2.5) | (0.2–0.7) | (0.3–1.0) | (0.1–0.5) | (0.3–1.3) | (0.1–0.9) | (0.3–0.8) | (0.2–1.2) | (0.2–1.0) | |
| Do not know | 0.3 | 0.2 | 0.1 | 0.1 | 0.2 | 0.2 | 0.2 | 0.1 | 0.2 | 0 | 0.3 |
| (0.2–0.5) | (0.0–0.5) | (0.1–0.4) | (0.1–0.4) | (0.1–0.3) | (0.0–0.6) | (0.0–0.6) | (0.0–0.7) | (0.0–0.6) | (0.0–0.1) | (0.1–1.4) | |
| BMI (kg/m2) | |||||||||||
| <24.9 | 53.1 | 28.4 | 27.1 | 27.8 | 30.6 | 22.7 * | 33.2 * | 24.5 | 26.5 | 23.1 | 24.5 |
| (51.9–54.3) | (25.0–32.1) | (24.0–30.5) | (25.5–30.2) | (28.3–33.0) | (19.9–25.8) | (29.6–37.0) | (21.9–27.4) | (24.0–29.2) | (20.4–26.2) | (21.7–27.6) | |
| 25–29.9 | 32.9 | 16.5 | 15.1 | 13.7 | 15.8 | 14.7 * | 16.2 * | 15.8 | 18.9 | 18.7 | 17 |
| (31.8–34.0) | (14.2–19.0) | (12.9–17.5) | (12.4–15.1) | (14.2–17.5) | (12.5–17.1) | (14.1–18.6) | (13.9–18.0) | (16.9–21.0) | (16.3–21.3) | (14.8–19.5) | |
| >30 | 14 | 5.5 | 7.4 | 5.7 | 6.5 | 5.1 * | 8.0 * | 6.8 | 7.5 | 8.2 | 8.4 |
| (13.2–14.8) | (4.5–6.8) | (5.9–9.2) | (4.8–6.6) | (5.7–7.4) | (4.2–6.3) | (6.6–9.7) | (5.7–8.1) | (6.3–8.8) | (6.8–9.9) | (7.0–10.1) | |
| Regions | Seasons | Effects/Interaction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| North | Northeast | Midwest | Southeast | South | Spring-Summer | Fall-Winter | Region | Season | Region x Season | |
| Chrononutritional Variables | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | F p-Value | F p-Value | F p-Value |
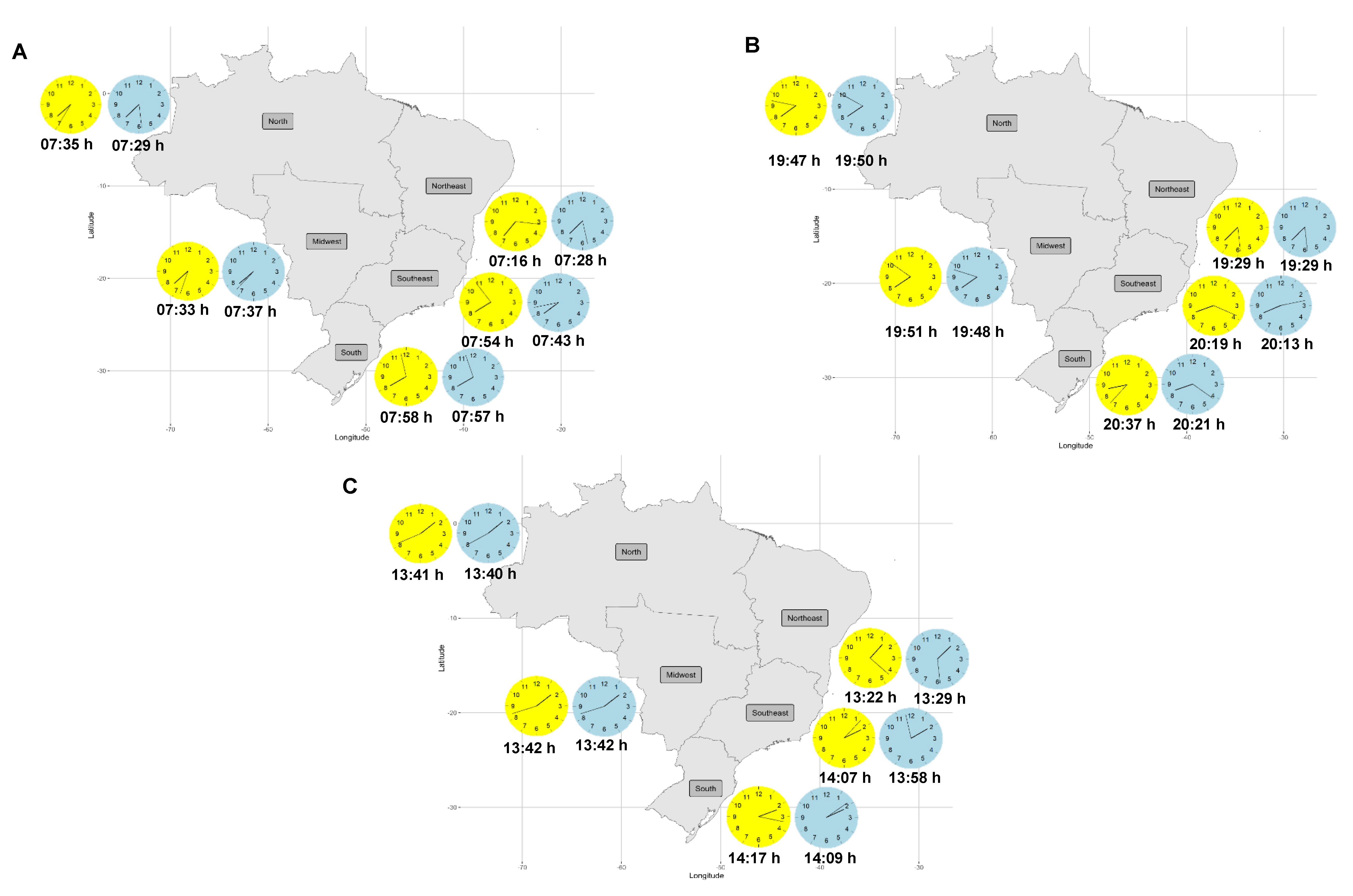
| First Food Intake Time | 07:32 (07:27–07:37) | 07:23 (07:19–07:26) | 07:35 (07:29–07:42) | 07:48 (07:43–07:54) | 07:57 (07:51–08:02) | 07:42 (07:38–07:46) | 07:39 (07:36–07:43) | 38.71 <0.001 * | 0.06 0.81 | 5.11 <0.001 * |
| Last Food Intake Time | 19:49 (19:43–19:54) | 19:29 (19:25–19:33) | 19:50 (19:43–19:57) | 20:16 (20:10–20:22) | 20:29 (20:23–20:35) | 20:04 (19:59–20:09) | 19:59 (19:55–20:03) | 77.80 <0.001 * | 2.77 0.09 | 1.45 0.21 |
| Eating Midpoint | 13:40 (13:36–13:45) | 13:26 (13:23–13:28) | 13:42 (13:38–13:47) | 14:02 (13:58–14:06) | 14:13 (14:09–14:17) | 13:53 (13:49–13:57) | 13:49 (13:46–13:52) | 102.76 <0.001 * | 1.84 0.17 | 3.17 0.01 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.S.; Skene, D.J.; Crispim, C.A.; Moreno, C.R.d.C. Seasonal and Regional Differences in Eating Times in a Representative Sample of the Brazilian Population. Nutrients 2023, 15, 4019. https://doi.org/10.3390/nu15184019
Santos JS, Skene DJ, Crispim CA, Moreno CRdC. Seasonal and Regional Differences in Eating Times in a Representative Sample of the Brazilian Population. Nutrients. 2023; 15(18):4019. https://doi.org/10.3390/nu15184019
Chicago/Turabian StyleSantos, Jefferson Souza, Debra Jean Skene, Cibele Aparecida Crispim, and Claudia Roberta de Castro Moreno. 2023. "Seasonal and Regional Differences in Eating Times in a Representative Sample of the Brazilian Population" Nutrients 15, no. 18: 4019. https://doi.org/10.3390/nu15184019
APA StyleSantos, J. S., Skene, D. J., Crispim, C. A., & Moreno, C. R. d. C. (2023). Seasonal and Regional Differences in Eating Times in a Representative Sample of the Brazilian Population. Nutrients, 15(18), 4019. https://doi.org/10.3390/nu15184019








