Potential Role for Diet in Mediating the Association of Olfactory Dysfunction and Cognitive Decline: A Nationally Representative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
2.2. Olfactory Assessment
2.3. Dietary Recall
2.4. Cognitive Assessments
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
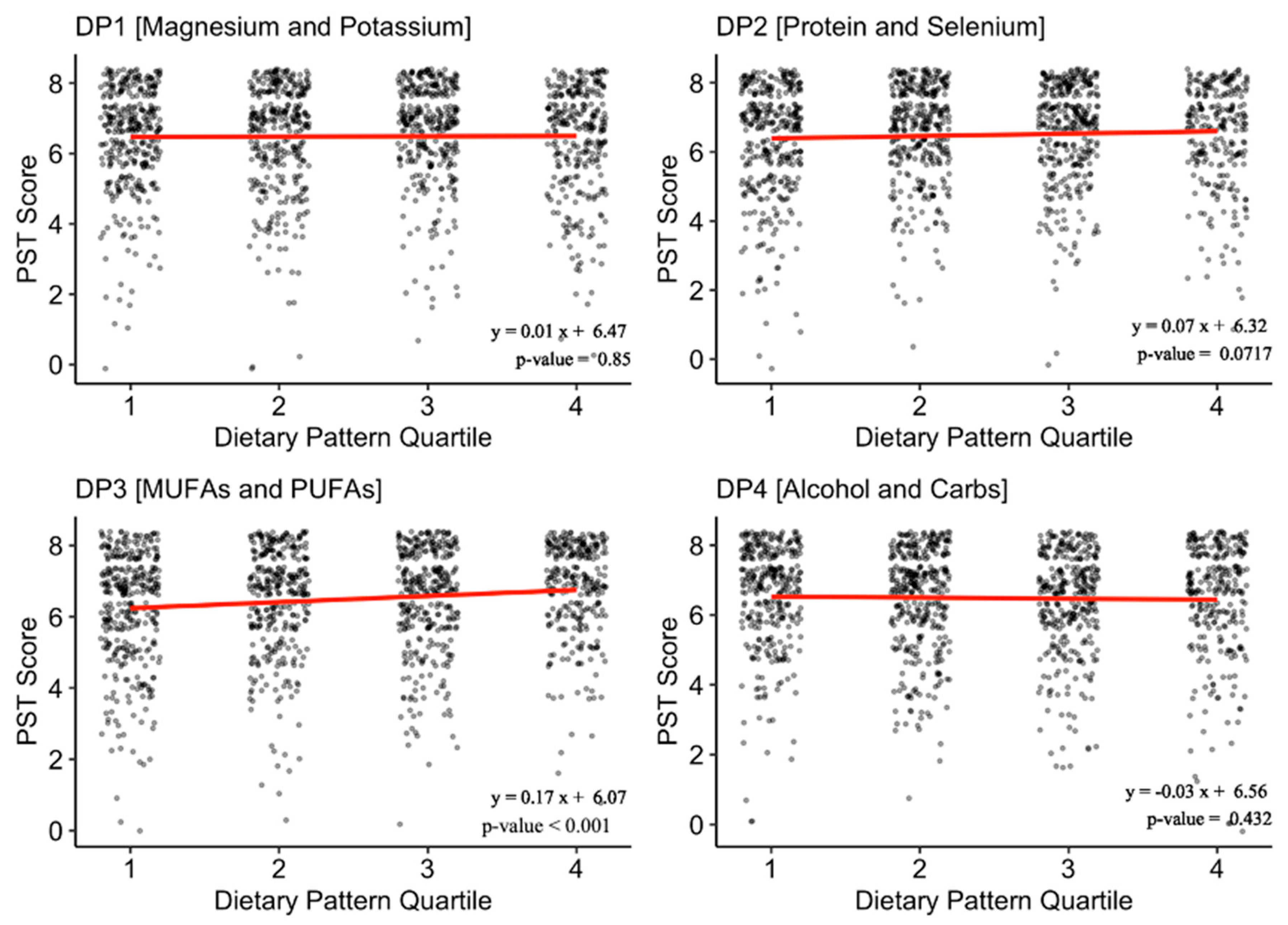
3.2. Dietary Patterns and Olfaction
3.3. Olfaction, Dietary Patterns, and Cognition: Multivariable Analysis
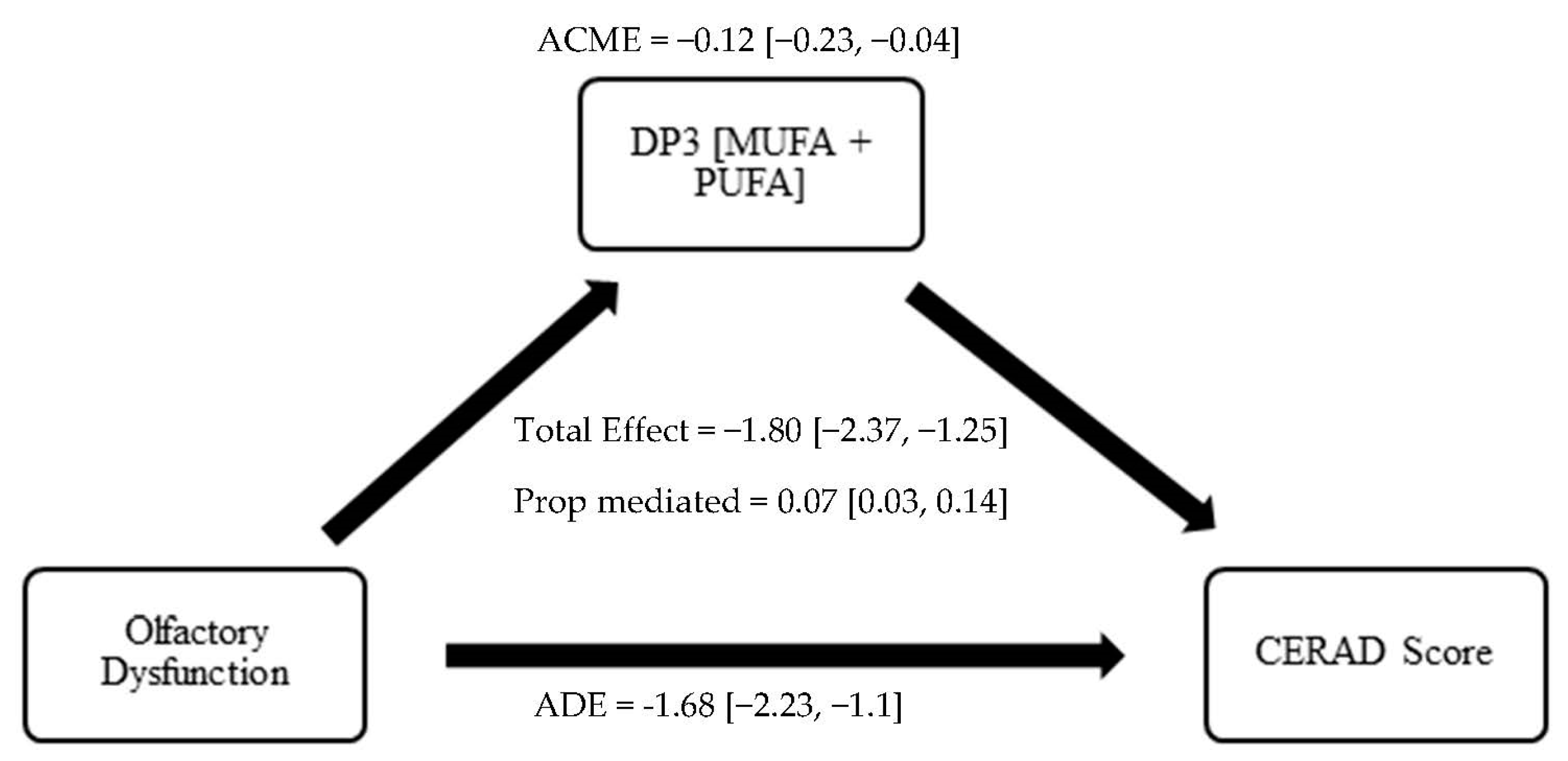
3.4. Olfaction, Dietary Patterns, and Cognition: Causal Mediation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, G.; Zong, G.; Doty, R.L.; Sun, Q. Prevalence and risk factors of taste and smell impairment in a nationwide representative sample of the US population: A cross-sectional study. BMJ Open 2016, 6, e013246. [Google Scholar] [CrossRef]
- Seubert, J.; Laukka, E.J.; Rizzuto, D.; Hummel, T.; Fratiglioni, L.; Bäckman, L.; Larsson, M. Prevalence and Correlates of Olfactory Dysfunction in Old Age: A Population-Based Study. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.J.; Rawal, S.; Li, C.M.; Duffy, V.B. New chemosensory component in the U.S. National Health and Nutrition Examination Survey (NHANES): First-year results for measured olfactory dysfunction. Rev. Endocr. Metab. Disord. 2016, 17, 221–240. [Google Scholar] [CrossRef]
- Murphy, C.; Schubert, C.R.; Cruickshanks, K.J.; Klein, B.E.K.; Klein, R.; Nondahl, D.M. Prevalence of olfactory impairment in older adults. JAMA 2002, 288, 2307–2312. [Google Scholar] [CrossRef]
- Dintica, C.S.; Marseglia, A.; Rizzuto, D.; Wang, R.; Seubert, J.; Arfanakis, K.; Bennett, D.A.; Xu, W. Impaired olfaction is associated with cognitive decline and neurodegeneration in the brain. Neurology 2019, 92, e700–e709. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, M.; Lessard-Beaudoin, M.; Busch, K.; Loudghi, A.; Gaudreau, P.; Graham, R.K. Olfactory Dysfunction Associated with Cognitive Decline in an Elderly Population. Exp. Aging Res. 2022, 1–16. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, H.; Yao, L.; Wei, Y. Olfactory impairment and the risk of cognitive decline and dementia in older adults: A meta-analysis. Braz. J. Otorhinolaryngol. 2020, 87, 94–102. [Google Scholar] [CrossRef]
- Knight, J.E.; Yoneda, T.; Lewis, N.A.; Muniz-Terrera, G.; Bennett, D.A.; Piccinin, A.M. Transitions between Mild Cognitive Impairment, Dementia, and Mortality: The Importance of Olfaction. J. Gerontol. Ser. A Biol. Sci. Med Sci. 2022, 78, 1284–1291. [Google Scholar] [CrossRef]
- Kershaw, J.C.; Mattes, R.D. Nutrition and taste and smell dysfunction. World J. Otorhinolaryngol. Head. Neck Surg. 2018, 4, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Rawal, S.; Duffy, V.B.; Berube, L.; Hayes, J.E.; Kant, A.K.; Li, C.-M.; Graubard, B.I.; Hoffman, H.J. Self-Reported Olfactory Dysfunction and Diet Quality: Findings from the 2011–2014 National Health and Nutrition Examination Survey (NHANES). Nutrients 2021, 13, 4561. [Google Scholar] [CrossRef] [PubMed]
- Aschenbrenner, K.; Hummel, C.; Teszmer, K.; Krone, F.; Ishimaru, T.; Seo, H.-S.; Hummel, T. The influence of olfactory loss on dietary behaviors. Laryngoscope 2008, 118, 135–144. [Google Scholar] [CrossRef]
- Duffy, V.B.; Backstrand, J.R.; Ferris, A.M. Olfactory dysfunction and related nutritional risk in free-living, elderly women. J. Am. Diet. Assoc. 1995, 95, 879–884. [Google Scholar] [CrossRef]
- Roxbury, C.R.; Bernstein, I.A.; Lin, S.Y.; Rowan, N.R. Association between Chemosensory Dysfunction and Diet Quality in United States Adults. Am. J. Rhinol. Allergy 2022, 36, 47–56. [Google Scholar] [CrossRef]
- Kong, I.G.; Kim, S.Y.; Kim, M.S.; Park, B.; Kim, J.H.; Choi, H.G. Olfactory Dysfunction Is Associated with the Intake of Macronutrients in Korean Adults. PLoS ONE 2016, 11, e0164495. [Google Scholar] [CrossRef]
- Taylor, M.K.; Sullivan, D.K.; Swerdlow, R.H.; Vidoni, E.D.; Morris, J.K.; Mahnken, J.D.; Burns, J.M. A high-glycemic diet is associated with cerebral amyloid burden in cognitively normal older adults. Am. J. Clin. Nutr. 2017, 106, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Otaegui-Arrazola, A.; Amiano, P.; Elbusto, A.; Urdaneta, E.; Martínez-Lage, P. Diet, cognition, and Alzheimer’s disease: Food for thought. Eur. J. Nutr. 2014, 53, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.K.; Mahnken, J.D.; Sullivan, D.K. NHANES 2011–2014 Reveals Cognition of US Older Adults may Benefit from Better Adaptation to the Mediterranean Diet. Nutrients 2020, 12, 1929. [Google Scholar] [CrossRef]
- González, L.M.; Bourissai, A.; Lessard-Beaudoin, M.; Lebel, R.; Tremblay, L.; Lepage, M.; Graham, R.K. Amelioration of Cognitive and Olfactory System Deficits in APOE4 Transgenic Mice with DHA Treatment. Mol. Neurobiol. 2023, 60, 5624–5641. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Hoffmann, K.; Kroke, A.; Boeing, H. An approach to construct simplified measures of dietary patterns from exploratory factor analysis. Br. J. Nutr. 2003, 89, 409–419. [Google Scholar] [CrossRef]
- Morris, J.C.; Heyman, A.; Mohs, R.C.; Hughes, J.P.; van Belle, G.; Fillenbaum, G.; Mellits, E.D.; Clark, C. The consortium to establish a registry for Alzheimer’s disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 1989, 39, 1159–1165. [Google Scholar]
- Strauss, E.; Sherman, E.M.S.; Spreen, O.; Spreen, O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary, 3rd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2006. [Google Scholar]
- Boake, C. From the Binet-Simon to the Wechsler-Bellevue: Tracing the history of intelligence testing. J. Clin. Exp. Neuropsychol. 2002, 24, 383–405. [Google Scholar] [CrossRef] [PubMed]
- Pacyna, R.R.; Han, S.D.; Wroblewski, K.E.; McClintock, M.K.; Pinto, J.M. Rapid olfactory decline during aging predicts dementia and GMV loss in AD brain regions. Alzheimer’s Dement. 2023, 19, 1479–1490. [Google Scholar] [CrossRef]
- Bathini, P.; Brai, E.; Auber, L.A. Olfactory dysfunction in the pathophysiological continuum of dementia. Ageing Res. Rev. 2019, 55, 100956. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C. The role of nutrition in Alzheimer’s disease: Epidemiological evidence. Eur. J. Neurol. 2009, 16 (Suppl. S1), 1–7. [Google Scholar] [CrossRef]
- Laitinen, M.H.; Ngandu, T.; Rovio, S.; Helkala, E.-L.; Uusitalo, U.; Viitanen, M.; Nissinen, A.; Tuomilehto, J.; Soininen, H.; Kivipelto, M. Fat intake at midlife and risk of dementia and Alzheimer’s disease: A population-based study. Dement. Geriatr. Cogn. Disord. 2006, 22, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzi, V.; Colacicco, A.M.; D’Introno, A.; Capurso, C.; Torres, F.; Rizzo, C.; Capurso, A.; Panza, F. Dietary intake of unsaturated fatty acids and age-related cognitive decline: A 8.5-year follow-up of the Italian Longitudinal Study on Aging. Neurobiol. Aging 2006, 27, 1694–1704. [Google Scholar] [CrossRef]
- Schubert, C.R.; Cruickshanks, K.J.; Fischer, M.E.; Klein, B.E.; Klein, R.; Pinto, A.A. Inflammatory and vascular markers and olfactory impairment in older adults. Age Ageing 2015, 44, 878–882. [Google Scholar] [CrossRef]
- Vercambre, M.N.; Boutron-Ruault, M.C.; Ritchie, K.; Clavel-Chapelon, F.; Berr, C. Long-term association of food and nutrient intakes with cognitive and functional decline: A 13-year follow-up study of elderly French women. Br. J. Nutr. 2009, 102, 419–427. [Google Scholar] [CrossRef]
- Solfrizzi, V.; Colacicco, A.M.; D’Introno, A.; Capurso, C.; Del Parigi, A.; Capurso, S.A.; Argentieri, G.; Capurso, A.; Panza, F. Dietary fatty acids intakes and rate of mild cognitive impairment. The Italian Longitudinal Study on Aging. Exp. Gerontol. 2006, 41, 619–627. [Google Scholar] [CrossRef]
- Panza, F.; Solfrizzi, V.; Colacicco, A.M.; D’Introno, A.; Capurso, C.; Torres, F.; Del Parigi, A.; Capurso, S.; Capurso, A. Mediterranean diet and cognitive decline. Public Health Nutr. 2004, 7, 959–963. [Google Scholar] [CrossRef]
- Allensworth, J.J.; Schlosser, R.J.; Smith, T.L.; Mace, J.C.; Soler, Z.M. Use of the Diet History Questionnaire III to determine the impact of dysosmia on dietary quality. Int. Forum Allergy Rhinol. 2022, 12, 849–858. [Google Scholar] [CrossRef]
- Lee, J.; Tucker, R.M.; Tan, S.Y.; Running, C.A.; Jones, J.B.; Mattes, R.D. Nutritional Implications of Taste and Smell Dysfunction. In Handbook of Olfaction and Gustation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 829–864. [Google Scholar] [CrossRef]
- Yan, C.H.; Rathor, A.; Krook, K.; Ma, Y.; Rotella, M.R.; Dodd, R.L.; Hwang, P.H.; Nayak, J.V.; Oyesiku, N.M.; DelGaudio, J.M.; et al. Effect of Omega-3 Supplementation in Patients with Smell Dysfunction Following Endoscopic Sellar and Parasellar Tumor Resection: A Multicenter Prospective Randomized Controlled Trial. Neurosurgery 2020, 87, E91–E98. [Google Scholar] [CrossRef] [PubMed]
- Laudisio, A.; Navarini, L.; Margiotta, D.P.E.; Fontana, D.O.; Chiarella, I.; Spitaleri, D.; Bandinelli, S.; Gemma, A.; Ferrucci, L.; Incalzi, R.A. The Association of Olfactory Dysfunction, Frailty, and Mortality Is Mediated by Inflammation: Results from the InCHIANTI Study. J. Immunol. Res. 2019, 2019, 3128231. [Google Scholar] [CrossRef]
- Amor, S.; Puentes, F.; Baker, D.; van der Valk, P. Inflammation in neurodegenerative diseases. Immunology 2010, 129, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Gardener, S.L.; Rainey-Smith, S.R.; Martins, R.N. Diet and Inflammation in Alzheimer’s Disease and Related Chronic Diseases: A Review. J. Alzheimer’s Dis. JAD 2016, 50, 301–334. [Google Scholar] [CrossRef] [PubMed]
- Riera, C.E.; Tsaousidou, E.; Halloran, J.; Follett, P.; Hahn, O.; Pereira, M.M.; Ruud, L.E.; Alber, J.; Tharp, K.; Anderson, C.M.; et al. The Sense of Smell Impacts Metabolic Health and Obesity. Cell Metab. 2017, 26, 198–211.e5. [Google Scholar] [CrossRef]
- Palouzier-Paulignan, B.; Lacroix, M.C.; Aimé, P.; Baly, C.; Caillol, M.; Congar, P.; Julliard, A.K.; Tucker, K.; Fadool, D.A. Olfaction under metabolic influences. Chem. Senses 2012, 37, 769–797. [Google Scholar] [CrossRef] [PubMed]
| Overall | No OD | OD | p-Value | |
|---|---|---|---|---|
| Number of observations: n (%) | 1310 (100) | 1025 (78.2) | 285 (21.8) | |
| Age: mean (sd) | 69.51 (6.69) | 68.80 (6.37) | 72.06 (7.19) | <0.001 *** |
| Gender: n (%) | <0.001 *** | |||
| Male | 626 (47.8) | 458 (46.7) | 168 (59.9) | |
| Female | 684 (52.2) | 567 (55.3) | 117 (41.1) | |
| Race: n (%) | 0.129 | |||
| Non-Hispanic White | 698 (53.3) | 559 (54.5) | 139 (48.8) | |
| Mexican American | 140 (10.7) | 114 (11.1) | 26 (9.1) | |
| Other Hispanic | 106 (8.1) | 75 (7.3) | 31 (10.9) | |
| Non-Hispanic black | 265 (20.2) | 202 (19.7) | 63 (22.1) | |
| Non-Hispanic Asian | 87 (6.6) | 63 (6.1) | 24 (8.4) | |
| Other | 14 (1.1) | 12 (1.2) | 2 (0.7) | |
| Education: n (%) | <0.001 *** | |||
| ≤High School Diploma | 594 (45.3) | 438 (42.7) | 168 (58.9) | |
| >High School Diploma | 716 (54.7) | 587 (57.3) | 117 (41.1) | |
| Income-to-Poverty ratio: mean (sd) | 2.69 (1.59) | 2.75 (1.62) | 2.49 (1.47) | 0.020 * |
| BMI: mean (sd) | 29.17 (6.26) | 29.33 (6.29) | 28.59 (6.16) | 0.079 |
| Current Smoker: n (%) | 0.990 | |||
| Yes | 145 (11.1) | 114 (11.1) | 31 (10.9) | |
| No | 2433 (0.83) | 911 (88.9) | 254 (89.1) | |
| Self-reported OD: n (%) | <0.001 *** | |||
| Yes | 251 (19.2) | 170 (16.6) | 81 (28.4) | |
| No | 1059 (81.8) | 855 (83.4) | 204 (79.6) | |
| History of Head/Face Injury | 0.99 | |||
| Yes | 179 (13.7) | 140 (13.7) | 39 (13.7) | |
| No | 1131 (86.3) | 885 (86.3) | 246 (86.3) | |
| History of Persistent Cold/Flu in last 12 months | 0.95 | |||
| Yes | 86 (6.6) | 68 (6.6) | 18 (6.3) | |
| No | 1224 (93.4) | 957 (93.4) | 267 (93.7) | |
| Cognitive Function | ||||
| CERAD-WL: mean (sd) | 19.85 (4.53) | 20.39 (4.28) | 17.88 (4.87) | <0.001 *** |
| CERAD-DR: mean (sd) | 6.35 (2.25) | 6.62 (2.12) | 5.38 (2.45) | <0.001 *** |
| DSST: mean (sd) | 47.68 (16.53) | 49.95 (15.98) | 39.52 (15.91) | <0.001 *** |
| Animal Fluency: mean (sd) | 17.01 (5.42) | 17.58 (5.44) | 14.94 (4.83) | <0.001 *** |
| Dietary Data | ||||
| Energy (kcal): mean (sd) | 1839.18 (679.25) | 1864 (679) | 1751 (673) | 0.013 * |
| DP1 (Magnesium and Potassium): n (%) | 0.111 | |||
| Q1 | 345 (26.3) | 269 (26.2) | 76 (26.7) | |
| Q2 | 338 (25.8) | 257 (25.1) | 81 (28.4) | |
| Q3 | 332 (25.3) | 275 (26.8) | 57 (20.0) | |
| Q4 | 295 (22.5) | 224 (21.9) | 71 (24.9) | |
| DP2 (Protein and Selenium): n (%) | 0.116 | |||
| Q1 | 351 (26.8) | 259 (25.3) | 92 (32.3) | |
| Q2 | 355 (27.1) | 287 (28.0) | 68 (23.9) | |
| Q3 | 347 (26.5) | 275 (26.8) | 72 (25.3) | |
| Q4 | 257 (19.6) | 204 (19.9) | 53 (18.6) | |
| DP3 (MUFA and PUFA): n (%) | 0.002 ** | |||
| Q1 | 350 (26.7) | 253 (24.7) | 97 (34.0) | |
| Q2 | 332 (25.3) | 258 (25.2) | 74 (26.0) | |
| Q3 | 338 (25.8) | 268 (26.1) | 70 (24.6) | |
| Q4 | 290 (22.1) | 246 (24.0) | 44 (15.4) | |
| DP4 (Alcohol and Carbs): n (%) | 0.786 | |||
| Q1 | 329 (25.1) | 262 (25.6) | 67 (23.5) | |
| Q2 | 357 (27.3) | 273 (26.6) | 84 (29.5) | |
| Q3 | 340 (26.0) | 267 (26.0) | 73 (25.6) | |
| Q4 | 284 (21.7) | 223 (21.8) | 61 (21.4) |
| Odds of Olfactory Dysfunction | |||
|---|---|---|---|
| OR | 95% CI | p-Value | |
| Quartiles of adherence to DP3 * | |||
| Q4 | ref | - | - |
| Q3 | 1.73 | (0.75, 3.98) | 0.16 |
| Q2 | 1.51 | (0.89, 2.56) | 0.1 |
| Q1 | 2.11 | (1.39, 3.18) | 0.003 ** |
| Difference in Cognitive Test Scores | |||
|---|---|---|---|
| β-Estimate | 95% CI | p-Value | |
| CERAD-WL | |||
| OD | −1.77 | (−2.79, −0.79) | 0.006 ** |
| DP3 | |||
| Q4 | ref | - | - |
| Q3 | −0.08 | (−0.88, 0.72) | 0.8 |
| Q2 | −0.88 | (−1.89, 0.13) | 0.076 |
| Q1 | −1.66 | (−2.7, −0.62) | 0.009** |
| CERAD-DR | |||
| OD | −0.93 | (−1.52, −0.35) | 0.005 ** |
| DP3 | |||
| Q4 | ref | - | - |
| Q3 | −0.17 | (−0.69, 0.35) | 0.46 |
| Q2 | −0.42 | (−0.9, 0.05) | 0.07 |
| Q1 | −0.57 | (−1.12, −0.013) | 0.045 * |
| DSST | |||
| OD | −5.56 | (−10.2, −0.88) | 0.026 ** |
| DP3 | |||
| Q4 | ref | - | - |
| Q3 | 0.96 | (−3.32, 5.25) | 0.61 |
| Q2 | −2.46 | (−5.7, 0.82) | 0.12 |
| Q1 | −4.31 | (−7.38, −1.23) | 0.013 * |
| AF | |||
| OD | −2.06 | (−3.7, −0.41) | 0.023 * |
| DP3 | |||
| Q4 | ref | - | - |
| Q3 | 0.19 | (−0.71, 1.55) | 0.61 |
| Q2 | −0.1 | (−1.23, 1.44) | 0.85 |
| Q1 | −1.82 | (−3.4, −0.26) | 0.03 * |
| Mediation Analysis | ||
|---|---|---|
| Beta (95% CI) | p-Value | |
| CERAD-WL | ||
| Model 1 | ||
| ACME | −0.12 (−0.23, −0.04) | <0.001 *** |
| ADE | −1.68 (−2.23, −1.1) | <0.001 *** |
| Total Effect | −1.80 (−2.37, −1.25) | <0.001 *** |
| Prop. Mediated | 0.07 (0.03, 0.14) | <0.001 *** |
| CERAD-DR | ||
| Model 2 | ||
| ACME | −0.05 (−0.09, −0.01) | 0.004 ** |
| ADE | −0.99 (−1.27, −0.72) | <0.001 *** |
| Total Effect | −1.04 (−1.32, −0.78) | <0.001 *** |
| Prop. Mediated | 0.05 (0.02, 0.12) | 0.003 ** |
| DSST | ||
| Model 3 | ||
| ACME | −0.35 (−0.55, −0.16) | <0.001 *** |
| ADE | −3.99 (−6.01, −1.9) | <0.001 *** |
| Total Effect | −4.34 (−6.44, −2.23) | <0.001 *** |
| Prop. Mediated | 0.08 (0.04, 0.14) | <0.001 *** |
| AF | ||
| Model 4 | ||
| ACME | −0.14 (−0.26, −0.04) | 0.008 ** |
| ADE | −2.05 (−2.75, −1.36) | <0.001 *** |
| Total Effect | −2.19 (−2.9, −1.49) | <0.001 *** |
| Prop. Mediated | 0.06 (0.02, 0.12) | 0.008 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vohra, V.; Assi, S.; Kamath, V.; Soler, Z.M.; Rowan, N.R. Potential Role for Diet in Mediating the Association of Olfactory Dysfunction and Cognitive Decline: A Nationally Representative Study. Nutrients 2023, 15, 3890. https://doi.org/10.3390/nu15183890
Vohra V, Assi S, Kamath V, Soler ZM, Rowan NR. Potential Role for Diet in Mediating the Association of Olfactory Dysfunction and Cognitive Decline: A Nationally Representative Study. Nutrients. 2023; 15(18):3890. https://doi.org/10.3390/nu15183890
Chicago/Turabian StyleVohra, Varun, Sahar Assi, Vidyulata Kamath, Zachary M. Soler, and Nicholas R. Rowan. 2023. "Potential Role for Diet in Mediating the Association of Olfactory Dysfunction and Cognitive Decline: A Nationally Representative Study" Nutrients 15, no. 18: 3890. https://doi.org/10.3390/nu15183890
APA StyleVohra, V., Assi, S., Kamath, V., Soler, Z. M., & Rowan, N. R. (2023). Potential Role for Diet in Mediating the Association of Olfactory Dysfunction and Cognitive Decline: A Nationally Representative Study. Nutrients, 15(18), 3890. https://doi.org/10.3390/nu15183890







