Body Composition Analysis in Patients with Metabolic Dysfunction-Associated Fatty Liver Disease
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Body Composition Analysis
2.3. Our Study
2.4. Statistics
3. Results
3.1. Baseline Characteristics
3.2. Percentage of Patients with FF Index Less than 18 kg/m2 in Males and FF Index Less than 15 kg/m2 in Females According to Age
3.3. Percentage of Patients with FF Index Less than 18 kg/m2 in Males and FF Index Less than 15 kg/m2 in Females According to BMI
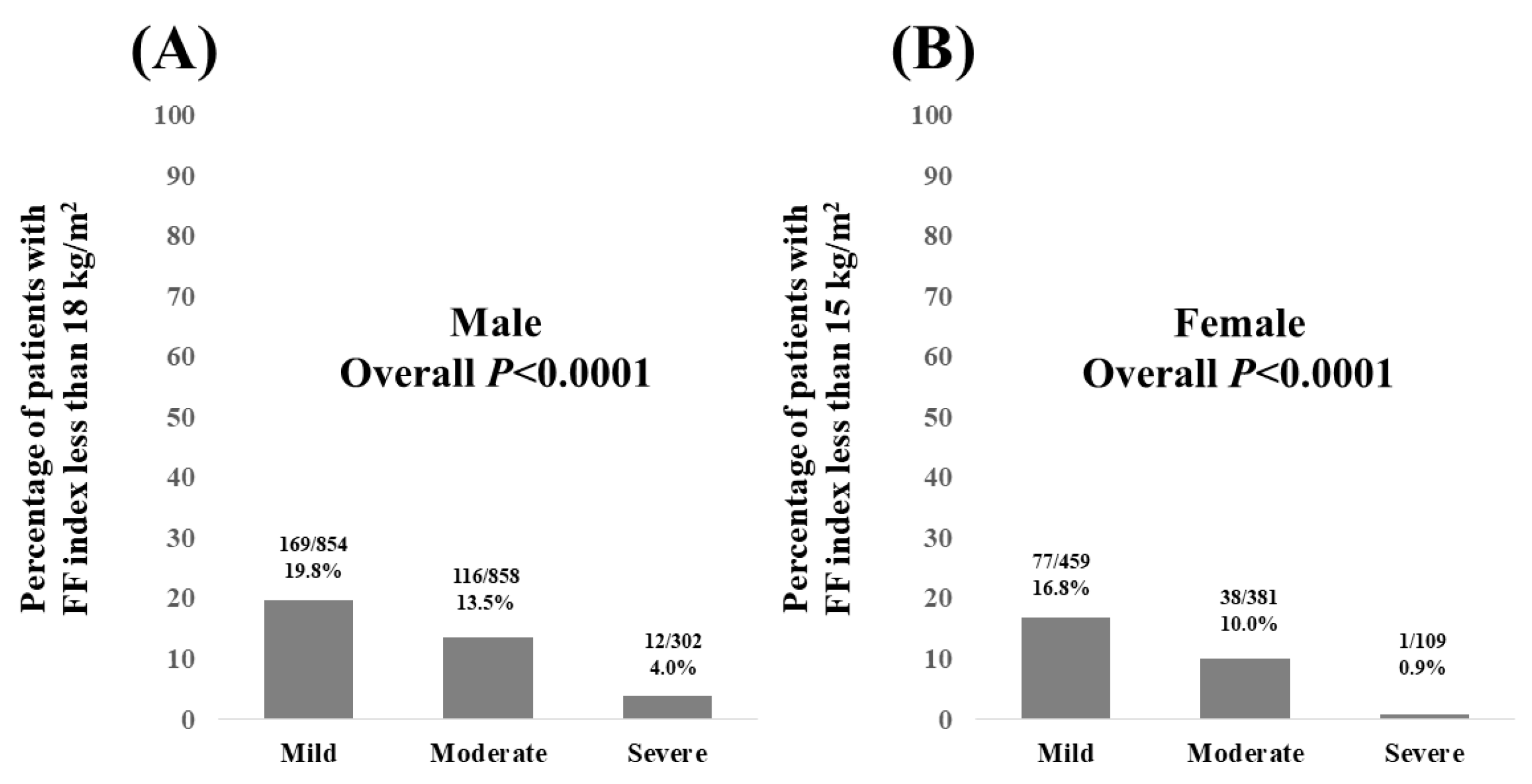
3.4. Percentage of Patients with FF Index Less than 18 kg/m2 in Males and FF Index Less than 15 kg/m2 in Females According to the Severity of FL on US
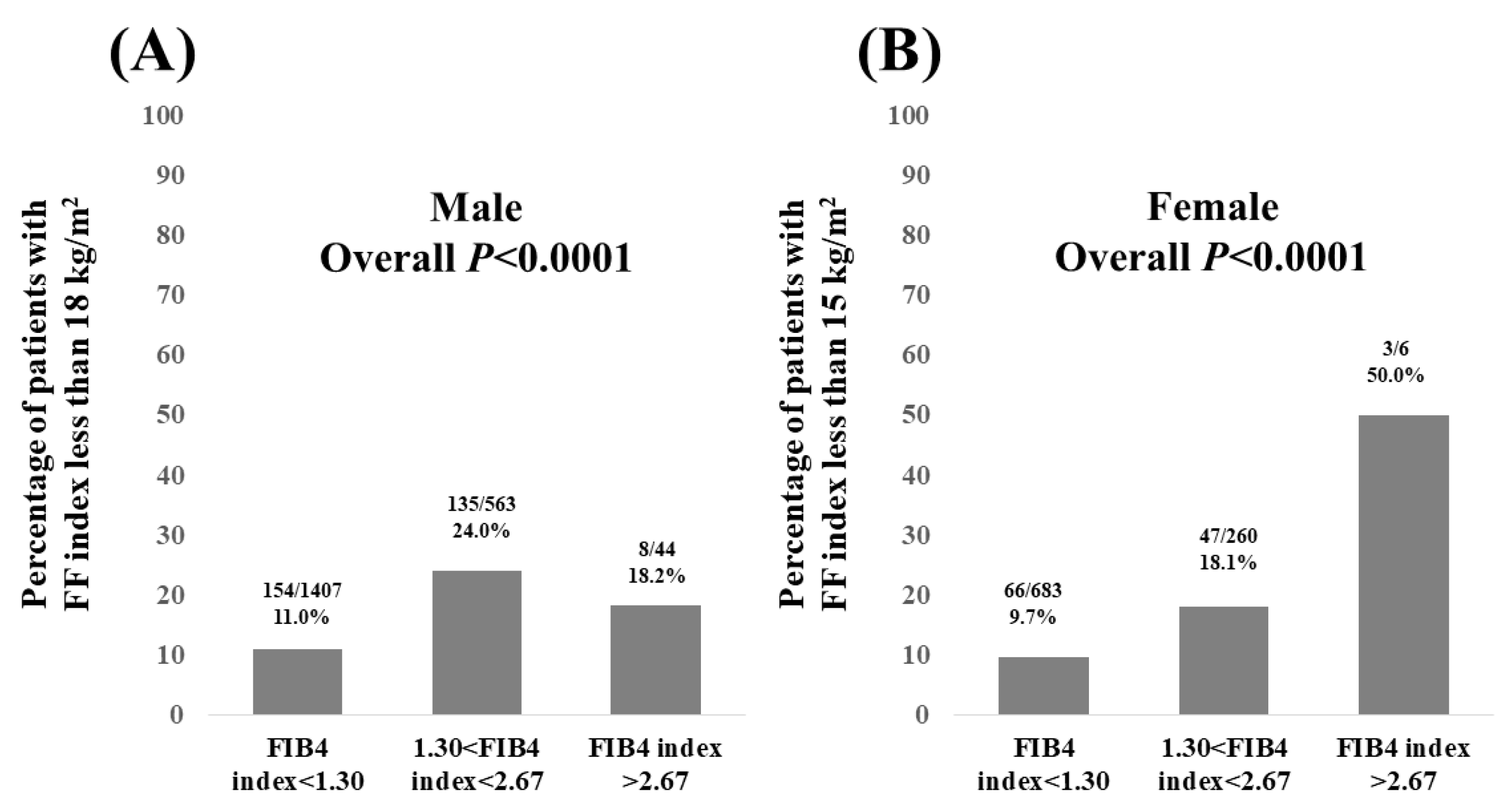
3.5. Percentage of Patients with FF Index Less than 18 kg/m2 in Males and FF Index Less than 15 kg/m2 in Females According to FIB4 Index
3.6. Univariate and Multivariate Analyses of Factors Linked to FF Index
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [PubMed]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya, K.; Hino, K.; Nishiguchi, S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar] [PubMed]
- Rinaldi, S.; Gilliland, J.; O’Connor, C.; Seabrook, J.A.; Mura, M.; Madill, J. Fat-Free Mass Index Controlled for Age and Sex and Malnutrition Are Predictors of Survival in Interstitial Lung Disease. Respiration 2021, 100, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Yago, A.; Haruta, S.; Ueno, M.; Ogawa, Y.; Shimoyama, H.; Ohkura, Y.; Udagawa, H. Identification of Preoperative Fat-Free Mass Index for the Prognosis of Curatively Resected Esophageal Cancer. World J. Surg. 2022, 46, 845–854. [Google Scholar] [PubMed]
- Van Venrooij, L.M.; de Vos, R.; Zijlstra, E.; Borgmeijer-Hoelen, M.M.; van Leeuwen, P.A.; de Mol, B.A. The impact of low preoperative fat-free body mass on infections and length of stay after cardiac surgery: A prospective cohort study. J. Thorac. Cardiovasc. Surg. 2011, 142, 1263–1269. [Google Scholar]
- Gotsman, I.; Keren, A.; Amir, O.; Zwas, D.R. Increased estimated fat-free mass and fat mass associated with improved clinical outcome in heart failure. Eur. J. Clin. Investig. 2022, 52, e13655. [Google Scholar]
- Ding, N.S.; Tassone, D.; Al Bakir, I.; Wu, K.; Thompson, A.J.; Connell, W.R.; Malietzis, G.; Lung, P.; Singh, S.; Choi, C.R.; et al. Systematic Review: The Impact and Importance of Body Composition in Inflammatory Bowel Disease. J. Crohn’s Colitis 2022, 16, 1475–1492. [Google Scholar]
- Vestbo, J.; Prescott, E.; Almdal, T.; Dahl, M.; Nordestgaard, B.G.; Andersen, T.; Sørensen, T.I.; Lange, P. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: Findings from the Copenhagen City Heart Study. Am. J. Respir. Crit. Care Med. 2006, 173, 79–83. [Google Scholar]
- Kawakami, R.; Tanisawa, K.; Ito, T.; Usui, C.; Miyachi, M.; Torii, S.; Midorikawa, T.; Ishii, K.; Muraoka, I.; Suzuki, K.; et al. Fat-Free Mass Index as a Surrogate Marker of Appendicular Skeletal Muscle Mass Index for Low Muscle Mass Screening in Sarcopenia. J. Am. Med. Dir. Assoc. 2022, 23, 1955–1961.e3. [Google Scholar]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun, W.V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar]
- Gutiérrez-Cuevas, J.; Santos, A.; Armendariz-Borunda, J. Pathophysiological Molecular Mechanisms of Obesity: A Link between MAFLD and NASH with Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 11629. [Google Scholar]
- Kawaguchi, T.; Tsutsumi, T.; Nakano, D.; Torimura, T. MAFLD: Renovation of clinical practice and disease awareness of fatty liver. Hepatol. Res. 2022, 52, 422–432. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, H.; Liu, Y.; Hou, X.; Wei, L.; Bao, Y.; Yang, C.; Zong, G.; Wu, J.; Jia, W. Association of MAFLD With Diabetes, Chronic Kidney Disease, and Cardiovascular Disease: A 4.6-Year Cohort Study in China. J. Clin. Endocrinol. Metab. 2022, 107, 88–97. [Google Scholar]
- Drożdż, K.; Nabrdalik, K.; Hajzler, W.; Kwiendacz, H.; Gumprecht, J.; Lip, G.Y.H. Metabolic-Associated Fatty Liver Disease (MAFLD), Diabetes, and Cardiovascular Disease: Associations with Fructose Metabolism and Gut Microbiota. Nutrients 2021, 14, 103. [Google Scholar]
- Yamamura, S.; Eslam, M.; Kawaguchi, T.; Tsutsumi, T.; Nakano, D.; Yoshinaga, S.; Takahashi, H.; Anzai, K.; George, J.; Torimura, T. MAFLD identifies patients with significant hepatic fibrosis better than NAFLD. Liver Int. 2020, 40, 3018–3030. [Google Scholar] [CrossRef]
- Bali, T.; Chrysavgis, L.; Cholongitas, E. Metabolic-Associated Fatty Liver Disease and Sarcopenia. Endocrinol. Metab. Clin. N. Am. 2023, 52, 497–508. [Google Scholar]
- Yamagishi, K.; Iso, H. The criteria for metabolic syndrome and the national health screening and education system in Japan. Epidemiol. Health 2017, 39, e2017003. [Google Scholar] [CrossRef]
- Tokushige, K.; Ikejima, K.; Ono, M.; Eguchi, Y.; Kamada, Y.; Itoh, Y.; Akuta, N.; Yoneda, M.; Iwasa, M.; Yoneda, M.; et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. J. Gastroenterol. 2021, 56, 951–963. [Google Scholar] [CrossRef]
- Shiha, G.; Korenjak, M.; Casanovas, T.; Mooney, V.; Sigurðardóttir, S.; Koulla, Y.; Soliman, R. MAFLD 2022: An ELPA/ALPA/EASO-ECPO joint statement on disease stigma. J. Hepatol. 2022, 77, 1717–1719. [Google Scholar] [CrossRef]
- Bredella, M.A. Sex Differences in Body Composition. Adv. Exp. Med. Biol. 2017, 1043, 9–27. [Google Scholar]
- Liu, Z.; Lin, C.; Suo, C.; Zhao, R.; Jin, L.; Zhang, T.; Chen, X. Metabolic dysfunction-associated fatty liver disease and the risk of 24 specific cancers. Metabolism 2022, 127, 154955. [Google Scholar] [CrossRef] [PubMed]
- Yokokawa, H.; Fukuda, H.; Saita, M.; Goto, K.; Kaku, T.; Miyagami, T.; Takahashi, Y.; Hamada, C.; Hisaoka, T.; Naito, T. An association between visceral or subcutaneous fat accumulation and diabetes mellitus among Japanese subjects. Diabetol. Metab. Syndr. 2021, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, Y.; Wong, G.; Lee, E.I.; Akhtar, O.; Lopes, R.; Sumida, Y. Epidemiology of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in Japan: A focused literature review. JGH Open 2020, 4, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Kamada, Y.; Takahashi, H.; Shimizu, M.; Kawaguchi, T.; Sumida, Y.; Fujii, H.; Seko, Y.; Fukunishi, S.; Tokushige, K.; Nakajima, A.; et al. Clinical practice advice on lifestyle modification in the management of nonalcoholic fatty liver disease in Japan: An expert review. J. Gastroenterol. 2021, 56, 1045–1061. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Yoneda, M.; Kessoku, T.; Ogawa, Y.; Tomeno, W.; Imajo, K.; Mawatari, H.; Fujita, K.; Hyogo, H.; Ueno, T.; et al. Characteristics of non-obese non-alcoholic fatty liver disease: Effect of genetic and environmental factors. Hepatol. Res. 2016, 46, 1011–1018. [Google Scholar] [CrossRef]
- Zembura, M.; Matusik, P. Sarcopenic Obesity in Children and Adolescents: A Systematic Review. Front. Endocrinol. 2022, 13, 914740. [Google Scholar] [CrossRef]
- Murdock, D.J.; Wu, N.; Grimsby, J.S.; Calle, R.A.; Donahue, S.; Glass, D.J.; Sleeman, M.W.; Sanchez, R.J. The prevalence of low muscle mass associated with obesity in the USA. Skelet. Muscle 2022, 12, 26. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Chalasani, N. Non-invasive assessment of non-alcoholic fatty liver disease: Clinical prediction rules and blood-based biomarkers. J. Hepatol. 2018, 68, 305–315. [Google Scholar] [CrossRef]
- Lee, J.; Vali, Y.; Boursier, J.; Spijker, R.; Anstee, Q.M.; Bossuyt, P.M.; Zafarmand, M.H. Prognostic accuracy of FIB-4, NAFLD fibrosis score and APRI for NAFLD-related events: A systematic review. Liver Int. 2021, 41, 261–270. [Google Scholar] [CrossRef]
- Angulo, P.; Kleiner, D.E.; Dam-Larsen, S.; Adams, L.A.; Bjornsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Keach, J.C.; Lafferty, H.D.; Stahler, A.; et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015, 149, 389–397.e10. [Google Scholar] [CrossRef]
- Tantai, X.; Liu, Y.; Yeo, Y.H.; Praktiknjo, M.; Mauro, E.; Hamaguchi, Y.; Engelmann, C.; Zhang, P.; Jeong, J.Y.; van Vugt, J.L.A.; et al. Effect of sarcopenia on survival. in patients with cirrhosis: A meta-analysis. J. Hepatol. 2022, 76, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.marriott.com/ja/hotels/osajw-jw-marriott-hotel-nara/events/ (accessed on 18 July 2023).
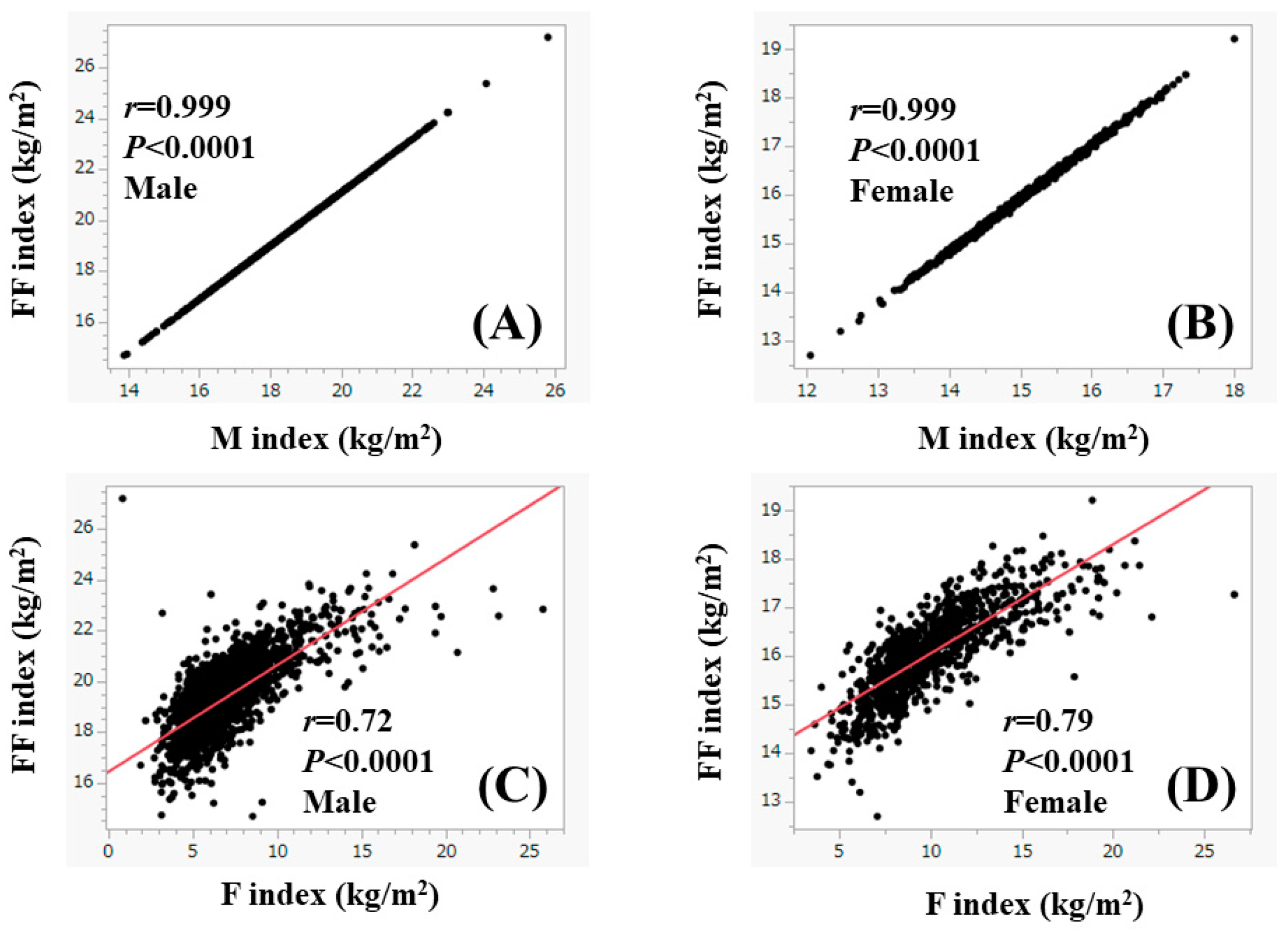
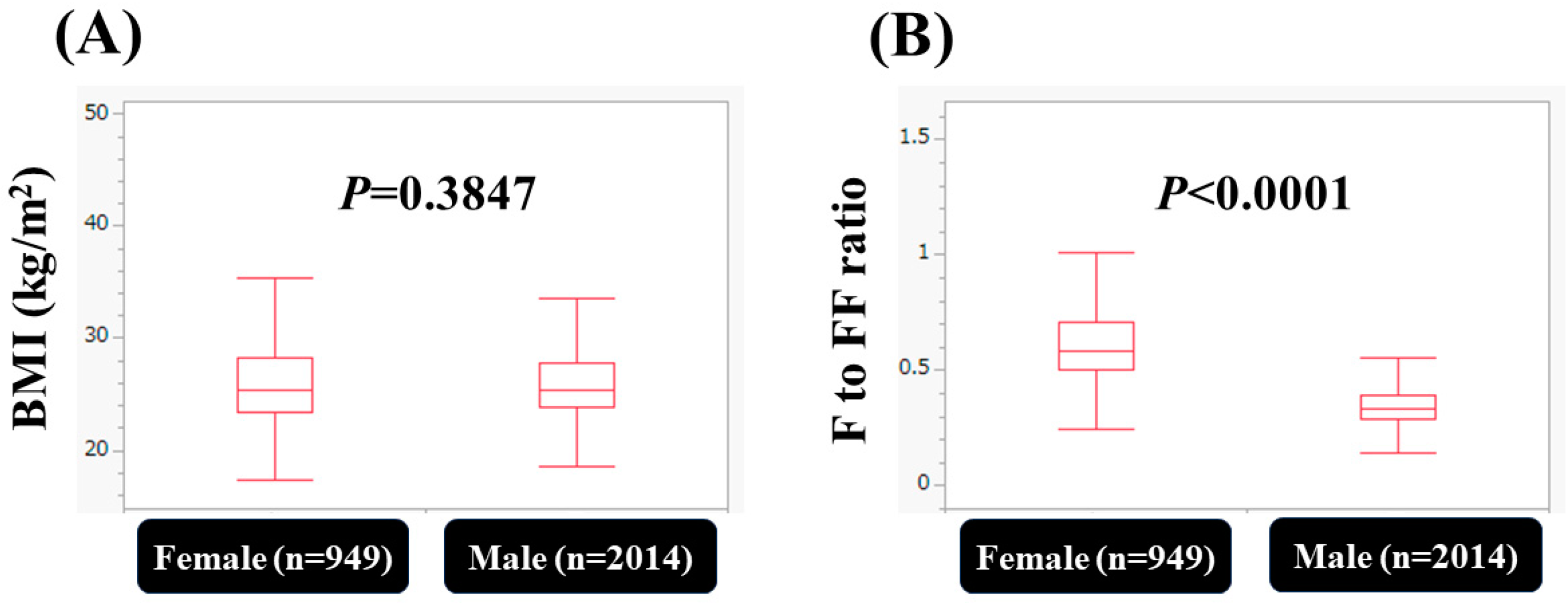
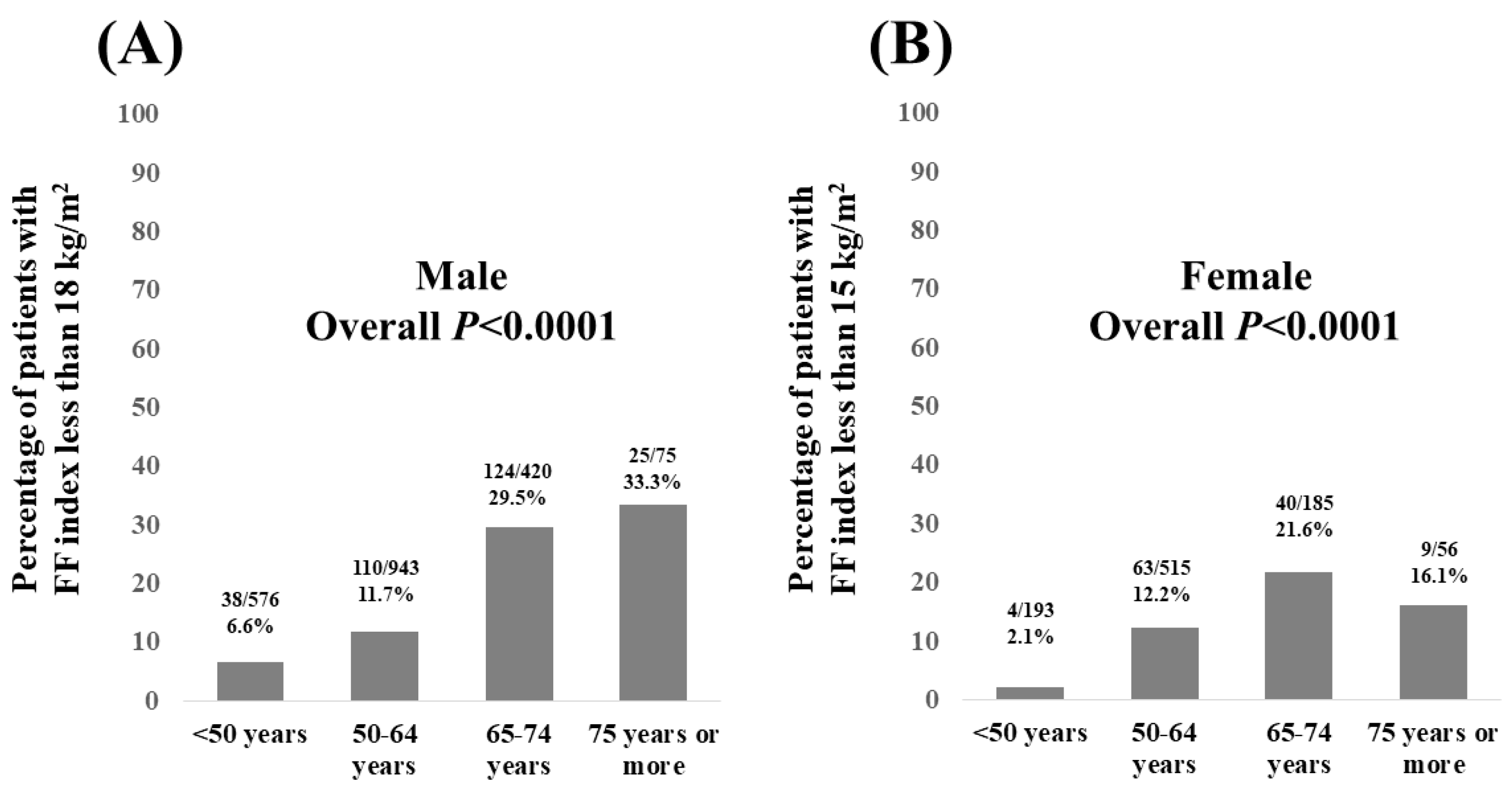
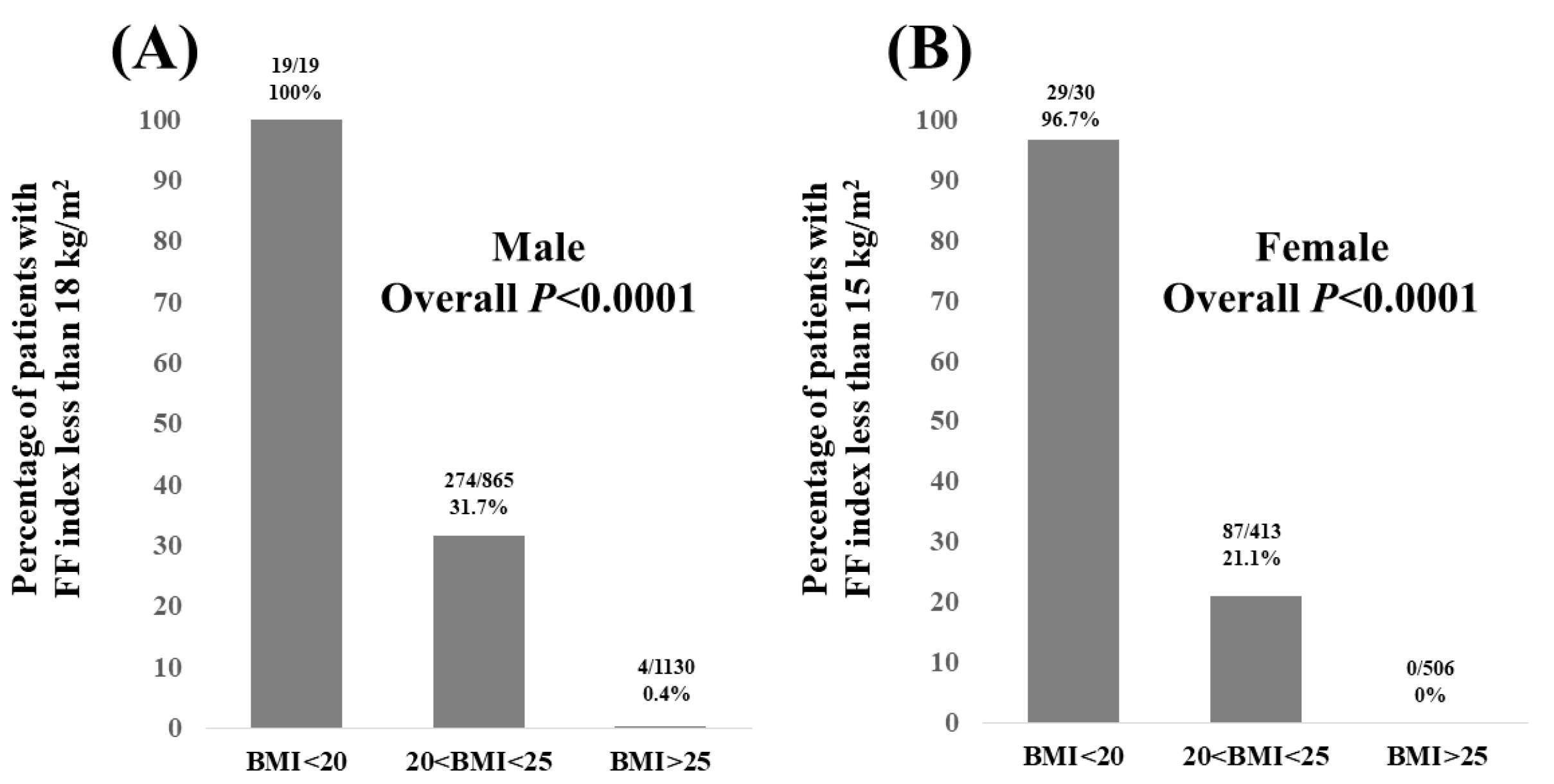
| Male (n = 2014) | Female (n = 949) | p Value | |
|---|---|---|---|
| Age (years) | 55 (27–88) | 57 (25–83) | 0.0052 |
| Body mass index (kg/m2) | 25.4 (17.9–48.6) | 25.4 (17.3–43.9) | 0.3847 |
| Waist circumference (cm) | 90.5 (70–141) | 89 (67–127) | <0.0001 |
| Systolic blood pressure (mmHg) | 127 (78–206) | 125 (78–188) | 0.0756 |
| Diastolic blood pressure (mmHg) | 82 (45–131) | 78 (43–129) | <0.0001 |
| Anti-hypertension drug, yes/no | 694/1320 | 256/693 | 0.0002 |
| Severity of FL (mild/moderate/severe) | 854/858/302 | 459/381/109 | 0.0029 |
| HbA1c (%) | 5.8 (4.1–13.9) | 5.8 (4.1–10.9) | 0.2109 |
| Fasting blood sugar (mg/dL) | 97 (67–336) | 94 (65–262) | <0.0001 |
| Platelet count (×104/μL) | 24.7 (10.6–48.6) | 26.9 (13.4–70.1) | <0.0001 |
| Serum albumin (g/dL) | 4.4 (3.5–5.2) | 4.3 (3.7–5.1) | <0.0001 |
| AST (IU/L) | 24 (11–175) | 21 (10–176) | <0.0001 |
| ALT (IU/L) | 27 (5–307) | 20 (5–195) | <0.0001 |
| ALP (IU/L) | 68 (22–187) | 72 (27–160) | <0.0001 |
| GGT (IU/L) | 39 (8–923) | 25 (8–612) | <0.0001 |
| FIB4 index | 1.03 (0.25–6.73) | 1.02 (0.26–3.89) | 0.0705 |
| Uric acid (mg/dL) | 6.4 (0.8–10.8) | 5.3 (2.4–9.9) | <0.0001 |
| Total cholesterol (mg/dL) | 207 (90–348) | 219 (113–380) | <0.0001 |
| Triglyceride (mg/dL) | 127 (24–1681) | 108 (27–1226) | <0.0001 |
| Anti-hyperlipidemia drug, yes/no | 563/1451 | 276/673 | 0.3400 |
| Habitual smoking, yes/no/unknown | 430/1515/69 | 70/828/50 | <0.0001 |
| Ethanol intake (g/day) | 648/397/397/378/ 125/69 | 583/187/66/53/ 10/50 | <0.0001 |
| 0/<20/20–40/40–60/>60/unknown | |||
| HCV antibody, positive/negative | 17/1997 | 8/941 | 0.9976 |
| HBs antigen, positive/negative | 12/2002 | 1/948 | 0.0595 |
| Fat-free mass index (kg/m2) | 19.2 (14.7–27.2) | 16.0 (12.7–19.2) | <0.0001 |
| Fat mass index (kg/m2) | 6.4 (0.84–25.8) | 9.4 (3.5–26.7) | <0.0001 |
| Muscle mass index (kg/m2) | 18.2 (13.9–25.8) | 15.1 (12.1–18.0) | <0.0001 |
| F index to FF index ratio | 0.344 (0.031–1.129) | 0.588 (0.247–1.544) | <0.0001 |
| (A) | |||
|---|---|---|---|
| Univariate | r | p Value | |
| Age | −0.326 | <0.0001 | |
| Body mass index | 0.882 | <0.0001 | |
| WC | 0.709 | <0.0001 | |
| Fat mass index | 0.719 | <0.0001 | |
| Body fat ratio | 0.592 | <0.0001 | |
| Platelet count | 0.027 | 0.2265 | |
| Serum albumin | 0.0137 | 0.5514 | |
| ALT | 0.315 | <0.0001 | |
| ALP | 0.0335 | 0.1385 | |
| GGT | 0.027 | 0.2260 | |
| FBS | 0.039 | 0.0788 | |
| Total cholesterol | −0.0057 | 0.7987 | |
| Triglyceride | 0.029 | 0.1935 | |
| FIB4 index | −0.166 | <0.0001 | |
| (B) | |||
| Multivariate | Estimates | Standard Error | pValue |
| Age | 9.256 × 10−5 | 8.986 × 10−5 | 0.3031 |
| Body mass index | 0.999 | 0.000905 | <0.0001 |
| WC | 0.0005014 | 0.000179 | 0.0050 |
| Fat mass index | −0.998 | 0.00229 | <0.0001 |
| Body fat ratio | −0.000989 | 0.000653 | 0.1303 |
| ALT | −4.653 × 10−5 | 0.000033 | 0.1598 |
| FIB4 index | −0.000467 | 0.001634 | 0.7749 |
| (A) | |||
|---|---|---|---|
| Univariate | r | p Value | |
| Age | −0.359 | <0.0001 | |
| Body mass index | 0.871 | <0.0001 | |
| WC | 0.654 | <0.0001 | |
| Fat mass index | 0.795 | <0.0001 | |
| Body fat ratio | 0.751 | <0.0001 | |
| Platelet count | 0.105 | 0.0012 | |
| Serum albumin | −0.182 | <0.0001 | |
| ALT | 0.216 | <0.0001 | |
| ALP | −0.011 | 0.7430 | |
| GGT | 0.052 | 0.1068 | |
| FBS | 0.042 | 0.1950 | |
| Total cholesterol | −0.100 | 0.0690 | |
| Triglyceride | 0.065 | 0.0542 | |
| FIB4 index | −0.311 | <0.0001 | |
| (B) | |||
| Multivariate | Estimates | Standard Error | pValue |
| Age | 0.0000958 | 0.000152 | 0.5295 |
| Body mass index | 0.996 | 0.002105 | <0.0001 |
| WC | −0.000233 | 0.000225 | 0.3014 |
| Fat mass index | −0.992 | 0.003502 | <0.0001 |
| Body fat ratio | −0.001285 | 0.000892 | 0.1501 |
| Platelet count | −0.000413 | 0.000225 | 0.0662 |
| Serum albumin | −0.002888 | 0.004628 | 0.5328 |
| ALT | −7.068 × 10−7 | 6.523 × 10−5 | 0.9914 |
| FIB4 index | −0.005708 | 0.004209 | 0.1754 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onishi, S.; Fukuda, A.; Matsui, M.; Ushiro, K.; Nishikawa, T.; Asai, A.; Kim, S.K.; Nishikawa, H. Body Composition Analysis in Patients with Metabolic Dysfunction-Associated Fatty Liver Disease. Nutrients 2023, 15, 3878. https://doi.org/10.3390/nu15183878
Onishi S, Fukuda A, Matsui M, Ushiro K, Nishikawa T, Asai A, Kim SK, Nishikawa H. Body Composition Analysis in Patients with Metabolic Dysfunction-Associated Fatty Liver Disease. Nutrients. 2023; 15(18):3878. https://doi.org/10.3390/nu15183878
Chicago/Turabian StyleOnishi, Saori, Akira Fukuda, Masahiro Matsui, Kosuke Ushiro, Tomohiro Nishikawa, Akira Asai, Soo Ki Kim, and Hiroki Nishikawa. 2023. "Body Composition Analysis in Patients with Metabolic Dysfunction-Associated Fatty Liver Disease" Nutrients 15, no. 18: 3878. https://doi.org/10.3390/nu15183878
APA StyleOnishi, S., Fukuda, A., Matsui, M., Ushiro, K., Nishikawa, T., Asai, A., Kim, S. K., & Nishikawa, H. (2023). Body Composition Analysis in Patients with Metabolic Dysfunction-Associated Fatty Liver Disease. Nutrients, 15(18), 3878. https://doi.org/10.3390/nu15183878







