The Long-Term Effect of Maternal Iron Levels in the Second Trimester on Mild Thinness among Preschoolers: The Modifying Effect of Small for Gestational Age
Abstract
1. Introduction
2. Methods
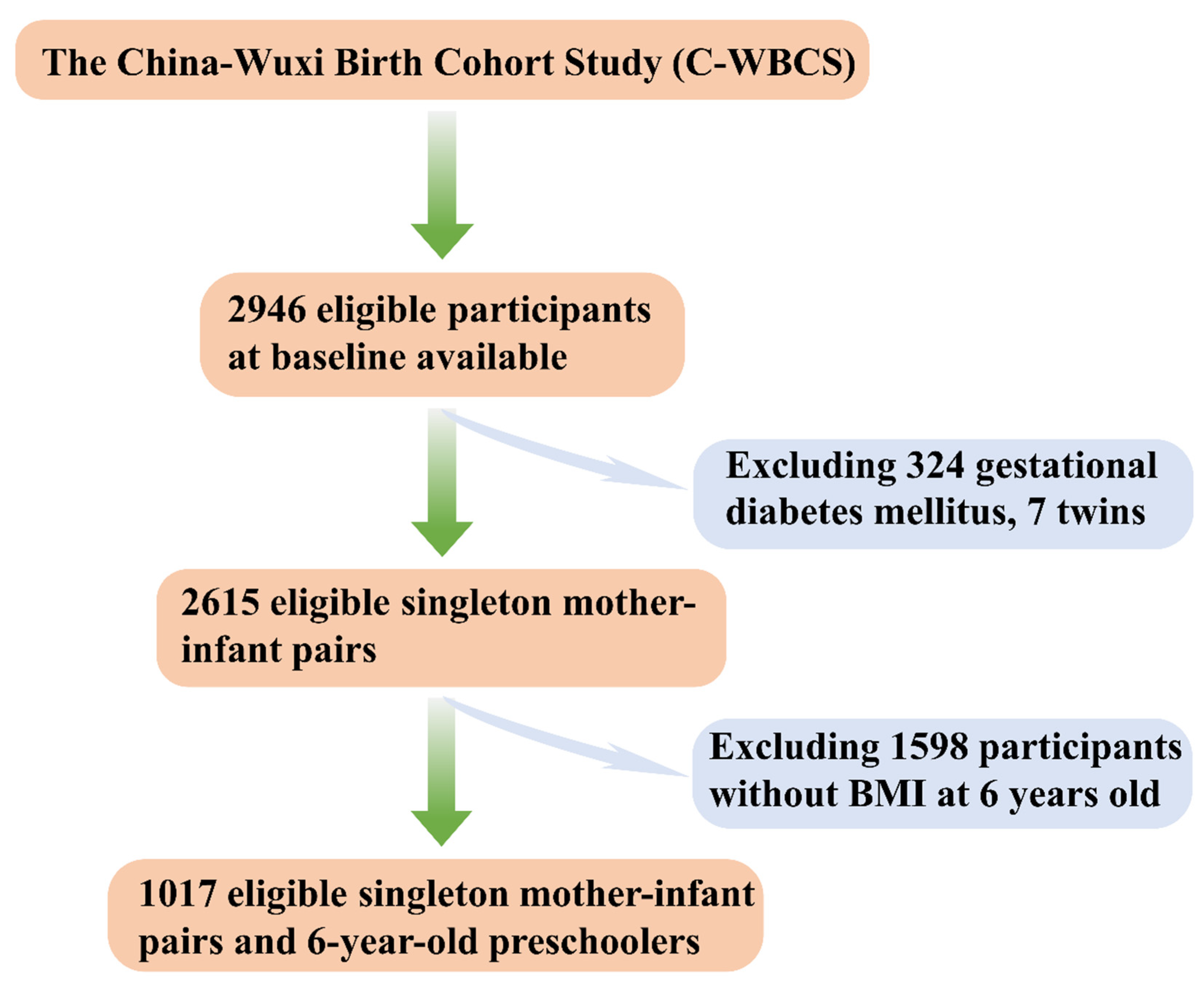
2.1. The Background of the Cohort
2.2. Participants
2.3. Measurement of Five Essential Metal Elements Concentrations
2.4. Definitions of SGA and LBW
2.5. Definitions of Mild Thinness of Preschoolers
2.6. Definitions of Gestational Diabetes Mellitus
2.7. Statistical Analysis
3. Results
3.1. Maternal Basic Characteristics and Neonatal Birth Outcomes
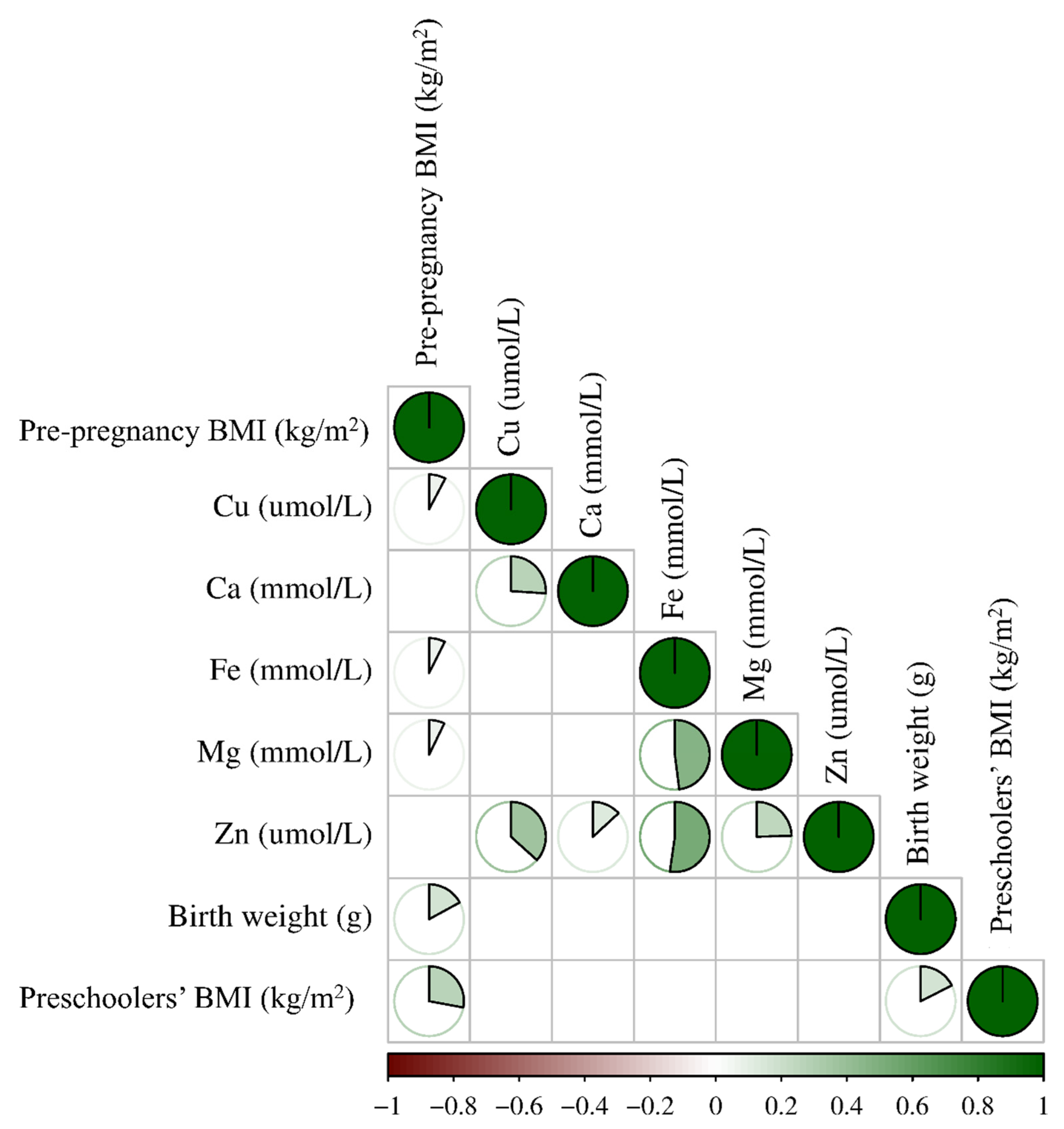
3.2. Association of Five Maternal Essential Metal Elements Levels at the Second Trimester with the Mild Thinness of 6-Year-Old Preschoolers
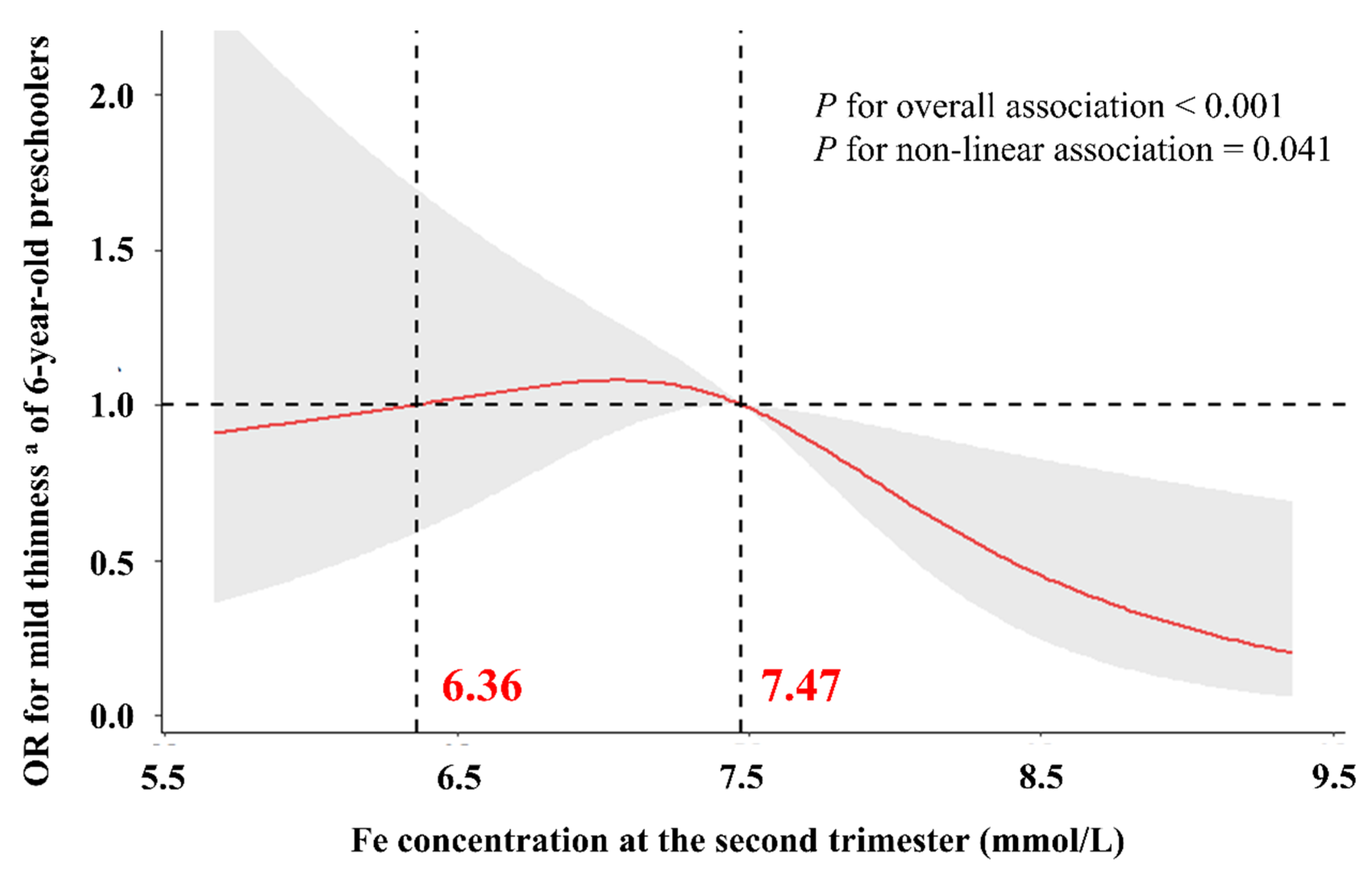
3.3. Dose–Response Relationship between Maternal Iron Concentrations in the Second Trimester and Mild Thinness of 6-Year-Old Preschoolers
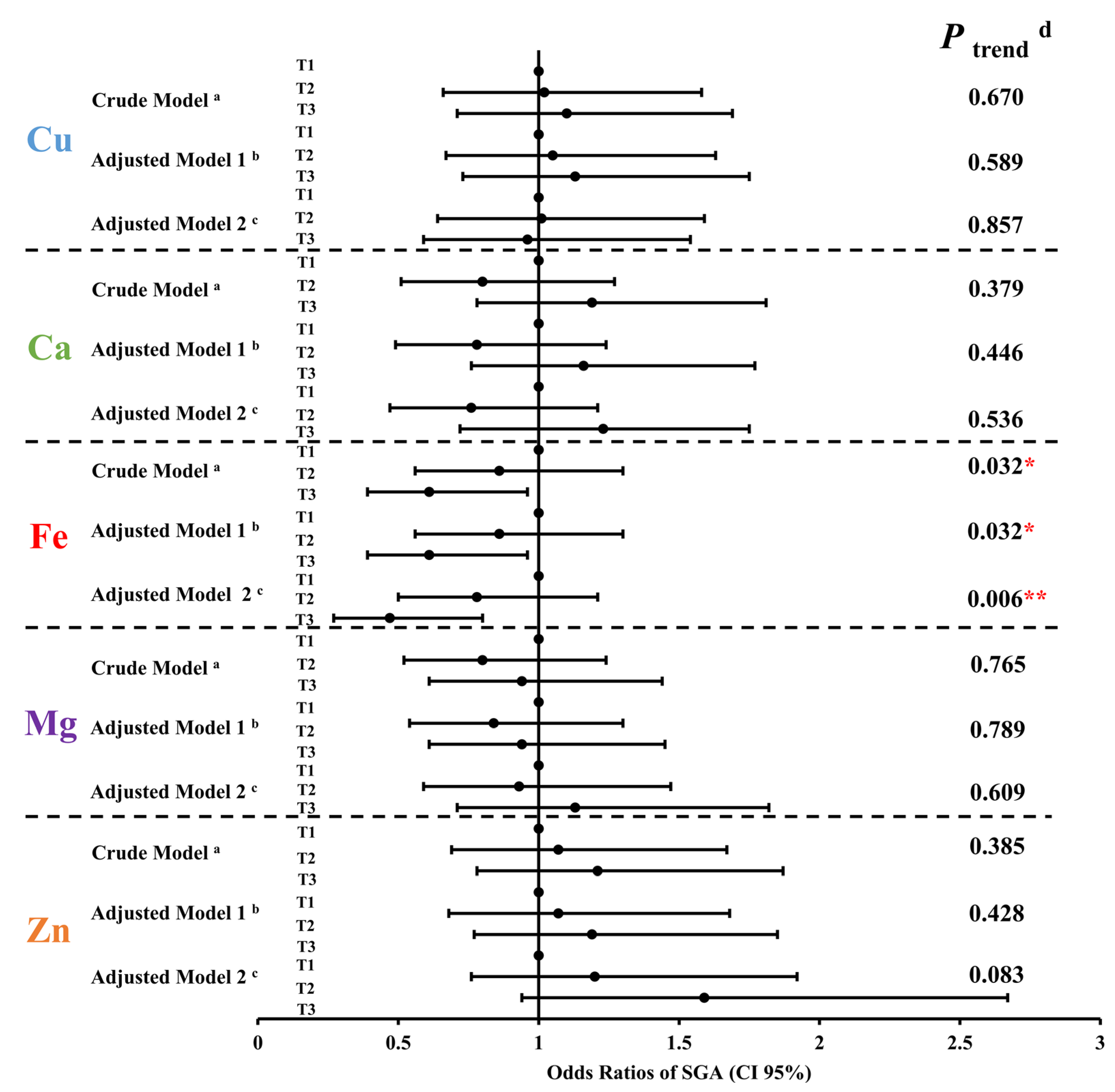
3.4. Relationship between Five Maternal Essential Metal Elements Levels at the Second Trimester and Small for Gestational Age
3.5. Association between Small for Gestational Age and Mild Thinness of 6-Year-Old Preschoolers
3.6. Subgroup and Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arigliani, M.; Canciani, M.C.; Mottini, G.; Altomare, M.; Magnolato, A.; Loa Clemente, S.V.; Tshilolo, L.; Cogo, P.; Quanjer, P.H. Evaluation of the Global Lung Initiative 2012 Reference Values for Spirometry in African Children. Am. J. Respir. Crit. Care Med. 2017, 195, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Flegal, K.M.; Nicholls, D.; Jackson, A.A. Body mass index cut offs to define thinness in children and adolescents: International survey. BMJ 2007, 335, 194. [Google Scholar] [CrossRef] [PubMed]
- Schoonees, A.; Lombard, M.J.; Musekiwa, A.; Nel, E.; Volmink, J. Ready-to-use therapeutic food (RUTF) for home-based nutritional rehabilitation of severe acute malnutrition in children from six months to five years of age. Cochrane Database Syst. Rev. 2019, 5, CD009000. [Google Scholar] [CrossRef] [PubMed]
- Mak, T.-N.; Angeles-Agdeppa, I.; Lenighan, Y.M.; Capanzana, M.V.; Montoliu, I. Diet Diversity and Micronutrient Adequacy among Filipino School-Age Children. Nutrients 2019, 11, 2197. [Google Scholar] [CrossRef]
- Stevens, G.A.; Finucane, M.M.; Paciorek, C.J.; Flaxman, S.R.; White, R.A.; Donner, A.J.; Ezzati, M. Trends in mild, moderate, and severe stunting and underweight, and progress towards MDG 1 in 141 developing countries: A systematic analysis of population representative data. Lancet 2012, 380, 824–834. [Google Scholar] [CrossRef] [PubMed]
- McGuire, S. World Health Organization. Comprehensive Implementation Plan on Maternal, Infant, and Young Child Nutrition. Geneva, Switzerland, 2014. Adv. Nutr. 2015, 6, 134–135. [Google Scholar] [CrossRef]
- Murray, C.J.L. Shifting to Sustainable Development Goals--Implications for Global Health. N. Engl. J. Med. 2015, 373, 1390–1393. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Griggs, D.; Visbeck, M. Policy: Map the interactions between Sustainable Development Goals. Nature 2016, 534, 320–322. [Google Scholar] [CrossRef]
- Collaborators, G.S. Measuring progress and projecting attainment on the basis of past trends of the health-related Sustainable Development Goals in 188 countries: An analysis from the Global Burden of Disease Study 2016. Lancet 2017, 390, 1423–1459. [Google Scholar]
- Viteri, F.E.; Gonzalez, H. Adverse outcomes of poor micronutrient status in childhood and adolescence. Nutr. Rev. 2002, 60, S77–S83. [Google Scholar] [CrossRef]
- Kapil, U.; Bhavna, A. Adverse effects of poor micronutrient status during childhood and adolescence. Nutr. Rev. 2002, 60, S84–S90. [Google Scholar] [CrossRef]
- Alderman, H.; Fernald, L. The Nexus Between Nutrition and Early Childhood Development. Annu. Rev. Nutr. 2017, 37, 447–476. [Google Scholar] [CrossRef]
- van der Kam, S.; Roll, S.; Swarthout, T.; Edyegu-Otelu, G.; Matsumoto, A.; Kasujja, F.X.; Casademont, C.; Shanks, L.; Salse-Ubach, N. Effect of Short-Term Supplementation with Ready-to-Use Therapeutic Food or Micronutrients for Children after Illness for Prevention of Malnutrition: A Randomised Controlled Trial in Uganda. PLoS Med. 2016, 13, e1001951. [Google Scholar]
- van der Kam, S.; Salse-Ubach, N.; Roll, S.; Swarthout, T.; Gayton-Toyoshima, S.; Jiya, N.M.; Matsumoto, A.; Shanks, L. Effect of Short-Term Supplementation with Ready-to-Use Therapeutic Food or Micronutrients for Children after Illness for Prevention of Malnutrition: A Randomised Controlled Trial in Nigeria. PLoS Med. 2016, 13, e1001952. [Google Scholar]
- Norris, S.A.; Frongillo, E.A.; Black, M.M.; Dong, Y.; Fall, C.; Lampl, M.; Liese, A.D.; Naguib, M.; Prentice, A.; Rochat, T.; et al. Nutrition in adolescent growth and development. Lancet 2022, 399, 172–184. [Google Scholar] [CrossRef] [PubMed]
- McArdle, H.J.; Ashworth, C.J. Micronutrients in fetal growth and development. Br. Med. Bull. 1999, 55, 499–510. [Google Scholar] [CrossRef]
- Hsu, C.-N.; Tain, Y.-L. The Good, the Bad, and the Ugly of Pregnancy Nutrients and Developmental Programming of Adult Disease. Nutrients 2019, 11, 894. [Google Scholar] [CrossRef] [PubMed]
- Gillman, M.W. Developmental origins of health and disease. N. Engl. J. Med. 2005, 353, 1848–1850. [Google Scholar] [CrossRef]
- Belizán, J.M.; Villar, J.; Bergel, E.; del Pino, A.; Di Fulvio, S.; Galliano, S.V.; Kattan, C. Long-term effect of calcium supplementation during pregnancy on the blood pressure of offspring: Follow up of a randomised controlled trial. BMJ 1997, 315, 281–285. [Google Scholar] [CrossRef]
- Skogheim, T.S.; Weyde, K.V.F.; Engel, S.M.; Aase, H.; Surén, P.; Øie, M.G.; Biele, G.; Reichborn-Kjennerud, T.; Caspersen, I.H.; Hornig, M.; et al. Metal and essential element concentrations during pregnancy and associations with autism spectrum disorder and attention-deficit/hyperactivity disorder in children. Environ. Int. 2021, 152, 106468. [Google Scholar] [CrossRef]
- Tsai, T.-L.; Hsieh, C.-J.; Wu, M.-T.; Chen, M.-L.; Kuo, P.-H.; Wang, S.-L. Co-exposure to toxic metals and phthalates in pregnant women and their children’s mental health problems aged four years-Taiwan Maternal and Infant Cohort Study (TMICS). Environ. Int. 2023, 173, 107804. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.; Shi, D.; Xu, W.; Zhu, L.; Hao, X.; Zhu, B.; Shu, Q.; Lozoff, B.; Geng, F.; Shao, J. Differentiation between fetal and postnatal iron deficiency in altering brain substrates of cognitive control in pre-adolescence. BMC Med. 2023, 21, 167. [Google Scholar] [CrossRef] [PubMed]
- Terstappen, F.; Calis, J.J.A.; Paauw, N.D.; Joles, J.A.; van Rijn, B.B.; Mokry, M.; Plosch, T.; Lely, A.T. Developmental programming in human umbilical cord vein endothelial cells following fetal growth restriction. Clin. Epigenetics 2020, 12, 185. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.L.; Shi, X.T.; Xu, X.F.; Zhou, G.X.; Xiong, Y.W.; Yi, S.J.; Liu, W.B.; Dai, L.M.; Cao, X.L.; Xu, D.X.; et al. Melatonin protects against environmental stress-induced fetal growth restriction via suppressing ROS-mediated GCN2/ATF4/BNIP3-dependent mitophagy in placental trophoblasts. Redox Biol. 2021, 40, 101854. [Google Scholar] [CrossRef]
- Sun, C.; Groom, K.M.; Oyston, C.; Chamley, L.W.; Clark, A.R.; James, J.L. The placenta in fetal growth restriction: What is going wrong? Placenta 2020, 96, 10–18. [Google Scholar] [CrossRef]
- Ota, E.; Ganchimeg, T.; Morisaki, N.; Vogel, J.P.; Pileggi, C.; Ortiz-Panozo, E.; Souza, J.P.; Mori, R. Risk factors and adverse perinatal outcomes among term and preterm infants born small-for-gestational-age: Secondary analyses of the WHO Multi-Country Survey on Maternal and Newborn Health. PLoS ONE 2014, 9, e105155. [Google Scholar] [CrossRef]
- Kahn, L.G.; Philippat, C.; Nakayama, S.F.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: Implications for human health. Lancet Diabetes Endocrinol. 2020, 8, 703–718. [Google Scholar] [CrossRef]
- Lee, A.C.; Katz, J.; Blencowe, H.; Cousens, S.; Kozuki, N.; Vogel, J.P.; Adair, L.; Baqui, A.H.; Bhutta, Z.A.; Caulfield, L.E.; et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob. Health 2013, 1, e26–e36. [Google Scholar] [CrossRef]
- Blencowe, H.; Krasevec, J.; de Onis, M.; Black, R.E.; An, X.; Stevens, G.A.; Borghi, E.; Hayashi, C.; Estevez, D.; Cegolon, L.; et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: A systematic analysis. Lancet Glob. Health 2019, 7, e849–e860. [Google Scholar] [CrossRef]
- Sovio, U.; White, I.R.; Dacey, A.; Pasupathy, D.; Smith, G.C.S. Screening for fetal growth restriction with universal third trimester ultrasonography in nulliparous women in the Pregnancy Outcome Prediction (POP) study: A prospective cohort study. Lancet 2015, 386, 2089–2097. [Google Scholar] [CrossRef]
- Georgieff, M.K. Iron deficiency in pregnancy. Am. J. Obstet. Gynecol. 2020, 223, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, Y.-F.; Hao, J.-H.; Chen, Y.-H.; Su, P.-Y.; Wang, Y.; Yu, Z.; Fu, L.; Xu, Y.-Y.; Zhang, C.; et al. Maternal zinc deficiency during pregnancy elevates the risks of fetal growth restriction: A population-based birth cohort study. Sci. Rep. 2015, 5, 11262. [Google Scholar] [CrossRef]
- Bermudez, L.; Garcia-Vicent, C.; Lopez, J.; Torro, M.I.; Lurbe, E. Assessment of ten trace elements in umbilical cord blood and maternal blood: Association with birth weight. J. Transl. Med. 2015, 13, 291. [Google Scholar] [CrossRef] [PubMed]
- Mericq, V.; Martinez-Aguayo, A.; Uauy, R.; Iñiguez, G.; Van der Steen, M.; Hokken-Koelega, A. Long-term metabolic risk among children born premature or small for gestational age. Nat. Rev. Endocrinol. 2017, 13, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, C.; Marino, C.; Nosarti, C.; Vieno, A.; Visentin, S.; Simonelli, A. Association of Intrauterine Growth Restriction and Small for Gestational Age Status With Childhood Cognitive Outcomes: A Systematic Review and Meta-analysis. JAMA Pediatr. 2020, 174, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Hokken-Koelega, A.C.S.; van der Steen, M.; Boguszewski, M.C.S.; Cianfarani, S.; Dahlgren, J.; Horikawa, R.; Mericq, V.; Rapaport, R.; Alherbish, A.; Braslavsky, D.; et al. International Consensus Guideline on Small for Gestational Age: Etiology and Management From Infancy to Early Adulthood. Endocr. Rev. 2023, 44, 539–565. [Google Scholar] [CrossRef]
- Aris, I.M.; Perng, W.; Dabelea, D.; Ganiban, J.M.; Liu, C.; Marceau, K.; Robertson, O.C.; Hockett, C.W.; Mihalopoulos, N.L.; Kong, X.; et al. Analysis of Early-Life Growth and Age at Pubertal Onset in US Children. JAMA Netw. Open 2022, 5, e2146873. [Google Scholar] [CrossRef]
- Liang, C.; Wu, X.; Huang, K.; Yan, S.; Li, Z.; Xia, X.; Pan, W.; Sheng, J.; Tao, R.; Tao, Y.; et al. Domain- and sex-specific effects of prenatal exposure to low levels of arsenic on children’s development at 6 months of age: Findings from the Ma’anshan birth cohort study in China. Environ. Int. 2020, 135, 105112. [Google Scholar] [CrossRef]
- Marks, K.J.; Howards, P.P.; Smarr, M.M.; Flanders, W.D.; Northstone, K.; Daniel, J.H.; Calafat, A.M.; Sjödin, A.; Marcus, M.; Hartman, T.J. Prenatal exposure to mixtures of persistent endocrine disrupting chemicals and early menarche in a population-based cohort of British girls. Environ. Pollut. 2021, 276, 116705. [Google Scholar] [CrossRef]
- Noble, R.M.N.; Holody, C.D.; Woodman, A.G.; Nie, C.; Liu, S.N.; Young, D.; Wiedemeyer, A.; Soni, S.; Dyck, J.R.B.; Graf, D.; et al. Perinatal iron restriction is associated with changes in neonatal cardiac function and structure in a sex-dependent manner. Clin. Sci. 2023, 137, 1115–1130. [Google Scholar] [CrossRef]
- Wiegersma, A.M.; Dalman, C.; Lee, B.K.; Karlsson, H.; Gardner, R.M. Association of Prenatal Maternal Anemia with Neurodevelopmental Disorders. JAMA Psychiatry 2019, 76, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Standard of Recommendation for Weight Gain during Pregnancy Period. Biomed. Environ. Sci. 2022, 35, 875–877.
- Mikolajczyk, R.T.; Zhang, J.; Betran, A.P.; Souza, J.P.; Mori, R.; Gülmezoglu, A.M.; Merialdi, M. A global reference for fetal-weight and birthweight percentiles. Lancet 2011, 377, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Weinert, L.S. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy: Comment to the International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Diabetes Care 2010, 33, e97. [Google Scholar] [CrossRef] [PubMed]
- Overholser, B.R.; Sowinski, K.M. Biostatistics primer: Part 2. Nutr. Clin. Pract. 2008, 23, 76–84. [Google Scholar] [CrossRef]
- Lawn, J.E.; Ohuma, E.O.; Bradley, E.; Idueta, L.S.; Hazel, E.; Okwaraji, Y.B.; Erchick, D.J.; Yargawa, J.; Katz, J.; Lee, A.C.C.; et al. Small babies, big risks: Global estimates of prevalence and mortality for vulnerable newborns to accelerate change and improve counting. Lancet 2023, 401, 1707–1719. [Google Scholar] [CrossRef]
- Ogrizek Pelkič, K.; Sobočan, M.; Takač, I. Low Selenium Levels in Amniotic Fluid Correlate with Small-For-Gestational Age Newborns. Nutrients 2020, 12, 3046. [Google Scholar] [CrossRef]
- Liu, D.; Li, S.; Zhang, B.; Kang, Y.; Cheng, Y.; Zeng, L.; Chen, F.; Mi, B.; Qu, P.; Zhao, D.; et al. Maternal Hemoglobin Concentrations and Birth Weight, Low Birth Weight (LBW), and Small for Gestational Age (SGA): Findings from a Prospective Study in Northwest China. Nutrients 2022, 14, 858. [Google Scholar] [CrossRef]
- Gong, L.; Yang, Q.; Liu, C.-W.-B.; Wang, X.; Zeng, H.-L. Assessment of 12 Essential and Toxic Elements in Whole Blood of Pregnant and Non-pregnant Women Living in Wuhan of China. Biol. Trace. Elem. Res. 2021, 199, 2121–2130. [Google Scholar] [CrossRef]
- Ashrap, P.; Aker, A.; Watkins, D.J.; Mukherjee, B.; Rosario-Pabón, Z.; Vélez-Vega, C.M.; Alshawabkeh, A.; Cordero, J.F.; Meeker, J.D. Psychosocial status modifies the effect of maternal blood metal and metalloid concentrations on birth outcomes. Environ. Int. 2021, 149, 106418. [Google Scholar] [CrossRef]
- Luo, J.; Wu, W.; Zhang, P.; Chen, X.; Feng, Y.; Ma, N.; Yang, H.; Wang, Y.; Li, M.; Xie, B.; et al. Zinc Levels and Birth Weight in Pregnant Women with Gestational Diabetes Mellitus: A Matched Cohort Study in China. J. Clin. Endocrinol. Metab. 2020, 105, e2337–e2345. [Google Scholar] [CrossRef] [PubMed]
- Christian, P.; Murray-Kolb, L.E.; Khatry, S.K.; Katz, J.; Schaefer, B.A.; Cole, P.M.; Leclerq, S.C.; Tielsch, J.M. Prenatal micronutrient supplementation and intellectual and motor function in early school-aged children in Nepal. JAMA 2010, 304, 2716–2723. [Google Scholar] [CrossRef] [PubMed]
- Devakumar, D.; Stocks, J.; Ayres, J.G.; Kirkby, J.; Yadav, S.K.; Saville, N.M.; Devereux, G.; Wells, J.C.K.; Manandhar, D.S.; Costello, A.; et al. Effects of antenatal multiple micronutrient supplementation on lung function in mid-childhood: Follow-up of a double-blind randomised controlled trial in Nepal. Eur. Respir. J. 2015, 45, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Ekström, E.-C.; Lindström, E.; Raqib, R.; El Arifeen, S.; Basu, S.; Brismar, K.; Selling, K.; Persson, L.-Å. Effects of prenatal micronutrient and early food supplementation on metabolic status of the offspring at 4.5 years of age. The MINIMat randomized trial in rural Bangladesh. Int. J. Epidemiol. 2016, 45, 1656–1667. [Google Scholar] [CrossRef] [PubMed]
- DeVilbiss, E.A.; Magnusson, C.; Gardner, R.M.; Rai, D.; Newschaffer, C.J.; Lyall, K.; Dalman, C.; Lee, B.K. Antenatal nutritional supplementation and autism spectrum disorders in the Stockholm youth cohort: Population based cohort study. BMJ 2017, 359, j4273. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Cheng, Y.; Zeng, L.; Elhoumed, M.; He, G.; Li, W.; Zhang, M.; Li, W.; Li, D.; Tsegaye, S.; et al. Association of Antenatal Micronutrient Supplementation With Adolescent Intellectual Development in Rural Western China: 14-Year Follow-up From a Randomized Clinical Trial. JAMA Pediatr. 2018, 172, 832–841. [Google Scholar] [CrossRef]
- Shaheen, S.O.; Gissler, M.; Devereux, G.; Erkkola, M.; Kinnunen, T.I.; McArdle, H.; Sheikh, A.; Hemminki, E.; Nwaru, B.I. Maternal iron supplementation in pregnancy and asthma in the offspring: Follow-up of a randomised trial in Finland. Eur. Respir. J. 2020, 55, 1902335. [Google Scholar] [CrossRef]
- Braithwaite, V.S.; Mwangi, M.N.; Jones, K.S.; Demir, A.Y.; Prentice, A.; Prentice, A.M.; Andang’o, P.E.A.; Verhoef, H. Antenatal iron supplementation, FGF23, and bone metabolism in Kenyan women and their offspring: Secondary analysis of a randomized controlled trial. Am. J. Clin. Nutr. 2021, 113, 1104–1114. [Google Scholar] [CrossRef]
- Liu, X.; An, H.; Li, N.; Li, Z.; Zhang, Y.; Zhang, L.; Li, H.; Liu, J.; Ye, R. Preconception Hemoglobin Concentration and Risk of Low Birth Weight and Small-for-Gestational-Age: A Large Prospective Cohort Study in China. Nutrients 2022, 14, 271. [Google Scholar] [CrossRef]
- Roberts, H.; Woodman, A.G.; Baines, K.J.; Jeyarajah, M.J.; Bourque, S.L.; Renaud, S.J. Maternal Iron Deficiency Alters Trophoblast Differentiation and Placental Development in Rat Pregnancy. Endocrinology 2021, 162, bqab215. [Google Scholar] [CrossRef]
- Wang, J.; Lan, P.; Gao, H.; Zheng, L.; Li, W.; Schmidt, W. Expression changes of ribosomal proteins in phosphate- and iron-deficient Arabidopsis roots predict stress-specific alterations in ribosome composition. BMC Genom. 2013, 14, 783. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; O’Brien, K.O. Pregnancy and iron homeostasis: An update. Nutr. Rev. 2013, 71, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Devireddy, L.R.; Hart, D.O.; Goetz, D.H.; Green, M.R. A mammalian siderophore synthesized by an enzyme with a bacterial homolog involved in enterobactin production. Cell 2010, 141, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Evstatiev, R.; Gasche, C. Iron sensing and signalling. Gut 2012, 61, 933–952. [Google Scholar] [CrossRef]
- Haase, M.; Bellomo, R.; Haase-Fielitz, A. Novel biomarkers, oxidative stress, and the role of labile iron toxicity in cardiopulmonary bypass-associated acute kidney injury. J. Am. Coll. Cardiol. 2010, 55, 2024–2033. [Google Scholar] [CrossRef]
- Maltepe, E.; Fisher, S.J. Placenta: The forgotten organ. Annu. Rev. Cell Dev. Biol. 2015, 31, 523–552. [Google Scholar] [CrossRef]
- Cao, P.; Maximov, A.; Südhof, T.C. Activity-dependent IGF-1 exocytosis is controlled by the Ca(2+)-sensor synaptotagmin-10. Cell 2011, 145, 300–311. [Google Scholar] [CrossRef]
- Elis, S.; Wu, Y.; Courtland, H.-W.; Sun, H.; Rosen, C.J.; Adamo, M.L.; Yakar, S. Increased serum IGF-1 levels protect the musculoskeletal system but are associated with elevated oxidative stress markers and increased mortality independent of tissue igf1 gene expression. Aging Cell 2011, 10, 547–550. [Google Scholar] [CrossRef][Green Version]
- Kamenický, P.; Mazziotti, G.; Lombès, M.; Giustina, A.; Chanson, P. Growth hormone, insulin-like growth factor-1, and the kidney: Pathophysiological and clinical implications. Endocr. Rev. 2014, 35, 234–281. [Google Scholar] [CrossRef]
- Schlotz, W.; Jones, A.; Phillips, D.I.W.; Gale, C.R.; Robinson, S.M.; Godfrey, K.M. Lower maternal folate status in early pregnancy is associated with childhood hyperactivity and peer problems in offspring. J. Child Psychol. Psychiatry 2010, 51, 594–602. [Google Scholar] [CrossRef]
- Caniglia, E.C.; Zash, R.; Swanson, S.A.; Smith, E.; Sudfeld, C.; Finkelstein, J.L.; Diseko, M.; Mayondi, G.; Mmalane, M.; Makhema, J.; et al. Iron, folic acid, and multiple micronutrient supplementation strategies during pregnancy and adverse birth outcomes in Botswana. Lancet Glob. Health 2022, 10, e850–e861. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xiong, S.; Jing, Q.; van Gestel, C.A.M.; van Straalen, N.M.; Roelofs, D.; Sun, L.; Qiu, H. Maternal exposure to polystyrene nanoparticles retarded fetal growth and triggered metabolic disorders of placenta and fetus in mice. Sci. Total Environ. 2022, 854, 158666. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, L.; Hu, Y.-F.; Hao, J.-H.; Chen, Y.-H.; Su, P.-Y.; Fu, L.; Yu, Z.; Zhang, G.-B.; Wang, L.; et al. Maternal serum cadmium level during pregnancy and its association with small for gestational age infants: A population-based birth cohort study. Sci. Rep. 2016, 6, 22631. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Zhang, X.; Tian, F.; Pan, B.; Peng, R.; Wang, Y.; Xia, M.; Yang, M.; Hu, J.; Kan, H.; et al. Maternal exposure to ambient PM2.5 causes fetal growth restriction via the inhibition of spiral artery remodeling in mice. Ecotoxicol. Environ. Saf. 2022, 237, 113512. [Google Scholar] [CrossRef]
- Hinney, A.; Körner, A.; Fischer-Posovszky, P. The promise of new anti-obesity therapies arising from knowledge of genetic obesity traits. Nat. Rev. Endocrinol. 2022, 18, 623–637. [Google Scholar] [CrossRef]
- Llewellyn, C.H.; Kininmonth, A.R.; Herle, M.; Nas, Z.; Smith, A.D.; Carnell, S.; Fildes, A. Behavioural susceptibility theory: The role of appetite in genetic susceptibility to obesity in early life. Philos. Trans. R. Soc. B Biol. Sci. 2023, 378, 20220223. [Google Scholar] [CrossRef]
- Chen, J.-R.; Samuel, H.A.; Shlisky, J.; Sims, C.R.; Lazarenko, O.P.; Williams, D.K.; Andres, A.; Badger, T.M. A longitudinal observational study of skeletal development between ages 3 mo and 6 y in children fed human milk, milk formula, or soy formula. Am. J. Clin. Nutr. 2023, 117, 1211–1218. [Google Scholar] [CrossRef]
- Stone, R.A.; Haycraft, E.; Blissett, J.; Farrow, C. Preschool-Aged Children’s Food Approach Tendencies Interact with Food Parenting Practices and Maternal Emotional Eating to Predict Children’s Emotional Eating in a Cross-Sectional Analysis. J. Acad Nutr. Diet. 2022, 122, 1465–1473. [Google Scholar] [CrossRef]

| Characteristics | Total | Mild Thinness a (n = 133) | Non-Mild Thinness (n = 884) | p-Value |
|---|---|---|---|---|
| Maters | ||||
| Maternal age [years, n (%)] | ||||
| <36 | 1010 (99.3) | 133 (100.0) | 877 (99.2) | 0.303 |
| ≥36 | 7 (0.7) | 0 (0) | 7 (0.8) | |
| Pre-pregnancy BMI [kg/m2, n (%)] | ||||
| <18.5 | 171 (16.8) | 38 (28.6) | 133 (15.0) | <0.001 *** |
| 18.5–25.0 | 793 (78.0) | 93 (69.9) | 700 (79.2) | |
| ≥25.0 | 53 (5.2) | 2 (1.5) | 51 (5.8) | |
| Gestational age [weeks, n (%)] | ||||
| <37 | 23 (2.3) | 4 (3.0) | 19 (2.1) | 0.710 |
| 37–42 | 972 (95.6) | 127 (95.5) | 845 (95.6) | |
| ≥42 | 22 (2.1) | 2 (1.5) | 20 (2.3) | |
| Parity [n (%)] | ||||
| 1 | 928 (91.2) | 125 (94.0) | 803 (90.8) | 0.231 |
| ≥2 | 89 (8.8) | 8 (6.0) | 81 (9.2) | |
| Gravidity [n (%)] | ||||
| 1 | 672 (66.1) | 84 (63.2) | 588 (66.5) | 0.446 |
| ≥2 | 345 (33.9) | 49 (36.8) | 296 (33.5) | |
| Cu (μmol/L) | ||||
| 1st tertile (<21.09) | 338 (33.3) | 44 (33.1) | 294 (33.3) | 0.719 |
| 2nd tertile (21.09–25.63) | 340 (33.4) | 41 (30.8) | 299 (33.8) | |
| 3rd tertile (≥25.63) | 339 (33.3) | 48 (36.1) | 291 (32.9) | |
| Ca (mmol/L) | ||||
| 1st tertile (<1.62) | 319 (31.4) | 41 (30.8) | 278 (31.5) | 0.875 |
| 2nd tertile (1.62–1.74) | 343 (33.7) | 43 (32.3) | 300 (33.9) | |
| 3rd tertile (≥1.74) | 355 (34.9) | 49 (36.9) | 306 (34.6) | |
| Fe (mmol/L) | ||||
| 1st tertile (<7.19) | 336 (33.1) | 54 (40.6) | 282 (31.9) | 0.009 ** |
| 2nd tertile (7.19–7.77) | 342 (33.6) | 50 (37.6) | 292 (33.0) | |
| 3rd tertile (≥7.77) | 339 (33.3) | 29 (21.8) | 310 (35.1) | |
| Mg (mmol/L) | ||||
| 1st tertile (<1.33) | 321 (31.6) | 39 (29.3) | 282 (31.9) | 0.819 |
| 2nd tertile (1.33–1.46) | 356 (35.0) | 49 (36.9) | 307 (34.7) | |
| 3rd tertile (≥1.46) | 340 (33.4) | 45 (33.8) | 295 (33.4) | |
| Zn (μmol/L) | ||||
| 1st tertile (<80.60) | 338 (33.3) | 50 (37.6) | 288 (32.6) | 0.241 |
| 2nd tertile (80.60–92.15) | 340 (33.4) | 47 (35.3) | 293 (33.1) | |
| 3rd tertile (≥92.15) | 339 (33.3) | 36 (27.1) | 303 (34.3) | |
| Infants | ||||
| Newborn gender [n (%)] | ||||
| Male | 535 (52.6) | 57 (42.9) | 478 (54.1) | 0.016 * |
| Female | 482 (47.4) | 76 (57.1) | 406 (45.9) | |
| SGA [n (%)] | ||||
| Yes | 143 (14.1) | 28 (21.1) | 115 (13.0) | 0.013 * |
| No | 874 (85.9) | 105 (78.9) | 769 (87.0) | |
| LBW [n (%)] | ||||
| Yes | 20 (2.0) | 2 (1.5) | 18 (2.0) | 0.680 |
| No | 997 (98.0) | 131 (98.5) | 866 (98.0) |
| Metal Elements | Percentile | |||||||
|---|---|---|---|---|---|---|---|---|
| Min | 5 | 25 | 50 | 75 | 95 | Max | IQR | |
| Cu (μmol/L) | 11.86 | 15.90 | 19.87 | 23.22 | 27.15 | 32.73 | 40.10 | 7.28 |
| Ca (mmol/L) | 1.18 | 1.47 | 1.59 | 1.68 | 1.78 | 1.96 | 2.13 | 0.19 |
| Fe (mmol/L) | 4.59 | 6.24 | 7.03 | 7.48 | 7.90 | 8.70 | 11.55 | 0.87 |
| Mg (mmol/L) | 1.13 | 1.20 | 1.31 | 1.40 | 1.49 | 1.65 | 1.91 | 0.18 |
| Zn (μmol/L) | 52.08 | 66.75 | 78.08 | 86.36 | 95.61 | 109.75 | 146.50 | 17.53 |
| BMI of Preschoolers | Crude Model a | p-Value | Adjusted Model 1 b | p-Value | Adjusted Model 2 c | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| Mild Thinness d | Non-Mild Thinness | OR (95% CI) | OR (95% CI) | OR (95% CI) | ||||
| SGA | 28 (21.1) | 115 (13.0) | 1.78 (1.12–2.83) | 0.014 * | 1.80 (1.12–2.89) | 0.014 * | 1.72 (1.07–2.78) | 0.026 * |
| Non-SGA | 105 (78.9) | 769 (87.0) | ||||||
| Subgroups | Crude Model a | P interaction | Adjusted Model 1 b | P interaction | Adjusted Model 2 c | P interaction |
|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||||
| Maters | ||||||
| Parity | ||||||
| 1 | 0.69 (0.55–0.88) | 0.325 | 0.69 (0.54–0.88) | 0.297 | 0.68 (0.52–0.90) | 0.280 |
| ≥2 | 1.02 (0.42–2.49) | 1.14 (0.44–2.99) | 0.64 (0.16–2.49) | |||
| Gravidity | ||||||
| 1 | 0.76 (0.57–1.00) | 0.618 | 0.76 (0.57–1.02) | 0.604 | 0.75 (0.54–1.05) | 0.672 |
| ≥2 | 0.64 (0.44–0.95) | 0.63 (0.42–0.95) | 0.56 (0.34–0.93) | |||
| Infants | ||||||
| Newborn gender | ||||||
| Male | 0.71 (0.50–0.99) | 0.684 | 0.81 (0.54–1.20) | 0.652 | 1.06 (0.62–1.80) | 0.631 |
| Female | 0.72 (0.52–0.98) | 0.71 (0.50–1.00) | 0.66 (0.45–0.96) | |||
| SGA | ||||||
| Yes | 0.37 (0.19–0.69) | 0.034 * | 0.29 (0.14–0.58) | 0.026 * | 0.34 (0.14–0.87) | 0.026 * |
| No | 0.83 (0.64–1.06) | 0.83 (0.64–1.07) | 0.76 (0.56–1.02) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.-W.; Ling, Z.-J.; Yuan, Z.; Zhang, J.; Yi, S.-J.; Xiong, Y.-W.; Chang, W.; Lin, Z.-J.; Zhu, H.-L.; Yang, L.; et al. The Long-Term Effect of Maternal Iron Levels in the Second Trimester on Mild Thinness among Preschoolers: The Modifying Effect of Small for Gestational Age. Nutrients 2023, 15, 3939. https://doi.org/10.3390/nu15183939
Wang K-W, Ling Z-J, Yuan Z, Zhang J, Yi S-J, Xiong Y-W, Chang W, Lin Z-J, Zhu H-L, Yang L, et al. The Long-Term Effect of Maternal Iron Levels in the Second Trimester on Mild Thinness among Preschoolers: The Modifying Effect of Small for Gestational Age. Nutrients. 2023; 15(18):3939. https://doi.org/10.3390/nu15183939
Chicago/Turabian StyleWang, Kai-Wen, Zheng-Jia Ling, Zhi Yuan, Jin Zhang, Song-Jia Yi, Yong-Wei Xiong, Wei Chang, Zhi-Jing Lin, Hua-Long Zhu, Lan Yang, and et al. 2023. "The Long-Term Effect of Maternal Iron Levels in the Second Trimester on Mild Thinness among Preschoolers: The Modifying Effect of Small for Gestational Age" Nutrients 15, no. 18: 3939. https://doi.org/10.3390/nu15183939
APA StyleWang, K.-W., Ling, Z.-J., Yuan, Z., Zhang, J., Yi, S.-J., Xiong, Y.-W., Chang, W., Lin, Z.-J., Zhu, H.-L., Yang, L., & Wang, H. (2023). The Long-Term Effect of Maternal Iron Levels in the Second Trimester on Mild Thinness among Preschoolers: The Modifying Effect of Small for Gestational Age. Nutrients, 15(18), 3939. https://doi.org/10.3390/nu15183939







