Predictive Value of the Hemoglobin-Geriatric Nutritional Risk Index in Patients with Heart Failure
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics
2.2. Data Source
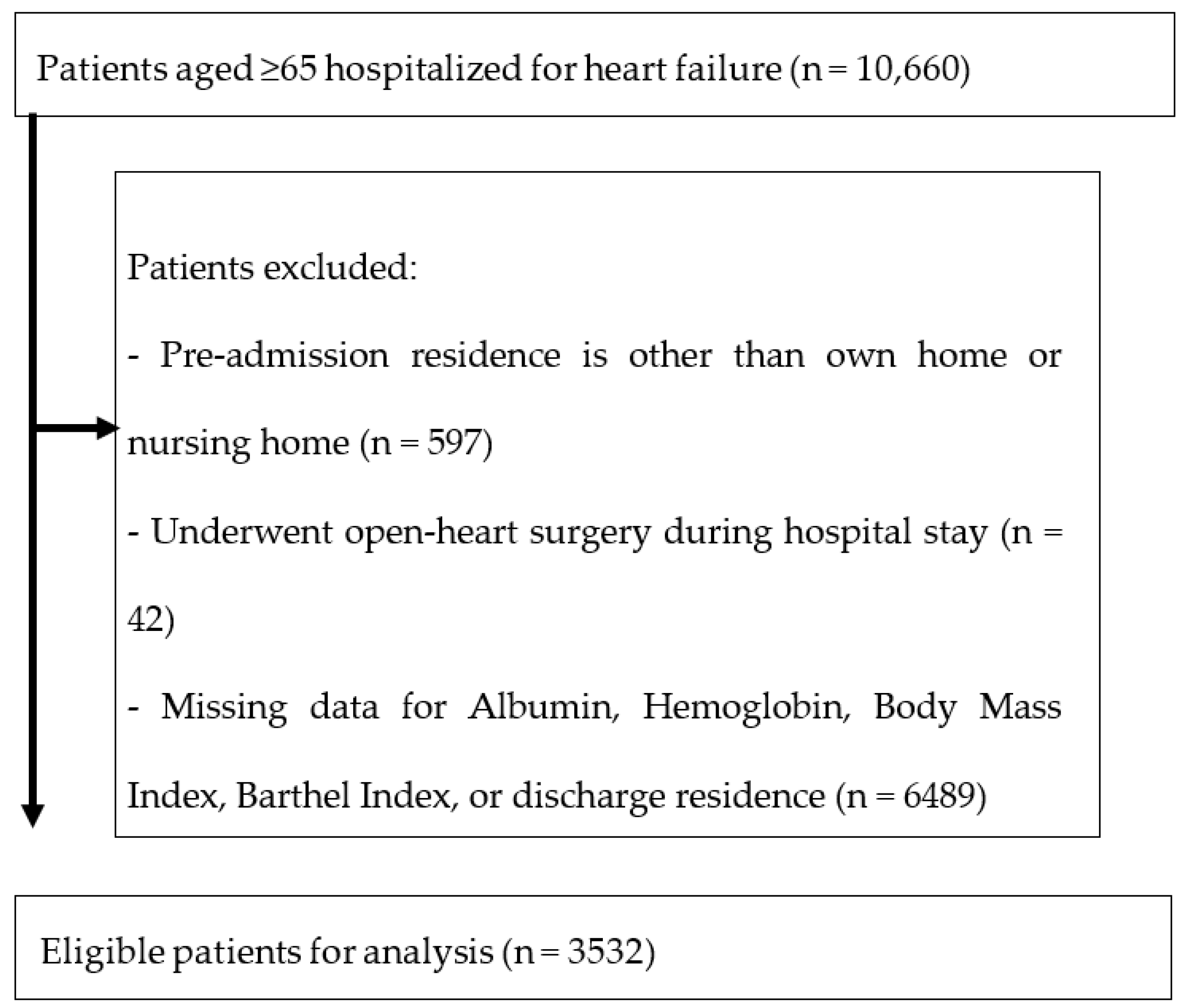
2.3. Participants
2.4. Geriatric Nutritional Risk Index (GNRI)
| GNRI =14.84 × Albumin (g/dL) + 41.7 × weight (kg)/ideal body weight (kg) =14.84 × albumin (g/dL) + 41.7 × weight (kg)/[(height)2(m2)/22] =14.84 × albumin (g/dL) + 41.7 × BMI/22. |
2.5. Hemoglobin-GNRI (H-GNRI)
- Low-risk group (H-GNRI score 2): normal GNRI and normal hemoglobin;
- Intermediate-risk group (H-GNRI score 1): low GNRI or low hemoglobin;
- High-risk group (H-GNRI score 0): low GNRI and low hemoglobin.
2.6. Outcomes
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- GBD. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Albert, N.M.; Allen, L.A.; Bluemke, D.A.; Butler, J.; Fonarow, G.C.; Ikonomidis, J.S.; Khavjou, O.; Konstam, M.A.; Maddox, T.M.; et al. Forecasting the impact of heart failure in the United States: A policy statement from the American Heart Association. Circ. Heart Fail. 2013, 6, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, S.; Kasahara, Y.; Izawa, K.P.; Watanabe, S.; Yoshizawa, K.; Takeichi, N.; Akao, K.; Watanabe, S.; Mizukoshi, K.; Suzuki, N.; et al. Hospital-acquired disability in older heart failure patients decreases independence and increases difficulties in activities of daily living. Eur. J. Cardiovasc. Nurs. 2023, 22, 355–363. [Google Scholar] [CrossRef]
- Uemura, Y.; Shibata, R.; Takemoto, K.; Koyasu, M.; Ishikawa, S.; Murohara, T.; Watarai, M. Prognostic impact of the preservation of Activities of Daily Living on post-discharge outcomes in patients with acute heart failure. Circ. J. 2018, 82, 2793–2799. [Google Scholar] [CrossRef]
- Ogawa, M.; Yoshida, N.; Nakai, M.; Kanaoka, K.; Sumita, Y.; Kanejima, Y.; Emoto, T.; Saito, Y.; Yamamoto, H.; Sakai, Y.; et al. Hospital-associated disability and hospitalization costs for acute heart failure stratified by body mass index- insight from the JROAD/JROAD-DPC database. Int. J. Cardiol. 2022, 367, 38–44. [Google Scholar] [CrossRef]
- Liang, L.; Zhao, X.; Huang, L.; Tian, P.; Huang, B.; Feng, J.; Zhou, P.; Wang, J.; Zhang, J.; Zhang, Y. Prevalence and prognostic importance of malnutrition, as assessed by four different scoring systems, in elder patients with heart failure. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 978–986. [Google Scholar] [CrossRef]
- Dong, C.H.; Chen, S.Y.; Zeng, H.L.; Yang, B.; Pan, J. Geriatric nutritional risk index predicts all-cause mortality in patients with heart failure: A systematic review and meta-analysis. Clinics 2021, 76, e2258. [Google Scholar] [CrossRef]
- Hirose, S.; Miyazaki, S.; Yatsu, S.; Sato, A.; Ishiwata, S.; Matsumoto, H.; Shitara, J.; Murata, A.; Kato, T.; Suda, S.; et al. Impact of the geriatric nutritional risk index on in-hospital mortality and length of hospitalization in patients with acute decompensated heart failure with preserved or reduced ejection fraction. J. Clin. Med. 2020, 9, 1169. [Google Scholar] [CrossRef]
- Kojima, I.; Tanaka, S.; Otobe, Y.; Suzuki, M.; Koyama, S.; Kimura, Y.; Ishiyama, D.; Maetani, Y.; Kusumi, H.; Terao, Y.; et al. What is the optimal nutritional assessment tool for predicting decline in the activity of daily living among older patients with heart failure? Heart Vessels 2022, 37, 1356–1362. [Google Scholar] [CrossRef]
- Honda, Y.; Nagai, T.; Iwakami, N.; Sugano, Y.; Honda, S.; Okada, A.; Asaumi, Y.; Aiba, T.; Noguchi, T.; Kusano, K.; et al. Usefulness of Geriatric Nutritional Risk Index for Assessing Nutritional Status and Its Prognostic Impact in Patients Aged ≥65 Years with Acute Heart Failure. Am. J. Cardiol. 2016, 118, 550–555. [Google Scholar] [CrossRef]
- Wang, B.; Xu, C.; Ying, K.; Chu, J.; Geng, W. Prognostic value of hemoglobin combined with Geriatric Nutritional Risk Index scores in patients undergoing postoperative radiotherapy for esophageal squamous cell carcinoma. Future Oncol. 2022, 18, 179–191. [Google Scholar] [CrossRef]
- Anand, I.S.; Gupta, P. Anemia and Iron Deficiency in Heart Failure: Current Concepts and Emerging Therapies. Circulation 2018, 138, 80–98. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ruiz, A.; Tornel-Osorio, P.L.; Sánchez-Más, J.; Pérez-Fornieles, J.; Vílchez, J.A.; Martínez-Hernández, P.; Pascual-Figal, D.A. Soluble TNFα receptor type I and hepcidin as determinants of development of anemia in the long-term follow-up of heart failure patients. Clin. Biochem. 2012, 45, 1455–1458. [Google Scholar] [CrossRef] [PubMed]
- Alexandrakis, M.G.; Tsirakis, G. Anemia in heart failure patients. Int. Sch. Res. Not. Hematol. 2012, 2012, 246915. [Google Scholar] [CrossRef]
- Anand, I.; McMurray, J.J.; Whitmore, J.; Warren, M.; Pham, A.; McCamish, M.A.; Burton, P.B. Anemia and its relationship to clinical outcome in heart failure. Circulation 2004, 110, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Polat, N.; Yıldız, A.; Bilik, M.Z.; Aydın, M.; Acet, H.; Kaya, H.; Demir, M.; Işık, M.A.; Alan, S.; Toprak, N. The importance of hematologic indices in the risk stratification of patients with acute decompensated systolic heart failure. Turk. Kardiyol. Dern. Ars. 2015, 43, 157–165. [Google Scholar] [CrossRef]
- Nagai, K.; Tanaka, T.; Kodaira, N.; Kimura, S.; Takahashi, Y.; Nakayama, T. Data resource profile: JMDC claims databases sourced from Medical Institutions. J. Gen. Fam. Med. 2020, 21, 211–218. [Google Scholar] [CrossRef]
- Yasunaga, H.; Ide, H.; Imamura, T.; Ohe, K. Impact of the Japanese Diagnosis Procedure Combination-based Payment System on cardiovascular medicine-related costs. Int. Heart J. 2005, 46, 855–866. [Google Scholar] [CrossRef]
- Yamana, H.; Moriwaki, M.; Horiguchi, H.; Kodan, M.; Fushimi, K.; Yasunaga, H. Validity of diagnoses, procedures, and laboratory data in Japanese administrative data. J. Epidemiol. 2017, 27, 476–482. [Google Scholar] [CrossRef]
- Ouchi, Y.; Rakugi, H.; Arai, H.; Akishita, M.; Ito, H.; Toba, K.; Kai, I.; Joint Committee of Japan Gerontological Society (JGLS); Japan Geriatrics Society (JGS) on the definition and classification of the elderly. Redefining the elderly as aged 75 years and older: Proposal from the Joint Committee of Japan Gerontological Society and the Japan Geriatrics Society. Geriatr. Gerontol. Int. 2017, 17, 1045–1047. [Google Scholar] [CrossRef] [PubMed]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel index: A simple index of independence useful in scoring improvement in the rehabilitation of the chronically ill. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Furuya, R.; Takita, T.; Maruyama, Y.; Yamaguchi, Y.; Ohkawa, S.; Kumagai, H. Simplified nutritional screening tools for patients on maintenance hemodialysis. Am. J. Clin. Nutr. 2008, 87, 106–113. [Google Scholar] [CrossRef]
- Tsutsumi, H.; Ohta, M. Diagnosis and treatment of anemia. 4. Anemia of the aged. Nihon Naika Gakkai Zasshi 2006, 10, 2021–2025. [Google Scholar] [CrossRef]
- Ohta, M. Management of anemia in the elderly. Nihon Naika Gakkai Zasshi. 2011, 48, 20–23. [Google Scholar] [CrossRef]
- Kinugasa, Y.; Kato, M.; Sugihara, S.; Hirai, M.; Yamada, K.; Yanagihara, K.; Yamamoto, K. Geriatric nutritional risk index predicts functional dependency and mortality in patients with heart failure with preserved ejection fraction. Circ. J. 2013, 77, 705–711. [Google Scholar] [CrossRef]
- Minamisawa, M.; Miura, T.; Motoki, H.; Ueki, Y.; Nishimura, H.; Shimizu, K.; Shoin, W.; Harada, M.; Mochidome, T.; Senda, K.; et al. Geriatric Nutritional Risk Index Predicts Cardiovascular Events in Patients at Risk for Heart Failure. Circ. J. 2018, 82, 1614–1622. [Google Scholar] [CrossRef]
- Nishi, I.; Seo, Y.; Hamada-Harimura, Y.; Yamamoto, M.; Ishizu, T.; Sugano, A.; Sato, K.; Sai, S.; Obara, K.; Suzuki, S.; et al. Geriatric nutritional risk index predicts all-cause deaths in heart failure with preserved ejection fraction. ESC Heart Fail. 2019, 6, 396–405. [Google Scholar] [CrossRef]
- Minamisawa, M.; Seidelmann, S.B.; Claggett, B.; Hegde, S.M.; Shah, A.M.; Desai, A.S.; Lewis, E.F.; Shah, S.J.; Sweitzer, N.K.; Fang, J.C.; et al. Impact of Malnutrition Using Geriatric Nutritional Risk Index in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2019, 7, 664–675. [Google Scholar] [CrossRef]
- Nakamura, T.; Matsumoto, M.; Haraguchi, Y.; Ishida, T.; Momomura, S.I. Prognostic impact of malnutrition assessed using geriatric nutritional risk index in patients aged ≥80 years with heart failure. Eur. J. Cardiovasc. Nurs. 2020, 19, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.W.; Wang, C.H.; Wu, S.C.; Chen, P.C.; Sheu, C.F.; Hsieh, C.L. Establishing the minimal clinically important difference of the Barthel Index in stroke patients. Neurorehabilit. Neural Repair. 2007, 21, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Unnanuntana, A.; Jarusriwanna, A.; Nepal, S. Validity and responsiveness of Barthel index for measuring functional recovery after hemiarthroplasty for femoral neck fracture. Arch. Orthop. Trauma Surg. 2018, 138, 1671–1677. [Google Scholar] [CrossRef]
- Maraldi, C.; Volpato, S.; Cesari, M.; Cavalieri, M.; Onder, G.; Mangani, I.; Woodman, R.C.; Fellin, R.; Pahor, M. Anemia and recovery from disability in activities of daily living in hospitalized older persons. J. Am. Geriatr. Soc. 2006, 54, 632–636. [Google Scholar] [CrossRef]
- Saitoh, M.; Ozawa, T.; Okamura, D.; Mabuchi, M.; Nakazawa, M.; Aoyagi, M.; Sakamoto, J.; Akiho, M. Effects of let ventricular systolic dysfunction and cardio-renal anemia syndrome on walking ability and Activities of Daily Living in patients with heart failure. Phys. Ther. Jpn. 2014, 17, 40. [Google Scholar] [CrossRef][Green Version]
- Saitoh, M.; Itoh, H.; Morotomi, N.; Ozawa, T.; Ishii, N.; Uewaki, R.; Hori, K.; Shiotani, Y.; Ando, M.; Nakashima, S.; et al. Impact of chronic kidney disease and anemia on physical function in patients with chronic heart failure. Cardiorenal Med. 2014, 4, 73–81. [Google Scholar] [CrossRef]
- Listerman, J.; Geisberg, C.; Nading, M.A.; Goring, J.; Huang, R.; Butler, J. Blunted hemodynamic response and reduced oxygen delivery with exercise in anemic heart failure patients with systolic dysfunction. Congest. Heart Fail. 2007, 13, 71–77. [Google Scholar] [CrossRef]
- Hirani, V.; Naganathan, V.; Blyth, F.; Le Couteur, D.G.; Seibel, M.J.; Waite, L.M.; Handelsman, D.J.; Hsu, B.; Cumming, R.G. Low hemoglobin concentrations are associated with sarcopenia, physical performance, and disability in older Australian men in cross-sectional and longitudinal analysis: The concord health and ageing in men project. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1667–1675. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Sashika, H. Malnutrition is associated with poor rehabilitation outcome in elderly inpatients with hospital-associated deconditioning a prospective cohort study. J. Rehabil. Med. 2014, 46, 277–282. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Sakuma, K. Rehabilitation nutrition for sarcopenia with disability: A combination of both rehabilitation and nutrition care management. J. Cachexia Sarcopenia Muscle 2014, 5, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Hsu, C.C.; Yu, P.C.; Peng, L.N.; Lin, M.H.; Chen, L.K. Hospitalization-associated muscle weakness and functional outcomes among oldest old patients: A hospital-based cohort study. Exp. Gerontol. 2021, 150, 111353. [Google Scholar] [CrossRef] [PubMed]
- de Luis, D.A.; Culebras, J.M.; Aller, R.; Eiros-Bouza, J.M. Surgical infection and malnutrition. Nutr. Hosp. 2014, 30, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Gladysheva, I.P.; Sullivan, R.D.; Pellicori, P. Editorial: Edema in heart failure with reduced ejection fraction. Front. Cardiovasc. Med. 2023, 10, 1141937. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, J.H.; Kang, J.; Cho, H.J.; Lee, H.Y. Association between Changes in Bioelectrical Impedance Analysis (BIA) Parameter and the Clinical Outcomes in Patients with Acute Heart Failure. J. Korean Med. Sci. 2023, 38, e276. [Google Scholar] [CrossRef]
- Saito, H.; Matsue, Y.; Maeda, D.; Kasai, T.; Kagiyama, N.; Endo, Y.; Zoda, M.; Mizukami, A.; Yoshioka, K.; Hashimoto, T.; et al. Prognostic values of muscle mass assessed by dual-energy X-ray absorptiometry and bioelectrical impedance analysis in older patients with heart failure. Geriatr. Gerontol. Int. 2022, 22, 610–615. [Google Scholar] [CrossRef]
| Characteristics | Overall | Low-Risk Group (H-GNRI Score 2) | Intermediate-Risk Group (H-GNRI Score 1) | High-Risk Group (H-GNRI Score 0) | p-Value |
|---|---|---|---|---|---|
| Age, years, n [%] | <0.001 | ||||
| - 65–74 | 460 [13.0] | 83 [34.0] | 153 [16.1] | 224 [9.6] | |
| - 75–89 | 2064 [58.4] | 139 [57.0] | 580 [60.9] | 1345 [57.6] | |
| - ≥90 | 1008 [28.5] | 22 [9.0] | 219 [23.0] | 767 [32.8] | |
| Female sex, n [%] | 1792 [50.7] | 65 [26.6] | 440 [46.2] | 1235 [52.9] | <0.001 |
| Body mass index, n [%] | <0.001 | ||||
| - <18.5 | 722 [20.4] | 7 [2.9] | 72 [7.6] | 643 [27.5] | |
| - 18.5–25 | 2158 [61.1] | 154 [63.1] | 640 [67.2] | 1364 [58.4] | |
| - 25–30 | 605 [17.1] | 80 [32.8] | 224 [23.5] | 301 [12.9] | |
| - ≥30 | 47 [1.3] | 3 [1.2] | 16 [1.7] | 28 [1.2] | |
| New York Heart Association class, n [%] | 0.937 | ||||
| - 1 | 173 [4.9] | 10 [4.1] | 43 [4.5] | 150 [5.1] | |
| - 2 | 689 [19.5] | 42 [17.2] | 188 [19.7] | 459 [19.6] | |
| - 3 | 1404 [39.8] | 103 [42.2] | 378 [39.7] | 923 [39.5] | |
| - 4 | 1129 [32.0] | 82 [33.6] | 306 [32.1] | 741 [31.7] | |
| - Unclear | 137 [3.9] | 7 [2.9] | 37 [3.9] | 93 [4.0] | |
| Admission to hospital by ambulance, n [%] | 1406 [39.8] | 102 [41.8] | 378 [39.7] | 926 [39.6] | 0.804 |
| Ventilator at admission, n [%] | 452 [12.8] | 41 [16.8] | 148 [15.5] | 263 [11.3] | 0.001 |
| Vasopressor at admission, n [%] | 542 [15.3] | 44 [18.0] | 156 [16.4] | 342 [14.6] | 0.218 |
| Years of admission, n [%] | 0.062 | ||||
| - 2017 | 136 [3.9] | 7 [2.9] | 37 [3.9] | 92 [3.9] | |
| - 2018 | 179 [5.1] | 5 [2.0] | 48 [5.0] | 126 [5.4] | |
| - 2019 | 266 [7.5] | 17 [7.0] | 71 [7.5] | 178 [7.6] | |
| - 2020 | 468 [13.3] | 38 [15.6] | 131 [13.8] | 299 [12.8] | |
| - 2021 | 469 [13.3] | 42 [17.2] | 146 [15.3] | 281 [12.0] | |
| - 2022 | 2014 [57.0] | 135 [55.3] | 519 [54.5] | 1360 [58.2] | |
| Number of beds, n [%] | 0.006 | ||||
| - 20–99 | 27 [0.8] | 3 [1.2] | 9 [0.9] | 15 [0.6] | |
| - 100–199 | 708 [20.0] | 29 [11.9] | 189 [19.9] | 490 [21.0] | |
| - 200–299 | 442 [12.5] | 43 [17.6] | 131 [13.8] | 268 [11.5] | |
| - 300–499 | 1179 [33.4] | 76 [31.1] | 310 [32.6] | 793 [33.9] | |
| - ≥500 | 1176 [33.3] | 93 [38.1] | 313 [32.9] | 770 [33.0] | |
| Barthel Index at admission, mean ± SD | 50.7 ± 40.29 | 67.6 ± 39.52 | 57.1 ± 39.81 | 46.2 ± 39.76 | <0.001 |
| Charlson Comorbidity Index, mean ± SD | 2.4 ± 1.48 | 2.1 ± 1.23 | 2.3 ± 1.37 | 2.5 ± 1.54 | <0.001 |
| Outcome Measures | Overall | Low-Risk Group (H-GNRI Score 2) | Intermediate-Risk Group (H-GNRI Score 1) | High-Risk Group (H-GNRI Score 0) | p-Value |
|---|---|---|---|---|---|
| Barthel Index at discharge, mean ± SD | 63.7 ± 40.14 | 86.2 ± 28.55 | 72.8 ± 36.26 | 57.6 ± 41.14 | <0.001 |
| Barthel Index gain, mean ± SD | 13.1 ± 36.90 | 18.6 ± 40.39 | 15.7 ± 36.23 | 11.4 ± 36.69 | <0.001 |
| Barthel Index efficiency, mean ± SD | 1.0 ± 4.95 | 1.7 ± 4.34 | 1.2 ± 5.34 | 0.9 ± 4.83 | <0.001 |
| Length of hospital stay, mean ± SD | 21.5 ± 22.3 | 15.4 ± 13.7 | 18.9 ± 19.0 | 23.3 ± 24.0 | <0.001 |
| In-hospital mortality, n [%] | 409 [11.6] | 10 [4.1] | 67 [7.0] | 332 [14.2] | <0.001 |
| Discharge to home or nursing home, n [%] | 2831 [80.2] | 224 [91.8] | 814 [85.5] | 1793 [76.8] | <0.001 |
| Hospitalization-associated disability, n [%] | 541 [15.3] | 23 [9.4] | 112 [11.8] | 406 [17.4] | <0.001 |
| Variables | Coefficient | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Barthel Index at Discharge | ||||
| Low-risk group (reference) | ― | ― | ― | |
| Intermediate-risk group | −3.87 | −8.27 | 0.51 | 0.084 |
| High-risk group | −11.08 | −15.29 | −6.87 | <0.001 |
| Barthel Index gain | ||||
| Low-risk group (reference) | ― | ― | ― | |
| Intermediate-risk group | −3.87 | −8.27 | 0.51 | 0.084 |
| High-risk group | −11.08 | −15.29 | −6.87 | <0.001 |
| Barthel Index efficiency | ||||
| Low-risk group (reference) | ― | ― | ― | |
| Intermediate-risk group | −0.60 | −1.27 | 0.05 | 0.073 |
| High-risk group | −1.23 | −1.87 | −0.59 | <0.001 |
| Length of hospital stay | ||||
| Low-risk group (reference) | ― | ― | ― | |
| Intermediate-risk group | 1.96 | −1.12 | 5.06 | 0.213 |
| High-risk group | 5.29 | 2.22 | 8.25 | <0.001 |
| Variables | Odds Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| In-hospital mortality | ||||
| Low-risk group (reference) | ― | ― | ― | |
| Intermediate-risk group | 1.37 | 0.68 | 2.77 | 0.372 |
| High-risk group | 2.51 | 1.28 | 4.90 | 0.007 |
| Discharge to home or nursing home | ||||
| Low-risk group (reference) | ― | ― | ― | |
| Intermediate-risk group | 0.64 | 0.38 | 1.07 | 0.091 |
| High-risk group | 0.42 | 0.25 | 0.69 | 0.001 |
| Hospitalization-associated disability | ||||
| Low-risk group (reference) | ― | ― | ― | |
| Intermediate-risk group | 1.13 | 0.70 | 1.84 | 0.602 |
| High-risk group | 1.87 | 1.18 | 2.97 | 0.007 |
| Variables | Coefficient | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age 65–74 | ||||
| Low-risk group (reference) | ― | ― | ― | |
| Intermediate-risk group | −2.38 | −9.63 | 4.87 | 0.519 |
| High-risk group | −4.59 | −11.59 | 2.41 | 0.198 |
| Age 75–89 | ||||
| Low-risk group (reference) | ― | ― | ― | |
| Intermediate-risk group | −5.66 | −11.64 | 0.30 | 0.063 |
| High-risk group | −14.11 | −19.82 | −8.41 | < 0.01 |
| Age ≥90 | ||||
| Low-risk group (reference) | ― | ― | ― | |
| Intermediate-risk group | −9.02 | −21.58 | 3.53 | 0.159 |
| High-risk group | −15.28 | −27.46 | −3.11 | 0.014 |
| Patients | Sample Size | Exclusion Criteria | GNRI Cut off | Outcomes | Conclusions | References |
|---|---|---|---|---|---|---|
| HFpEF | 152 | ・Patients with cancer ・Patients with liver cirrhosis ・Patients on dialysis ・Patients with missing data | 92 | ・all-cause mortality ・HF re-hospitalization ・ADL at discharge | GNRI may be a useful index for predicting functional dependency and mortality | Kinugasa et al. [28] |
| Acute HF | 490 | ・Patients with acute coronary syndrome ・Patients aged <65 ・Patients with missing for GNRI data | 92 | ・all-cause death ・cardiovascular death ・non-cardiovascular death | GNRI is helpful for risk stratification | Honda et al. [11] |
| At risk of HF | 1823 | ・Patients without HF risk ・Patients on dialysis | 107.1 | ・cardiovascular events | GNRI may be useful for predicting cardiovascular events | Minamisawa et al. [29] |
| HFpEF | 110 | ・Patients aged <65 years ・Patients transferred elsewhere ・Patients who died in hospital ・Patients on dialysis ・Patients with missing GNRI data ・Patients other than HFpEF | 92 | ・all-cause mortality | GNRI at discharge is helpful in predicting the long-term prognosis | Nishi et al. [30] |
| HFpEF | 1677 | ・Patients with missing GNRI data | 98 | ・cardiovascular events ・all-cause death ・HF hospitalization | GNRI was associated with an increased risk for cardiovascular events | Minamisawa et al. [31] |
| HF | 213 | ・Patients aged <80 years | 92 | ・all-cause death | GNRI could predict poor prognosis in HF hospitalized patients aged ≧80 years | Nakamura et al. [32] |
| HFpEF/ HFrEF | 451 | ・Patients with acute coronary syndrome ・Patients with active malignancy ・Patients on dialysis ・Patients undergoing surgery during hospitalization ・Patients with missing data | 92 | ・in-hospital mortality ・length of hospital stay | GNRI was not associated with increased in-hospital mortality, GNRI is useful for stratifying patients at high risk for longer length of hospital stay in HFpEF but not in HFrEF | Hirose et al. [9] |
| HF | 91 | ・Patients with cognitive impairment ・Patients with exercise restrictions ・Patients with a pre-admission Barthel Index of less than 85 ・Patients with missing data | 92 | ・ADL decline | GNRI was not associated with ADL | Kojima et al. [10] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tohyama, M.; Shirai, Y.; Shimizu, M.; Kato, Y.; Kokura, Y.; Momosaki, R. Predictive Value of the Hemoglobin-Geriatric Nutritional Risk Index in Patients with Heart Failure. Nutrients 2023, 15, 4789. https://doi.org/10.3390/nu15224789
Tohyama M, Shirai Y, Shimizu M, Kato Y, Kokura Y, Momosaki R. Predictive Value of the Hemoglobin-Geriatric Nutritional Risk Index in Patients with Heart Failure. Nutrients. 2023; 15(22):4789. https://doi.org/10.3390/nu15224789
Chicago/Turabian StyleTohyama, Momoko, Yuka Shirai, Miho Shimizu, Yuki Kato, Yoji Kokura, and Ryo Momosaki. 2023. "Predictive Value of the Hemoglobin-Geriatric Nutritional Risk Index in Patients with Heart Failure" Nutrients 15, no. 22: 4789. https://doi.org/10.3390/nu15224789
APA StyleTohyama, M., Shirai, Y., Shimizu, M., Kato, Y., Kokura, Y., & Momosaki, R. (2023). Predictive Value of the Hemoglobin-Geriatric Nutritional Risk Index in Patients with Heart Failure. Nutrients, 15(22), 4789. https://doi.org/10.3390/nu15224789









