The First 1000 Days of Life: How Changes in the Microbiota Can Influence Food Allergy Onset in Children
Abstract
:1. Introduction
2. Methods
3. Epigenetics and the Gut Microbiota
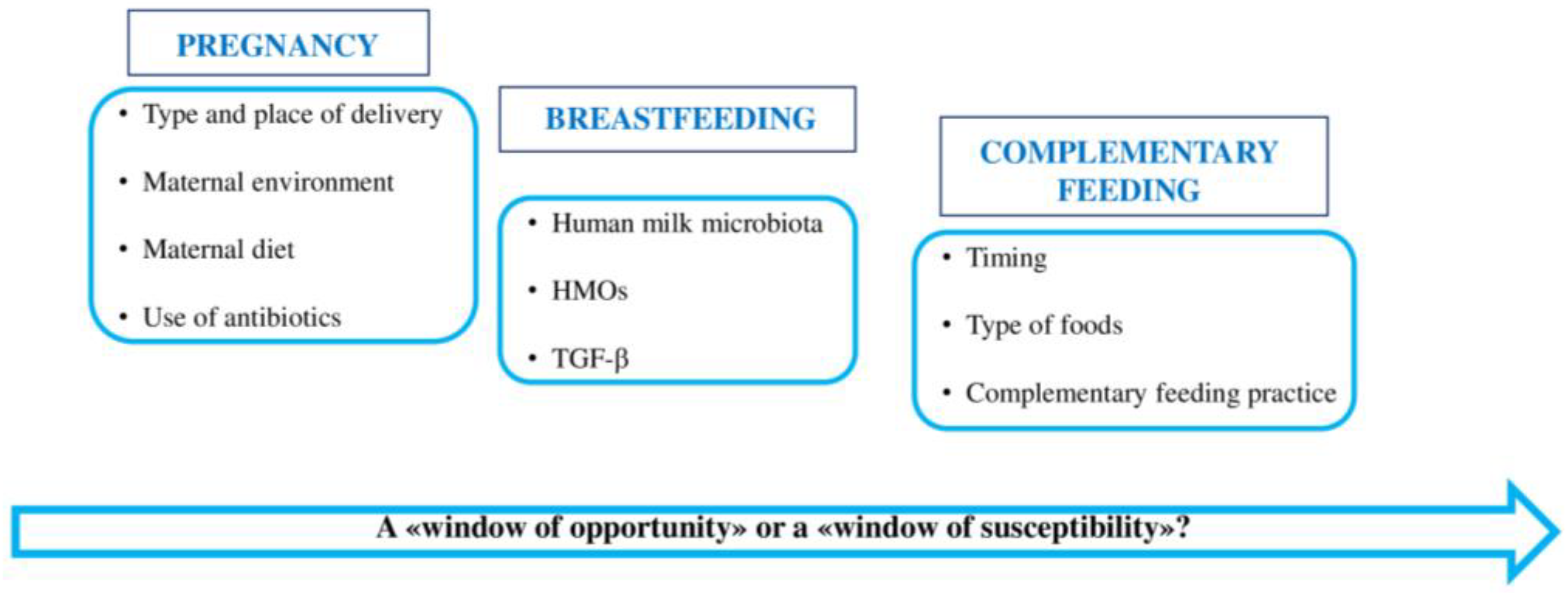
4. First 1000 Days of Life: “A Window of Opportunity” in the Gut Microbiota
4.1. Gut Microbiota of Pregnant Women
4.2. Gut Microbiota in the First Months of Life
4.2.1. Role of Human Milk Microbiota
4.2.2. Role of Human Milk Oligosaccharides (HMOs)
4.3. Gut Microbiota during Complementary Feeding
5. Microbiota Dysbiosis: Mechanisms Associated with FA
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, T.; Dong, Y.; Yang, C.; Zhao, M.; He, Q. Pathogenesis of Children’s Allergic Diseases: Refocusing the Role of the Gut Microbiota. Front. Physiol. 2021, 12, 749544. [Google Scholar] [CrossRef] [PubMed]
- Molloy, J.; Allen, K.; Collier, F.; Tang, M.L.K.; Ward, A.C.; Vuillermin, P. The potential link between gut microbiota and IgE-mediated food allergy in early life. Int. J. Environ. Res. Public Health 2013, 10, 7235–7256. [Google Scholar] [CrossRef] [PubMed]
- Brzozowska, A.; Podlecka, D.; Jankowska, A.; Król, A.; Kaleta, D.; Trafalska, E.; Nowakowska-Swirta, E.; Kaluzny, P.; Hanke, W.; Bal-Gierańczyk, K.; et al. Maternal diet during pregnancy and risk of allergic diseases in children up to 7–9 years old from Polish Mother and Child Cohort study. Environ. Res. 2022, 208, 112682. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.C.; Jackson, C.M.; Ting, T.; Harizaj, A.; Järvinen, K.M. Predictors and biomarkers of food allergy and sensitization in early childhood. Ann. Allergy Asthma Immunol. 2022, 129, 292–300. [Google Scholar] [CrossRef]
- Poole, A.; Song, Y.; Brown, H.; Hart, P.H.; Zhang, G.B. Cellular and molecular mechanisms of vitamin D in food allergy. J. Cell Mol. Med. 2018, 22, 3270–3277. [Google Scholar] [CrossRef]
- Acevedo, N.; Alhamwe, B.A.; Caraballo, L.; Ding, M.; Ferrante, A.; Garn, H.; Garssen, J.; Hii, C.S.; Irvine, J.; Llinás-Caballero, K.; et al. Perinatal and Early-Life Nutrition, Epigenetics, and Allergy. Nutrients 2021, 13, 724. [Google Scholar] [CrossRef]
- Rackaityte, E.; Halkias, J. Mechanisms of Fetal T Cell Tolerance and Immune Regulation. Front. Immunol. 2020, 11, 588. [Google Scholar] [CrossRef]
- Tsabouri, S.; Priftis, K.N.; Chaliasos, N.; Siamopoulou, A. Modulation of gut microbiota downregulates the development of food allergy in infancy. Allergol. Immunopathol. 2014, 42, 69–77. [Google Scholar] [CrossRef]
- Vuillermin, P.J.; O’Hely, M.; Collier, F.; Allen, K.J.; Tang, M.L.K.; Harrison, L.C.; Carlin, J.B.; Saffery, R.; Ranganathan, S.; Sly, P.D.; et al. Maternal carriage of Prevotella during pregnancy associates with protection against food allergy in the offspring. Nat. Commun. 2020, 11, 1452. [Google Scholar] [CrossRef]
- Netting, M.J.; Middleton, P.F.; Makrides, M. Does maternal diet during pregnancy and lactation affect outcomes in offspring? A systematic review of food-based approaches. Nutrition 2014, 30, 1225–1241. [Google Scholar] [CrossRef]
- Gao, Y.; Nanan, R.; Macia, L.; Tan, J.; Sominsky, L.; Quinn, T.P.; O’Hely, M.; Ponsonby, A.; Tang, M.L.K.; Collier, F.; et al. The maternal gut microbiome during pregnancy and offspring allergy and asthma. J. Allergy Clin. Immunol. 2021, 148, 669–678. [Google Scholar] [CrossRef]
- Hirsch, A.G.; Pollak, J.; Glass, T.A.; Poulsen, M.N.; Bailey-Davis, L.; Mowery, J.; Schwartz, B.S. Early Life Antibiotic Use and Subsequent Diagnosis of Food Allergy and Allergic Diseases. Clin. Exp. Allergy 2017, 47, 236–244. [Google Scholar] [CrossRef]
- Ferrante, G.; Carta, M.; Montante, C.; Notarbartolo, V.; Corsello, G.; Giuffrè, M. Current Insights on Early Life Nutrition and Prevention of Allergy. Front. Pediatr. 2020, 8, 448. [Google Scholar] [CrossRef]
- Akkerman, R.; Faas, M.M.; de Vos, P. Non-digestible carbohydrates in infant formula as substitution for human milk oligosaccharide functions: Effects on microbiota and gut maturation. Crit. Rev. Food Sci. Nutr. 2019, 59, 1486–1497. [Google Scholar] [CrossRef]
- Rodríguez, J.M.; Fernández, L.; Verhasselt, V. The Gut-Breast Axis: Programming Health for Life. Nutrients 2021, 13, 606. [Google Scholar] [CrossRef]
- Groer, M.W.; Morgan, K.H.; Louis-Jacques, A.; Miller, E.M. A scoping review of research on the human milk microbiome. J. Hum. Lact. 2020, 36, 628–643. [Google Scholar] [CrossRef]
- Notarbartolo, V.; Giuffrè, M.; Montante, C.; Corsello, G.; Carta, M. Composition of Human Breast Milk Microbiota and Its Role in Children’s Health. Pediatr. Gastroenterol. Hepatol. Nutr. 2022, 25, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Renz, H.; Skevaki, C. Early life microbial exposures and allergy risks: Opportunities for prevention. Nat. Rev. Immunol. 2021, 21, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Gabbianelli, R.; Bordoni, L.; Morano, S.; Calleja-Agius, J.; Lalor, J.G. Nutri-Epigenetics and Gut Microbiota: How Birth Care, Bonding and Breastfeeding Can Influence and Be Influenced? Int. J. Mol. Sci. 2020, 21, 5032. [Google Scholar] [CrossRef] [PubMed]
- Kers, J.G.; Saccenti, E. The Power of Microbiome Studies: Some Considerations on Which Alpha and Beta Metrics to Use and How to Report Results. Front. Microbiol. 2022, 12, 796025. [Google Scholar] [CrossRef] [PubMed]
- Finotello, F.; Mastrorilli, E.; Di Camillo, B. Measuring the diversity of the human microbiota with targeted next-generation sequencing. Brief. Bioinform. 2018, 19, 679–692. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Infuence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Su, Q.; Liu, Q. Factors Affecting Gut Microbiome in Daily Diet. Front. Nutr. 2021, 8, 644138. [Google Scholar] [CrossRef]
- Woo, V.; Alenghat, T. Epigenetic regulation by gut microbiota. Gut Microbes 2022, 14, 2022407. [Google Scholar] [CrossRef]
- Hong, X.; Wang, X. Epigenetics and Development of Food Allergy (FA) in Early Childhood. Curr. Allergy Asthma Rep. 2014, 14, 460. [Google Scholar] [CrossRef] [PubMed]
- Di Costanzo, M.; De Paulis, N.; Capra, M.E.; Biasucci, G. Nutrition during Pregnancy and Lactation: Epigenetic Effects on Infants’ Immune System in Food Allergy. Nutrients 2022, 14, 1766. [Google Scholar] [CrossRef] [PubMed]
- Verduci, E.; Banderali, G.; Barberi, S.; Radaelli, G.; Lops, A.; Betti, F.; Riva, E.; Giovannini, M. Epigenetic Effects of Human Breast Milk. Nutrients 2014, 6, 1711–1724. [Google Scholar] [CrossRef] [PubMed]
- Caesar, R.; Tremaroli, V.; Kovatcheva-Datchary, P.; Cani, P.D.; Bäckhed, F. Crosstalk between gut microbiota and dietary lipids aggravates WAT infammation through TLR signaling. Cell Metab. 2015, 22, 658–668. [Google Scholar] [CrossRef]
- Hartwig, F.P.; Loret de Mola, C.; Davies, N.M.; Victora, C.G.; Relton, C.L. Breastfeeding effects on DNA methylation in the offspring: A systematic literature review. PLoS ONE 2017, 12, e0173070. [Google Scholar] [CrossRef]
- Van Esch, B.C.A.M.; Porbahaie, M.; Abbring, S.; Garssen, J.; Potaczek, D.P.; Savelkoul, H.F.J.; Joost van Neerven, R.J. The Impact of Milk and Its Components on Epigenetic Programming of Immune Function in Early Life and Beyond: Implications for Allergy and Asthma. Front. Immunol. 2020, 11, 2141. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, I.V.; Davidson, E.J.; Joetham, A.; Takeda, K.; O’Connor, B.P.; Gelfand, E.W. Forkhead Box Protein 3 (FoxP3) Demethylation is Associated with Tolerance Induction in Peanut-Induced Intestinal allergy. J. Allergy Clin. Immunol. 2018, 141, 659–670.e2. [Google Scholar] [CrossRef]
- Briollais, L.; Rustand, D.; Allard, C.; Wu, Y.; Xu, J.; Rajan, S.G.; Hivert, M.F.; Doyon, M.; Bouchard, L.; McGowan, P.O.; et al. DNA methylation mediates the association between breastfeeding and early-life growth trajectories. Clin. Epigenet. 2021, 13, 231. [Google Scholar] [CrossRef]
- Barker, D.J.P. Sir Richard Doll Lecture. Developmental origins of chronic disease. Public Health 2012, 126, 185–189. [Google Scholar] [CrossRef]
- Di Costanzo, M.; De Paulis, N.; Biasucci, G. Butyrate: A Link between Early Life Nutrition and Gut Microbiome in the Development of Food Allergy. Life 2021, 11, 384. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Konya, T.; Guttman, D.S.; Fiedl, C.J.; Sears, M.R.; HayGlass, K.T.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; Becker, A.B.; et al. Infant gut microbiota and food sensitization: Associations in the first year of life. Clin. Exp. Allergy 2015, 45, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Roduit, C.; Frei, R.; Ferstl, R.; Loeliger, S.; Westermann, P.; Ehyner, C.; Schiavi, E.; Barcik, W.; Rodriguez-Perez, N.; Wawrzyniak, M.; et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy 2019, 74, 799–809. [Google Scholar] [CrossRef]
- Lee, S.E.; Kim, H. Update on Early Nutrition and Food Allergy in Children. Yonsei Med. J. 2016, 57, 542–548. [Google Scholar] [CrossRef]
- Junge, K.M.; Bauer, T.; Geissler, S.; Hirche, F.; Thürmann, L.; Bauer, M.; Trump, S.; Bieg, M.; Weichenhan, D.; Gu, L.; et al. Increased vitamin D levels at birth and in early infancy increase offspring allergy risk-evidence for involvement of epigenetic mechanisms. J. Allergy Clin. Immunol. 2016, 137, 610–613. [Google Scholar] [CrossRef]
- Weisse, K.; Winkler, S.; Hirche, F.; Herberth, G.; Hinz, D.; Bauer, M.; Röder, S.; Rolle-Kampczyk, U.; von Bergern, M.; Olek, S.; et al. Maternal and newborn vitamin D status and its impact on food allergy development in the German LINA cohort study. Allergy 2013, 68, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Agostoni, C.; Arshad, S.H.; Ben-Abdallah, M.; Du Toit, G.; Fleischer, D.M.; Greenhawt, M.; Glueck, D.H.; Groetch, M.; Lunjani, N.; et al. Dietary factors during pregnancy and atopic outcomes in childhood: A systematic review from the European Academy of Allergy and Clinical Immunology. Pediatr. Allergy Immunol. 2020, 31, 889–912. [Google Scholar] [CrossRef]
- Järvinen, K.M.; Martin, H.; Oyoshi, M.K. Immunomodulatory Effects of Breast Milk on Food Allergy. Ann. Allergy Asthma Immunol. 2019, 123, 133–143. [Google Scholar] [CrossRef]
- Best, K.P.; Gold, M.; Kennedy, D.; Martin, J.; Makrides, M. Omega-3 long-chain PUFA intake during pregnancy and allergic disease outcomes in the offspring: A systematic review and meta-analysis of observational studies and randomized controlled trials. Am. J. Clin. Nutr. 2016, 103, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Brown, K.R.; Maslin, K.; Palmer, D.J. Maternal dietary intake in pregnancy and lactation and allergic disease outcomes in offspring. Pediatr. Allergy Immunol. 2017, 28, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, R.; Li, X.; Gao, Y.; Dai, N.; We, Y.; Liu, L.; Xing, Y.; Li, Z. Relationship between maternal-infant gut microbiota and infant food allergy. Front. Microbiol. 2022, 13, 933152. [Google Scholar] [CrossRef] [PubMed]
- Tuokkola, J.; Luukkainen, P.; Tapanainen, H.; Kaila, M.; Vaarala, O.; Kenward, M.G.; Virta, L.J.; Veijola, R.; Simell, O.; Ilonen, J.; et al. Maternal diet during pregnancy and lactation and cow’s milk allergy in offspring. Eur. J. Clin. Nutr. 2016, 70, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Scientific Opinion on the Evaluation of Allergenic Foods and Food Ingredients for Labelling Purposes. Available online: https://www.efsa.europa.eu/it/efsajournal/pub/3894 (accessed on 19 August 2023).
- Vuillermin, P.J.; Macia, L.; Nanan, R.; Lk Tang, M.; Collier, F.; Brix, S. The maternal microbiome during pregnancy and allergic disease in the offspring. Semin. Immunopathol. 2017, 39, 669–675. [Google Scholar] [CrossRef]
- Venter, C.; Palumbo, M.P.; Glueck, D.H.; Sauder, K.A.; O’Mahony, L.; Fleischer, D.M.; Ben-Abdallah, M.; Ringham, B.M.; Dabelea, D. The maternal diet index in pregnancy is associated with offspring allergic diseases: The Healthy Start study. Allergy 2022, 77, 162–172. [Google Scholar] [CrossRef]
- Asnicar, F.; Berry, S.E.; Valdes, A.M.; Nguyen, L.H.; Piccinno, G.; Drew, D.A.; Leeming, E.; Gibson, R.; Le Roy, C.; Al Khatib, H.; et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat. Med. 2021, 27, 321–332. [Google Scholar] [CrossRef]
- Russell, W.R.; Gratz, S.W.; Duncan, S.H.; Holtrop, G.; Ince, J.; Scobbie, L.; Duncan, G.; Johnstone, A.M.; Lobley, G.E.; Wallace, R.J.; et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profles likely to be detrimental to colonic health. Am. J. Clin. Nutr. 2011, 93, 1062–1072. [Google Scholar] [CrossRef]
- Eid, N.; Enani, S.; Walton, G.; Corona, G.; Costabile, A.; Gibson, G.; Rowland, I.; Spencer, J.P.E. The impact of date palm fruits and their component polyphenols, on gut microbial ecology, bacterial metabolites and colon cancer cell proliferation. J. Nutr. Sci. 2014, 3, e46. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zeevi, D.; Ziberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artifcial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Shen, X.; Wang, M.; Zhang, X.; He, M.; Li, M.; Cheng, G.; Wan, C.; He, F. Dynamic construction of gut microbiota may influence allergic diseases of infants in Southwest China. BMC Microbiol. 2019, 19, 123. [Google Scholar] [CrossRef] [PubMed]
- Méndez, C.S.; Bueno, S.M.; Kalergis, A.M. Contribution of Gut Microbiota to Immune Tolerance in Infants. J. Immunol. Res. 2021, 2021, 7823316. [Google Scholar] [CrossRef]
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Bjorksten, B.; Engstrand, L.; Jenmalm, M.C. Low diversity of the gut microbiota in infants with atopic eczema. J. Allergy Clin. Immunol. 2012, 129, 434–440.e2. [Google Scholar] [CrossRef] [PubMed]
- Penders, J.; Thijs, C.; van den Brandt, P.A.; Kummeling, I.; Snijders, B.; Stelma, F.; Adams, H.; van Ree, R.; Stobberingh, E.E. Gut microbiota composition and development of atopic manifestations in infancy: The KOALA Birth Cohort Study. Gut 2007, 56, 661–667. [Google Scholar] [CrossRef]
- Ling, Z.; Li, Z.; Liu, X.; Cheng, Y.; Luo, Y.; Tong, X.; Yuan, L.; Wang, Y.; Sun, J.; Li, L.; et al. Altered fecal microbiota composition for food allergy in infants. Appl. Environ. Microbiol. 2014, 80, 2546–2554. [Google Scholar] [CrossRef] [PubMed]
- Kielleniva, K.; Ainonen, S.; Vänni, P.; Paalanne, N.; Renko, M.; Salo, J.; Tejesvi, M.V.; Pokka, T.; Pirttilä, A.M.; Tapiainen, T. Microbiota of the first-pass meconium and subsequent atopic and allergic disorders in children. Clin. Exp. Allergy 2022, 52, 684–696. [Google Scholar] [CrossRef]
- Shu, S.A.; Yuen, A.W.T.; Woo, E.; Chu, K.H.; Kwan, H.S.; Yang, G.X.; Yang, Y.; Leung, P.S.C. Microbiota and Food Allergy. Clin. Rev. Allergy Immunol. 2019, 57, 83–97. [Google Scholar] [CrossRef]
- Leyva, L.L.; Brereton, N.J.B.; Koski, K.G. Emerging frontiers in human milk microbiome research and suggested primers for 16S rRNA gene analysis. Comput. Struct. Biotechnol. J. 2020, 19, 121–133. [Google Scholar] [CrossRef]
- Moubareck, C.A. Human Milk Microbiota and Oligosaccharides: A Glimpse into Benefits, Diversity, and Correlations. Nutrients 2021, 13, 1123. [Google Scholar] [CrossRef]
- Demmelmair, H.; Jiménez, E.; Collado, M.C.; Salminen, S.; McGuire, M.K. Maternal and Perinatal Factors Associated with the Human Milk Microbiome. Curr. Dev. Nutr. 2020, 4, nzaa027. [Google Scholar] [CrossRef] [PubMed]
- Verduci, E.; Giannì, M.L.; Vizzari, G.; Vizzuso, S.; Cesarani, J.; Mosca, F.; Zuccotti, G.V. The Triad Mother-Breast Milk-Infant as Predictor of Future Health: A Narrative Review. Nutrients 2021, 13, 486. [Google Scholar] [CrossRef]
- Wang, S.; Wei, Y.; Liu, L.; Li, Z. Association Between Breastmilk Microbiota and Food Allergy in Infants. Front. Cell Infect. Microbiol. 2022, 11, 770913. [Google Scholar] [CrossRef]
- Rey-Mariňo, A.; Pilar Francino, M. Nutrition, Gut Microbiota, and Allergy Development in Infants. Nutrients 2022, 14, 4316. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.L.Y.; Petersen, C.; Hoskinson, C.; Del Bel, K.L.; Becker, A.B.; Moraes, T.J.; Mandhane, P.J.; Finlay, B.B.; Simons, E.; Kozyrskyj, A.L.; et al. Breastfeeding enrichment of B. longum subsp. infantis mitigates the effect of antibiotics on the microbiota and childhood asthma risk. Med 2023, 4, 92–112.e5. [Google Scholar] [CrossRef]
- Charbonneau, M.R.; O’Donnell, D.; Blanton, L.V.; Totten, S.M.; Davis, J.C.C.; Barratt, M.J.; Cheng, J.; Guruge, J.; Talcott, M.; Bain, J.R.; et al. Sialylated Milk Oligosaccharides Promote Microbiota-Dependent Growth in Models of Infant Undernutrion. Cell 2016, 164, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.Y.; Chan, Y.L.; Tsai, Y.S.; Chen, S.A.; Wang, C.J.; Chen, K.F.; Chung, I.F. Airway Microbial Diversity is Inversely Associated with Mite-Sensitized Rhinitis and Asthma in Early Childhood. Sci. Rep. 2017, 7, 1820. [Google Scholar] [CrossRef]
- Yokanovich, L.T.; Newberry, R.D.; Knoop, K.A. Regulation of oral antigen delivery early in life: Implications for oral tolerance and food allergy. Clin. Exp. Allergy 2021, 51, 518–526. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakayama, J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol. Int. 2017, 66, 515–522. [Google Scholar] [CrossRef]
- Laursen, M.F. Gut Microbiota Development: Influence of Diet from Infancy to Toddlerhood. Ann. Nutr. Metab. 2021, 30, 1–14. [Google Scholar] [CrossRef]
- Di Profio, E.; Magenes, V.C.; Fiore, G.; Agostinelli, M.; La Mendola, A.; Acunzo, M.; Francavilla, R.; Indrio, F.; Bosetti, A.; D’Auria, E.; et al. Special Diets in Infants and Children and Impact on Gut Microbioma. Nutrients 2022, 14, 3198. [Google Scholar] [CrossRef]
- Differding, M.K.; Benjamin-Neelon, S.E.; Hoyo, C.; Østbye, T.; Mueller, N.T. Timing of complementary feeding is associated with gut microbiota diversity and composition and short chain fatty acid concentrations over the first year of life. BMC Microbiol. 2020, 20, 56. [Google Scholar] [CrossRef] [PubMed]
- De la Cuesta-Zuluaga, J.; Mueller, N.T.; Alvarez-Quintero, R.; Velasquez-Mejia, E.P.; Sierra, J.A.; Corrales-Agudelo, V.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Higher Fecal Short-Chain Fatty Acid Levels Are Associated with Gut Microbiome Dysbiosis, Obesity, Hypertension and Cardiometabolic Disease Risk Factors. Nutrients 2018, 11, 51. [Google Scholar] [CrossRef]
- Kim, K.N.; Yao, Y.; Ju, S.Y. Short Chain Fatty Acids and Fecal Microbiota Abundance in Humans with Obesity: A Systematic Review and MetaAnalysis. Nutrients 2019, 11, 2512. [Google Scholar] [CrossRef] [PubMed]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr. 2017, 171, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.L.; Monteagudo-Mera, A.; Cadenas, M.B.; Lampl, M.L.; Azcarate-Peril, M.A. Milk- and solid-feeding practices and daycare attendance are associated with differences in bacterial diversity, predominant communities, and metabolic and immune function of the infant gut microbiome. Front. Cell Infect. Microbiol. 2015, 5, 3. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Knusten, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Appropriate age range for introduction of complementary feeding into an infant’s diet. EFSA J. 2019, 17, e05780. [Google Scholar] [CrossRef]
- Comberiati, P.; Costagliola, G.; D’Elios, S.; Peroni, D. Prevention of Food Allergy: The Significance of Early Introduction. Medicina 2019, 55, 323. [Google Scholar] [CrossRef]
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Bahnson, H.T.; Radulovic, S.; Santos, A.F.; Brough, H.A.; Phippard, D.; Basting, M.; Feeney, M.; et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N. Engl. J. Med. 2015, 372, 803–813. [Google Scholar] [CrossRef]
- Soriano, V.X.; Peters, R.L.; Moreno-Betancur, M.; Ponsonby, A.L.; Gell, G.; Odoi, A.; Perrett, K.P.; Tang, M.L.K.; Gurrin, L.C.; Allen, K.J.; et al. Association Between Earlier Introduction of Peanut and Prevalence of Peanut Allergy in Infants in Australia. JAMA 2022, 328, 48–56. [Google Scholar] [CrossRef]
- Grimshaw, K.E.C.; Maskell, J.; Oliver, E.M.; Morris, R.C.G.; Foote, K.D.; Clare Mills, E.N.; Roberts, G.; Margetts, B.M. Introduction of complementary foods and the relationship to food allergy. Pediatrics 2013, 132, e1529–e1538. [Google Scholar] [CrossRef] [PubMed]
- Leong, C.; Haszard, J.J.; Lawley, B.; Otal, A.; Taylor, R.W.; Szymlek-Gay, E.A.; Fleming, E.A.; Daniels, L.; Fangupo, L.J.; Tannock, G.W.; et al. Mediation Analysis as a Means of Identifying Dietary Components That Differentially Affect the Fecal Microbiota of Infants Weaned by Modified Baby-Led and Traditional Approaches. Appl. Environ. Microbiol. 2018, 84, e00914-18. [Google Scholar] [CrossRef] [PubMed]
- Fazlollahi, M.; Chun, Y.; Grishin, A.; Wood, R.A.; Burks, A.W.; Dawson, P.; Jones, S.M.; Leung, D.Y.M.; Sampson, H.A.; Sicherer, S.H.; et al. Early-life gut microbiome and egg allergy. Allergy 2018, 73, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Augustine, T.; Kumar, M.; Al Khodor, S.; van Panhuys, N. Microbial Dysbiosis Tunes the Immune Response Towards Allergic Disease Outcomes. Clin. Rev. Allergy Immunol. 2023, 65, 43–71. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, L.; Wang, J.; Hao, M.; Che, H. Antibiotic-Induced Gut Microbiota Dysbiosis Damages the Intestinal Barrier, Increasing Food Allergy in Adult Mice. Nutrients 2021, 13, 3315. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, X.; Chen, F.; Rolnik, B.M.; Chleilat, F.; Ling, Z.; Snyder, M.P.; Zhou, X. The Roles and Mechanisms of Gut Microbiota in Food Allergy. Adv. Gut Microbiome Res. 2023, 2023, 9575410. [Google Scholar] [CrossRef]
- American Academy of Allergy, Asthma & Immunology. Available online: https://www.aaaai.org (accessed on 19 August 2023).
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- Fava, F.; Gitau, R.; Griffin, B.A.; Gibson, G.R.; Tuohy, K.M.; Lovegrove, J.A. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome “at-risk” population. Int. J. Obes. 2013, 37, 216–223. [Google Scholar] [CrossRef]
- Nance, C.L.; Deniskin, R.; Diaz, V.C.; Paul, M.; Anvari, S.; Anagnostou, A. The Role of the Microbiome in Food Allergy: A Review. Children 2020, 7, 50. [Google Scholar] [CrossRef] [PubMed]
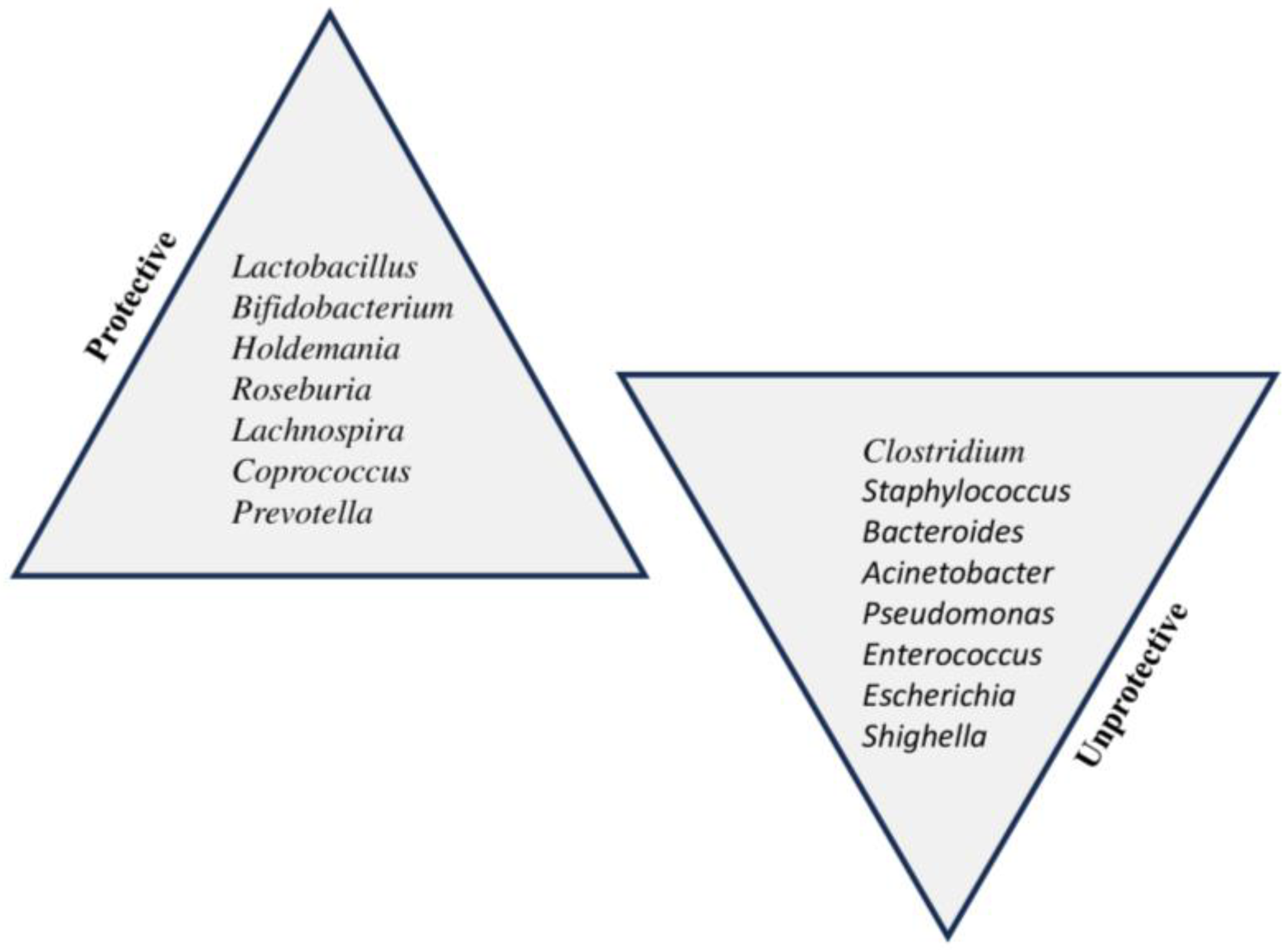
| Phylum | Family | Genus |
|---|---|---|
| Firmicutes [44,54,70] | Lactobacillaceae | Lactobacillus [8,19,24,41,54,69] |
| Clostridiaceae | Clostridium [8,14,26,35,65,70] Anaerobacter [57,65] | |
| Staphylococcaceae | Staphylococcus [8,19,61,62] | |
| Lachnospiraceae | Roseburia [11] Lachnospira [11,54,71,83] Coprococcus [11] | |
| Streptococcaceae | Streptococcus [61,62] | |
| Actinomycetota | Propionibacteriaceae | Propionibacterium [19,61,62] |
| Bacteroidota [54,70] | Bacteroidaceae | Bacteroides [26,34,41,57,77] |
| Prevotellaceae | Prevotella [11,19,44,47] | |
| Actinobacteria [44] | Bifidobacteriaceae | Bifidobacterium [2,4,8,14,24,41,54,57,66,67,71,77] |
| Corynebacteriaceae | Corynebacterium [61,62] | |
| Bacillota | Erysipelotrichaceae | Holdemania [11,44] |
| Enterococcaceae [54] | Enterococcus [26,41] | |
| Ruminococcaceae [71] | - | |
| Pseudomonadota | Sphyngomonadaceae | Sphyngomonas [61,62] |
| Nitrobacteraceae | Bradyrhizobium [61,62] | |
| Proteobacteria [35,44,57] | Enterobacteriaceae [35,41,54] | Escherichia, Shighella [Azad] |
| Yersiniaceae | Serratia [61,62] | |
| Pseudomonadaceae [44,61,62] | Pseudomonas [61,62] | |
| Ralstoniaceae | Ralstonia [61,62] | |
| Moraxellaceae | Acinetobacter [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Notarbartolo, V.; Carta, M.; Accomando, S.; Giuffrè, M. The First 1000 Days of Life: How Changes in the Microbiota Can Influence Food Allergy Onset in Children. Nutrients 2023, 15, 4014. https://doi.org/10.3390/nu15184014
Notarbartolo V, Carta M, Accomando S, Giuffrè M. The First 1000 Days of Life: How Changes in the Microbiota Can Influence Food Allergy Onset in Children. Nutrients. 2023; 15(18):4014. https://doi.org/10.3390/nu15184014
Chicago/Turabian StyleNotarbartolo, Veronica, Maurizio Carta, Salvatore Accomando, and Mario Giuffrè. 2023. "The First 1000 Days of Life: How Changes in the Microbiota Can Influence Food Allergy Onset in Children" Nutrients 15, no. 18: 4014. https://doi.org/10.3390/nu15184014
APA StyleNotarbartolo, V., Carta, M., Accomando, S., & Giuffrè, M. (2023). The First 1000 Days of Life: How Changes in the Microbiota Can Influence Food Allergy Onset in Children. Nutrients, 15(18), 4014. https://doi.org/10.3390/nu15184014





