Evaluation of Serum Diamine Oxidase as a Diagnostic Test for Histamine Intolerance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Determination of Serum DAO Concentration
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population and Serum DAO Levels
3.2. Serum Level of DAO
3.3. Validity of DAO Determination for Diagnosis of HIT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Comas-Basté, O.; Sánchez-Pérez, S.; Veciana-Nogués, M.T.; Latorre-Moratalla, M.; Vidal-Carou, M.D.C. Histamine Intolerance: The Current State of the Art. Biomolecules 2020, 10, 1181. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, S.; Jutel, M.; Crameri, R.; O’Mahony, L. Histamine and gut mucosal immune regulation. Allergy 2014, 69, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Ezema, J.N.; Agbo, E.C.; Eye, E.A. Microbial production of histamine and the imperatives of processed food consumption. Bio-Research 2021, 19, 1317–1327. [Google Scholar] [CrossRef]
- Wüthrich, B. Allergic and intolerance reactions to wine. Allergol. Select 2018, 2, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Wolvekamp, M.C.; de Bruin, R.W. Diamine oxidase: An overview of historical, biochemical and functional aspects. Dig. Dis. 1994, 12, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Manzotti, G.; Breda, D.; Di Gioacchino, M.; Burastero, S.E. Serum diamine oxidase activity in patients with histamine intolerance. Int. J. Immunopathol. Pharmacol. 2016, 29, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Feillet-Coudray, C.; Coudray, C.; Bayle, D.; Rock, E.; Rayssiguier, Y.; Mazur, A. Response of diamine oxidase and other plasma copper biomarkers to various dietary copper intakes in the rat and evaluation of copper absorption with a stable isotope. Br. J. Nutr. 2000, 83, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Hrubisko, M.; Danis, R.; Huorka, M.; Wawruch, M. Histamine Intolerance-The More We Know the Less We Know. A Review. Nutrients 2021, 13, 2228. [Google Scholar] [CrossRef] [PubMed]
- Durak-Dados, A.; Michalski, M.; Osek, J. Histamine and Other Biogenic Amines in Food. J. Vet. Res. 2020, 64, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.M. Severe histamine mediated reactions to intravenous drugs used in anaesthesia. Anaesth. Intensive Care 1975, 3, 180–197. [Google Scholar] [CrossRef] [PubMed]
- Leitner, R.; Zoernpfenning, E.; Missbichler, A. Evaluation of the inhibitory effect of various drugs / active ingredients on the activity of human diamine oxidase in vitro. Clin. Transl. Allergy 2014, 4 (Suppl. 3), P23. [Google Scholar] [CrossRef]
- Mušič, E.; Korošec, P.; Šilar, M.; Adamič, K.; Košnik, M.; Rijavec, M. Serum diamine oxidase activity as a diagnostic test for histamine intolerance. Wien. Klin. Wochenschr. 2013, 125, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pérez, S.; Comas-Basté, O.; Rabell-González, J.; Veciana-Nogués, M.T.; Latorre-Moratalla, M.L.; Vidal-Carou, M.C. Biogenic Amines in Plant-Origin Foods: Are They Frequently Underestimated in Low-Histamine Diets? Foods 2018, 7, 205. [Google Scholar] [CrossRef] [PubMed]
- Comas-Basté, O.; Sánchez-Pérez, S.; Veciana-Nogués, M.T.; Latorre-Moratalla, M.; Vidal-Carou, M.C. Concept, Etiology and Current Diagnostic and Treatment Approaches of Histamine Intolerance: A Review. In Prime Archives in Nutrition; Zepeda-Carrillo, E.A., Ed.; Vide Leaf: Hyderabad, India, 2021; pp. 1–49. [Google Scholar]
- Schnedl, W.J.; Enko, D. Considering histamine in functional gastrointestinal disorders. Crit. Rev. Food Sci. Nutr. 2021, 61, 2960–2967. [Google Scholar] [CrossRef] [PubMed]
- Cucca, V.; Ramirez, G.A.; Pignatti, P.; Asperti, C.; Russo, M.; Della-Torre, E.; Breda, D.; Burastero, S.E.; Lorenzo Dagna, L.; Yacoub, M.-R. Basal Serum Diamine Oxidase Levels as a Biomarker of Histamine Intolerance: A Retrospective Cohort Study. Nutrients 2022, 14, 1513. [Google Scholar] [CrossRef] [PubMed]
- van Odijk, J.; Weisheit, A.; Arvidsson, M.; Miron, N.; Nwaru, B.; Ekerljung, L. The Use of DAO as a Marker for Histamine Intolerance: Measurements and Determinants in a Large Random Population-Based Survey. Nutrients 2023, 15, 2887. [Google Scholar] [CrossRef] [PubMed]
- Töndury, B.; Wüthrich, B.; Schmid-Grendelmeier, P.; Seifert, B.; Ballmer-Weber, B. Histamine intolerance: Is the determination of diamine oxidase activity in the serum useful in routine clinical practice? Allergologie 2008, 31, 350–356. [Google Scholar] [CrossRef]
- Kofler, H.; Aberer, W.; Deibl, M.; Hawranek, T.; Klein, G.; Reider, N.; Fellner, N. Diamine oxidase (DAO) serum activity: Not a useful marker for diagnosis of histamine intolerance. Allergologie 2009, 32, 105. [Google Scholar] [CrossRef]
- Schnoor, H.S.J.; Mosbech, H.; Skov, P.S.; Poulsen, L.K.; Jensen, B.M. Diamine oxidase determination in serum. Allergo J. 2013, 22, 108–111. [Google Scholar] [CrossRef]
- Pinzer, T.C.; Tietz, E.; Waldmann, E.; Schink, M.; Neurath, M.F.; Zopf, Y. Circadian profiling reveals higher histamine plasma levels and lower diamine oxidase serum activities in 24% of patients with suspected histamine intolerance compared to food allergy and controls. Allergy 2018, 73, 949–957. [Google Scholar] [CrossRef] [PubMed]
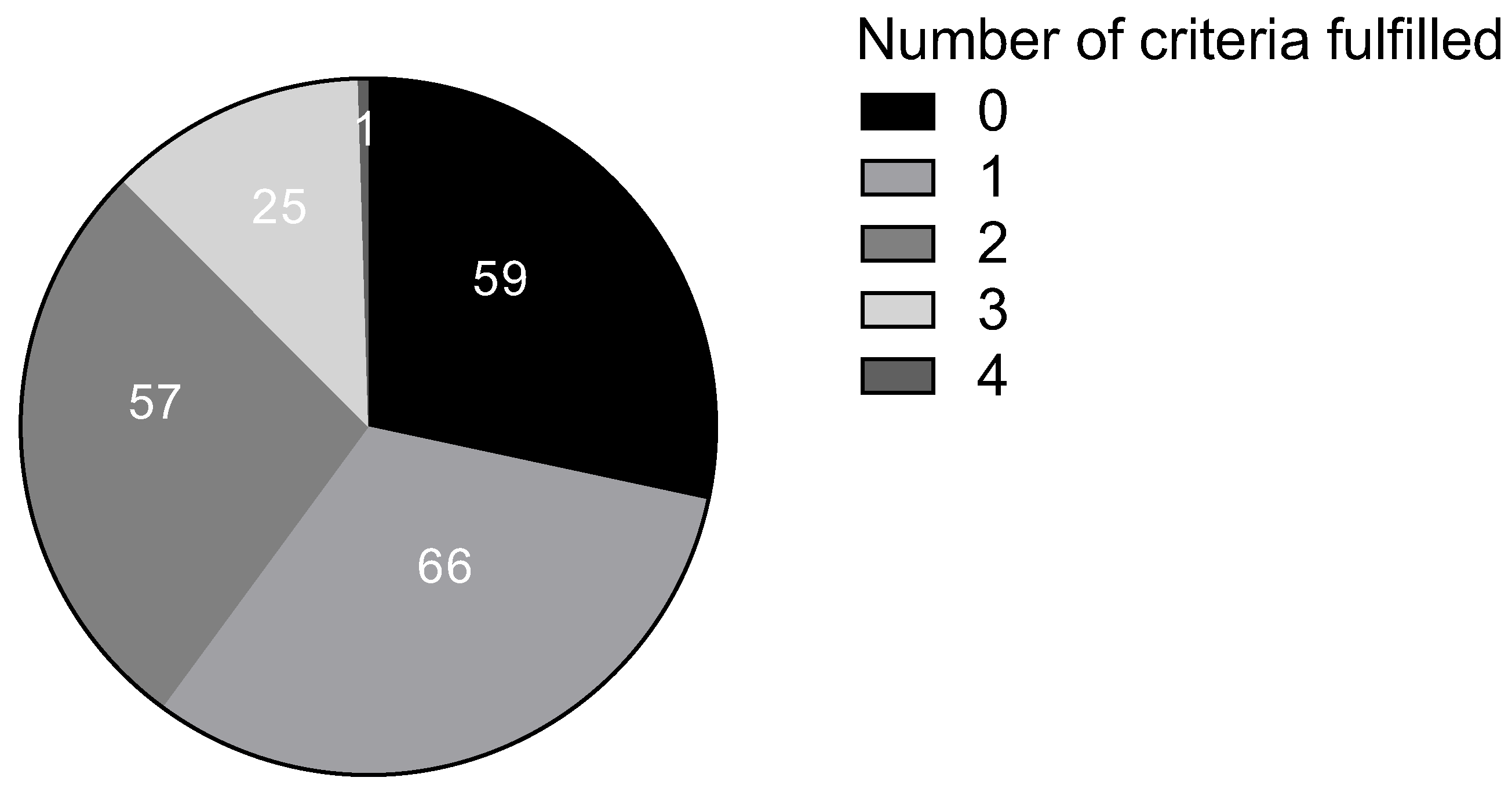
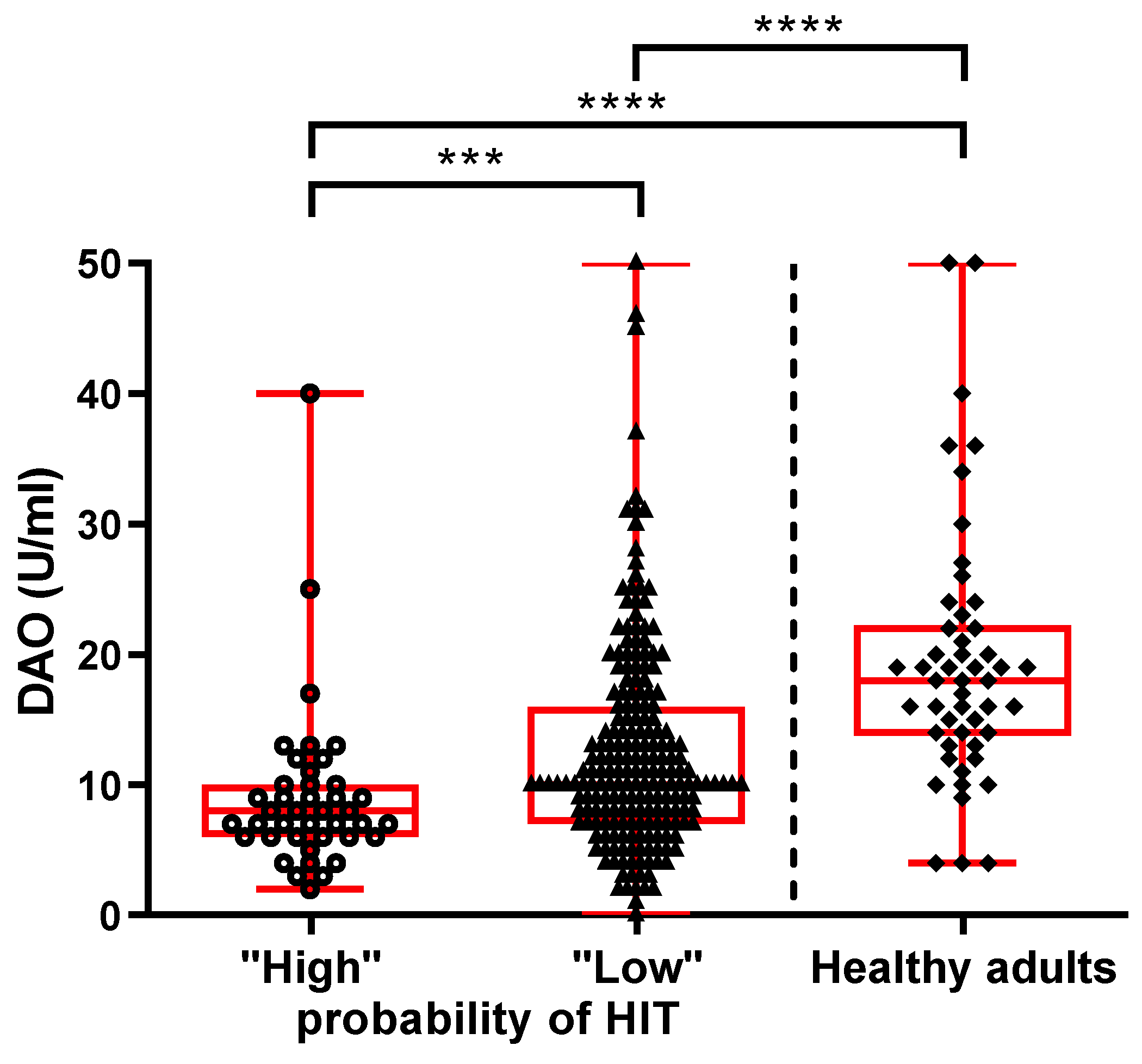

| All Patients (N = 249) | ‘‘High Probability of HIT’’ (N = 41) | ‘‘Low Probability of HIT’’ (N = 208) | Healthy Adults (N = 50) | p Value | |
|---|---|---|---|---|---|
| Age (years): median (IQR) | 47 (38–61) | 51 (42–66) | 47 (37–60) | 44 (36–55) | 0.427 a |
| Women: N (%) | 181 (73) | 33 (81) | 148 (71) | 37 (74) | 0.672 b |
| Men: N (%) | 68 (27) | 8 (19) | 60 (29) | 13 (26) | |
| DAO (U/mL): median (IQR) | 10 (7–14) | 8 (6–10) | 10 (7– 16) | 18 (14–22) | <0.0001 a |
| Criterion | Number (%) of “Low Probability Patients” |
|---|---|
| Typical clinical manifestation | 118 (57) |
| The appearance of symptoms after consumption of biogenic amine-rich food | 30 (14) |
| The appearance of symptoms within 2 h to 1 day | 44 (21) |
| Improvement of symptoms while following a low-biogenic amine diet | 1 (0.5) |
| Improvement of symptoms through treatment (with antihistamines or exogenous DAO supplementation) | 64 (31) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arih, K.; Đorđević, N.; Košnik, M.; Rijavec, M. Evaluation of Serum Diamine Oxidase as a Diagnostic Test for Histamine Intolerance. Nutrients 2023, 15, 4246. https://doi.org/10.3390/nu15194246
Arih K, Đorđević N, Košnik M, Rijavec M. Evaluation of Serum Diamine Oxidase as a Diagnostic Test for Histamine Intolerance. Nutrients. 2023; 15(19):4246. https://doi.org/10.3390/nu15194246
Chicago/Turabian StyleArih, Kristina, Nina Đorđević, Mitja Košnik, and Matija Rijavec. 2023. "Evaluation of Serum Diamine Oxidase as a Diagnostic Test for Histamine Intolerance" Nutrients 15, no. 19: 4246. https://doi.org/10.3390/nu15194246
APA StyleArih, K., Đorđević, N., Košnik, M., & Rijavec, M. (2023). Evaluation of Serum Diamine Oxidase as a Diagnostic Test for Histamine Intolerance. Nutrients, 15(19), 4246. https://doi.org/10.3390/nu15194246






