Abstract
The role of overall diet on longevity among cancer survivors (CS) needs further elucidation. We performed a systematic review of the literature and a meta-analysis of related cohort studies published up to October 2022 investigating post-diagnosis a priori (diet quality indices) and a posteriori (data-driven) dietary patterns (DPs) in relation to all-cause and cancer-specific mortality. Pooled hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using random-effects meta-analyses comparing highest versus lowest categories of adherence to DPs. We assessed heterogeneity and risk of bias in the selected studies. A total of 19 cohort studies with 38,846 adult CS, some assessing various DPs, were included in the meta-analyses. Higher adherence to a priori DPs was associated with lower all-cause mortality by 22% (HR = 0.78, 95% CI: 0.73–0.83, I2 = 22.6%) among all CS, by 22% (HR = 0.78, 95% CI: 0.73–0.84, I2 = 0%) among breast CS and by 27% (HR = 0.73, 95% CI: 0.62–0.86, I2 = 41.4%) among colorectal CS. Higher adherence to a “prudent/healthy” DP was associated with lower all-cause mortality (HR = 0.79, 95% CI: 0.64–0.97 I2 = 49.3%), whereas higher adherence to a “western/unhealthy” DP was associated with increased all-cause mortality (HR = 1.48, 95% CI: 1.26–1.74, I2 = 0%) among all CS. Results for cancer-specific mortality were less clear. In conclusion, higher adherence to a “healthy” DP, either a priori or a posteriori, was inversely associated with all-cause mortality among CS. A “healthy” overall diet after cancer diagnosis could protect and promote longevity and well-being.
1. Introduction
Cancer survivors form a fast-growing segment of the population worldwide. In 2018, 43.8 million people were diagnosed with cancer within the previous five years [1]. Although improvement in cancer survival, observed during the past decades for many cancer sites, is considered a great achievement, cancer survivors have important concerns and face several challenges, such as the late and long-term effects of cancer and its treatment on their survival and quality of life [2,3].
Lifestyle habits and modifications related to a healthy diet and regular physical activity after cancer diagnosis are potentially important behaviors through which cancer survivors could protect and promote their well-being and longevity [4,5].
Several studies among cancer survivors have highlighted that their diet is often characterized by poor dietary habits, unfavorable consumption of specific food groups or nutrients, such as low intake of whole grains and healthy fatty acids, unwanted weight gain and overuse of dietary supplements [6,7,8,9,10]. Furthermore, cancer survivors have consistently expressed their need for additional nutrition guidance and focused dietary advice [11]. Due to a lack of sufficient evidence and shortage of studies conducted among cancer survivors worldwide, currently, dietary recommendations for cancer survivors are the same as those addressed to apparently healthy adults for the primary prevention of cancer, according to the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer [12,13]. More specifically, cancer survivors are encouraged, unless otherwise advised, to eat more whole grains, vegetables, fruits and legumes, to limit consumption of red meat, “fast foods” and processed foods high in fat, starches or sugars and to avoid processed meat, alcohol and sugary drinks. The guidelines stress that although adherence to each of the individual recommendations is endorsed, there is potentially more benefit, if these are treated as an integrated pattern, which, combined with regular physical activity and avoidance of obesity, will have a major impact on cancer risk.
In that respect, studying the role of dietary patterns, defined as the combinations, quantities and frequencies foods and beverages are habitually consumed, in relation to cancer survivors’ health, is especially appealing since these patterns capture the influence of overall diet on health and well-being, while they also offer an intuitive understanding and an easier interpretation of research findings [14,15,16]. Moreover, the study of dietary patterns, in addition to the study of individual foods and nutrients, is a key element in the process of reviewing the scientific evidence and formulating dietary guidelines for the general population, providing easy to translate, real-life dietary recommendations [17,18]. Two main research approaches have been used for the study of dietary patterns and their association with disease risk [15,16]. The hypothesis-oriented approach uses a priori-defined indices, also called dietary quality indices, to express adherence to a distinct existing dietary pattern, such as the Mediterranean diet, or the level of compliance to formal dietary guidelines, such as guidelines issued by the WCRF/American Institute for Cancer Research (AICR). The data-driven, exploratory approach uses a posteriori-defined dietary patterns, which derive empirically through the application of mathematical/statistical methods, such as principal component and factor analysis, to identify the major dietary pattern/s of a particular study population. Both approaches allow ranking of study participants and quantifying adherence to the specific patterns.
Several systematic reviews, with [19,20,21,22,23,24] or without meta-analyses [25,26,27,28,29], have been conducted so far among cancer survivors, investigating the association between a priori and/or a posteriori dietary patterns, before or after the diagnosis of cancer [20,22] or in both periods [19,21,23,24], in relation to all-cause mortality, cancer-specific mortality or other health outcomes, as well as quality of life. Some of the systematic reviews have focused on studies with survivors from one specific cancer site, such as breast or colorectal cancer survivors, which apparently are the most in numbers so far [21,25,26,27,28], whereas others have included studies with survivors from all cancer sites [19,20,22,24,29].
The majority of findings point out to an inverse association between closer adherence to high-quality diets, as assessed by various a priori dietary patterns or closer adherence to a “prudent/healthy” dietary pattern, as assessed by a posteriori dietary patterns, with all-cause or cancer-specific mortality among cancer survivors [20,21,22,23,24,25,28,29]. In the context of the latest relevant systematic review, a meta-analysis was also performed comparing high versus low adherence to diet quality indices, as assessed by the Healthy Eating Index (HEI) 2005, the HEI 2015 and the Alternative HEI, which found a 23% reduction in overall mortality among breast cancer survivors [23].
Based on the above, the purpose of this study was to synthesize the latest evidence regarding the association of a priori and a posteriori dietary patterns with robust outcome measures, such as total mortality and cancer-specific mortality, expanding our search chronologically, including more databases, implementing robust risk of bias tools and focusing exclusively on the post-diagnosis period, an extremely important period for cancer survivors.
2. Materials and Methods
2.1. Search Strategy
The reporting of this systematic review and meta-analysis followed the updated PRISMA guidelines [30]. We focused on specific cancer sites for which there is prior evidence that a diet-related component may be involved in their etiology [12]. We specifically focused on cancer in the mouth, pharynx, larynx, nasopharynx, oesophagus, lung, stomach, pancreas, liver, gallbladder, colorectum (colon), breast, ovaries, endometrium, cervix, prostate, kidney, bladder and skin. The literature search was performed in three electronic databases, MEDLINE, Scopus and Web of Science from January 2000 up to 9 October 2022 (Supplementary S1). Reference lists of previous meta-analyses and systematic reviews, as well as the identified articles in the present review, were also hand-searched to retrieve any additional relevant articles.
2.2. Study Selection
Studies were eligible to be included if: (i) they had a cohort design, prospective or retrospective, (ii) examined the association of a priori or a posteriori dietary pattern or patterns after cancer diagnosis with at least one of the primary endpoints of interest, all-cause mortality and cancer-specific mortality, (iii) the study population consisted of cancer survivors, defined as women and men aged 18 years and older with a diagnosis of a primary cancer (from the time of diagnosis through the remainder of their lives), (iv) the minimum sample size was 100 participants, (v) the length of follow-up was at least six months, and (vi) provided a measure of association, such as Hazard Ratio (HR), and the corresponding 95% confidence intervals (CIs), or sufficient information for their calculation, for the comparison between the highest versus the lowest category of adherence to one a priori or a posteriori dietary pattern. Abstracts and full-texts were independently screened by two authors (ME-S, IB) and disagreement was resolved by consensus with the authors (VB, ES). Following the literature search, studies were screened and the non-relevant ones were excluded: studies with a population not consisting of cancer survivors, cross-sectional studies, case-control studies, studies using as endpoints cancer incidence or quality of life, investigating physical activity and survival, studies that did not use a priori or a posteriori dietary patterns but intake of individual foods, food groups or macro- and micronutrients, studies with changes in dietary intake or low energy reporting, studies investigating the glycemic load index or indices based on biomarkers of diet or inflammation. The search excluded editorials, letters to the editor, comments, conference abstracts, systematic reviews and meta-analyses, and it was limited to English articles.
2.3. Data Extraction
All data were extracted in a standard pre-determined format including information on first author, publication year, study location, cancer site, cancer stage (where available), study population, age and sex distribution, follow-up duration, outcome assessed (all-cause mortality and cancer-specific mortality), types of dietary patterns and dietary assessment method used, increments or categories used for the analysis of dietary patterns (i.e., values from quartiles/quintiles used to define the highest category and the lowest category taken as reference), adjustment covariates, and the reported measures of associations (i.e., HR with associated 95% CIs).
We extracted the effect estimates comparing the highest vs. the lowest categories of adherence to a priori dietary patterns assessed in each study (e.g., quartiles, tertiles, good vs. poor adherence, adherers vs. non-adherers, high vs. low score) with the aim to assess the association of adherence to a higher quality diet in relation to all-cause and cancer-specific mortality [20,21,22,31]. In the case which higher adherence to a DP indicated a diet of lower quality, as in the case of the empirical dietary inflammatory pattern (EDIP), the inverse HR comparing those least adhering with those most adhering to that pattern was calculated in order to assess adherence to a higher quality diet. For the a posteriori dietary patterns, based on their characterization by the authors in the primary studies, we created two major groups, the “prudent/healthy” and the “western/unhealthy” group, again comparing the highest vs. the lowest categories of adherence to each pattern. Reported measures of association such as HR, or equivalent estimates, adjusted for the largest number of confounders, were selected and extracted from each study.
2.4. Risk of Bias Assessment of Included Studies
The methodological rigor of the included studies was critically appraised using the ROBINS-I (Risk of Bias In Non-randomized Studies of Interventions) tool, which is proposed for non-randomized studies of interventions/exposures [32]. The ROBINS-I tool comprises seven domains of bias: confounding, selection of participants into the study, classification of exposures, deviations from intended exposures during follow-up, missing data, outcome measurement, and selection of reported result. Each domain is characterized as low, moderate, serious and critical risk of bias, as well as no information (NI), while the overall risk across domains is low only if all domains are characterized as low, moderate if at least one is moderate and high risk of bias if at least one is high. The assessment was performed by two researchers independently, resolving any potential discrepancies by consensus.
2.5. Statistical Analysis
The pooled estimate regarding the association of the highest vs. the lowest categories of adherence to post-diagnosis a priori and a posteriori dietary patterns, grouped by cancer site and overall, with each of the outcomes of interest, i.e., all-cause and cancer-specific mortality, was estimated by random effects meta-analysis models to take into account the between-study heterogeneity. The between-studies variance was estimated using the approach by Der Simonian and Laird [33]. Heterogeneity was assessed by the I2 statistic, with values >0.50% considered as substantial heterogeneity, and graphically by Galbraith plots [34,35]. Publication bias was assessed by funnel plots. Egger’s test was used to investigate the asymmetry in the case of more than 10 studies. We further applied subgroup analysis among studies by their assessment of the overall risk of bias. A cumulative meta-analysis by year of publication was also performed for all-cause mortality and cancer-specific mortality. A sensitivity analysis with influence plots investigating the impact of a priori dietary patterns on overall mortality and cancer-specific mortality by omitting one study at a time and assessing its effect on the overall estimate was also applied. All analyses were performed using the STATA software and the statistical package metan (version 13.1; StataCorp, College Station, TX, USA) [36,37,38,39].
3. Results
3.1. Characteristics of Included Studies and Risk of Bias
In this meta-analysis, we included only prospective or retrospective cohort studies and we focused on the dietary patterns that cancer survivors followed after the diagnosis of cancer. Of the 18,964 studies, 41 studies were assessed for eligibility. From those, 22 studies were excluded because of the following reasons: one had a case-control design, one study did not include cancer survivors, five were referring to pre-diagnosis dietary patterns, 10 studies assessed food groups, one study referred to dietary patterns incorporating physical activity, one study included macronutrients, one study included glycemic index, one study included dietary inflammatory index, one study referred to pre-treatment DP (Figure 1). The list of the excluded studies can be found in the Supplementary Materials (Supplementary S2).
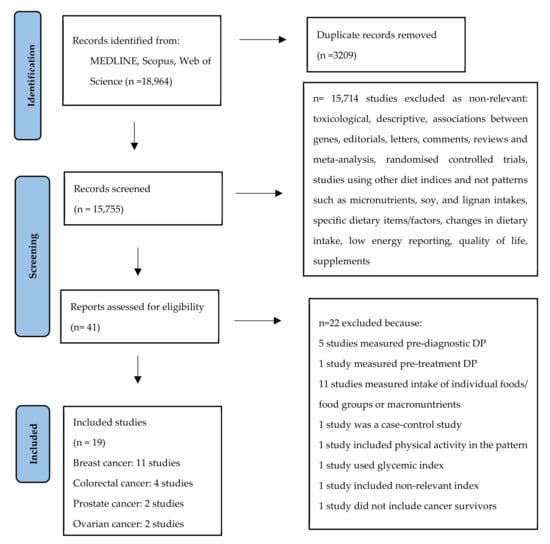
Figure 1.
Flow chart of the literature search process.
Overall, 19 cohort studies, some of them assessing more than one DP, involving 38,846 cancer survivors, men and women, aged ≥ 18 years old, from four specific cancer sites were included in the meta-analysis. More specifically, 11 studies were conducted among breast cancer survivors, four studies among colorectal cancer survivors, two studies among prostate cancer survivors and two studies among ovarian cancer survivors. The studies on breast cancer survivors included 27,161 women with an average follow-up ranging from 4 to 12 years [40,41,42,43,44,45,46,47,48,49,50]; the studies on colorectal cancer survivors included 4935 men and women with an average follow-up ranging from 5 to 11 years [51,52,53,54]; the studies on prostate cancer survivors included 5464 men with follow-up from 8.7 to 24 years [55,56]; and studies on ovarian cancer survivors included 1286 women with follow-up from 4.4 to 20 years [57,58]. Out of the 19 studies, 16 were conducted in North America, one in Europe, one in Asia and one in Australia. All studies used a validated food frequency questionnaire for the assessment of diet, except one that used a 24-hour recall. The main characteristics of the included studies by cancer site are presented in Table 1.

Table 1.
Main characteristics of the cohort studies included in this meta-analysis by cancer survivor site.
The dietary patterns reported included 17 a priori distinct dietary patterns and 2 categories of a posteriori dietary patterns characterized by the authors in the published papers as: (a) “prudent/healthy” and (b) “western/unhealthy” dietary patterns.
With respect to the a priori dietary patterns, these were assessed by the following indices or scores: (1) the Dietary Approaches to Stop Hypertension (DASH) diet, (2) the Healthy Eating Index-2005 (HEI-2005), (3) the Healthy Eating Index-2010 (HEI-2010), (4) the Healthy Eating Index 2015 (HEI-2015), (5) the Alternate Healthy Eating Index (AHEI), (6) the Alternate Healthy Eating Index-2010 (AHEI-2010), (7) the Mediterranean Diet Score (MDS), (8) the alternate Mediterranean Diet Score (aMED), (9) the modified Mediterranean Diet Score (MMDS), (10) the Chinese Food Pagoda-2007 (CHFP-2007), (11) the Chinese Food Pagoda-2016 (CHFP-2016), (12) the Recommended Food Score (RFS), (13) the Diet Quality Index-Revised (DQIR), (14) the American Cancer Society nutrition score (ACS), (15) the Australian Dietary Guidelines Index (DGI), (16) the Empirical dietary inflammatory pattern (EDIP) and (17) the Healthy Nordic Food Index (HNFI). The main characteristics of the indices/scores used to measure adherence to the above mentioned a priori dietary patterns, and more specifically, their characteristic components and short description, are presented in Table 2.

Table 2.
Main characteristics of a priori dietary patterns used in the studies included in this meta-analysis.
With respect to a posteriori dietary patterns, the category of “prudent/healthy” dietary pattern was generally characterized by high intakes of fruits and vegetables, whole grains, legumes and fish, while the category of “western/unhealthy” pattern was described in general as a pattern with high intakes of refined grains, red and processed meats, eggs, solid fats, salty snacks and sweets.
The overall risk of bias using the ROBINS-I tool was considered low for 10 of the studies and moderate for nine of the studies (Table 3).

Table 3.
Assessment of Risk of bias based on the ROBINS-I tool.
3.2. All-Cause Mortality
3.2.1. A Priori DPs and All-Cause Mortality
Τhe results of the random-effects meta-analysis assessing diet quality through a priori dietary patterns with all-cause mortality by cancer site (breast, colorectal, ovarian and prostate cancer survivors) and overall combined are presented in Figure 2.
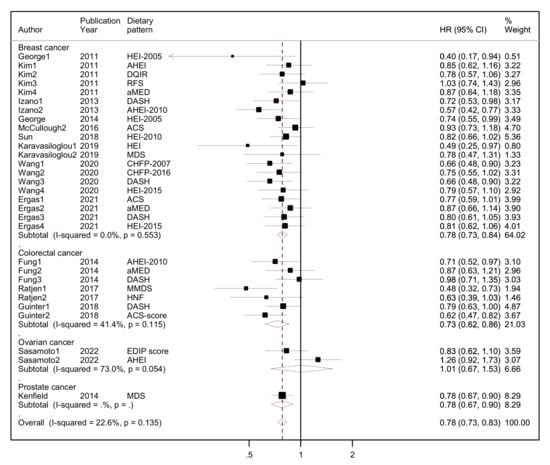
Figure 2.
Forest plot showing the association between highest versus lowest adherence to a priori dietary patterns with all-cause mortality by cancer site and overall, among cancer survivors. Abbreviations: CHFP-2007: Chinese Food Pagoda-2007, CHFP-2016: Chinese Food Pagoda-2016, MDS: Mediterranean Diet Score, HEI-2015: Healthy Eating Index-2015, HEI-2005: Healthy Eating Index-2005, HEI-2010: Healthy Eating Index-2010, aMED, altMed: alternate Mediterranean Diet Score, MMDS: Modified Mediterranean Diet Score, HNFI: Healthy Nordic Food Index, RFS: Recommended Food Score, DASH: Dietary Approaches to Stop Hypertension, EDIP: Empirical dietary inflammatory pattern, ACS: American Cancer Society, DQIR: Diet Quality Index-Revised, AHEI: Alternate Healthy Eating Index, AHEI-2010: Alternate Healthy Eating Index-2010, DGI: Australian Dietary Guideline Index.
Overall, a protective association was found between higher adherence to diet quality indices (highest quintile/quartile) versus lower adherence (lowest quintile/quartile) and all-cause mortality, reaching 22% lower risk (HR = 0.78, 95% CI: 0.73–0.83, I2 = 23%, p-value for heterogeneity = 0.135) among all cancer survivors in 14 unique studies (10 of them assessing more than one a priori pattern). No statistical heterogeneity was observed at the overall effect size or indicated by the Galbraith plot (Supplementary S3, Supplementary Figure S1). There was no evidence of asymmetry in the funnel plot, as also indicated by Egger’s test (Supplementary S3, Supplementary Figure S2).
Among breast cancer survivors, those who reported higher adherence to these dietary patterns compared to those who reported lower adherence had 22% lower risk of all-cause mortality (HR = 0.78, 95% CI: 0.73–0.84, I2 = 0%) in nine unique studies (five of them assessing more than one a priori pattern). Among colorectal cancer survivors, those with higher adherence had 27% lower all-cause mortality (HR = 0.73, 95% CI: 0.62–0.86, I2 = 41.4%, p-value for heterogeneity = 0.115) compared to those with lower adherence in three unique studies (all assessing more than one a priori pattern). No association was found among ovarian cancer survivors comparing high adherence to a priori DPs vs. low adherence and all-cause mortality (HR = 1.01, 95% CI: 0.67–1.53, I2 = 73%, p-value for heterogeneity = 0.054) in one study assessing two a priori DPs. With respect to the one study among prostate cancer survivors, the association was inverse, similar to breast and colorectal cancer survivors (HR = 0.78, 95% CI: 0.67–0.90, I2 = 0%).
3.2.2. A Posteriori DPs and All-Cause Mortality
The results of the random-effects meta-analyses concerning a posteriori-derived “prudent/healthy” and “western/unhealthy” DPs in relation to all-cause mortality by cancer site (breast, colorectal and prostate cancer survivors) and overall combined are presented in Figure 3 (for the “prudent/healthy” DP) and Figure 4 (for the “western/unhealthy” DP).
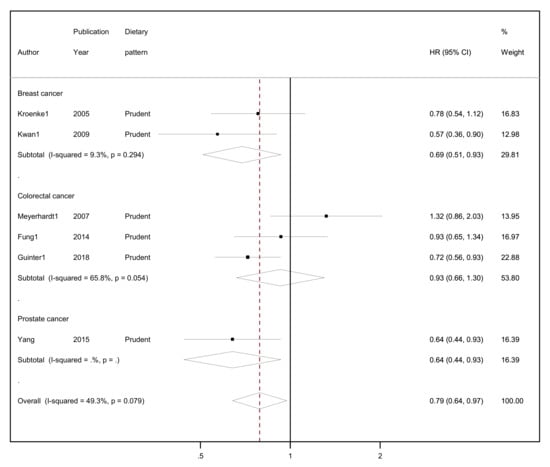
Figure 3.
Forest plot showing the association between highest versus lowest adherence to “prudent/healthy” dietary patterns with all-cause mortality by cancer site and overall, among cancer survivors.
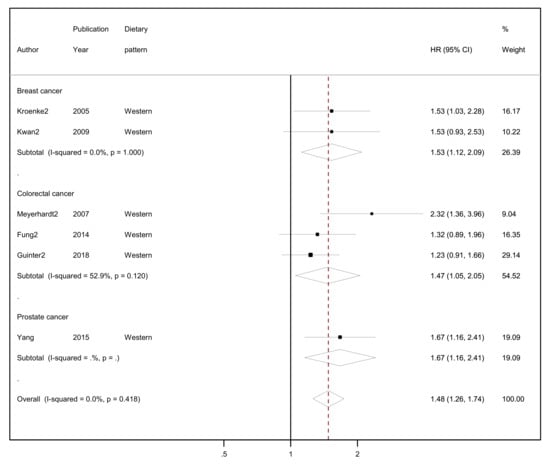
Figure 4.
Forest plot showing the association between highest versus lowest adherence to “western/unhealthy” dietary patterns with all-cause mortality by cancer site and overall, among cancer survivors.
A protective association was found between higher adherence to the “prudent/healthy” dietary patterns versus lower adherence and all-cause mortality, reaching 21% lower risk (HR = 0.79, 95% CI: 0.64–0.97, I2 = 49.3%, p-value for heterogeneity = 0.079; Figure 3) among all cancer survivors. Evidence of heterogeneity was substantial, but the number of studies was small (n = 6). Breast cancer survivors who reported higher adherence in comparison to those who reported lower adherence to a “prudent/healthy” dietary pattern had a 31% lower risk of all-cause mortality (HR = 0.69, 95% CI: 0.51–0.93, I2 = 9.3%, p-value for heterogeneity = 0.294). No heterogeneity was observed regarding the two studies included in the meta-analysis among breast cancer survivors. Similarly, adherence to a “prudent/healthy” dietary pattern was negatively associated with all-cause mortality among colorectal cancer survivors, but the association was not statistically significant (HR = 0.93, 95% CI: 0.66–1.30, I2 = 65.8%, p-value for heterogeneity = 0.054), and although substantial heterogeneity was observed, this was based on few studies (n = 3). The one study on prostate cancer survivors reported 36% lower risk (HR = 0.64, 95% CI: 0.44–0.93) comparing highest with lowest adherence to a “prudent/healthy” dietary pattern.
Regarding the “western/unhealthy” dietary pattern, among cancer survivors from all cancer sites in six studies, higher adherence to this pattern had 48% higher risk of all-cause mortality compared to lower adherence (HR = 1.48, 95% CI: 1.26–1.74, I2 = 0%; Figure 4) with no evidence of heterogeneity. In the two studies among breast cancer survivors who reported highest adherence in comparison to lowest adherence, a 53% higher risk of all-cause mortality was observed (HR = 1.53, 95% CI: 1.12–2.09, I2 = 0%). In three studies with colorectal cancer survivors, the highest adherence to a “western/unhealthy” dietary pattern compared to lowest adherence had a 47% higher risk of all-cause mortality (HR = 1.47, 95% CI: 1.05–2.05, I2 = 52.9%, p-value for heterogeneity = 0.120). Only one study reported results among prostate cancer survivors where the highest level of adherence to a “western” dietary pattern had a 67% higher risk of all-cause mortality compared to the lowest (HR = 1.67, 95% CI: 1.16–2.41).
3.3. Cancer-Specific Mortality
3.3.1. A Priori DPs and Cancer-Specific Mortality
Although an inverse association was noted between higher adherence to a priori dietary patterns and cancer-specific mortality among cancer survivors from all sites, this did not reach statistical significance (HR = 0.91, 95% CI: 0.82–1.01, I2 = 47.2%, p-value for heterogeneity = 0.003; 13 studies; Supplementary S4, Supplementary Figure S3). There was no evidence of asymmetry in the funnel plot, as also indicated by Egger’s test (Supplementary S4, Supplementary Figure S4).
Among breast cancer survivors, higher adherence to a diet quality index was associated with lower breast cancer mortality, but again the association was not statistically significant (HR = 0.90, 95% CI: 0.78–1.03, I2 = 48.9%, p-value for heterogeneity = 0.010; seven studies; Supplementary S4, Supplementary Figure S3). We observed moderate heterogeneity and no evidence of asymmetry in the funnel plot. Results from Egger’s test indicated that there was no evidence of the small study effect in breast cancer studies (Supplementary S5, Supplementary Figure S5).
On the other hand, among colorectal cancer survivors, colorectal cancer mortality was statistically significantly lower, by 33%, among those with high adherence to a priori dietary patterns compared to lower (HR = 0.67, 95% CI: 0.50–0.88, I2 = 26.1%, p-value for heterogeneity = 0.248; Supplementary S4, Supplementary Figure S3). There was no evidence of asymmetry or heterogeneity, but the number of studies was small (two unique studies assessing more than one pattern; Supplementary S6, Supplementary Figure S6). Among ovarian cancer survivors in two unique studies assessing more than one pattern, high adherence to a priori DPs was not statistically associated with cancer mortality (HR = 1.09, 95% CI: 0.92–1.28, I2 = 0%, p-value = 0.434; Supplementary S4, Supplementary Figure S3). The one study among prostate cancer survivors reported no association (HR = 0.98; 95% CI: 0.75–1.29).
3.3.2. A Posteriori DPs and Cancer-Specific Mortality
With respect to adherence to a “prudent/healthy” dietary pattern, an inverse association was found among cancer survivors from all sites (HR = 0.76, 95% CI: 0.58–0.99, I2 = 0%, p-value for heterogeneity = 0.451; Supplementary S7, Supplementary Figure S7) among five studies. Among colorectal cancer survivors, in two studies, higher adherence to a “prudent/healthy” dietary pattern was associated with 36% lower colorectal cancer mortality (HR = 0.64, 95% CI: 0.43–0.95, I2 = 0%, p-value for heterogeneity = 0.848). No association was evident among breast cancer survivors and prostate cancer survivors (Supplementary S7, Supplementary Figure S7).
Regarding the association between the “western/unhealthy” dietary pattern and cancer-specific mortality, higher adherence was associated with 41% higher cancer mortality among cancer survivors from all sites (HR = 1.41, 95% CI: 1.06–1.88, I2 = 0%, p-value for heterogeneity = 0.408; five studies; Supplementary S7, Supplementary Figure S8), with 69% higher colorectal cancer mortality (HR = 1.69, 95% CI: 1.09–2.64, I2 = 0%, p-value for heterogeneity = 0.938; two studies) among colorectal cancer survivors, whereas results were not statistically significant for breast cancer survivors (HR = 1.08, 95% CI: 0.72–1.62, I2 = 0%, p-value for heterogeneity = 0.688; two studies).
3.4. Sensitivity Analysis
In sensitivity analysis, the pooled estimate for all-cause mortality in relation to a priori DPs decreased by 1.3%, when the studies by Kim et al., 2011 [42], by McCullough et al., 2016 [46] and by Sasamoto et al. 2022 [58] were omitted, and increased by a range between 1.3% to 2.6% when the studies by Izano et al., 2013 [44], Ratjen et al., 2017 [53] and Guinter et al., 2018 [54] were omitted (Supplementary S8, Supplementary Figure S9). The pooled estimate for breast cancer mortality regarding a priori DPs, decreased by 3% when the study by Kim et al., 2011 [42] and McCullough et al., 2016 [46] were omitted and by 2% when the study by Izano et al., 2013 [44] was omitted, and increased by 2% when the study by Wang et al., 2020 [49] was omitted (Supplementary S8, Supplementary Figure S10).
Cumulative meta-analysis for a priori dietary patterns and all-cause mortality by year of publication indicated a change in the estimates of HR, with the HR increasing and moving away from the null (HR2011 = 0.85, 95% CI: 0.62–1.16; HR2022 = 0.78, 95% CI: 0.73–0.83) (Supplementary S8, Supplementary Figure S11). Regarding breast cancer mortality, cumulative meta-analysis by year of publication indicated the opposite change, since the overall estimate decreased in magnitude among studies published between 2011 and 2021 (HR2011 = 0.12, 95% CI: 0.02–0.84; HR2021 = 0.89, 95% CI: 0.77–1.03) (Supplementary S8, Supplementary Figure S12).
3.5. Subgroup Analysis
A subgroup analysis for the association between a priori DPs and overall mortality by risk of bias showed a similar association both among the nine studies with moderate risk (HR = 0.72, 95% CI: 0.63–0.82, I2 = 46%, p-value for heterogeneity = 0.035) and among the 10 studies with low risk of bias (HR = 0.81, 95% CI: 0.76-0.86, I2 = 0%) (Supplementary S9, Supplementary Figure S13). Regarding the association of a priori DPs and breast cancer mortality, the subgroup analysis revealed an inverse association only among studies with moderate risk of bias (HR = 0.67, 95% CI: 0.53–0.83, I2 = 24.3%, p-value for heterogeneity = 0.259) (Supplementary S9, Supplementary Figure S14).
4. Discussion
In this systematic review and meta-analysis of cohort studies focusing on the role of post-diagnosis dietary patterns in survival among cancer survivors, greater adherence to diets of higher quality, as assessed by a priori dietary patterns, or greater adherence to a “prudent/healthy” a posteriori dietary pattern, were associated with a significant reduction in all-cause mortality. On the other hand, greater adherence to a “western/unhealthy” a posteriori dietary pattern was associated with a significant increase in all-cause mortality. Among cancer survivors of different sites, the inverse association observed between adherence to a priori dietary patterns and all-cause mortality was more pronounced for colorectal cancer survivors compared to breast cancer survivors or survivors from other sites. Adherence to a “western/unhealthy” dietary pattern was associated with higher all-cause mortality in cancer survivors from all cancer sites, whereas adherence to a “prudent/healthy” dietary pattern was associated with lower all-cause mortality mainly among breast cancer survivors.
With respect to cancer-specific mortality, findings were less clear and were based on few studies; thus, they should be interpreted with caution. Nevertheless, higher adherence to a “western/unhealthy” dietary pattern was associated with increased cancer mortality and higher adherence to a “prudent/healthy” dietary pattern with decreased cancer mortality among all cancer survivors and among colorectal cancer survivors. Cumulative meta-analysis by year of publication revealed stronger inverse associations between a priori dietary patterns and all-cause mortality among cancer survivors of all sites during the more recent years. This could be partly attributed to updates and time-dependent improvements in the characteristics of a priori dietary patterns used in these studies, such as the Healthy Eating Index [22].
Our findings are, in general, in agreement with previous systematic reviews and meta-analyses in which adherence to various a priori dietary patterns was associated with longer survival among cancer survivors [19,20,21,22,23,24]. The a priori dietary patterns investigated in this meta-analysis have differences in terms of their characteristic components, construction of scores and final scoring, as shown in Table 2. Yet, their association with all-cause mortality was evident when they were meta-analyzed together. A careful inspection of their individual components can lead to the observation that all of them reflect core constituents of a “healthy” diet [15]. This “healthy” diet is mostly plant-based, characterized by a high consumption of vegetables, fruits, whole grains, nuts, legumes and preference of plant oils, and less animal-based, characterized by low consumption of red and/or processed meat and moderate dairy consumption. Consumption of foods with low salt content and limited added sugars are also common characteristics of these dietary patterns. The same applies for the “prudent” and “western” a posteriori-derived dietary patterns, which all consist of food group combinations that have been associated with better health for the former, and with worse health for the latter. Thus, the “prudent/healthy” dietary patterns are usually characterized by high intake of fruits, vegetables, whole grains, legumes, fish and low intake of red and processed meat, whereas the ‘‘unhealthy/western’’ dietary patterns are characterized by high intakes of animal-based products, processed meats, refined grains, sweets and desserts, sweetened beverages and salty snacks [52,54,56]. The plausible biological pathways through which the above mentioned a priori and a posteriori “prudent” dietary patterns, may exert their beneficial effects on health, overall and cancer-specific survival, could be attributed to the anti-inflammatory, antithrombotic, antioxidative and antioncogenic properties of vitamins, antioxidants, phytochemicals, potassium, folate, minerals, fiber and healthy fatty acids, constituents abundant in their characteristic food groups [12,76]. On the contrary, the a posteriori “western/unhealthy” dietary patterns may exert their detrimental effects through their high content in saturated and trans fatty acids, added sugars, salt and refined grains and low content in fiber, vitamins, minerals and antioxidants [76].
Compared to previous meta-analyses, our meta-analysis includes solely observational studies, cohort studies in particular, and focuses exclusively on the post-diagnosis period. Although intervention studies can provide the strongest and most direct epidemiologic evidence for the existence of a cause-effect association, they have unique challenges in terms of feasibility, compliance and cost in the context of nutritional epidemiology and especially in the context of investigating “hard” primary outcomes such as mortality [77]. Most randomized controlled trials (RCTs) conducted among cancer survivors investigating the role of diet in survivors’ prognosis so far have used indices of quality of life as primary outcomes and not survival [23]. Carefully conducted observational studies, with low risk of bias, like most of the cohorts included in this meta-analysis, can provide reliable and reproducible evidence on diet and health relationships, whereas well-designed RCTs, of course, can contribute substantially [78]. The focus on the post-diagnosis period in this meta-analysis was deemed necessary to highlight the relative importance of adhering to specific dietary patterns after cancer diagnosis and be able to proceed to specific dietary recommendations for this period. Also, we decided to meta-analyze different a priori indices in relation to all-cause and cancer-specific mortality, as was done in a previous meta-analysis [20], with the rationale that all were constructed based on adherence to a dietary pattern characterized by healthy food groups and/or dietary recommendations to achieve better health.
Limitations of our analysis include the relatively small number of cohort studies conducted during the post-diagnosis period among cancer survivors. Also, the available studies to conduct meta-analysis were limited to breast and colorectal cancer survivors, whereas those on prostate and ovarian cancer survivors were too few to make reliable conclusions. Studies among cancer survivors from other sites with some evidence of diet involvement in their etiology during the post-diagnosis period were not found. The meta-analysis of different a priori dietary patterns can be considered a limitation due to their differences in construction and heterogeneous nature and the inability to attribute and translate the findings to a single dietary pattern. On the other hand, an inherent strength of our study is the investigation of dietary patterns instead of individual food groups in relation to health and disease, which has several advantages, such as better capture of the nutrient-nutrient interactions and food synergies between the individual components of the patterns consumed. Another strength is the search in three databases and the extent of the search until October 2022 with the inclusion of seven additional cohort studies in relation to previous meta-analyses with respect to post-diagnosis a priori or a posteriori dietary patterns among cancer survivors diagnosed with cancers with a probable nutrition-related aspect in their etiology [19,20,21,22,23,24]. Although inherent methodological limitations of observational studies should be considered when interpreting this data, such as residual confounding (e.g by cancer treatment or pre-diagnosis exposures), selection bias or measurement error (e.g self-reported assessment of diet only at one point in time), cohort studies included in this meta-analysis had low to moderate risk of bias assessed by the ROBINS-I tool and results were similar both among low and moderate risk of bias studies. The existence of publication bias and heterogeneity were unlikely based on the results from specific tests and plots.
In conclusion, this systematic review and meta-analysis supports the beneficial role of “healthy” dietary patterns, either a priori or a posteriori, during the post-diagnosis period, in relation to all-cause mortality among cancer survivors. Continuous research on the role of dietary patterns after cancer diagnosis is needed in order to confirm the important role of overall diet and issue evidence-based dietary recommendations that will preserve and promote the health and well-being of cancer survivors.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nu15173860/s1. References [79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100] are cited in the Supplementary Materials. Figure S1: Galbraith’s plot; Figure S2: Funnel plot of a priori dietary patterns and all-cause mortality; Figure S3: Forest plot showing the association between highest versus lowest adherence to a priori dietary patterns and cancer-specific mortality by cancer site and overall; Figure S4: Funnel plot of a priori dietary patterns and cancer-specific mortality among all studies; Figure S5: Funnel plot of a priori dietary patterns and breast cancer mortality; Figure S6: Funnel plot of a priori dietary patterns and colorectal cancer mortality; Figure S7: Forest plot showing the association between highest versus lowest adherence to a “prudent/healthy” dietary pattern and cancer-specific mortality by cancer site and overall, among cancer survivors; Figure S8: Forest plot showing the association between highest versus lowest adherence to “western/unhealthy” dietary pattern and cancer-specific mortality by cancer type and overall, among cancer survivors; Figure S9: Influence plot of a priori dietary patterns and all-cause mortality-all studies; Figure S10: Influence plot of a priori dietary patterns and breast cancer mortality; Figure S11: Cumulative meta-analysis of a priori dietary patterns and all-cause mortality; Figure S12: Cumulative meta-analysis a priori dietary patterns and breast cancer mortality; Figure S13: Forest plot showing the association between highest vs lowest adherence to a priori dietary patterns and all-cause mortality among cancer survivors by risk of bias; Figure S14: Funnel plot of a-priori dietary patterns and all-cause mortality by risk of bias.
Author Contributions
Study protocol M.-E.S., V.B. and E.S.; Study selection M.-E.S. and I.B.; validation, V.B. and E.S.; formal analysis M.-E.S.; writing-original draft preparation, M.-E.S.; writing-review and editing; M.-E.S., V.B., I.B. and E.S.; supervision, V.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval was not required since no individual patient data were collected. This is a meta-analysis based on published data.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created. The data used in this study were found in published articles identified in the references. All results of meta-analyses are shown in tables and figures of the main text and Supplementary Materials.
Acknowledgments
We dedicate this paper to Christina Bamia, who initially proposed to work on this subject.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jemal, A. Cancer Survivorship. In The Cancer Atlas, 2nd ed.; American Cancer Society: Atlanta, GA, USA, 2015; p. 136. [Google Scholar]
- Leach, C.R.; Weaver, K.E.; Aziz, N.M.; Alfano, C.M.; Bellizzi, K.M.; Kent, E.E.; Forsythe, L.P.; Rowland, J.H. The complex health profile of long-term cancer survivors: Prevalence and predictors of comorbid conditions. J. Cancer Surviv. 2015, 9, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Mayer, D.K.; Nasso, S.F.; Earp, J.A. Defining cancer survivors, their needs, and perspectives on survivorship health care in the USA. Lancet Oncol. 2017, 18, e11–e18. [Google Scholar] [CrossRef] [PubMed]
- Basen-Engquist, K.; Alfano, C.M.; Maitin-Shepard, M.; Thomson, C.A.; Stein, K.; Syrjala, K.L.; Fallon, E.; Pinto, B.M.; Schmitz, K.H.; Zucker, D.S.; et al. Moving Research into Practice: Physical Activity, Nutrition, and Weight Management for Cancer Patients and Survivors. NAM Perspect. 2018. [Google Scholar] [CrossRef]
- McTiernan, A.; Friedenreich, C.M.; Katzmarzyk, P.T.; Powell, K.E.; Macko, R.; Buchner, D.; Pescatello, L.S.; Bloodgood, B.; Tennant, B.; Vaux-Bjerke, A.; et al. Physical Activity in Cancer Prevention and Survival: A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, N.; Douglas, P.; Keaver, L. Nutrition Practices among Adult Cancer Survivors Living on the Island of Ireland: A Cross-Sectional Study. Nutrients 2022, 14, 767. [Google Scholar] [CrossRef]
- Lee, E.; Zhu, J.; Velazquez, J.; Bernardo, R.; Garcia, J.; Rovito, M.; Hines, R.B. Evaluation of Diet Quality Among American Adult Cancer Survivors: Results From 2005–2016 National Health and Nutrition Examination Survey. J. Acad. Nutr. Diet. 2021, 121, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.F.; Liu, S.; John, E.M.; Must, A.; Demark-Wahnefried, W. Diet quality of cancer survivors and noncancer individuals: Results from a national survey. Cancer 2015, 121, 4212–4221. [Google Scholar] [CrossRef]
- Marian, M.J. Dietary Supplements Commonly Used by Cancer Survivors: Are There Any Benefits? Nutr. Clin. Pract. 2017, 32, 607–627. [Google Scholar] [CrossRef]
- Kanellopoulou, A.; Riza, E.; Samoli, E.; Benetou, V. Dietary Supplement Use after Cancer Diagnosis in Relation to Total Mortality, Cancer Mortality and Recurrence: A Systematic Review and Meta-Analysis. Nutr. Cancer 2021, 73, 16–30. [Google Scholar] [CrossRef]
- Benetou, V. Nutrition for Cancer Survivors. Nutrients 2022, 14, 4093. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Continuous Project Expert Report 2018. Available online: https://www.wcrf.org/wp-content/uploads/2021/02/Summary-of-Third-Expert-Report-2018.pdf (accessed on 2 July 2022).
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J. Nutr. 2020, 150, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Benetou, V. Impact of Mediterranean Diet on Longevity. In Centenarians: An Example of Positive Biology; Caruso, C., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 161–168. [Google Scholar]
- Bamia, C. Dietary patterns in association to cancer incidence and survival: Concept, current evidence, and suggestions for future research. Eur. J. Clin. Nutr. 2018, 72, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Snetselaar, L.G.; de Jesus, J.M.; DeSilva, D.M.; Stoody, E.E. Dietary Guidelines for Americans, 2020-2025: Understanding the Scientific Process, Guidelines, and Key Recommendations. Nutr. Today 2021, 56, 287–295. [Google Scholar] [CrossRef]
- Tapsell, L.C.; Neale, E.P.; Satija, A.; Hu, F.B. Foods, Nutrients, and Dietary Patterns: Interconnections and Implications for Dietary Guidelines. Adv. Nutr. 2016, 7, 445–454. [Google Scholar] [CrossRef]
- Molina-Montes, E.; Salamanca-Fernandez, E.; Garcia-Villanova, B.; Sanchez, M.J. The Impact of Plant-Based Dietary Patterns on Cancer-Related Outcomes: A Rapid Review and Meta-Analysis. Nutrients 2020, 12, 2010. [Google Scholar] [CrossRef]
- Schwedhelm, C.; Boeing, H.; Hoffmann, G.; Aleksandrova, K.; Schwingshackl, L. Effect of diet on mortality and cancer recurrence among cancer survivors: A systematic review and meta-analysis of cohort studies. Nutr. Rev. 2016, 74, 737–748. [Google Scholar] [CrossRef]
- Hou, R.; Wei, J.; Hu, Y.; Zhang, X.; Sun, X.; Chandrasekar, E.K.; Voruganti, V.S. Healthy dietary patterns and risk and survival of breast cancer: A meta-analysis of cohort studies. Cancer Causes Control 2019, 30, 835–846. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Bogensberger, B.; Hoffmann, G. Diet Quality as Assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and Health Outcomes: An Updated Systematic Review and Meta-Analysis of Cohort Studies. J. Acad. Nutr. Diet 2018, 118, 74–100.e111. [Google Scholar] [CrossRef]
- Castro-Espin, C.; Agudo, A. The Role of Diet in Prognosis among Cancer Survivors: A Systematic Review and Meta-Analysis of Dietary Patterns and Diet Interventions. Nutrients 2022, 14, 348. [Google Scholar] [CrossRef]
- Morze, J.; Danielewicz, A.; Przybylowicz, K.; Zeng, H.; Hoffmann, G.; Schwingshackl, L. An updated systematic review and meta-analysis on adherence to mediterranean diet and risk of cancer. Eur. J. Nutr. 2021, 60, 1561–1586. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jeon, J.Y.; Meyerhardt, J.A. Diet and lifestyle in survivors of colorectal cancer. Hematol. Oncol. Clin. North Am. 2015, 29, 1–27. [Google Scholar] [CrossRef]
- Van Blarigan, E.L.; Meyerhardt, J.A. Role of physical activity and diet after colorectal cancer diagnosis. J. Clin. Oncol. 2015, 33, 1825–1834. [Google Scholar] [CrossRef]
- van Zutphen, M.; Kampman, E.; Giovannucci, E.L.; van Duijnhoven, F.J.B. Lifestyle after Colorectal Cancer Diagnosis in Relation to Survival and Recurrence: A Review of the Literature. Curr. Colorectal. Cancer Rep. 2017, 13, 370–401. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Mele, M.C.; Cintoni, M.; Raoul, P.; Ianiro, G.; Salerno, L.; Pozzo, C.; Bria, E.; Muscaritoli, M.; Molfino, A.; et al. The Facts about Food after Cancer Diagnosis: A Systematic Review of Prospective Cohort Studies. Nutrients 2020, 12, 2345. [Google Scholar] [CrossRef]
- Jochems, S.H.J.; Van Osch, F.H.M.; Bryan, R.T.; Wesselius, A.; van Schooten, F.J.; Cheng, K.K.; Zeegers, M.P. Impact of dietary patterns and the main food groups on mortality and recurrence in cancer survivors: A systematic review of current epidemiological literature. BMJ Open 2018, 8, e014530. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Spei, M.E.; Samoli, E.; Bravi, F.; La Vecchia, C.; Bamia, C.; Benetou, V. Physical activity in breast cancer survivors: A systematic review and meta-analysis on overall and breast cancer survival. Breast 2019, 44, 144–152. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernan, M.A.; Reeves, B.C.; Savovic, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Kacker, R. Random-effects model for meta-analysis of clinical trials: An update. Contemp. Clin. Trials 2007, 28, 105–114. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, R.F. A note on graphical presentation of estimated odds ratios from several clinical trials. Stat. Med. 1988, 7, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef]
- Sterne, J.A.; Sutton, A.J.; Ioannidis, J.P.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef]
- Viechtbauer, W. Publication bias in meta-analysis: Prevention, assessment and adjustments. Psychometrika 2007, 72, 269–271. [Google Scholar] [CrossRef]
- Kroenke, C.H.; Fung, T.T.; Hu, F.B.; Holmes, M.D. Dietary patterns and survival after breast cancer diagnosis. J. Clin. Oncol. 2005, 23, 9295–9303. [Google Scholar] [CrossRef]
- Kwan, M.L.; Weltzien, E.; Kushi, L.H.; Castillo, A.; Slattery, M.L.; Caan, B.J. Dietary patterns and breast cancer recurrence and survival among women with early-stage breast cancer. J. Clin. Oncol. 2009, 27, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Willett, W.C.; Fung, T.; Rosner, B.; Holmes, M.D. Diet quality indices and postmenopausal breast cancer survival. Nutr. Cancer 2011, 63, 381–388. [Google Scholar] [CrossRef]
- George, S.M.; Irwin, M.L.; Smith, A.W.; Neuhouser, M.L.; Reedy, J.; McTiernan, A.; Alfano, C.M.; Bernstein, L.; Ulrich, C.M.; Baumgartner, K.B.; et al. Postdiagnosis diet quality, the combination of diet quality and recreational physical activity, and prognosis after early-stage breast cancer. Cancer Causes Control 2011, 22, 589–598. [Google Scholar] [CrossRef]
- Izano, M.A.; Fung, T.T.; Chiuve, S.S.; Hu, F.B.; Holmes, M.D. Are diet quality scores after breast cancer diagnosis associated with improved breast cancer survival? Nutr. Cancer 2013, 65, 820–826. [Google Scholar] [CrossRef]
- George, S.M.; Ballard-Barbash, R.; Shikany, J.M.; Caan, B.J.; Freudenheim, J.L.; Kroenke, C.H.; Vitolins, M.Z.; Beresford, S.A.; Neuhouser, M.L. Better postdiagnosis diet quality is associated with reduced risk of death among postmenopausal women with invasive breast cancer in the women’s health initiative. Cancer Epidemiol. Biomark. Prev. 2014, 23, 575–583. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McCullough, M.L.; Gapstur, S.M.; Shah, R.; Campbell, P.T.; Wang, Y.; Doyle, C.; Gaudet, M.M. Pre- and postdiagnostic diet in relation to mortality among breast cancer survivors in the CPS-II Nutrition Cohort. Cancer Causes Control 2016, 27, 1303–1314. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Bao, W.; Liu, B.; Caan, B.J.; Lane, D.S.; Millen, A.E.; Simon, M.S.; Thomson, C.A.; Tinker, L.F.; Van Horn, L.V.; et al. Changes in Overall Diet Quality in Relation to Survival in Postmenopausal Women with Breast Cancer: Results from the Women’s Health Initiative. J. Acad. Nutr. Diet 2018, 118, 1855–1863.e1856. [Google Scholar] [CrossRef] [PubMed]
- Karavasiloglou, N.; Pestoni, G.; Faeh, D.; Rohrmann, S. Post-Diagnostic Diet Quality and Mortality in Females with Self-Reported History of Breast or Gynecological Cancers: Results from the Third National Health and Nutrition Examination Survey (NHANES III). Nutrients 2019, 11, 2558. [Google Scholar] [CrossRef]
- Wang, F.; Cai, H.; Gu, K.; Shi, L.; Yu, D.; Zhang, M.; Zheng, W.; Zheng, Y.; Bao, P.; Shu, X.O. Adherence to Dietary Recommendations among Long-Term Breast Cancer Survivors and Cancer Outcome Associations. Cancer Epidemiol. Biomark. Prev. 2020, 29, 386–395. [Google Scholar] [CrossRef]
- Ergas, I.J.; Cespedes Feliciano, E.M.; Bradshaw, P.T.; Roh, J.M.; Kwan, M.L.; Cadenhead, J.; Santiago-Torres, M.; Troeschel, A.N.; Laraia, B.; Madsen, K.; et al. Diet Quality and Breast Cancer Recurrence and Survival: The Pathways Study. JNCI Cancer Spectr. 2021, 5. [Google Scholar] [CrossRef]
- Meyerhardt, J.A.; Niedzwiecki, D.; Hollis, D.; Saltz, L.B.; Hu, F.B.; Mayer, R.J.; Nelson, H.; Whittom, R.; Hantel, A.; Thomas, J.; et al. Association of Dietary Patterns With Cancer Recurrence and Survival in Patients With Stage III Colon Cancer. JAMA 2007, 298, 754–764. [Google Scholar] [CrossRef]
- Fung, T.T.; Kashambwa, R.; Sato, K.; Chiuve, S.E.; Fuchs, C.S.; Wu, K.; Giovannucci, E.; Ogino, S.; Hu, F.B.; Meyerhardt, J.A. Post diagnosis diet quality and colorectal cancer survival in women. PLoS ONE 2014, 9, e115377. [Google Scholar] [CrossRef]
- Ratjen, I.; Schafmayer, C.; di Giuseppe, R.; Waniek, S.; Plachta-Danielzik, S.; Koch, M.; Nothlings, U.; Hampe, J.; Schlesinger, S.; Lieb, W. Postdiagnostic Mediterranean and Healthy Nordic Dietary Patterns Are Inversely Associated with All-Cause Mortality in Long-Term Colorectal Cancer Survivors. J. Nutr. 2017, 147, 636–644. [Google Scholar] [CrossRef]
- Guinter, M.A.; McCullough, M.L.; Gapstur, S.M.; Campbell, P.T. Associations of Pre- and Postdiagnosis Diet Quality With Risk of Mortality Among Men and Women With Colorectal Cancer. J. Clin. Oncol. 2018, 36, 3404–3410. [Google Scholar] [CrossRef]
- Kenfield, S.A.; DuPre, N.; Richman, E.L.; Stampfer, M.J.; Chan, J.M.; Giovannucci, E.L. Mediterranean diet and prostate cancer risk and mortality in the Health Professionals Follow-up Study. Eur. Urol. 2014, 65, 887–894. [Google Scholar] [CrossRef]
- Yang, M.; Kenfield, S.A.; Van Blarigan, E.L.; Batista, J.L.; Sesso, H.D.; Ma, J.; Stampfer, M.J.; Chavarro, J.E. Dietary patterns after prostate cancer diagnosis in relation to disease-specific and total mortality. Cancer Prev. Res. 2015, 8, 545–551. [Google Scholar] [CrossRef]
- Al Ramadhani, R.M.; Nagle, C.M.; Ibiebele, T.I.; Grant, P.; Friedlander, M.; DeFazio, A.; Webb, P.M.; Ovarian Cancer, P.; Lifestyle Study, G. Pre- and Post-Diagnosis Diet Quality and Ovarian Cancer Survival. Cancer Epidemiol. Biomark. Prev. 2021, 30, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Sasamoto, N.; Wang, T.; Townsend, M.K.; Eliassen, A.H.; Tabung, F.K.; Giovannucci, E.L.; Matulonis, U.A.; Terry, K.L.; Tworoger, S.S.; Harris, H.R. Pre-diagnosis and post-diagnosis dietary patterns and survival in women with ovarian cancer. Br. J. Cancer 2022, 127, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Guenther, P.M.; Reedy, J.; Krebs-Smith, S.M. Development of the Healthy Eating Index-2005. J. Am. Diet Assoc. 2008, 108, 1896–1901. [Google Scholar] [CrossRef]
- McCullough, M.L.; Feskanich, D.; Stampfer, M.J.; Giovannucci, E.L.; Rimm, E.B.; Hu, F.B.; Spiegelman, D.; Hunter, D.J.; Colditz, G.A.; Willett, W.C. Diet quality and major chronic disease risk in men and women: Moving toward improved dietary guidance. Am. J. Clin. Nutr. 2002, 76, 1261–1271. [Google Scholar] [CrossRef]
- Guenther, P.M.; Casavale, K.O.; Reedy, J.; Kirkpatrick, S.I.; Hiza, H.A.; Kuczynski, K.J.; Kahle, L.L.; Krebs-Smith, S.M. Update of the Healthy Eating Index: HEI-2010. J. Acad. Nutr. Diet 2013, 113, 569–580. [Google Scholar] [CrossRef]
- Chiuve, S.E.; Fung, T.T.; Rimm, E.B.; Hu, F.B.; McCullough, M.L.; Wang, M.; Stampfer, M.J.; Willett, W.C. Alternative dietary indices both strongly predict risk of chronic disease. J. Nutr. 2012, 142, 1009–1018. [Google Scholar] [CrossRef]
- Kirkpatrick, S.I.; Reedy, J.; Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Wilson, M.M.; Lerman, J.L.; Tooze, J.A. Applications of the Healthy Eating Index for Surveillance, Epidemiology, and Intervention Research: Considerations and Caveats. J. Acad. Nutr. Diet 2018, 118, 1603–1621. [Google Scholar] [CrossRef] [PubMed]
- Haines, P.S.; Siega-Riz, A.M.; Popkin, B.M. The Diet Quality Index revised: A measurement instrument for populations. J. Am. Diet Assoc. 1999, 99, 697–704. [Google Scholar] [CrossRef]
- Mai, V.; Kant, A.K.; Flood, A.; Lacey, J.V., Jr.; Schairer, C.; Schatzkin, A. Diet quality and subsequent cancer incidence and mortality in a prospective cohort of women. Int. J. Epidemiol. 2005, 34, 54–60. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Fung, T.T.; McCullough, M.L.; Newby, P.K.; Manson, J.E.; Meigs, J.B.; Rifai, N.; Willett, W.C.; Hu, F.B. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2005, 82, 163–173. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Orfanos, P.; Norat, T.; Bueno-de-Mesquita, B.; Ocké, M.C.; Peeters, P.H.; van der Schouw, Y.T.; Boeing, H.; Hoffmann, K.; Boffetta, P.; et al. Modified Mediterranean diet and survival: EPIC-elderly prospective cohort study. Bmj 2005, 330, 991. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Chiuve, S.E.; McCullough, M.L.; Rexrode, K.M.; Logroscino, G.; Hu, F.B. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch. Intern. Med. 2008, 168, 713–720. [Google Scholar] [CrossRef]
- Kushi, L.H.; Doyle, C.; McCullough, M.; Rock, C.L.; Demark-Wahnefried, W.; Bandera, E.V.; Gapstur, S.; Patel, A.V.; Andrews, K.; Gansler, T. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: Reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J. Clin. 2012, 62, 30–67. [Google Scholar] [CrossRef]
- Tabung, F.K.; Smith-Warner, S.A.; Chavarro, J.E.; Wu, K.; Fuchs, C.S.; Hu, F.B.; Chan, A.T.; Willett, W.C.; Giovannucci, E.L. Development and Validation of an Empirical Dietary Inflammatory Index. J. Nutr. 2016, 146, 1560–1570. [Google Scholar] [CrossRef]
- McNaughton, S.A.; Ball, K.; Crawford, D.; Mishra, G.D. An index of diet and eating patterns is a valid measure of diet quality in an Australian population. J. Nutr. 2008, 138, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Lay, S.; Yu, H.N.; Shen, S.R. Dietary Guidelines for Chinese Residents (2016): Comments and comparisons. J. Zhejiang Univ. Sci. B 2016, 17, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.Q.; Li, F.; Wu, H.; Wang, Y.C.; Chen, J.S.; He, G.S.; Li, S.G.; Chen, B. Evaluation of the Validity and Reliability of the Chinese Healthy Eating Index. Nutrients 2018, 10, 114. [Google Scholar] [CrossRef]
- Olsen, A.; Egeberg, R.; Halkjær, J.; Christensen, J.; Overvad, K.; Tjønneland, A. Healthy aspects of the Nordic diet are related to lower total mortality. J. Nutr. 2011, 141, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Schulze, M.B.; Martínez-González, M.A.; Fung, T.T.; Lichtenstein, A.H.; Forouhi, N.G. Food based dietary patterns and chronic disease prevention. BMJ 2018, 361, k2396. [Google Scholar] [CrossRef]
- Satija, A.; Stampfer, M.J.; Rimm, E.B.; Willett, W.; Hu, F.B. Perspective: Are Large, Simple Trials the Solution for Nutrition Research? Adv. Nutr. 2018, 9, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Wu, K.; Meyerhardt, J.A.; Yilmaz, O.; Wang, M.; Ogino, S.; Fuchs, C.S.; Giovannucci, E.L.; Chan, A.T. Low-Carbohydrate Diet Score and Macronutrient Intake in Relation to Survival After Colorectal Cancer Diagnosis. JNCI Cancer Spectr. 2018, 2, pky077. [Google Scholar] [CrossRef] [PubMed]
- Kunnavuttivanich, V.; Pramyothin, P.; Ithimakin, S. Association between dietary patterns and disease recurrence in Thai colorectal cancer patients. Medicine 2020, 99, e19522. [Google Scholar] [CrossRef]
- Van Blarigan, E.L.; Fuchs, C.S.; Niedzwiecki, D.; Zhang, S.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; Hantel, A.; Benson, A.; et al. Association of Survival With Adherence to the American Cancer Society Nutrition and Physical Activity Guidelines for Cancer Survivors After Colon Cancer Diagnosis: The CALGB 89803/Alliance Trial. JAMA Oncol. 2018, 4, 783–790, Erratum in JAMA Oncol. 2019, 5, 579. [Google Scholar] [CrossRef]
- Meyerhardt, J.A.; Sato, K.; Niedzwiecki, D.; Ye, C.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; Hantel, A.; Benson, A.; et al. Dietary glycemic load and cancer recurrence and survival in patients with stage III colon cancer: Findings from CALGB 89803. J. Natl. Cancer Inst. 2012, 104, 1702–1711. [Google Scholar] [CrossRef]
- Jacobs, S.; Harmon, B.E.; Ollberding, N.J.; Wilkens, L.R.; Monroe, K.R.; Kolonel, L.N.; Le Marchand, L.; Boushey, C.J.; Maskarinec, G. Among 4 Diet Quality Indexes, Only the Alternate Mediterranean Diet Score Is Associated with Better Colorectal Cancer Survival and Only in African American Women in the Multiethnic Cohort. J. Nutr. 2016, 146, 1746–1755. [Google Scholar] [CrossRef]
- Shen, G.P.; Xu, F.H.; He, F.; Ruan, H.L.; Cui, C.; Chen, L.Z.; Zeng, Y.X.; Jia, W.H. Pretreatment lifestyle behaviors as survival predictors for patients with nasopharyngeal carcinoma. PLoS ONE 2012, 7, e36515. [Google Scholar] [CrossRef]
- van den Berg, M.G.; Rütten, H.; Rasmussen-Conrad, E.L.; Knuijt, S.; Takes, R.P.; van Herpen, C.M.; Wanten, G.J.; Kaanders, J.H.; Merkx, M.A. Nutritional status, food intake, and dysphagia in long-term survivors with head and neck cancer treated with chemoradiotherapy: A cross-sectional study. Head Neck 2014, 36, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Kenfield, S.A.; Van Blarigan, E.L.; Wilson, K.M.; Batista, J.L.; Sesso, H.D.; Ma, J.; Stampfer, M.J.; Chavarro, J.E. Dairy intake after prostate cancer diagnosis in relation to disease-specific and total mortality. Int. J. Cancer 2015, 137, 2462–2469. [Google Scholar] [CrossRef] [PubMed]
- Ratjen, I.; Shivappa, N.; Schafmayer, C.; Burmeister, G.; Nöthlings, U.; Hampe, J.; Hébert, J.R.; Lieb, W.; Schlesinger, S. Association between the dietary inflammatory index and all-cause mortality in colorectal cancer long-term survivors. Int. J. Cancer 2019, 144, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Arthur, A.E.; Peterson, K.E.; Rozek, L.S.; Taylor, J.M.; Light, E.; Chepeha, D.B.; Hébert, J.R.; Terrell, J.E.; Wolf, G.T.; Duffy, S.A.; et al. Pretreatment dietary patterns, weight status, and head and neck squamous cell carcinoma prognosis. Am. J. Clin. Nutr. 2013, 97, 360–368. [Google Scholar] [CrossRef]
- Vrieling, A.; Buck, K.; Seibold, P.; Heinz, J.; Obi, N.; Flesch-Janys, D.; Chang-Claude, J. Dietary patterns and survival in German postmenopausal breast cancer survivors. Br. J. Cancer 2013, 108, 188–192. [Google Scholar] [CrossRef]
- Thomson, C.A.; ECrane, T.; Wertheim, B.C.; Neuhouser, M.L.; Li, W.; Snetselaar, L.G.; Basen-Engquist, K.M.; Zhou, Y.; Irwin, M.L. Diet quality and survival after ovarian cancer: Results from the Women’s Health Initiative. J. Natl. Cancer Inst. 2014, 106, dju314. [Google Scholar] [CrossRef]
- Sharma, I.; Roebothan, B.; Zhu, Y.; Woodrow, J.; Parfrey, P.S.; Mclaughlin, J.R.; Wang, P.P. Hypothesis and data-driven dietary patterns and colorectal Cancer survival: Findings from Newfoundland and Labrador colorectal Cancer cohort. Nutr. J. 2018, 17, 55. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, H.; Wang, P.P.; Savas, S.; Woodrow, J.; Wish, T.; Jin, R.; Green, R.; Woods, M.; Roebothan, B.; et al. Dietary patterns and colorectal cancer recurrence and survival: A cohort study. BMJ Open 2013, 3, e002270. [Google Scholar] [CrossRef]
- Pelser, C.; Arem, H.; Pfeiffer, R.M.; Elena, J.W.; Alfano, C.M.; Hollenbeck, A.R.; Park, Y. Prediagnostic lifestyle factors and survival after colon and rectal cancer diagnosis in the National Institutes of Health (NIH)-AARP Diet and Health Study. Cancer 2014, 120, 1540–1547. [Google Scholar] [CrossRef]
- Carr, P.R.; Jansen, L.; Walter, V.; Kloor, M.; Roth, W.; Bläker, H.; Chang-Claude, J.; Brenner, H.; Hoffmeister, M. Associations of red and processed meat with survival after colorectal cancer and differences according to timing of dietary assessment. Am. J. Clin. Nutr. 2016, 103, 192–200. [Google Scholar] [CrossRef]
- Yang, B.; McCullough, M.L.; Gapstur, S.M.; Jacobs, E.J.; Bostick, R.M.; Fedirko, V.; Flanders, W.D.; Campbell, P.T. Calcium, vitamin D, dairy products, and mortality among colorectal cancer survivors: The Cancer Prevention Study-II Nutrition Cohort. J. Clin. Oncol. 2014, 32, 2335–2343. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.L.; Gapstur, S.M.; Shah, R.; Jacobs, E.J.; Campbell, P.T. Association between red and processed meat intake and mortality among colorectal cancer survivors. J. Clin. Oncol. 2013, 31, 2773–2782. [Google Scholar] [CrossRef]
- Lang, S.; Schimansky, S.; Beynon, R.; Penfold, C.; Davies, A.; Waylen, A.; Thomas, S.; Pring, M.; Pawlita, M.; Waterboer, T.; et al. Dietary behaviors and survival in people with head and neck cancer: Results from Head and Neck 5000. Head Neck 2019, 41, 2074–2084, Epub 2019 Jan 30. PMID: 30698303; PMCID: PMC7116031. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, M.; Font, R.; Mañós, M.; Dicenta, M.; Quintana, M.J.; Bosch, F.X.; Castellsagué, X. The role of vegetable and fruit consumption and other habits on survival following the diagnosis of oral cancer: A prospective study in Spain. Int. J. Oral. Maxillofac. Surg. 2009, 38, 31–39. [Google Scholar] [CrossRef]
- Chan, J.M.; Holick, C.N.; Leitzmann, M.F.; Rimm, E.B.; Willett, W.C.; Stampfer, M.J.; Giovannucci, E.L. Diet after diagnosis and the risk of prostate cancer progression, recurrence, and death (United States). Cancer Causes Control. 2006, 17, 199–208. [Google Scholar] [CrossRef]
- Dikshit, R.P.; Boffetta, P.; Bouchardy, C.; Merletti, F.; Crosignani, P.; Cuchi, T.; Ardanaz, E.; Brennan, P. Lifestyle habits as prognostic factors in survival of laryngeal and hypopharyngeal cancer: A multicentric European study. Int. J. Cancer 2005, 117, 992–995. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).