The Impact of Prenatal Vitamin D on Enamel Defects and Tooth Erosion: A Systematic Review
Abstract
1. Introduction
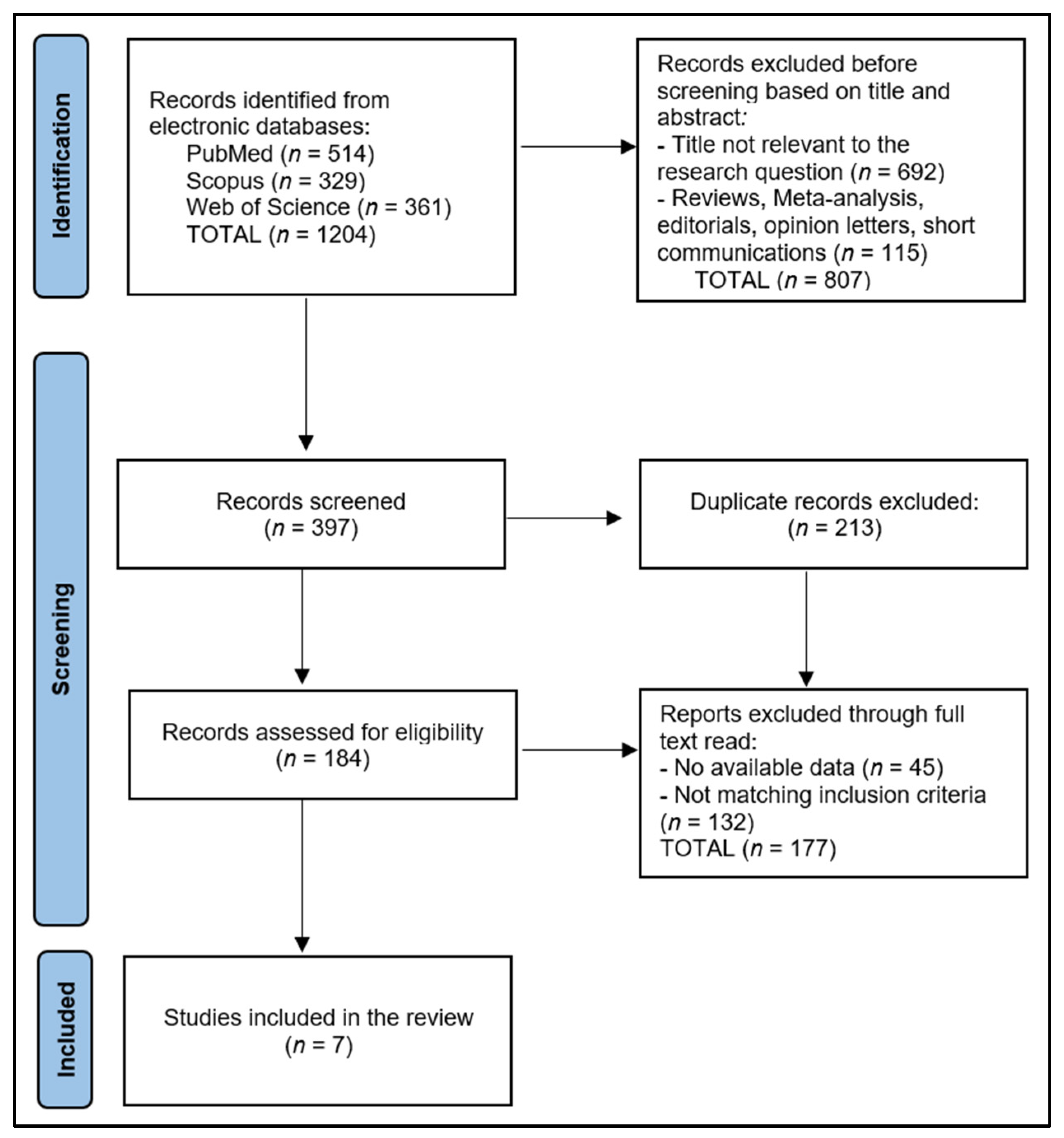
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Data Collection Process
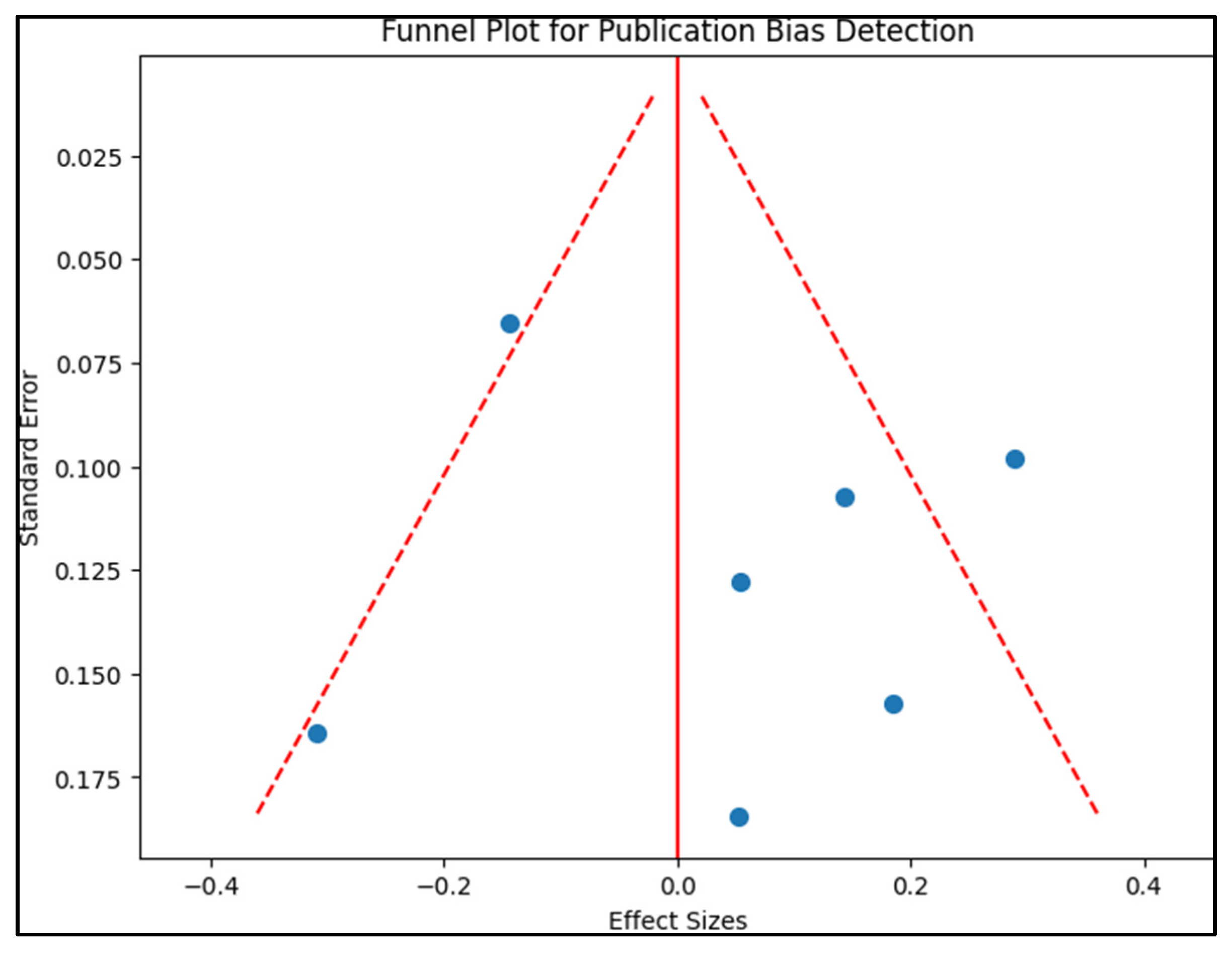
2.4. Risk of Bias
3. Results
3.1. Study Characteristics
3.2. Maternal and Children Characteristics
3.3. Outcomes
4. Discussion
4.1. Summary of Evidence
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Moynihan, P.; Petersen, P.E. Diet, nutrition and the prevention of dental diseases. Public Health Nutr. 2004, 7, 201–226. [Google Scholar] [CrossRef]
- Tungare, S.; Paranjpe, A.G. Diet and Nutrition to Prevent Dental Problems. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK534248/ (accessed on 14 July 2023).
- Hujoel, P.P. Vitamin D and dental caries in controlled clinical trials: Systematic review and meta-analysis. Nutr. Rev. 2013, 71, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Botelho, J.; Machado, V.; Proença, L.; Delgado, A.S.; Mendes, J.J. Vitamin D Deficiency and Oral Health: A Comprehensive Review. Nutrients 2020, 12, 1471. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Diachkova, E.; Trifonova, D.; Morozova, E.; Runova, G.; Ashurko, I.; Ibadulaeva, M.; Fadeev, V.; Tarasenko, S. Vitamin D and Its Role in Oral Diseases Development. Scoping Rev. Dent. J. 2021, 9, 129. [Google Scholar] [CrossRef]
- Suckling, G.W. Developmental defects of enamel-historical and present-day perspectives of their pathogenesis. Adv. Dent. Res. 1989, 3, 87–94. [Google Scholar] [CrossRef]
- Wright, J.T. Enamel Phenotypes: Genetic and Environmental Determinants. Genes 2023, 14, 545. [Google Scholar] [CrossRef] [PubMed]
- Lussi, A.; Carvalho, T.S. Erosive tooth wear: A multifactorial condition of growing concern and increasing knowledge. Erosive Tooth Wear 2014, 25, 1–15. [Google Scholar] [CrossRef]
- Johansson, A.K.; Omar, R.; Carlsson, G.E.; Johansson, A. Dental erosion and its growing importance in clinical practice: From past to present. Int. J. Dent. 2012, 2012, 632907. [Google Scholar] [CrossRef]
- Madi, M.; Pavlic, V.; Mongith Alammar, S.; Mohammad Alsulaimi, L.; Shaker Alotaibi, R.; Mohammed AlOtaibi, G.; Zakaria, O. The association between vitamin D level and periodontal disease in Saudi population, a preliminary study. Saudi Dent. J. 2021, 33, 595–600. [Google Scholar] [CrossRef]
- Chhonkar, A.; Gupta, A.; Arya, V. Comparison of Vitamin D Level of Children with Severe Early Childhood Caries and Children with No Caries. Int J Clin Pediatr Dent. 2018, 11, 199–204. [Google Scholar] [CrossRef]
- Dudding, T.; Thomas, S.J.; Duncan, K.; Lawlor, D.A.; Timpson, N.J. Re-Examining the Association between Vitamin D and Childhood Caries. PLoS ONE 2015, 10, e0143769. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moher, D. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, J.H. PROSPERO: An International Register of Systematic Review Protocols. Med. Ref. Serv. Q. 2019, 38, 171–180. [Google Scholar] [CrossRef]
- Foster, E.D.; Deardorff, A. Open Science Framework (OSF). J. Med. Libr. Assoc. 2017, 105, 203. [Google Scholar] [CrossRef]
- Mortensen, N.B.; Haubek, D.; Dalgård, C.; Nørgaard, S.M.; Christoffersen, L.; Cantio, E.; Rasmussen, A.; Möller, S.; Christesen, H.T. Vitamin D status and tooth enamel hypomineralization are not associated in 4-y-old children: An Odense Child Cohort study. J. Steroid Biochem. Mol. Biol. 2022, 221, 106130. [Google Scholar] [CrossRef] [PubMed]
- Beckett, D.M.; Broadbent, J.M.; Loch, C.; Mahoney, E.K.; Drummond, B.K.; Wheeler, B.J. Dental Consequences of Vitamin D Deficiency during Pregnancy and Early Infancy—An Observational Study. Int. J. Environ. Res. Public Health 2022, 19, 1932. [Google Scholar] [CrossRef]
- Børsting, T.; Schuller, A.; van Dommelen, P.; Stafne, S.N.; Skeie, M.S.; Skaare, A.B.; Mørkved, S.; Salvesen, K.Å.; Stunes, A.K.; Mosti, M.P.; et al. Maternal vitamin D status in pregnancy and molar incisor hypomineralisation and hypomineralised second primary molars in the offspring at 7–9 years of age: A longitudinal study. Eur. Arch. Paediatr. Dent. 2022, 23, 557–566. [Google Scholar] [CrossRef]
- Nørrisgaard, P.E.; Haubek, D.; Kühnisch, J.; Chawes, B.L.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H. Association of High-Dose Vitamin D Supplementation during Pregnancy with the Risk of Enamel Defects in Offspring: A 6-Year Follow-up of a Randomized Clinical Trial. JAMA Pediatr. 2019, 173, 924–930. [Google Scholar] [CrossRef]
- van der Tas, J.T.; Elfrink, M.E.C.; Heijboer, A.C.; Rivadeneira, F.; Jaddoe, V.W.V.; Tiemeier, H.; Schoufour, J.D.; Moll, H.A.; Ongkosuwito, E.M.; Wolvius, E.B.; et al. Foetal, neonatal and child vitamin D status and enamel hypomineralization. Community Dent. Oral. Epidemiol. 2018, 46, 343–351. [Google Scholar] [CrossRef]
- Reed, S.G.; Voronca, D.; Wingate, J.S.; Murali, M.; Lawson, A.B.; Hulsey, T.C.; Ebeling, M.D.; Hollis, B.W.; Wagner, C.L. Prenatal vitamin D and enamel hypoplasia in human primary maxillary central incisors: A pilot study. Pediatr. Dent. J. 2017, 27, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Schroth, R.J.; Lavelle, C.; Tate, R.; Bruce, S.; Billings, R.J.; Moffatt, M.E. Prenatal vitamin D and dental caries in infants. Pediatrics 2014, 133, e1277–e1284. [Google Scholar] [CrossRef]
- Chen, Z.; Lv, X.; Hu, W.; Qian, X.; Wu, T.; Zhu, Y. Vitamin D Status and Its Influence on the Health of Preschool Children in Hangzhou. Front. Public Health 2021, 9, 675403. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.; Creed, S.; Alexander, S.; Barnard, K.; Bridges, N.; Hancock, M. Vitamin D deficiency in children with dental caries—A prevalence study. Arch. Dis. Child. 2012, 97, A103. [Google Scholar] [CrossRef]
- Suárez-Calleja, C.; Aza-Morera, J.; Iglesias-Cabo, T.; Tardón, A. Vitamin D, pregnancy and caries in children in the INMA-Asturias birth cohort. BMC Pediatr. 2021, 21, 380. [Google Scholar] [CrossRef]
- Duarte, M.B.S.; Carvalho, V.R.; Hilgert, L.A.; Ribeiro, A.P.D.; Leal, S.C.; Takeshita, E.M. Is there an association between dental caries, fluorosis, and molar-incisor hypomineralization? J. Appl. Oral Sci. 2021, 29, e20200890. [Google Scholar] [CrossRef]
- Dang, M.H.; Jung, J.E.; Lee, D.W.; Song, K.Y.; Jeon, J.G. Recovery of acid production in Streptococcus mutans biofilms after short-term fluoride treatment. Caries Res. 2016, 50, 363–371. [Google Scholar] [CrossRef]
- Plonka, K.A.; Pukallus, M.L.; Barnett, A.G.; Holcombe, T.F.; Walsh, L.J.; Seow, W.K. A longitudinal case-control study of caries development from birth to 36 months. Caries Res. 2013, 47, 117–127. [Google Scholar] [CrossRef]
- Philip, N.; Suneja, B.; Walsh, L.J. Ecological approaches to dental caries prevention: Paradigm shift or shibboleth? Caries Res. 2018, 52, 153–165. [Google Scholar] [CrossRef]
- Nikiforuk, G.; Fraser, D. The etiology of enamel hypoplasia: A unifying concept. J. Pediatr. 1981, 98, 888–893. [Google Scholar] [CrossRef]
- Tanaka, K.; Hitsumoto, S.; Miyake, Y.; Okubo, H.; Sasaki, S.; Miyatake, N.; Arakawa, M. Higher vitamin D intake during pregnancy is associated with reduced risk of dental caries in young Japanese children. Ann. Epidemiol. 2015, 25, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Gyll, J.; Ridell, K.; Öhlund, I.; Karlsland Åkeson, P.; Johansson, I.; Lif, H.P. Vitamin D status and dental caries in healthy Swedish children. Nutr. J. 2018, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Schroth, R.J.; Levi, J.A.; Sellers, E.A.; Friel, J.; Kliewer, E.; Moffatt, M.E. Vitamin D status of children with severe early childhood caries: A case-control study. BMC Pediatr. 2013, 13, 174. [Google Scholar] [CrossRef] [PubMed]
| Study & Author | Country | Study Year | Study Design | Study Quality |
|---|---|---|---|---|
| 1 Mortensen et al. [17] | Denmark | 2022 | Retrospective Cohort | Good |
| 2 Beckett et al. [18] | New Zealand | 2022 | Retrospective Cohort | Good |
| 3 Nørrisgaard et al. [20] | Denmark | 2019 | Randomized Trial | Excellent |
| 4 van der Tas et al. [21] | The Netherlands | 2018 | Prospective Cohort | Good |
| 5 Reed et al. [22] | USA | 2017 | Randomized Trial | Excellent |
| 6 Schroth et al. [23] | USA | 2014 | Prospective Cohort | Good |
| 7 Børsting et al. [19] | Norway | 2022 | Randomized Trial | Excellent |
| Study Number | Number of Participants | Study Groups | Average Age (Years) | Vitamin D Assessment |
|---|---|---|---|---|
| 1 Mortensen et al. [17] | 1241 | HSPM: 679 (54.7%) No-HSPM: 562 (55.3%) | 30.6 | Supplementation: 83.7% Median: 26.0 ng/mL |
| 2 Beckett et al. [18] | 81 | Sufficient: 43 (53.1%) Insufficient: 14 (17.3%) | 32.9 | Supplementation: 0.0% Mean/Median: NR |
| 3 Nørrisgaard et al. [20] | 496 | High-dose supplementation: 244 (49.2%) Standard-dose supplementation: 252 (50.8%) | 32.5 | Mean: 43.4 ng/mL vs. 28.9 ng/mL |
| 4 van der Tas et al. [21] | 4750 | HSPM: 4278 (90.0%) MIH: | 30.4 | Mean/Median: NR |
| 5 Reed et al. [22] | 29 | With enamel hypoplasia: 13 (44.8%) Without enamel hypoplasia: 16 (55.2%) | 28.6 | Mean: 32.1 ng/mL vs. 33.6 ng/mL |
| 6 Schroth et al. [23] | 205 | Insufficient: 57 (27.8%) | 19.0 | Mean: 19.2 ng/mL |
| 7 Børsting et al. [19] | 176 | Gestational week 18–22 vs. Gestational week 32–36 | 31.2 | Mean: 27.4 ng/mL |
| Study Number | Age at Examination | Sex (Female, %) | Skin Color/Race | Vitamin D Assessment |
|---|---|---|---|---|
| 1 Mortensen et al. [17] | 4.1 years | 47.1% | Caucasian: 96.9% | Deficient: 58.4% Insufficient: 16.1% Median: 18.0 ng/mL |
| 2 Beckett et al. [18] | 6.6 years | 48.1% | Caucasian: 88.0% Maori: 7.4% | Deficient: 34.6% Insufficient: 30.9% |
| 3 Nørrisgaard et al. [20] | 6 years | 49.8% | Caucasian: 95.2% | NR |
| 4 van der Tas et al. [21] | 6.2 years | 50.3% | Caucasian: 65.0% Moroccan and Turkish: 14.0% African: 14.8% | Deficient: 26.5% Insufficient: 23.4% |
| 5 Reed et al. [22] | 3.6 years | 51.2% | Caucasian: 44.9% Hispanic: 31.0% Black: 24.1% | NR |
| 6 Schroth et al. [23] | 6 years | NR | Canadian aboriginal: 90.7% | NR |
| 7 Børsting et al. [19] | 8.1 years | 48.3% | NR | NR |
| Study Number | Outcomes | Risk Assessment (OR) | Other Particularities |
|---|---|---|---|
| 1 Mortensen et al. [17] | HSPM: 54.7% Opacities: 79.5% | Length of gestation: 0.82 * Maternal education: 1.57 * | yellow/brown opacities: 14.9%; post-eruptive breakdown: 5.2%; atypical restoration: 0.4% |
| 2 Beckett et al. [18] | Enamel defects: 64% Opacities: 58% | Maternal Vitamin D insufficiency: 3.55 Vitamin D insufficiency in the children: 1.64 | yellow/brown opacities: 49.4%; Vitamin D insufficiency was not significantly associated with enamel defect prevalence. |
| 3 Nørrisgaard et al. [20] | Enamel defects: 21.1% | Decidious dentition: 2.5 * | Decidious dentition: 12.3% There was no difference in the number of erupted permanent molars between the intervention and the control group. |
| 4 van der Tas et al. [21] | HSPM: 8.9% MIH: 8.2% | High Vitamin D: 0.84 for HSPM High Vitamin D: 0.95 for MIH | The fetal 25(OH)D concentration was not associated with the presence of MIH in children. Children with Vitamin D insufficiency in umbilical cord blood had significantly lower odds of having HSPM than children with sufficient to optimal levels. |
| 5 Reed et al. [22] | Enamel hypoplasia: 44.8% | Low Vitamin D: 1.29 | The fetal 25(OH)D concentration was not associated with the presence of enamel hypoplasia. |
| 6 Schroth et al. [23] | Enamel hypoplasia: 22% Opacities: 36% | Low Vitamin D: 2.18 * | There was a significant inverse relationship between the average number of decayed teeth and prenatal 25(OH)D levels. |
| 7 Børsting et al. [19] | HSPM: 22% MIH: 32% | Maternal Vitamin D insufficiency: 1.82 * | The presence of insufficient maternal Vitamin D levels at mid-pregnancy was related with a larger proportion of MIH in kids at 7–9 years of age. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tapalaga, G.; Bumbu, B.A.; Reddy, S.R.; Vutukuru, S.D.; Nalla, A.; Bratosin, F.; Fericean, R.M.; Dumitru, C.; Crisan, D.C.; Nicolae, N.; et al. The Impact of Prenatal Vitamin D on Enamel Defects and Tooth Erosion: A Systematic Review. Nutrients 2023, 15, 3863. https://doi.org/10.3390/nu15183863
Tapalaga G, Bumbu BA, Reddy SR, Vutukuru SD, Nalla A, Bratosin F, Fericean RM, Dumitru C, Crisan DC, Nicolae N, et al. The Impact of Prenatal Vitamin D on Enamel Defects and Tooth Erosion: A Systematic Review. Nutrients. 2023; 15(18):3863. https://doi.org/10.3390/nu15183863
Chicago/Turabian StyleTapalaga, Gianina, Bogdan Andrei Bumbu, Sandhya Rani Reddy, Sai Diksha Vutukuru, Akhila Nalla, Felix Bratosin, Roxana Manuela Fericean, Catalin Dumitru, Doru Ciprian Crisan, Nicoleta Nicolae, and et al. 2023. "The Impact of Prenatal Vitamin D on Enamel Defects and Tooth Erosion: A Systematic Review" Nutrients 15, no. 18: 3863. https://doi.org/10.3390/nu15183863
APA StyleTapalaga, G., Bumbu, B. A., Reddy, S. R., Vutukuru, S. D., Nalla, A., Bratosin, F., Fericean, R. M., Dumitru, C., Crisan, D. C., Nicolae, N., & Luca, M. M. (2023). The Impact of Prenatal Vitamin D on Enamel Defects and Tooth Erosion: A Systematic Review. Nutrients, 15(18), 3863. https://doi.org/10.3390/nu15183863






