Sex Differences in the Relationship between Chronotype and Eating Behaviour: A Focus on Binge Eating and Food Addiction
Abstract
:1. Introduction
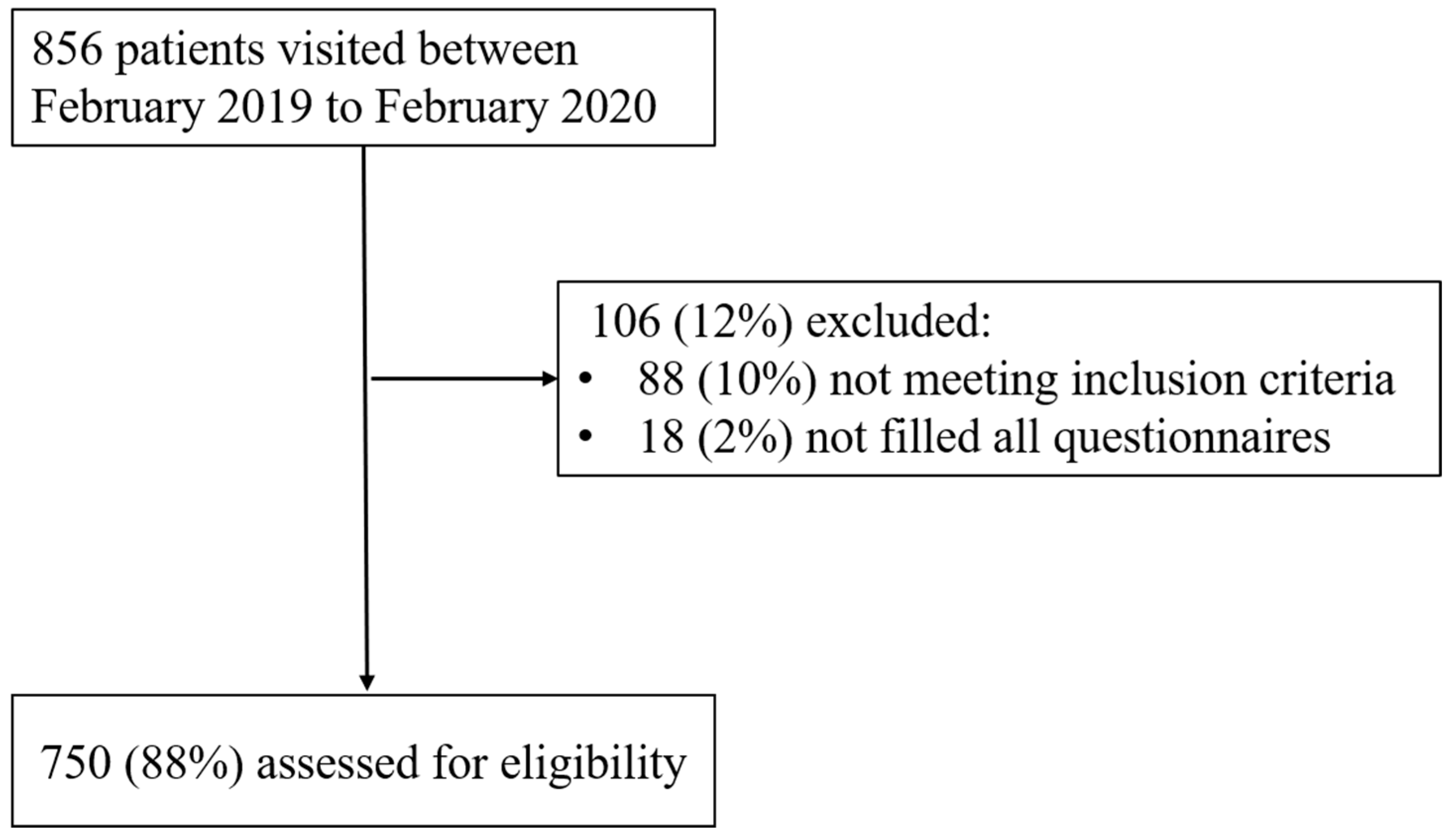
2. Materials and Methods
2.1. Study Design
2.2. Clinical and Anthropometrical Assessment
2.3. Eating Behaviour
2.4. Chronotype
2.5. Adherence to the Mediterranean Diet
2.6. Statistical Analysis
- sex (categorical: 0 = female, 1 = male)
- age (continuous, years)
- smoking (categorical: 0 = never smoked, 1 = smoker, ex-smoker)
- physical activity (categorical: 0 = sedentary, 1 = physically active)
- body mass index (continuous, kg/m2)
- Mediterranean diet adherence score (continuous)
- reduced Morningness-Eveningness Questionnaire score (continuous)
- sex × reduced Morningness-Eveningness Questionnaire score interaction
3. Results
3.1. Association between Chronotype and Binge-Eating Behaviour
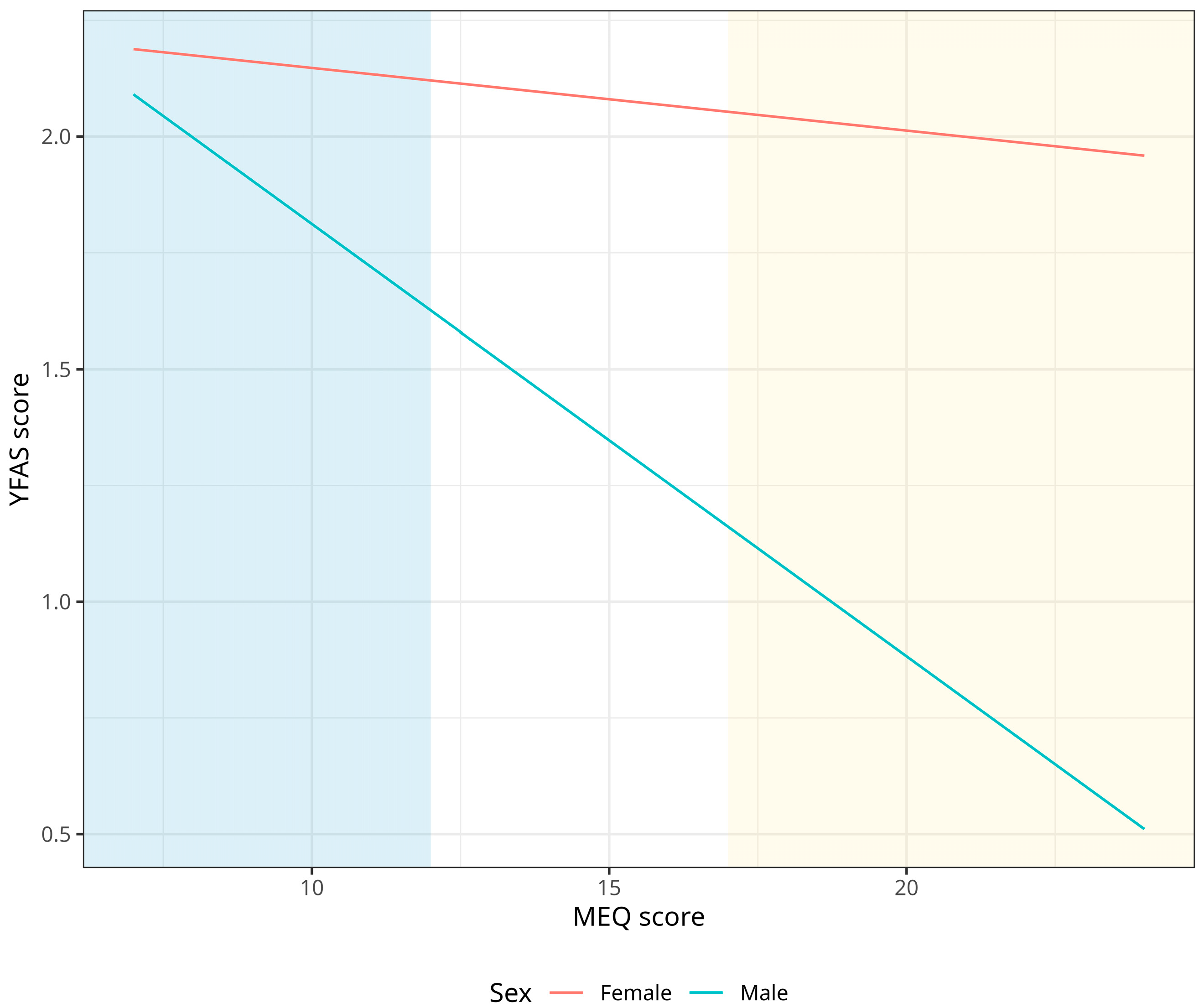
3.2. Association between Chronotype and Food Addiction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moore, C.F.; Sabino, V.; Koob, G.F.; Cottone, P. Pathological Overeating: Emerging Evidence for a Compulsivity Construct. Neuropsychopharmacology 2017, 42, 1375–1389. [Google Scholar] [PubMed]
- Goldschmidt, A.B.; Wall, M.M.; Zhang, J.; Loth, K.A.; Neumark-Sztainer, D. Overeating and Binge Eating in Emerging Adulthood: 10-Year Stability and Risk Factors. Dev. Psychol. 2016, 52, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, P.S.; Blacker, D.; Blazer, D.G.; Ganguli, M.; Jeste, D.V.; Paulsen, J.S.; Petersen, R.C. Classifying Neurocognitive Disorders: The DSM-5 Approach. Nat. Rev. Neurol. 2014, 10, 634–642. [Google Scholar] [CrossRef]
- Finlayson, G. Food Addiction and Obesity: Unnecessary Medicalization of Hedonic Overeating. Nat. Rev. Endocrinol. 2017, 13, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Kaya Cebioğlu, İ.; Dumlu Bilgin, G.; Kavsara, H.K.; Gül Koyuncu, A.; Sarioğlu, A.; Aydin, S.; Keküllüoğlu, M. Food Addiction among University Students: The Effect of Mindful Eating. Appetite 2022, 177, 106133. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; White, M.A.; Masheb, R.M.; Morgan, P.T.; Crosby, R.D.; Grilo, C.M. An Examination of the Food Addiction Construct in Obese Patients with Binge Eating Disorder. Int. J. Eat. Disord. 2012, 45, 657–663. [Google Scholar] [CrossRef]
- De Amicis, R.; Mambrini, S.P.; Pellizzari, M.; Foppiani, A.; Bertoli, S.; Battezzati, A.; Leone, A. Ultra-Processed Foods and Obesity and Adiposity Parameters among Children and Adolescents: A Systematic Review. Eur. J. Nutr. 2022, 61, 2297–2311. [Google Scholar] [PubMed]
- Wiss, D.A.; Criscitelli, K.; Gold, M.; Avena, N. Preclinical Evidence for the Addiction Potential of Highly Palatable Foods: Current Developments Related to Maternal Influence. Appetite 2017, 115, 19–27. [Google Scholar] [CrossRef]
- Lydecker, J.A.; Grilo, C.M. Psychiatric Comorbidity as Predictor and Moderator of Binge-Eating Disorder Treatment Outcomes: An Analysis of Aggregated Randomized Controlled Trials. Psychol. Med. 2022, 52, 4085–4093. [Google Scholar] [CrossRef]
- Leone, A.; Bedogni, G.; Ponissi, V.; Battezzati, A.; Beggio, V.; Magni, P.; Ruscica, M.; Bertoli, S. Contribution of Binge Eating Behaviour to Cardiometabolic Risk Factors in Subjects Starting a Weight Loss or Maintenance Programme. Br. J. Nutr. 2016, 116, 1984–1992. [Google Scholar] [CrossRef]
- Kandeger, A.; Selvi, Y.; Tanyer, D.K. The Effects of Individual Circadian Rhythm Differences on Insomnia, Impulsivity, and Food Addiction. Eat. Weight Disord. 2019, 24, 47–55. [Google Scholar] [CrossRef]
- da Silva Júnior, A.E.; de Lima Macena, M.; de Oliveira, A.D.S.; Praxedes, D.R.S.; de Oliveira Maranhão Pureza, I.R.; de Menezes Toledo Florêncio, T.M.; Gearhardt, A.N.; Bueno, N.B. Prevalence of Food Addiction and Its Association with Anxiety, Depression, and Adherence to Social Distancing Measures in Brazilian University Students during the COVID-19 Pandemic: A Nationwide Study. Eat. Weight Disord. 2022, 27, 2027–2035. [Google Scholar] [CrossRef]
- Almoosawi, S.; Vingeliene, S.; Gachon, F.; Voortman, T.; Palla, L.; Johnston, J.D.; Van Dam, R.M.; Darimont, C.; Karagounis, L.G. Chronotype: Implications for Epidemiologic Studies on Chrono-Nutrition and Cardiometabolic Health. Adv. Nutr. 2019, 10, 30–42. [Google Scholar] [CrossRef]
- Randler, C.; Faßl, C.; Kalb, N. From Lark to Owl: Developmental Changes in Morningness-Eveningness from New-Borns to Early Adulthood. Sci. Rep. 2017, 7, 45874. [Google Scholar] [CrossRef] [PubMed]
- Urbán, R.; Magyaródi, T.; Rigó, A. Morningness-Eveningness, Chronotypes and Health-Impairing Behaviors in Adolescents. Chronobiol. Int. 2011, 28, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Yun, C.H.; Ahn, J.H.; Suh, S.; Cho, H.J.; Lee, S.K.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Choi, K.M.; et al. Evening Chronotype Is Associated with Metabolic Disorders and Body Composition in Middle-Aged Adults. J. Clin. Endocrinol. Metab. 2015, 100, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Kanerva, N.; Kronholm, E.; Partonen, T.; Ovaskainen, M.-L.; Kaartinen, N.E.; Konttinen, H.; Broms, U.; Männistö, S. Tendency toward Eveningness Is Associated with Unhealthy Dietary Habits. Chronobiol. Int. 2012, 29, 920–927. [Google Scholar] [CrossRef]
- Castelli, L.; Galasso, L.; Mulè, A.; Ciorciari, A.; Esposito, F.; Roveda, E.; Montaruli, A. Physical Activity and Morningness: A Helpful Combination in Improving the Sleep Quality of Active Italian University Students. Chronobiol. Int. 2023, 40, 1028–1038. [Google Scholar] [CrossRef]
- Randler, C.; Engelke, J. Gender Differences in Chronotype Diminish with Age: A Meta-Analysis Based on Morningness/Chronotype Questionnaires. Chronobiol. Int. 2019, 36, 888–905. [Google Scholar] [CrossRef]
- van der Merwe, C.; Münch, M.; Kruger, R. Chronotype Differences in Body Composition, Dietary Intake and Eating Behavior Outcomes: A Scoping Systematic Review. Adv. Nutr. 2022, 13, 2357–2405. [Google Scholar] [CrossRef]
- Rosi, A.; Lotti, S.; Vitale, M.; Pagliai, G.; Madarena, M.P.; Bonaccio, M.; Esposito, S.; Ferraris, C.; Guglielmetti, M.; Angelino, D.; et al. Association between Chronotype, Sleep Pattern, and Eating Behaviours in a Group of Italian Adults. Int. J. Food Sci. Nutr. 2022, 73, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Vizmanos, B.; Muela, T.; Betancourt-Núñez, A.; Bonmatí-Carrión, M.Á.; Vetter, C.; Dashti, H.S.; Saxena, R.; Scheer, F.A.J.L. Evening Types as Determined by Subjective and Objective Are More Emotional Eaters. Obesity 2023, 5, 1192–1203. [Google Scholar] [CrossRef]
- Aoun, C.; Nassar, L.; Soumi, S.; El Osta, N.; Papazian, T.; Rabbaa Khabbaz, L. The Cognitive, Behavioral, and Emotional Aspects of Eating Habits and Association With Impulsivity, Chronotype, Anxiety, and Depression: A Cross-Sectional Study. Front. Behav. Neurosci. 2019, 13, 204. [Google Scholar] [CrossRef]
- De Amicis, R.; Galasso, L.; Leone, A.; Vignati, L.; De Carlo, G.; Foppiani, A.; Montaruli, A.; Roveda, E.; Cè, E.; Esposito, F.; et al. Is Abdominal Fat Distribution Associated with Chronotype in Adults Independently of Lifestyle Factors? Nutrients 2020, 12, 592. [Google Scholar] [CrossRef] [PubMed]
- Hasler, B.P.; Smith, L.J.; Cousins, J.C.; Bootzin, R.R. Circadian Rhythms, Sleep, and Substance Abuse. Sleep Med. Rev. 2012, 16, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Escobar, C.; Espitia-Bautista, E.; Golombek, D.A. Is the Binge-Eating Disorder a Circadian Disorder? Front. Nutr. 2022, 9, 964491. [Google Scholar]
- Zou, H.; Zhou, H.; Yan, R.; Yao, Z.; Lu, Q. Chronotype, Circadian Rhythm, and Psychiatric Disorders: Recent Evidence and Potential Mechanisms. Front. Neurosci. 2022, 16, 811771. [Google Scholar] [CrossRef]
- Culbert, K.M.; Sisk, C.L.; Klump, K.L. A Narrative Review of Sex Differences in Eating Disorders: Is There a Biological Basis? Clin. Ther. 2021, 43, 95–111. [Google Scholar] [CrossRef]
- Mento, C.; Rizzo, A.; Bruno, A.; Silvestri, M.C.; Cedro, C.; Komaei, I.; Navarra, G.; Muscatello, M.R.A. Sex Differences in Emotions and Eating Behaviors among People Affected by Obesity. Brain Sci. 2022, 12, 1663. [Google Scholar] [CrossRef]
- Lohman, T.G.; Roche, A.F.; Martorell, R. Anthropometric Standardization Reference Manual; Human Kinetics Books: Champaign, IL, USA, 1988; ISBN 0873221214 9780873221214. [Google Scholar]
- Marcus, M.D.; Wing, R.R.; Hopkins, J. Obese Binge Eaters: Affect, Cognitions, and Response to Behavioral Weight Control. J. Consult. Clin. Psychol. 1988, 56, 433–439. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; Corbin, W.R.; Brownell, K.D. Development of the Yale Food Addiction Scale Version 2.0. Psychol. Addict. Behav. 2016, 30, 113. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H. Multicollinearity and Misleading Statistical Results. Korean J. Anesthesiol. 2019, 72, 558–569. [Google Scholar] [CrossRef]
- Černelič-Bizjak, M.; Guiné, R.P.F. Predictors of Binge Eating: Relevance of BMI, Emotional Eating and Sensivity to Environmental Food Cues. Nutr. Food Sci. 2022, 52, 171–180. [Google Scholar] [CrossRef]
- Wittekind, D.A.; Kratzsch, J.; Mergl, R.; Baber, R.; Wirkner, K.; Schroeter, M.L.; Witte, A.V.; Villringer, A.; Kluge, M. Leptin, but Not Ghrelin, Is Associated with Food Addiction Scores in a Population-Based Subject Sample. Front. Psychiatry 2023, 14, 1200021. [Google Scholar] [CrossRef]
- Riley, R.D.; Ensor, J.; Snell, K.I.E.; Harrell, F.E.; Martin, G.P.; Reitsma, J.B.; Moons, K.G.M.; Collins, G.; Van Smeden, M. Calculating the Sample Size Required for Developing a Clinical Prediction Model. BMJ 2020, 368, m441. [Google Scholar] [CrossRef]
- Maukonen, M.; Kanerva, N.; Partonen, T.; Kronholm, E.; Konttinen, H.; Wennman, H.; Männistö, S. The Associations between Chronotype, a Healthy Diet and Obesity. Chronobiol. Int. 2016, 33, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cortés, F.J.; Morales-Cané, I.; Rodríguez-Muñoz, P.M.; Cappadona, R.; De Giorgi, A.; Manfredini, R.; Rodríguez-Borrego, M.A.; Fabbian, F.; López-Soto, P.J. Individual Circadian Preference, Eating Disorders and Obesity in Children and Adolescents: A Dangerous Liaison? A Systematic Review and a Meta-Analysis. Children 2022, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Menculini, G.; Brufani, F.; Del Bello, V.; Moretti, P.; Tortorella, A. Circadian Rhythms Disruptions and Eating Disorders: Clinical Impact and Possible Psychopathological Correlates. Psychiatr. Danub. 2019, 31, 497–502. [Google Scholar]
- Kim, S.; Lee, H.J. Sleep and Circadian Rhythm Disturbances in Eating Disorders. Chronobiol. Med. 2020, 2, 141–147. [Google Scholar] [CrossRef]
- Parikh, S.; Parikh, R.; Michael, K.; Bikovski, L.; Barnabas, G.; Mardamshina, M.; Hemi, R.; Manich, P.; Goldstein, N.; Malcov-Brog, H.; et al. Food-Seeking Behavior Is Triggered by Skin Ultraviolet Exposure in Males. Nat. Metab. 2022, 4, 883–900. [Google Scholar] [CrossRef]
- Joye, D.A.M.; Evans, J.A. Sex Differences in Daily Timekeeping and Circadian Clock Circuits. Semin. Cell Dev. Biol. 2022, 126, 45–55. [Google Scholar] [CrossRef] [PubMed]
- De Young, K.P.; Bottera, A.R. A Biobehavioral Circadian Model of Restrictive Eating and Binge Eating. Int. J. Eat. Disord. 2022, 55, 1291–1295. [Google Scholar] [CrossRef] [PubMed]
- De Amicis, R.; Foppiani, A.; Galasso, L.; Montaruli, A.; Roveda, E.; Esposito, F.; Battezzati, A.; Bertoli, S.; Leone, A. Weight Loss Management and Lifestyle Changes during COVID-19 Lockdown: A Matched Italian Cohort Study. Nutrients 2022, 14, 2897. [Google Scholar] [CrossRef]
- Messika, A.; Toledano, Y.; Hadar, E.; Shmuel, E.; Tauman, R.; Shamir, R.; Froy, O. Relationship among Chrononutrition, Sleep, and Glycemic Control in Women with Gestational Diabetes Mellitus: A Randomized Controlled Trial. Am. J. Obstet. Gynecol. MFM 2022, 4, 100660. [Google Scholar] [CrossRef]
- Verde, L.; Barrea, L.; Docimo, A.; Savastano, S.; Colao, A.; Muscogiuri, G. Chronotype as a Predictor of Weight Loss and Body Composition Improvements in Women with Overweight or Obesity Undergoing a Very Low-Calorie Ketogenic Diet (VLCKD). Clin. Nutr. 2023, 42, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lozano, T.; Vidal, J.; de Hollanda, A.; Canteras, M.; Garaulet, M.; Izquierdo-Pulido, M. Evening Chronotype Associates with Obesity in Severely Obese Subjects: Interaction with CLOCK 3111T/C. Int. J. Obes. 2016, 40, 1550–1557. [Google Scholar] [CrossRef]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef]
- Harrell, F.E. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis; Springer: New York City, NY, USA, 2001; Volume 608. [Google Scholar]

| Women (70%, n = 525) | Men (30%, n = 225) | |||||
|---|---|---|---|---|---|---|
| M-Type | N-Type | E-Type | M-Type | N-Type | E-Type | |
| Age (y) | 51 ± 11 | 49 ± 13 | 47 ± 14 | 52 ± 12 | 45 ± 13 | 46 ± 14 |
| BMI (kg/m2) | 29.6 ± 6.3 | 28.9 ± 6.6 | 28.2 ± 5.9 | 30.6 ± 5.3 | 30.4 ± 5.5 | 30.7 ± 4.4 |
| rMEQ score | 19 ± 1 | 15 ± 2 ° | 10 ± 1 * | 19 ± 1 | 15 ± 2 ° | 10 ± 1 * |
| MEDAS | 7 ± 2 | 7 ± 2 | 7 ± 2 | 7 ± 2 | 7 ± 2 | 6 ± 2 * |
| BES score | 10 ± 7 | 10 ± 8 | 9 ± 6 | 7 ± 5 | 8 ± 6 | 9 ± 6 |
| YFAS score | 2 ± 3 | 2 ± 3 | 2 ± 3 | 2 ± 2 | 2 ± 2 | 2 ± 2 |
| β | 95% CI | p | |
|---|---|---|---|
| Sex | 0.360 | −0.26–0.98 | 0.254 |
| Age | −0.104 | −0.17–−0.04 | <0.001 |
| Smoking | 0.555 | −14.71–25.80 | 0.591 |
| Physical activity | 0.134 | −14.31–16.91 | 0.866 |
| BMI | 0.150 | 0.04–0.26 | 0.008 |
| MEDAS | −0.142 | −0.49–0.20 | 0.420 |
| rMEQ score | 0.125 | −0.09–0.34 | 0.262 |
| Sex × rMEQ | −0.406 | −0.79–−0.02 | 0.037 |
| β | 95% CI | p | |
|---|---|---|---|
| Sex | 0.001 | −0.05–0.05 | 0.970 |
| Age | −0.015 | −0.03–−0.01 | 0.042 |
| Smoking | 0.121 | −0.48–0.72 | 0.691 |
| Physical activity | −0.026 | −0.63–0.68 | 0.933 |
| BMI | 0.113 | 0.09–0.14 | 0.000 |
| MEDAS | −0.124 | −0.23–0.02 | 0.019 |
| rMEQ score | 0.129 | −0.12–0.34 | 0.273 |
| Sex × rMEQ | −0.244 | −0.18–−0.31 | 0.039 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amicis, R.D.; Galasso, L.; Cavallaro, R.; Mambrini, S.P.; Castelli, L.; Montaruli, A.; Roveda, E.; Esposito, F.; Leone, A.; Foppiani, A.; et al. Sex Differences in the Relationship between Chronotype and Eating Behaviour: A Focus on Binge Eating and Food Addiction. Nutrients 2023, 15, 4580. https://doi.org/10.3390/nu15214580
Amicis RD, Galasso L, Cavallaro R, Mambrini SP, Castelli L, Montaruli A, Roveda E, Esposito F, Leone A, Foppiani A, et al. Sex Differences in the Relationship between Chronotype and Eating Behaviour: A Focus on Binge Eating and Food Addiction. Nutrients. 2023; 15(21):4580. https://doi.org/10.3390/nu15214580
Chicago/Turabian StyleAmicis, Ramona De, Letizia Galasso, Riccardo Cavallaro, Sara Paola Mambrini, Lucia Castelli, Angela Montaruli, Eliana Roveda, Fabio Esposito, Alessandro Leone, Andrea Foppiani, and et al. 2023. "Sex Differences in the Relationship between Chronotype and Eating Behaviour: A Focus on Binge Eating and Food Addiction" Nutrients 15, no. 21: 4580. https://doi.org/10.3390/nu15214580
APA StyleAmicis, R. D., Galasso, L., Cavallaro, R., Mambrini, S. P., Castelli, L., Montaruli, A., Roveda, E., Esposito, F., Leone, A., Foppiani, A., Battezzati, A., & Bertoli, S. (2023). Sex Differences in the Relationship between Chronotype and Eating Behaviour: A Focus on Binge Eating and Food Addiction. Nutrients, 15(21), 4580. https://doi.org/10.3390/nu15214580









