Association between Maternal Body Composition in Second Trimester and Risk of Fetal Macrosomia: A Population-Based Retrospective Study in China
Abstract
1. Introduction
2. Materials and Methods
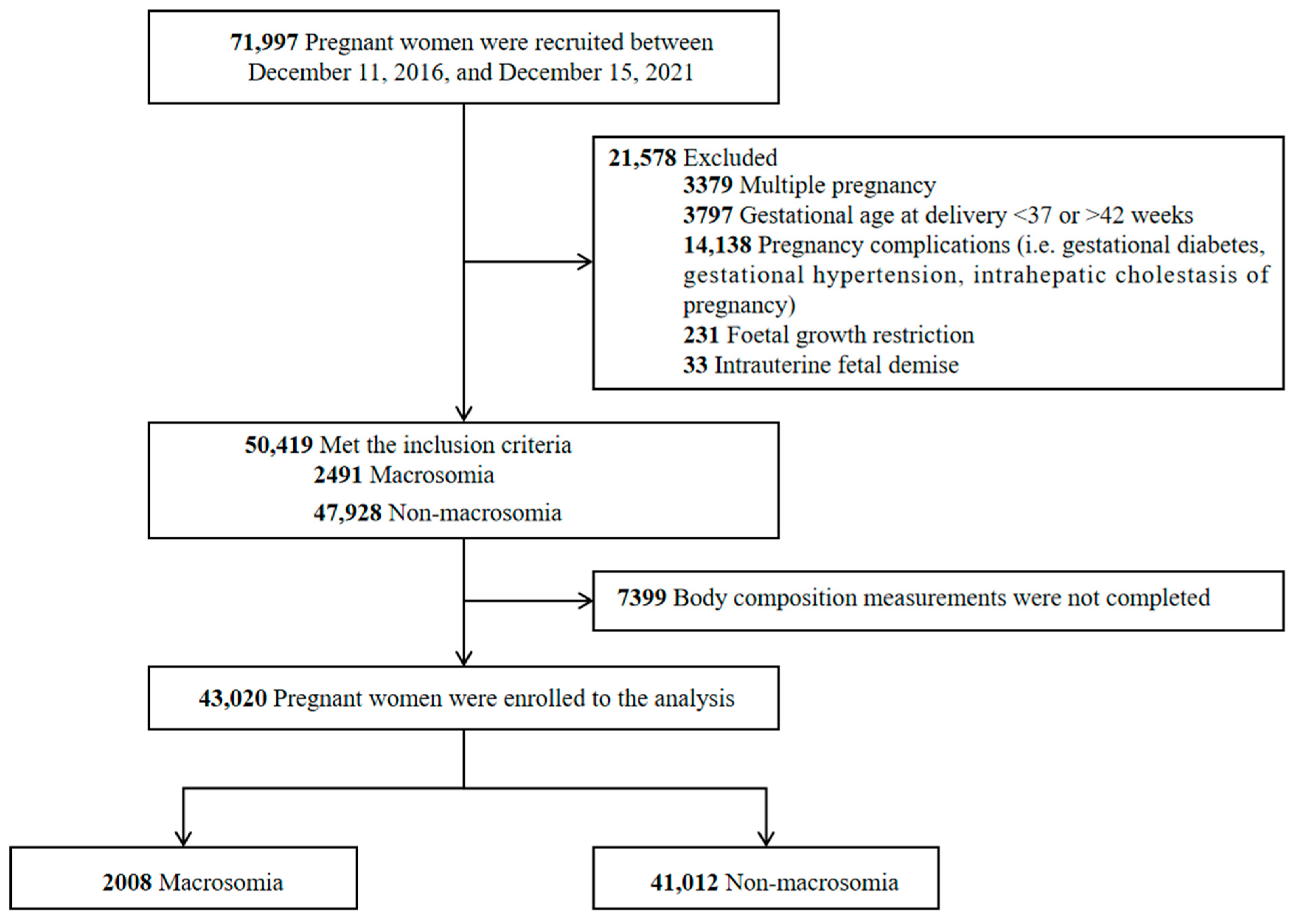
2.1. Study Design and Participants
2.2. Measures and Procedure
2.2.1. Body Composition
2.2.2. Clinical and Sociodemographic Characteristics
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beta, J.; Khan, N.; Khalil, A.; Fiolna, M.; Ramadan, G.; Akolekar, R. Maternal and neonatal complications of fetal macrosomia: Systematic review and meta-analysis. Ultrasound Obs. Gynecol. 2019, 54, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Barth, W.H., Jr.; Jackson, R. Macrosomia: ACOG Practice Bulletin, Number 216. Obs. Gynecol. 2020, 135, e18–e35. [Google Scholar] [CrossRef]
- Koyanagi, A.; Zhang, J.; Dagvadorj, A.; Hirayama, F.; Shibuya, K.; Souza, J.P.; Gülmezoglu, A.M. Macrosomia in 23 developing countries: An analysis of a multicountry, facility-based, cross-sectional survey. Lancet 2013, 381, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Chen, L.; Zhang, S.; Liu, Y.; Wei, J.; Wang, T.; Qin, J. Gestational diabetes mellitus and high triglyceride levels mediate the association between pre-pregnancy Overweight/Obesity and macrosomia: A prospective cohort study in central China. Nutrients 2022, 14, 3347. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.J.; Li, H.T.; Zhang, Y.L.; Zhou, Y.B.; Liu, J.M. Mobile terminal-based survey on the birth characteristics for Chinese newborns. J. Peking Univ. (Health Sci.) 2019, 51, 813–818. [Google Scholar] [CrossRef]
- Juan, J.; Yang, H.; Wei, Y.; Song, G.; Su, R.; Chen, X.; Shan, R.; Yan, J.; Xiao, M.; Li, Y.; et al. Prevalence and characteristics of macrosomia in the first and subsequent pregnancy: A multi-center retrospective study. Chin. Med. J. 2022, 135, 1492–1494. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Cabacungan, E.T. Neonatal Birth Trauma: Analysis of Yearly Trends, Risk Factors, and Outcomes. J. Pediatr. 2021, 238, 174–180.e173. [Google Scholar] [CrossRef]
- Aji, A.S.; Lipoeto, N.I.; Yusrawati, Y.; Malik, S.G.; Kusmayanti, N.A.; Susanto, I.; Majidah, N.M.; Nurunniyah, S.; Alfiana, R.D.; Wahyuningsih, W.; et al. Association between pre-pregnancy body mass index and gestational weight gain on pregnancy outcomes: A cohort study in Indonesian pregnant women. BMC Pregnancy Childbirth 2022, 22, 492. [Google Scholar] [CrossRef]
- Rao, J.; Fan, D.; Wu, S.; Lin, D.; Zhang, H.; Ye, S.; Luo, X.; Wang, L.; Yang, J.; Pang, M.; et al. Trend and risk factors of low birth weight and macrosomia in south China, 2005–2017: A retrospective observational study. Sci. Rep. 2018, 8, 3393. [Google Scholar] [CrossRef]
- Song, X.; Shu, J.; Zhang, S.; Chen, L.; Diao, J.; Li, J.; Li, Y.; Wei, J.; Liu, Y.; Sun, M.; et al. Pre-pregnancy body mass index and risk of macrosomia and large for gestational age births with gestational diabetes mellitus as a mediator: A prospective cohort study in Central China. Nutrients 2022, 14, 1072. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.; Boyle, J.A.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; Rode, L.; et al. Association of Gestational Weight Gain With Maternal and Infant Outcomes: A Systematic Review and Meta-analysis. JAMA 2017, 317, 2207–2225. [Google Scholar] [CrossRef] [PubMed]
- Institute of M. National Research Council Committee to Reexamine IOMPWG. The National Academies Collection: Reports Funded by National Institutes of Health. In Weight Gain During Pregnancy: Reexamining the Guidelines; Rasmussen, K.M., Yaktine, A.L., Eds.; National Academies Press (US): Washington, DC, USA, 2020. [Google Scholar]
- Frankenfield, D.C.; Rowe, W.A.; Cooney, R.N.; Smith, J.S.; Becker, D. Limits of body mass index to detect obesity and predict body composition. Nutrition 2001, 17, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Brunani, A.; Perna, S.; Soranna, D.; Rondanelli, M.; Zambon, A.; Bertoli, S.; Vinci, C.; Capodaglio, P.; Lukaski, H.; Cancello, R. Body composition assessment using bioelectrical impedance analysis (BIA) in a wide cohort of patients affected with mild to severe obesity. Clin. Nutr. 2021, 40, 3973–3981. [Google Scholar] [CrossRef]
- Kuriyan, R. Body composition techniques. Indian J. Med. Res. 2018, 148, 648. [Google Scholar] [CrossRef] [PubMed]
- Ceniccola, G.D.; Castro, M.G.; Piovacari, S.M.F.; Horie, L.M.; Corrêa, F.G.; Barrere, A.P.N.; Toledo, D.O. Current technologies in body composition assessment: Advantages and disadvantages. Nutrition 2019, 62, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Campa, F.; Toselli, S.; Mazzilli, M.; Gobbo, L.A.; Coratella, G. Assessment of Body Composition in Athletes: A Narrative Review of Available Methods with Special Reference to Quantitative and Qualitative Bioimpedance Analysis. Nutrients 2021, 13, 1620. [Google Scholar] [CrossRef]
- Most, J.; Marlatt, K.L.; Altazan, A.D.; Redman, L.M. Advances in assessing body composition during pregnancy. Eur. J. Clin. Nutr. 2018, 72, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Kakade, S.S.; Jagadale, A.B. Development of System for Estimation of Total Body Water (TBW), Fat Mass (FM), Fat Free Mass (FFM) Using Bioimpedance Analysis Technique. In Proceedings of the 2016 International Conference on Communication and Signal Processing (ICCSP), Melmaruvathur, India, 6–8 April 2016; pp. 0224–0226. [Google Scholar]
- Seo, Y.G.; Kim, J.H.; Kim, Y.; Lim, H.; Ju, Y.S.; Kang, M.J.; Lee, K.; Lee, H.J.; Jang, H.B.; Park, S.I.; et al. Validation of body composition using bioelectrical impedance analysis in children according to the degree of obesity. Scand. J. Med. Sci. Sports 2018, 28, 2207–2215. [Google Scholar] [CrossRef]
- Liu, S.; Yao, L.; Chen, Y.; Liu, Z.; Sun, M. Study on the trend of changes in fetal macrosomia in Yantai during the past 30 years. Zhonghua Fu Chan Ke Za Zhi 2002, 37, 469–471. [Google Scholar]
- Bao, C.; Zhou, Y.; Jiang, L.; Sun, C.; Wang, F.; Xia, W.; Han, F.; Zhao, Y.; Wu, L. Reasons for the increasing incidence of macrosomia in Harbin, China. BJOG 2011, 118, 93–98. [Google Scholar] [CrossRef][Green Version]
- Lin, S.; Chai, J.; Li, J.; Shang, X.; Pei, L.; Jiang, L.; Zhang, J.; Sun, P.; Dong, W.; Wang, Y.; et al. Incidence of Macrosomia in Rural Areas-Henan Province, China, 2013–2017. China CDC Wkly. 2021, 3, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Chen, L.; Zhang, S.; Liu, Y.; Wei, J.; Sun, M.; Shu, J.; Wang, T.; Qin, J. High Maternal Triglyceride Levels Mediate the Association between Pre-Pregnancy Overweight/Obesity and Macrosomia among Singleton Term Non-Diabetic Pregnancies: A Prospective Cohort Study in Central China. Nutrients 2022, 14, 2075. [Google Scholar] [CrossRef] [PubMed]
- Nkwabong, E.; Nzalli Tangho, G.R. Risk factors for macrosomia. J. Obstet. Gynecol. India 2015, 65, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Bowers, K.; Laughon, S.; Kiely, M.; Brite, J.; Chen, Z.; Zhang, C. Gestational diabetes, pre-pregnancy obesity and pregnancy weight gain in relation to excess fetal growth: Variations by race/ethnicity. Diabetologia 2013, 56, 1263–1271. [Google Scholar] [CrossRef]
- Terada, M.; Matsuda, Y.; Ogawa, M.; Matsui, H.; Satoh, S. Effects of Maternal Factors on Birth Weight in Japan. J. Pregnancy 2013, 2013, 172395. [Google Scholar] [CrossRef]
- Li, G.; Kong, L.; Li, Z.; Zhang, L.; Fan, L.; Zou, L.; Chen, Y.; Ruan, Y.; Wang, X.; Zhang, W. Prevalence of Macrosomia and Its Risk Factors in C hina: A Multicentre Survey Based on Birth Data Involving 101 723 Singleton Term Infants. Paediatr. Perinat. Epidemiol. 2014, 28, 345–350. [Google Scholar] [CrossRef]
- Guzman-Ortiz, E.; Bueno-Hernandez, N.; Melendez-Mier, G.; Roldan-Valadez, E. Quantitative systematic review: Methods used for the in vivo measurement of body composition in pregnancy. J. Adv. Nurs. 2021, 77, 537–549. [Google Scholar] [CrossRef]
- Ghezzi, F.; Franchi, M.; Balestreri, D.; Lischetti, B.; Mele, M.C.; Alberico, S.; Bolis, P. Bioelectrical impedance analysis during pregnancy and neonatal birth weight. Eur. J. Obs. Gynecol. Reprod. Biol. 2001, 98, 171–176. [Google Scholar] [CrossRef]
- Gernand, A.D. Maternal Nutritional Status, Total Body Ad Extracellular Water, and Placental Weight in Rural Bangladesh: A Pathway to Birth Weight; The Johns Hopkins University: Baltimore, MD, USA, 2011. [Google Scholar] [CrossRef]
- Butte, N.F.; Ellis, K.J.; Wong, W.W.; Hopkinson, J.M.; Smith, E.O.B. Composition of gestational weight gain impacts maternal fat retention and infant birth weight. Am. J. Obstet. Gynecol. 2003, 189, 1423–1432. [Google Scholar] [CrossRef]
- Kent, E.; O’Dwyer, V.; Fattah, C.; Farah, N.; O’Connor, C.; Turner, M.J. Correlation between birth weight and maternal body composition. Obs. Gynecol. 2013, 121, 46–50. [Google Scholar] [CrossRef]
- Forsum, E.; Löf, M.; Olausson, H.; Olhager, E. Maternal body composition in relation to infant birth weight and subcutaneous adipose tissue. Br. J. Nutr. 2006, 96, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Papageorghiou, A.T.; Ohuma, E.O.; Altman, D.G.; Todros, T.; Cheikh Ismail, L.; Lambert, A.; Jaffer, Y.A.; Bertino, E.; Gravett, M.G.; Purwar, M.; et al. International standards for fetal growth based on serial ultrasound measurements: The Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project. Lancet 2014, 384, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Butte, N.F. Carbohydrate and lipid metabolism in pregnancy: Normal compared with gestational diabetes mellitus. Am. J. Clin. Nutr. 2000, 71, 1256s–1261s. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E. Lipid metabolism in pregnancy and its consequences in the fetus and newborn. Endocrine 2002, 19, 43–55. [Google Scholar] [CrossRef]
- Gernand, A.D.; Christian, P.; Paul, R.R.; Shaikh, S.; Labrique, A.B.; Schulze, K.J.; Shamim, A.A.; West, K.P. Maternal weight and body composition during pregnancy are associated with placental and birth weight in rural Bangladesh. J. Nutr. 2012, 142, 2010–2016. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Ouzounian, J.G. Evaluation and management of fetal macrosomia. Obstet. Gynecol. Clin. 2021, 48, 387–399. [Google Scholar] [CrossRef]
- Zafman, K.B.; Bergh, E.; Fox, N.S. Accuracy of sonographic estimated fetal weight in suspected macrosomia: The likelihood of overestimating and underestimating the true birthweight. J. Matern. Fetal Neonatal Med. 2020, 33, 967–972. [Google Scholar] [CrossRef]
- Kayem, G.; Grangé, G.; Bréart, G.; Goffinet, F. Comparison of fundal height measurement and sonographically measured fetal abdominal circumference in the prediction of high and low birth weight at term. Ultrasound Obs. Gynecol. 2009, 34, 566–571. [Google Scholar] [CrossRef]
- Bai, M.; Susic, D.; O’Sullivan, A.; Henry, A. Reproducibility of Bioelectrical Impedance Analysis in Pregnancy and the Association of Body Composition with the Risk of Gestational Diabetes: A Substudy of MUMS Cohort. J. Obes. 2020, 2020, 3128767. [Google Scholar] [CrossRef]

| Variables | Total (N = 43,020) | Macrosomia Group (N = 2008) | Non-Macrosomia Group (N = 41,012) | t/χ2 | p |
|---|---|---|---|---|---|
| Age (years) | 31.27 ± 3.91 | 31.32 ± 3.85 | 31.26 ± 3.92 | 0.65 | 0.517 |
| Nationality | 7.33 | 0.007 | |||
| Han | 42,134 (97.94%) | 1974 (98.31%) | 40,160 (97.92%) | ||
| Minority | 886 (2.06%) | 34 (1.69%) | 852 (2.08%) | ||
| Height (m) | 160.49 ± 4.60 | 161.41 ± 4.85 | 160.26 ± 4.51 | 8.67 | <0.001 |
| Weight (kg) | 56.83 ± 7.43 | 59.23 ± 8.32 | 56.23 ± 7.06 | 14.88 | <0.001 |
| BMI (kg/m2) | 22.06 ± 2.64 | 22.80 ± 2.83 | 21.87 ± 2.56 | 12.14 | <0.001 |
| Gravidity (times) | 2.15 ± 1.34 | 2.20 ± 1.32 | 2.10 ± 1.33 | 2.92 | 0.003 |
| Parity (times) | 1.08 ± 0.67 | 1.08 ± 0.70 | 1.08 ± 0.66 | 0.03 | 0.980 |
| Gestational age | 38.87 ± 2.25 | 39.23 ± 2.28 | 38.78 ± 2.23 | 7.61 | <0.001 |
| GWG (kg) | 14.15 ± 3.69 | 15.64 ± 3.99 | 13.77 ± 3.52 | 16.19 | <0.001 |
| Delivery mode | 249.65 | <0.001 | |||
| Vaginal delivery | 16,872 (39.22%) | 450 (22.40%) | 16,422 (40.04%) | ||
| Cesarean section | 26,148 (60.78%) | 1558 (77.60%) | 24,590 (59.96%) | ||
| Newborn sex | 0.65 | 0.419 | |||
| Female | 24,392 (56.70%) | 1121 (55.83%) | 23,271 (56.74%) | ||
| Male | 18,628 (43.30%) | 887 (44.17%) | 17,741 (43.26%) | ||
| Postpartum hemorrhage | 1856 (4.31%) | 112 (5.58%) | 1744 (4.25%) | 8.14 | 0.004 |
| Total body water (kg) | 28.36 ± 2.83 | 29.31 ± 3.13 | 28.12 ± 2.70 | 14.14 | <0.001 |
| Protein (kg) | 7.52 ± 0.76 | 7.77 ± 0.84 | 7.46 ± 0.73 | 13.81 | <0.001 |
| Minerals (kg) | 2.84 ± 0.28 | 2.93 ± 0.31 | 2.82 ± 0.27 | 13.77 | <0.001 |
| Fat mass (kg) | 18.12 ± 4.65 | 19.46 ± 4.99 | 17.79 ± 4.51 | 12.36 | <0.001 |
| Soft lean mass (kg) | 36.34 ± 3.64 | 37.56 ± 4.02 | 36.04 ± 3.47 | 14.06 | <0.001 |
| Fat-free mass (kg) | 38.72 ± 3.85 | 40.02 ± 4.27 | 38.39 ± 3.68 | 14.11 | <0.001 |
| Skeletal muscle mass (kg) | 20.69 ± 2.30 | 21.45 ± 2.54 | 20.50 ± 2.20 | 13.83 | <0.001 |
| Bone minerals (kg) | 2.38 ± 0.24 | 2.45 ± 0.26 | 2.36 ± 0.22 | 13.96 | <0.001 |
| Percent body fat (%) | 31.44 ± 4.73 | 32.26 ± 4.52 | 31.23 ± 4.76 | 8.16 | <0.001 |
| Waist–hip ratio | 0.86 ± 0.04 | 0.87 ± 0.04 | 0.86 ± 0.04 | 10.40 | <0.001 |
| Visceral fat level | 85.90 ± 27.01 | 92.67 ± 28.62 | 84.18 ± 26.35 | 10.90 | <0.001 |
| Basal metabolic rate (kcal/day) | 1206.39 ± 83.25 | 1234.38 ± 92.17 | 1199.32 ± 79.47 | 14.11 | <0.001 |
| Variables | B-Value | SE | Wald χ2 | p-Value | OR (95%CI) |
|---|---|---|---|---|---|
| Gravidity (times) | 0.091 | 0.019 | 22.919 | <0.001 | 1.096 (1.056, 1.138) |
| Gestational week | 0.076 | 0.006 | 179.025 | <0.001 | 1.079 (1.067, 1.091) |
| BMI (kg/m2) | 0.115 | 0.022 | 27.369 | <0.001 | 1.122 (1.074, 1.171) |
| GWG (kg) | 0.423 | 0.024 | 304.403 | <0.001 | 1.527 (1.456, 1.602) |
| Total body water (kg) | 0.792 | 0.044 | 318.683 | <0.001 | 2.207 (2.023, 2.407) |
| Fat mass (kg) | 0.022 | 0.005 | 20.235 | <0.001 | 1.023 (1.013, 1.033) |
| Fat-free mass (kg) | 1.121 | 0.195 | 33.148 | <0.001 | 3.068 (2.095, 4.493) |
| Skeletal muscle mass (kg) | 0.060 | 0.020 | 8.828 | <0.001 | 1.062 (1.021, 1.105) |
| Visceral fat level | 0.036 | 0.007 | 24.545 | <0.001 | 1.037 (1.022, 1.052) |
| Variables | AUC | 95%CI | p | Cutoff Points | Sensitivity | Specificity | Youden Index |
|---|---|---|---|---|---|---|---|
| Gravidity (times) | 0.530 | 0.516–0.544 | <0.001 | 4.00 | 0.632 | 0.431 | 0.063 |
| Gestational week | 0.654 | 0.641–0.667 | <0.001 | 37.00 | 0.530 | 0.713 | 0.243 |
| BMI (kg/m2) | 0.586 | 0.571–0.600 | <0.001 | 24.50 | 0.251 | 0.856 | 0.107 |
| GWG (kg) | 0.705 | 0.692–0.718 | <0.001 | 14.00 | 0.397 | 0.831 | 0.228 |
| Total body water (kg) | 0.729 | 0.716–0.742 | <0.001 | 37.20 | 0.474 | 0.823 | 0.297 |
| Fat mass (kg) | 0.611 | 0.597–0.624 | <0.001 | 19.50 | 0.745 | 0.405 | 0.150 |
| Fat-free mass (kg) | 0.742 | 0.730–0.755 | <0.001 | 29.90 | 0.505 | 0.831 | 0.336 |
| Skeletal muscle mass (kg) | 0.684 | 0.670–0.697 | <0.001 | 23.90 | 0.515 | 0.752 | 0.276 |
| Visceral fat level | 0.647 | 0.634–0.660 | <0.001 | 85.40 | 0.510 | 0.750 | 0.260 |
| Model 1 | 0.770 | 0.758–0.781 | <0.001 | 0.24 | 0.608 | 0.780 | 0.388 |
| Model 2 | 0.774 | 0.761–0.786 | <0.001 | 0.22 | 0.607 | 0.781 | 0.388 |
| Model 3 | 0.848 | 0.838–0.858 | <0.001 | 0.22 | 0.737 | 0.812 | 0.549 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Huang, C.; Luo, B.; Liao, S. Association between Maternal Body Composition in Second Trimester and Risk of Fetal Macrosomia: A Population-Based Retrospective Study in China. Nutrients 2023, 15, 3879. https://doi.org/10.3390/nu15183879
He Y, Huang C, Luo B, Liao S. Association between Maternal Body Composition in Second Trimester and Risk of Fetal Macrosomia: A Population-Based Retrospective Study in China. Nutrients. 2023; 15(18):3879. https://doi.org/10.3390/nu15183879
Chicago/Turabian StyleHe, Yirong, Chuanya Huang, Biru Luo, and Shujuan Liao. 2023. "Association between Maternal Body Composition in Second Trimester and Risk of Fetal Macrosomia: A Population-Based Retrospective Study in China" Nutrients 15, no. 18: 3879. https://doi.org/10.3390/nu15183879
APA StyleHe, Y., Huang, C., Luo, B., & Liao, S. (2023). Association between Maternal Body Composition in Second Trimester and Risk of Fetal Macrosomia: A Population-Based Retrospective Study in China. Nutrients, 15(18), 3879. https://doi.org/10.3390/nu15183879





