Ultrasound-Treated and Thermal-Pasteurized Hawthorn Vinegar: Antioxidant and Lipid Profiles in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
- Group: Control (no oral HV).
- Group: HVN0.5 (0.5 mL/kg of untreated hawthorn vinegar consumed orally).
- Group: HVN1 (1 mL /kg of untreated hawthorn vinegar consumed orally).
- Group: HVP0.5 (0.5 mL/kg of thermal-pasteurized hawthorn vinegar consumed orally).
- Group: HVP1 (1 mL/kg of thermal-pasteurized hawthorn vinegar consumed orally).
- Group: HVU0.5 (0.5 mL/kg of ultrasound-treated hawthorn vinegar consumed orally).
- Group: HVU1 (1 mL/kg of ultrasound-treated hawthorn vinegar consumed orally).
2.2. Obtaining Vinegar Material
2.3. Processing of Vinegar Group Treatments
- Sample without any treatment (groups HVN0.5 and HVN1).
- Thermal pasteurization of samples in a jar in a water bath at 65 °C for 30 min (groups HVP0.5 and HVP1).
- The samples were treated with the Response Surface Method and Ultrasound and the application of temperature and time with the best bioactive components as a result of optimization (groups HVU0.5 and HVU1).
2.4. Determination of Vinegar’s Bioactive Compounds
2.5. Blood Samples
2.6. Tissue Sample Preparation
2.7. Antioxidant Analyses
2.8. Biochemical Parameters
2.9. Immunohistochemistry Method
2.10. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Medicines Agency. Herbal Medicine: Summary for the Public: Hawthorn Leaf and Flower. 2016. Available online: https://www.ema.europa.eu/en/documents/herbal-summary/hawthorn-leaf-flower-summary-public_en-0.pdf (accessed on 22 August 2023).
- Food and Drug Administration (FDA). Acetic Acid—Use in Foods—Labeling of Foods in Which Used. 1989. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cpg-sec-562100-acetic-acid-use-foods-labeling-foods-which-used (accessed on 22 August 2023).
- Lund, J.A.; Brown, P.N.; Shipley, P.R. Differentiation of Crataegus spp. Guided by Nuclear Magnetic Resonance Spectrometry with Chemometric Analyses. Phytochemistry 2017, 141, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Ljubuncic, P.; Portnaya, I.; Cogan, U.; Azaizeh, H.; Bomzon, A. Antioxidant activity of Crataegus aronia aqueous extract used in traditional Arab medicine in Israel. J. Ethnopharmacol. 2005, 101, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Bahorun, T.; Trotin, F. Antioxidant activities of Crataegus monogyna extracts. Planta Med. 1994, 60, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, G.B.; Özdemir, N.; Ertekin-Filiz, B.; Gökırmaklı, C.; Kök Taş, T.; Budak, N.H. Volatile Aroma Compounds and Bioactive Compounds of Hawthorn Vinegar Produced from Hawthorn Fruit (Crataegus tanacetifolia (lam.) pers.). J. Food Biochem. 2022, 46, e13676. [Google Scholar] [CrossRef]
- Tassell, M.C.; Kingston, R.; Gilroy, D.; Lehane, M.; Furey, A. Hawthorn (Crataegus spp.) in the treatment of cardiovascular disease. Pharm. Rev. 2010, 4, 32–41. [Google Scholar] [CrossRef]
- Lin, Y.G.; Vermeer, M.A.; Trautwein, E.A. Triterpenic acids present in hawthorn lower plasma cholesterol by inhibiting intestinal ACAT activity in hamsters. Evid. Based Complement. Altern. Med. 2011, 2011, 801272. [Google Scholar] [CrossRef] [PubMed]
- Aierken, A.; Buchholz, T.; Chen, C.; Zhang, X.; Melzig, M.F. Hypoglycemic effect of hawthorn in type II diabetes mellitus rat model. J. Sci. Food Agric. 2017, 97, 4557–4561. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, X.; Ji, Y. Total Flavonoid Extract from Hawthorn (Crataegus pinnatifida) Improves Inflammatory Cytokines-Evoked Epithelial Barrier Deficit. Med. Sci. Monit. 2020, 26, e920170. [Google Scholar] [CrossRef]
- Rao, H.; Li, P.; Wu, H.; Liu, C.; Peng, W.; Su, W. Simultaneous Determination of Six Compounds in Destructive Distillation Extracts of Hawthorn Seed by GC-MS and Evaluation of Their Antimicrobial Activity. Molecules 2019, 24, 4328. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Peng, Y.; Zhang, H.; Wang, Z.; Gao, Y.; Liu, Y.; Zhang, H. Chemical Constituents, Antioxidant and Gastrointestinal Transit Accelerating Activities of Dried Fruit of Crataegus dahurica. Food Chem. 2018, 246, 41–47. [Google Scholar] [CrossRef]
- Lis, M.; Szczypka, M.; Suszko-Pawłowska, A.; Sokół-Łetowska, A.; Kucharska, A.; Obminska-Mrukowicz, B. Hawthorn (Crataegus monogyna) Phenolic Extract Modulates Lymphocyte Subsets and Humoral Immune Response in Mice. Planta Med. 2020, 86, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.R.; Guo, X.B.; Liu, R.H.; You, L.J.; Abbasi, A.M.; Fu, X. Phenolic contents and cellular antioxidant activity of Chinese hawthorn “Crataegus pinnatifida”. Food Chem. 2015, 186, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Z.S.; Guo, Y.; Sun, P.; Lv, X.L.; Zuo, Y.B. Hawthorn fruit increases the antioxidant capacity and reduces lipid peroxidation in senescence-accelerated mice. Eur. Food Res. Technol. 2011, 232, 743–751. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.Q. Author Correction: Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 652. [Google Scholar] [CrossRef]
- Giudici, P.; De Vero, L.; Gullo, M. Vinegars in Acetic Acid Bacteria: Fundamentals and Food Applications; Sengun, I.Y., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 261–287. [Google Scholar]
- Joshi, V.K.; Thakur, N.S. Vinegar: Composition and Production. In Postharvest Technology of Fruits and Vegetables; Verma, L.R., Joshi, V.K., Eds.; The Indus Publication: New Delhi, India, 2000; pp. 1128–1170. [Google Scholar]
- Plessi, M. Vinegar. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Trugo, L.C., Finglas, P.M., Eds.; Academic Press: Oxford, UK, 2003; pp. 5996–6003. [Google Scholar]
- Budak, N.H.; Kumbul Doguc, D.; Savas, C.M.; Seydim, A.C.; Kok Tas, T.; Ciris, M.I.; Guzel Seydim, Z.B. Effects of apple cider vinegars produced with different techniques on blood lipids in high-cholesterol-fed rats. J. Agric. Food Chem. 2011, 59, 6638–6644. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.X.; Jia, M.; Gui, Y.; Ma, Y. Comparison of the effects of novel processing technologies and conventional thermal pasteurisation on the nutritional quality and aroma of mandarin (Citrus unshiu) juice. Innov. Food Sci. Emerg. Technol. 2020, 64, 102425. [Google Scholar] [CrossRef]
- Yıkmış, S. Investigation of the effects of non-thermal, combined and thermal treatments on the physicochemical parameters of pomegranate (Punica granatum L.) juice. Food Sci. Technol. Res. 2019, 25, 341–350. [Google Scholar] [CrossRef]
- Zhai, X.; Wang, X.; Wang, X.; Zhang, H.; Ji, Y.; Ren, D.; Lu, J. An efficient method using ultrasound to accelerate aging in crabapple (Malus asiatica) vinegar produced from fresh fruit and its influencing mechanism investigation. Ultrason. Sonochem. 2021, 72, 105464. [Google Scholar] [CrossRef]
- Yıkmış, S.; Erdal, B.; Bozgeyik, E.; Levent, O.; Yinanç, A. Evaluation of purple onion waste from the perspective of sustainability in gastronomy: Ultrasound-treated vinegar. Int. J. Gastron. Food Sci. 2022, 29, 100574. [Google Scholar] [CrossRef]
- Ali, M.; Manzoor, M.F.; Goksen, G.; Aadil, R.M.; Zeng, X.A.; Iqbal, M.W.; Lorenzo, J.M. High-intensity ultrasonication impact on the chlorothalonil fungicide and its reduction pathway in spinach juice. Ultrason. Sonochem. 2023, 94, 106303. [Google Scholar] [CrossRef]
- Yıkmış, S.; Ozer, H.; Levent, O.; Çöl, B.G.; Erdal, B. Effect of thermosonication and thermal treatments on antidiabetic, antihypertensive, mineral elements and in vitro bioaccessibility of bioactive compounds in freshly squeezed pomegranate juice. J. Food Meas. Charact. 2022, 16, 3023–3041. [Google Scholar] [CrossRef]
- Çöl, B.G.; Akhan, M.; Sancar, B.Ç.; Türkol, M.; Yıkmış, S.; Hecer, C. Effect of Thermosonication on Amino Acids, Phenolic Compounds, Sensory Properties and Microbial Quality in Freshly Squeezed Verjuice. Foods 2023, 12, 2167. [Google Scholar] [CrossRef] [PubMed]
- Golmohamadi, A.; Möller, G.; Powers, J.; Nindo, C. Effect of ultrasound frequency on antioxidant activity, total phenolic and anthocyanin content of red raspberry puree. Ultrason. Sonochem. 2013, 20, 1316–1323. [Google Scholar] [CrossRef]
- Erdal, B.; Akalın, R.B.; Yılmaz, B.; Bozgeyik, E.; Yıkmış, S. Application of ultrasound to the organic cornelian cherry (Cornus mas L.) vinegar: Changes in antibacterial, antidiabetic, antihypertensive, and anticancer activities. J. Food Process. Preserv. 2022, 46, e16952. [Google Scholar] [CrossRef]
- Khalil, A.A.; Khan, A.A.; Khalid, A.; Abid, Z.; Proestos, C.; Bhat, Z.F.; Aadil, R.M. Comparing the antioxidant properties and volatile compounds of carrot-orange juice blend processed through varied chemical, pasteurization and ultrasound conditions. Ultrason. Sonochem. 2023, 98, 106534. [Google Scholar] [CrossRef]
- Erdal, B.; Yıkmış, S.; Demirok, N.T.; Bozgeyik, E.; Levent, O. Effects of non-thermal treatment on gilaburu vinegar (Viburnum opulus L.): Polyphenols, amino acid, antimicrobial, and anticancer properties. Biology 2022, 11, 926. [Google Scholar] [CrossRef]
- Tomar, O.; Çağlar, A.; Akarca, G.; Vatansever, H. Physicochemical and sensory quality properties of yellow hawthorn fruit (Crataegus tanacetifolia) vinegar produced by traditional fermentation method. Eur. J. Sci. Technol. 2020, 19, 176–181. [Google Scholar] [CrossRef]
- Natić, M.; Pavlović, A.; Bosco, F.L.; Stanisavljević, N.; Zagorac, D.D.; Akšić, M.F.; Papetti, A. Nutraceutical properties and phytochemical characterization of wild Serbian fruits. Eur. Food Res. Technol. 2019, 245, 469–478. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Ho, W.K.K.; Huang, Y.; Chen, Z.Y. Hypocholesterolemic activity of hawthorn fruit is mediated by regulation of cholesterol-7alpha-hydroxylase and acyl CoA: Cholesterol acyltransferase. Food Res. Int. 2002, 35, 885–891. [Google Scholar] [CrossRef]
- Kadas, Z.; Evrendilek, G.A.; Heper, G. The metabolic effects of hawthorn vinegar in patients with high cardiovascular risk group. J. Food Nutr. Res. 2014, 2, 539–545. [Google Scholar] [CrossRef]
- Yang, S.; Liu, L.; Meng, L.; Hu, X. Capsaicin is beneficial to hyperlipidemia, oxidative stress, endothelial dysfunction, and atherosclerosis in Guinea pigs fed on a high-fat diet. Chem. Biol. Interact. 2019, 297, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hatipoglu, M.; Saglam, M.; Koseoglu, S.; Koksal, E.; Keles, A.; Esen, H.H. The Effectiveness of Crataegus orientalis M Bieber. (Hawthorn) extract administration in preventing alveolar bone loss in rats with experimental periodontitis. PLoS ONE 2015, 10, e0128134. [Google Scholar] [CrossRef] [PubMed]
- Yıkmış, S.; Aksu, H.; Çöl, B.G.; Alpaslan, M. Thermosonication processing of quince (Cydonia oblonga) juice: Effects on total phenolics, ascorbic acid, antioxidant capacity, color and sensory properties. Ciência Agrotecnologia 2019, 43, e019919. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagent. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- AOAC Official Methods of Analysis. Methods 925.10, 65.17, 974.24, 992.16, 17th ed.; Association of Official Analytical Chemists, AOAC: Gaithersburg, MD, USA, 2000; Volume II. [Google Scholar]
- Apak, R.; Güçlü, K.; Özyürek, M.; Esin Karademir, S.; Erçağ, E. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int. J. Food Sci. Nutr. 2006, 57, 292–304. [Google Scholar] [CrossRef]
- Grajeda-Iglesias, C.; Salas, E.; Barouh, N.; Baréa, B.; Panya, A.; Figueroa-Espinoza, M.C. Antioxidant activity of protocatechuates evaluated by DPPH, ORAC, and CAT methods. Food Chem. 2016, 194, 749–757. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell. Biol. 2007, 39, 44–84. [Google Scholar]
- Blumberg, J. Use of biomarkers of oxidative stress in research studies. J. Nutr. 2004, 134, 3188S–3189S. [Google Scholar] [CrossRef]
- Liu, S.; Willet, W.C.; Stampfer, M.J.; Hu, F.B.; Franz, M.; Sampson, L.; Hennekens, C.H.; Manson, J.E. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am. J. Clin. Nutr. 2000, 71, 1455–1461. [Google Scholar] [CrossRef]
- Bahorun, T.; Gressier, B.; Trotin, F.; Brunet, C.; Dine, T.; Luyckx, M.; Vasseur, J.; Cazin, M.; Cazin, J.C.; Pinkas, M. Oxygen species scavenging activity of phenolic extracts from hawthorn fresh plant organs and pharmaceutical preparations. Arzneimittel-Forschung 1996, 46, 1086–1089. [Google Scholar]
- Feng, Y.; Gao, S.; Ting, Z.; Sun, G.; Zhang, P. Hawthorn fruit acid consumption attenuates hyperlipidemia-associated oxidative damage in rats. Front. Nutr. 2022, 9, 936229. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; McDonald, J.; Chandrasekara, A.; Zhong, Y. Phytochemicals of foods, beverages and fruit vinegars: Chemistry and health effects. Asia Pac. J. Clin. Nutr. 2008, 17, 380–382. [Google Scholar] [PubMed]
- Panetta, C.; Jonk, Y.; Shapiro, A. Prospective randomized clinical trial evaluating the impact of vinegar on lipids in non-diabetics. World J. Cardiovas. Dis. 2013, 3, 191–196. [Google Scholar] [CrossRef][Green Version]
- Zhang, Z.; Chang, Q.; Zhu, M.; Huang, Y.; Ho, W.K.K.; Chen, Z.Y. Characterization of antioxidants present in hawthorn fruits. J. Nutr. Biochem. 2001, 12, 144–152. [Google Scholar] [CrossRef]
- Zhang, J.; Chai, X.; Zhao, F.; Hou, G.; Meng, Q. Food Applications and Potential Health Benefits of Hawthorn. Foods 2022, 11, 2861. [Google Scholar] [CrossRef]
- Demirok, N.T.; Yikmiş, S. Combined Effect of Ultrasound and Microwave Power in Tangerine Juice Processing: Bioactive Compounds, Amino Acids, Minerals, and Pathogen. Processes 2022, 10, 2100. [Google Scholar] [CrossRef]
- Alves, L.D.L.; Dos Santos, R.L.; Bayer, B.L.; Devens, A.L.M.; Cichoski, A.J.; Mendonça, C.R.B. Thermosonication of tangerine juice: Effects on quality characteristics, bioactive compounds, and antioxidant activity. J. Food Process 2020, 44, e14914. [Google Scholar] [CrossRef]


| Samples | Total Phenolic Compound (TPC) (mg GAE/100 mL) | Total Flavonoid Compound (TFC) (mg CE/100 mL) | Ascorbic Acid (mg/100 mL) | Antioxidant DPPH (% inhibition) | Antioxidant CUPRAC (% inhibition) |
|---|---|---|---|---|---|
| Ultrasound-treated (HVU) | 116.99 | 15.89 | 3.97 | 62.35 | 67.39 |
| Normal (HVN) | 110.58 | 14.22 | 4.22 | 57.39 | 63.55 |
| Thermal Pasteurization (HVP) | 104.22 | 13.18 | 3.45 | 54.86 | 60.22 |
| Antioxidant Parameters | Control | Groups | |||||
|---|---|---|---|---|---|---|---|
| HVN0.5 | HVN1 | HVP0.5 | HVP1 | HVU0.5 | HVU1 | ||
| Plasma MDA (nmol/mL) | 1.41 ± 0.07 | 1.48 ± 0.08 | 1.65 ± 0.08 | 1.60 ± 0.07 | 1.62 ± 0.02 | 1.67 ± 0.03 | 1.57 ± 0.05 |
| Plasma CAT (ng/mL) | 39.51 ± 3.18 | 37.21 ± 1.96 | 37.89 ± 1.44 | 42.42 ± 1.66 | 40.71 ± 0.94 | 40.94 ± 2.06 | 37.51 ± 2.90 |
| Plasma SOD (ng/mL) | 12.36 ± 0.80 | 14.62 ± 0.64 | 11.16 ± 0.23 * | 13.64 ± 1.06 | 14.08 ± 0.40 | 13.18 ± 0.91 | 13.30 ± 0.62 |
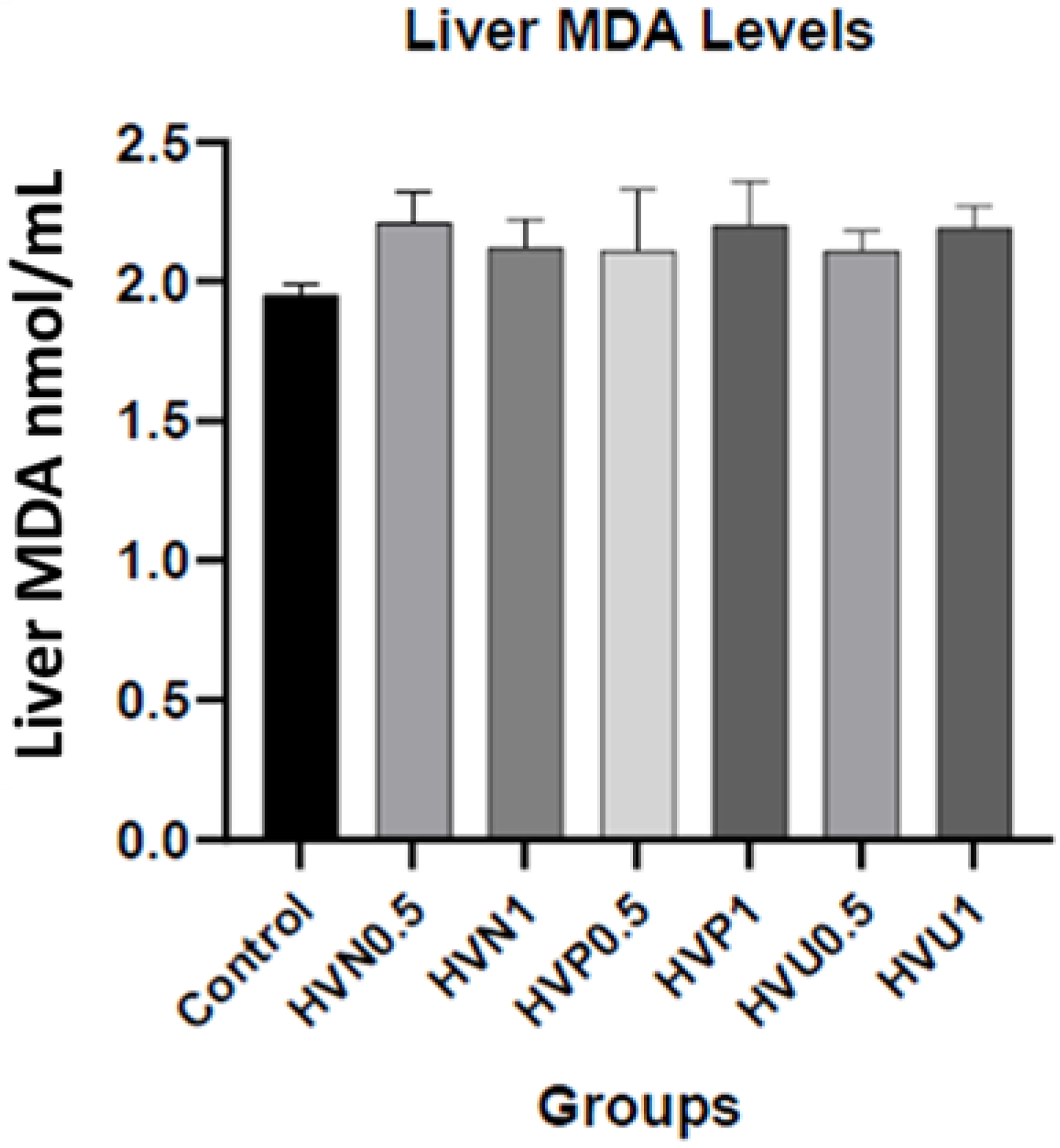
| Liver MDA (nmol/mL) | 1.95 ± 0.04 | 2.21 ± 0.11 | 2.12 ± 0.10 | 2.11 ± 0.22 | 2.20 ± 0.16 | 2.11 ± 0.07 | 2.19 ± 0.08 |
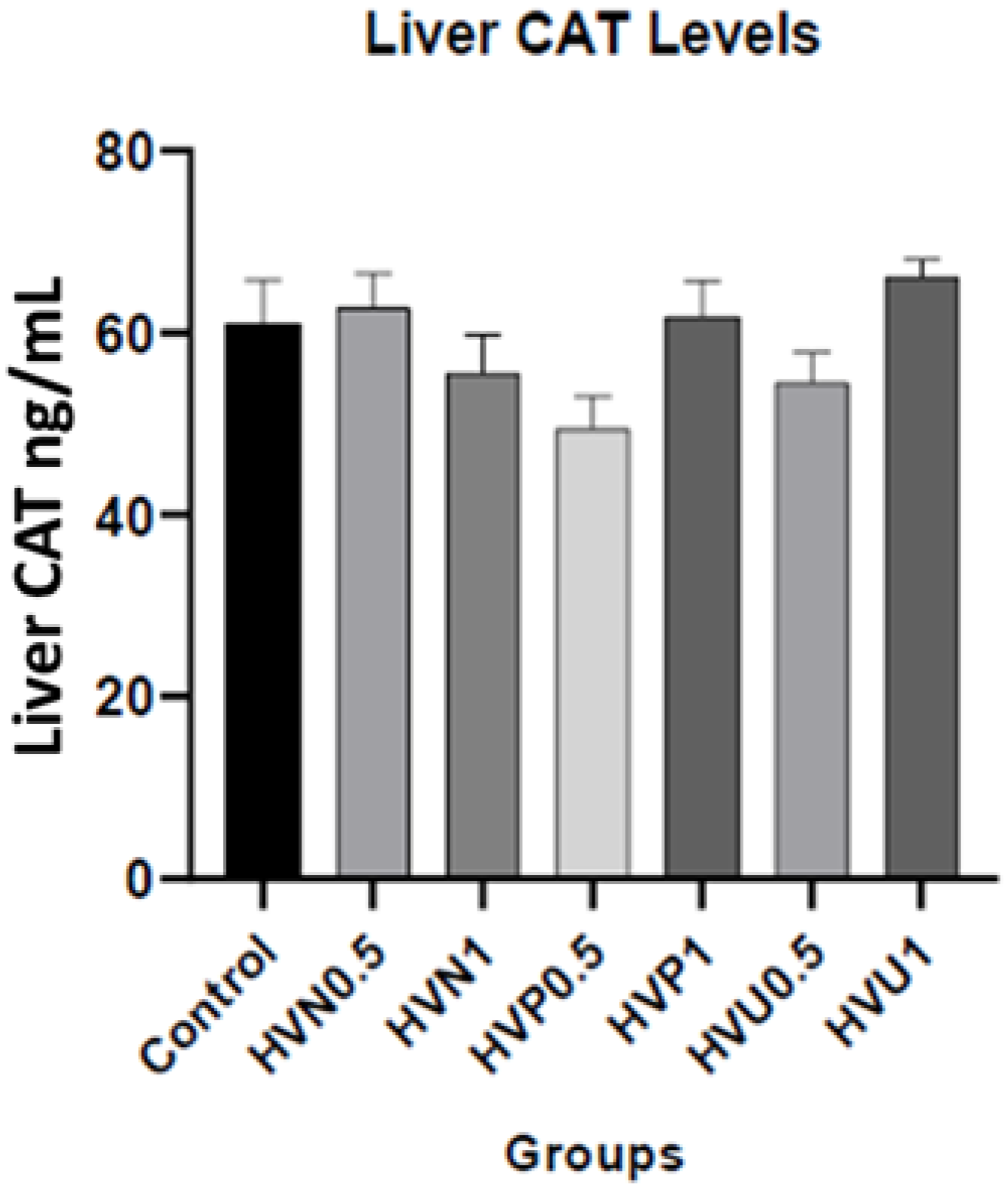
| Liver CAT (ng/mL) | 61.01 ± 4.73 | 62.72 ± 3.78 | 55.53 ± 4.22 | 49.42 ± 3.57 | 61.77 ± 3.91 | 54.48 ± 3.35 | 66.10 ± 1.94 |
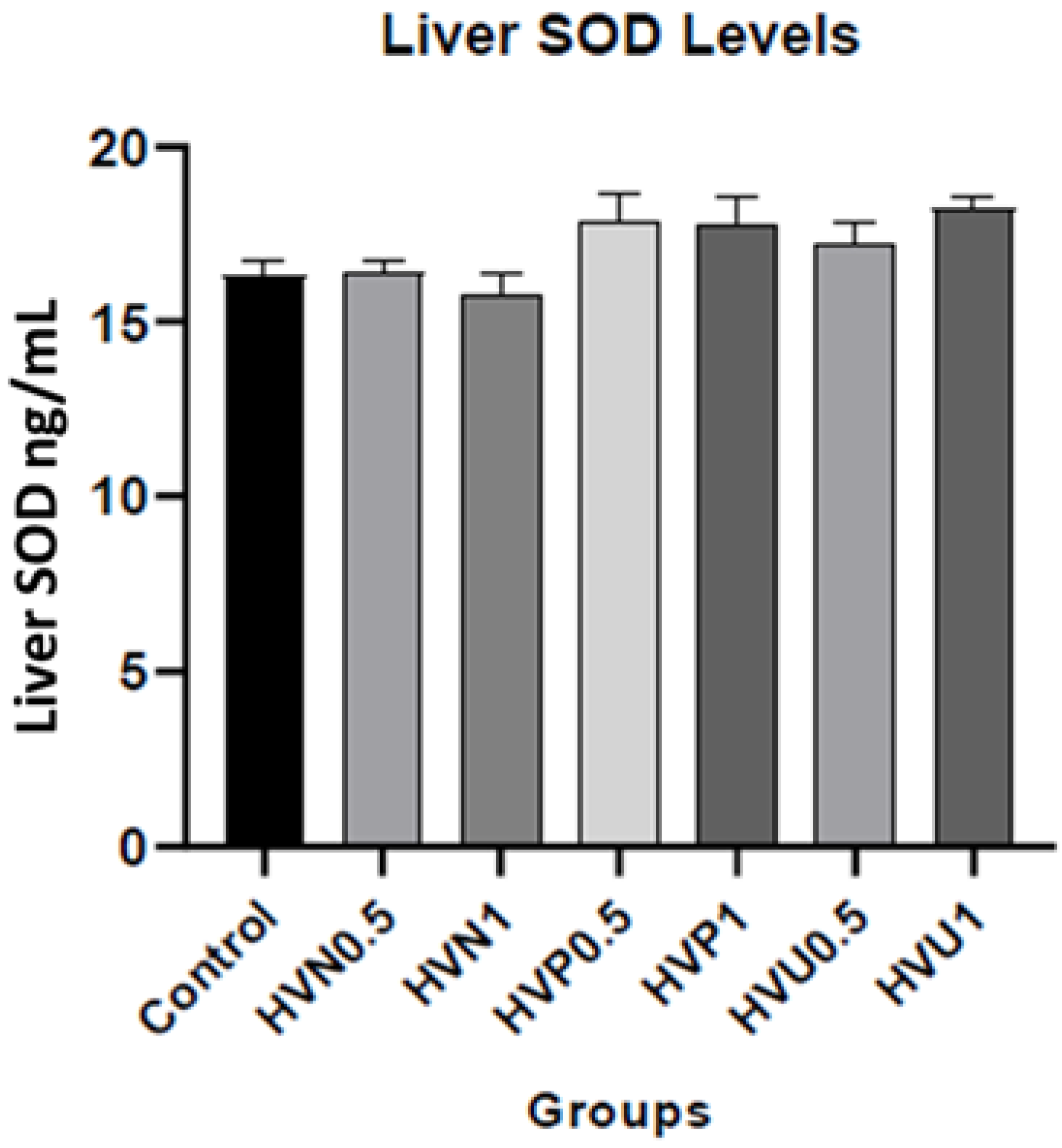
| Liver SOD (ng/mL) | 16.34 ± 0.38 | 16.38 ± 0.39 | 15.72 ± 0.69 | 17.82 ± 0.80 | 17.80 ± 0.78 | 17.24 ± 0.62 | 18.24 ± 0.35 |
| Biochemical Parameters | Control | Groups | |||||
|---|---|---|---|---|---|---|---|
| HVN0.5 | HVN1 | HVP0.5 | HVP1 | HVU0.5 | HVU1 | ||
| Total Cholesterol (mg/dL) | 86.34 ± 9.51 | 72.75 ± 6.69 | 60.69 ± 6.47 | 59.05 ± 3.17 | 63.36 ± 6.64 | 64.49 ± 4.23 | 67.83 ± 3.99 |
| HDL (mg/dL) | 18.36 ± 3.45 | 35.24 ± 5.08 | 28.54 ± 5.58 | 28.54 ± 4.87 | 32.26 ± 4.44 | 15.38 ± 3.46 | 34.00 ± 1.22 |
| LDL (mg/dL) | 48.56 ± 11.75 | 18.20 ± 3.80 a | 20.17 ± 4.83 b | 10.91 ± 3.11 c | 10.06 ± 2.98 d | 28.99 ± 3.98 | 13.69 ± 3.10 e |
| Triglyceride (mg/dL) | 97.12 ± 3.65 | 96.56 ± 4.28 | 89.30 ± 3.06 | 98.02 ± 5.95 | 105.17 ± 7.07 | 100.59 ± 5.86 | 100.70 ± 6.23 |
| ALT (IU/L) | 29.98 ± 2.37 | 32.65 ± 2.43 | 32.30 ± 2.45 | 31.43 ± 5.86 | 31.43 ± 2.26 | 32.65 ± 2.35 | 23.75 ± 3.13 |
| AST (IU/L) | 68.79 ± 6.01 | 59.51 ± 8.89 | 53.60 ± 8.83 | 76.65 ± 1.90 | 54.65 ± 6.46 | 53.77 ± 4.56 | 84.86 ± 16.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakçı, D.; Bakır, B.; Seyidoglu, N.; Yıkmış, S. Ultrasound-Treated and Thermal-Pasteurized Hawthorn Vinegar: Antioxidant and Lipid Profiles in Rats. Nutrients 2023, 15, 3933. https://doi.org/10.3390/nu15183933
Karakçı D, Bakır B, Seyidoglu N, Yıkmış S. Ultrasound-Treated and Thermal-Pasteurized Hawthorn Vinegar: Antioxidant and Lipid Profiles in Rats. Nutrients. 2023; 15(18):3933. https://doi.org/10.3390/nu15183933
Chicago/Turabian StyleKarakçı, Deniz, Buket Bakır, Nilay Seyidoglu, and Seydi Yıkmış. 2023. "Ultrasound-Treated and Thermal-Pasteurized Hawthorn Vinegar: Antioxidant and Lipid Profiles in Rats" Nutrients 15, no. 18: 3933. https://doi.org/10.3390/nu15183933
APA StyleKarakçı, D., Bakır, B., Seyidoglu, N., & Yıkmış, S. (2023). Ultrasound-Treated and Thermal-Pasteurized Hawthorn Vinegar: Antioxidant and Lipid Profiles in Rats. Nutrients, 15(18), 3933. https://doi.org/10.3390/nu15183933






