Exploring the Antioxidant Potential of Talisia esculenta Using In Vitro and In Vivo Approaches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Material
2.3. Preparation of Extracts
2.4. Antioxidant Capacity In Vitro
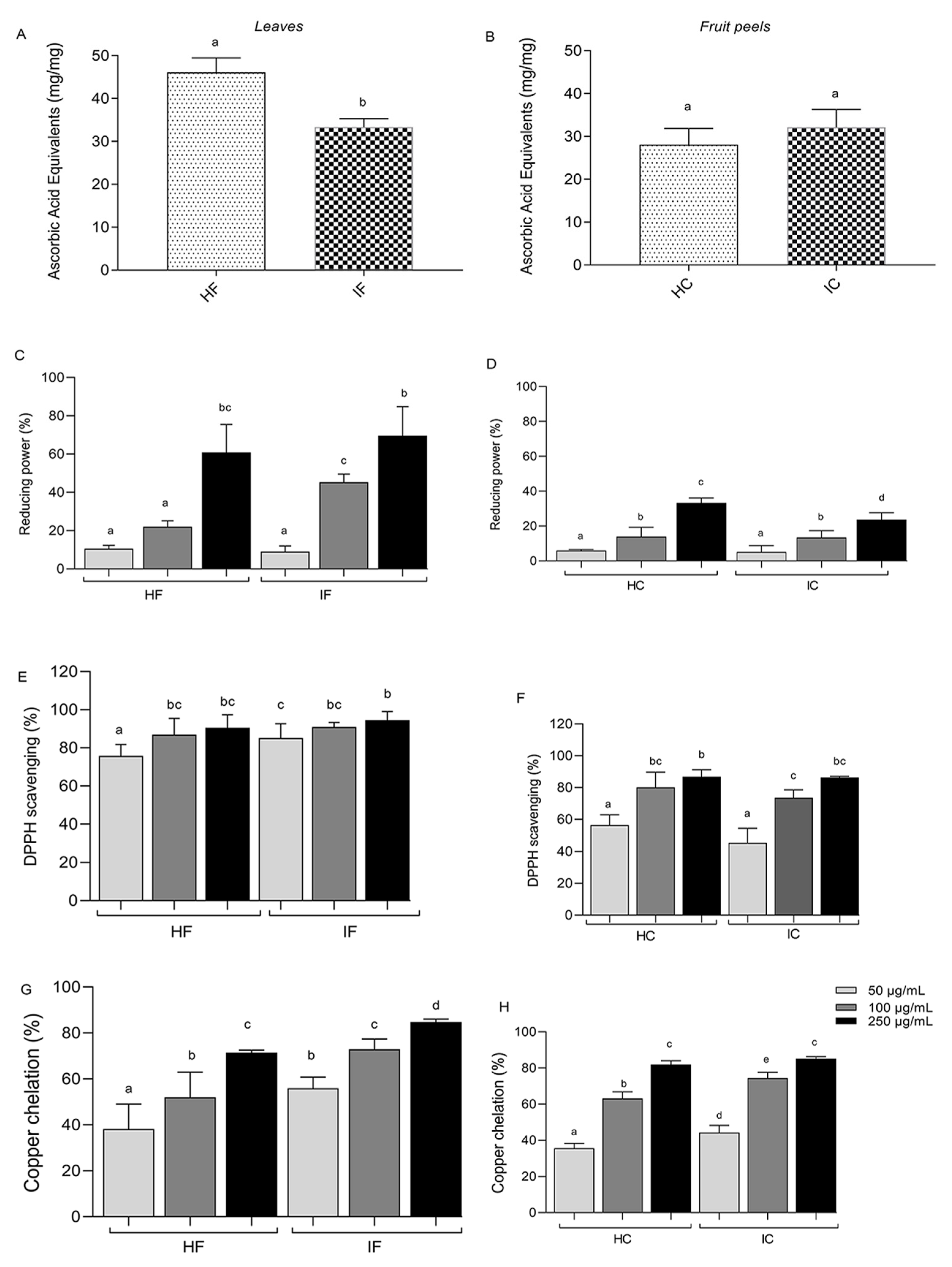
2.4.1. Total Antioxidant Capacity (TAC)
2.4.2. Reducing Power
2.4.3. DPPH Radical Scavenging
2.4.4. Copper Chelation
2.5. Cell Line Assays
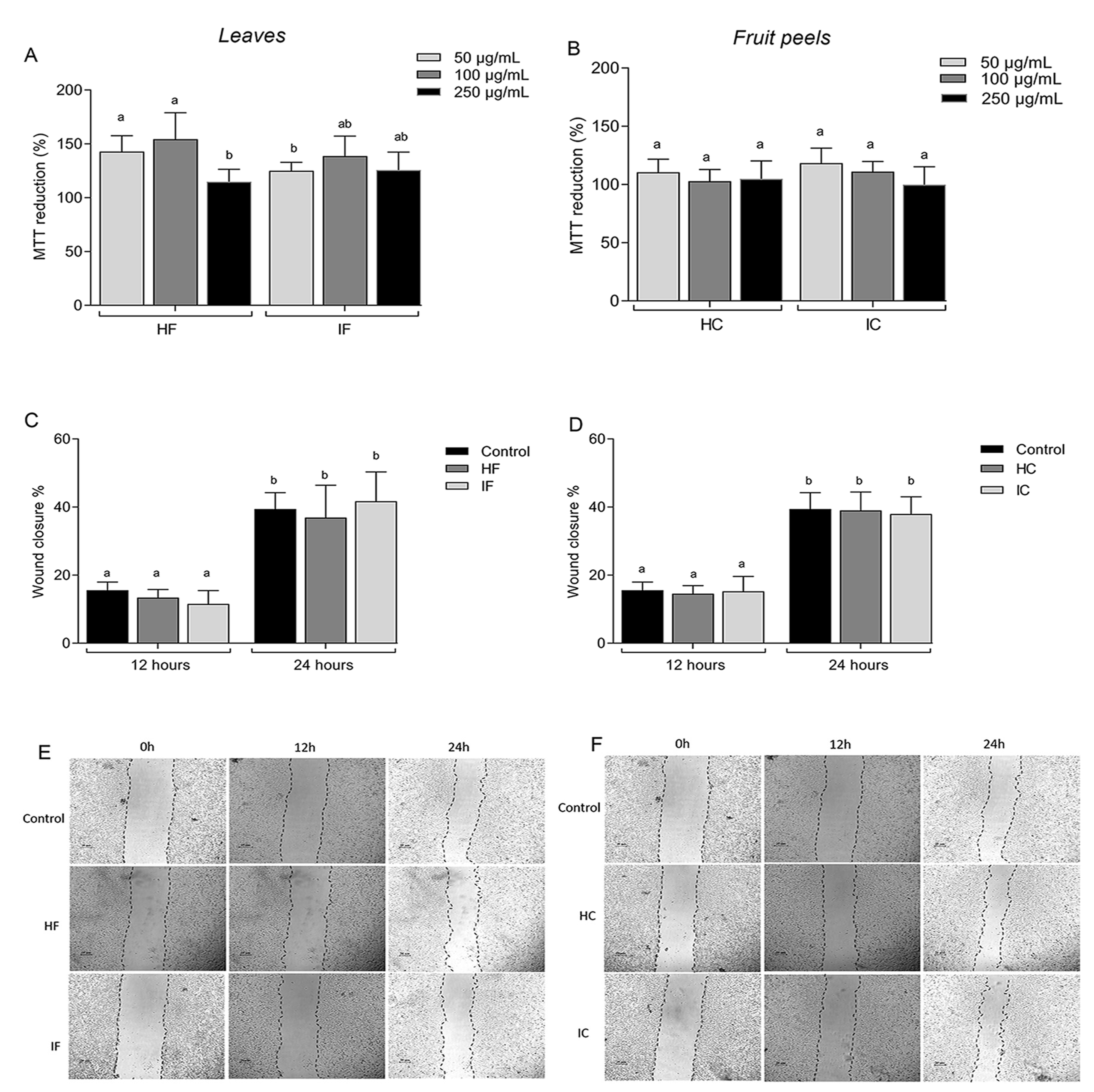
2.5.1. MTT Reduction Assay
2.5.2. Migration Assay
2.5.3. In Vivo Oxidative Stress Assay Using CuSO4 and Ascorbate-Induced in a NIH/3T3 Cell Line
2.6. Antioxidant Capacity In Vivo Using Tenebrio molitor as an Animal Model
2.6.1. Tenebrio molitor Maintenance
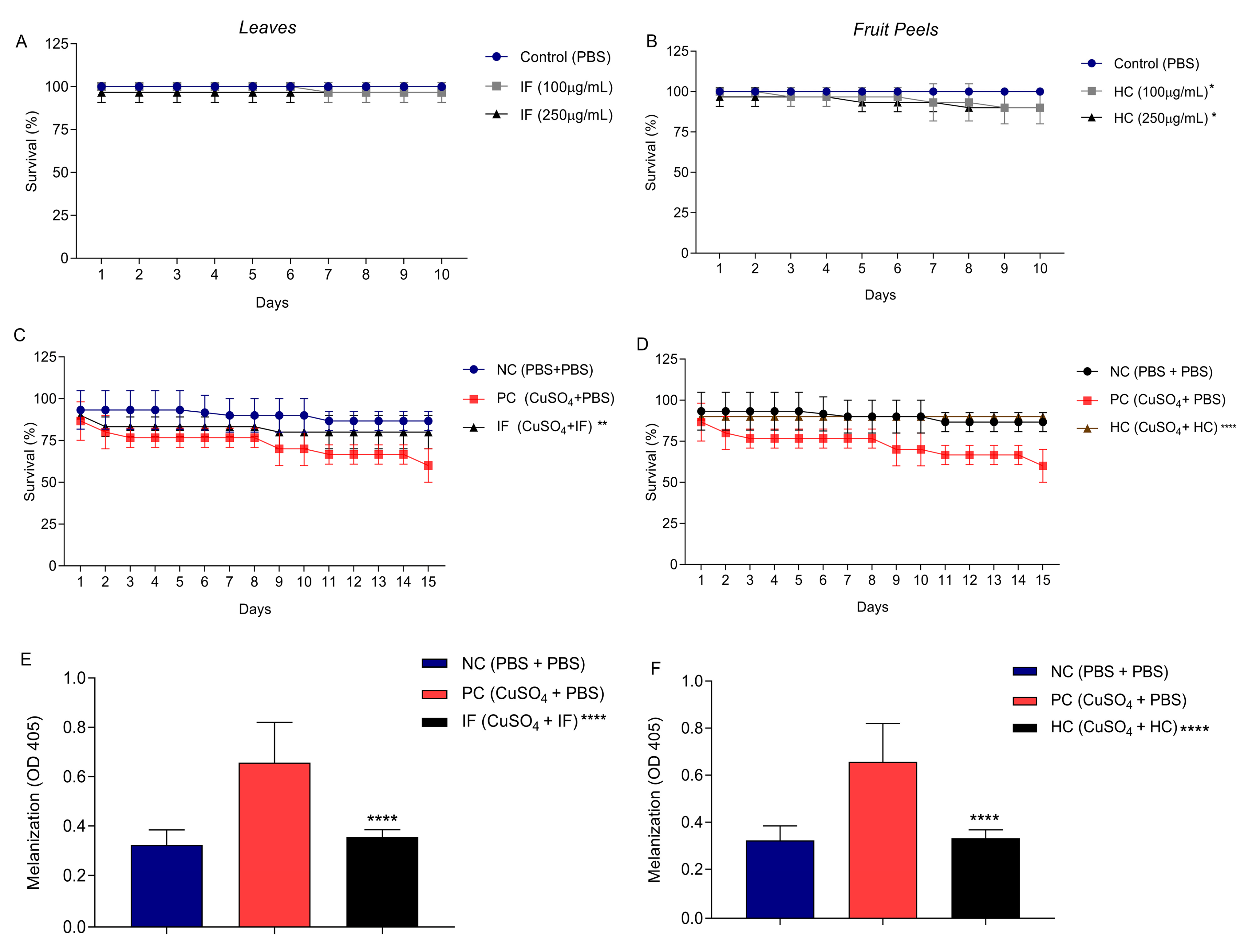
2.6.2. Effect of the Extract on T. molitor Survival
2.6.3. Effect of IF and HC on Tenebrio molitor Survival after CuSO4 Induced Oxidative Stress
2.6.4. Analysis of Melanization after CuSO4 Oxidative Induced Stress
2.7. Chemical Constituents of T. esculenta Extracts
2.7.1. Quantification of Total Phenolic Compound Content
2.7.2. Quantification of the Total Flavonoid Content
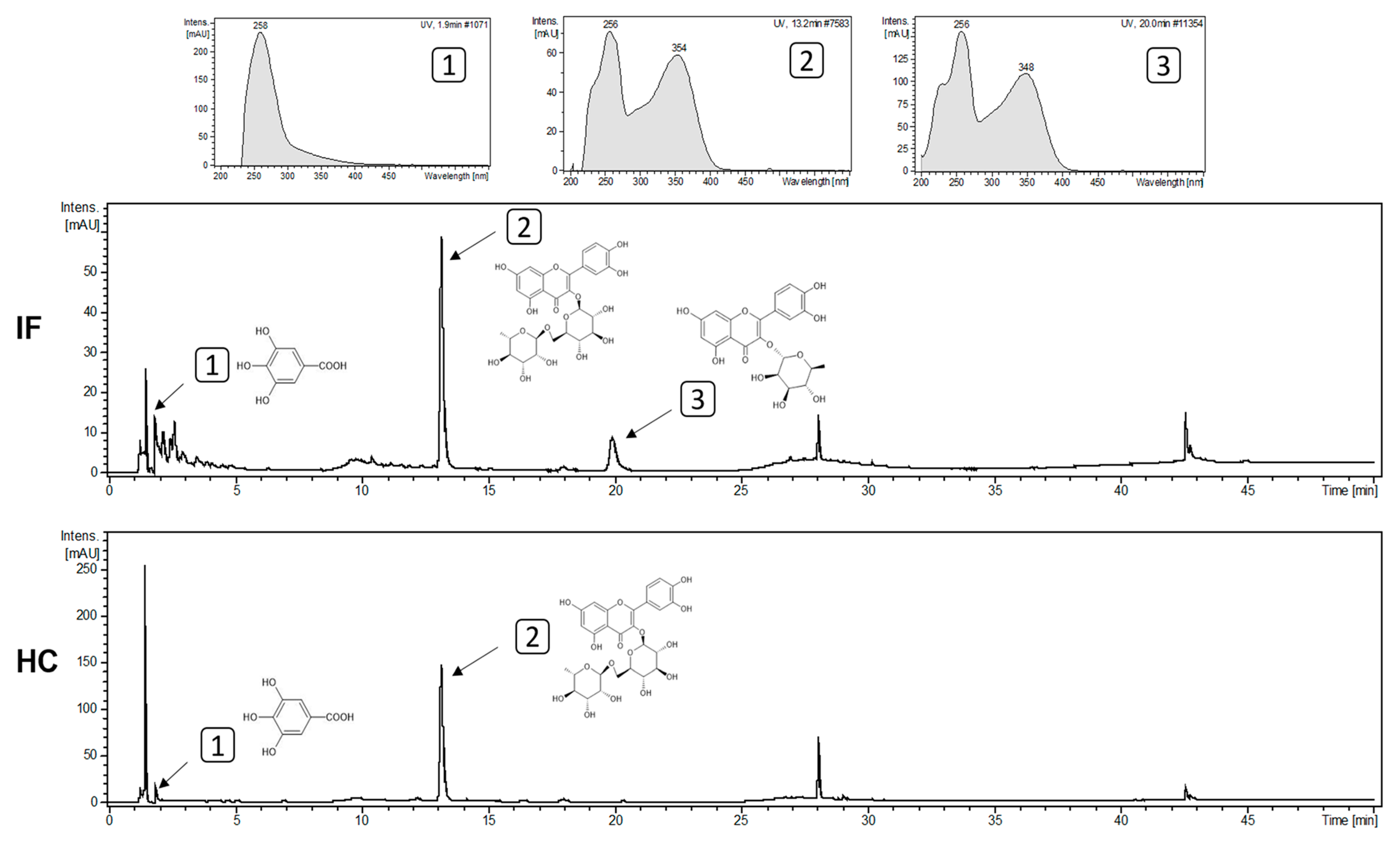
2.7.3. High-Performance Liquid Chromatography with Diode Array Detection (CLAE-DAD)
2.8. Statistical Analysis
3. Results
3.1. In Vitro Antioxidant Activity of TE Extracts
3.2. Effect of T. esculenta Extracts on the NIH/3T3 Cell Line
3.3. Effect of T. esculenta Extracts on the NIH/3T3 Fibroblast Cell Line Exposed to Oxidative Stress
3.4. Effect of Leaf Infusion and Fruit Peels Hydroethanolic Extract of T. esculenta on Tenebrio molitor
3.5. Detection of Biomolecules Present in the Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Kumar, V.; Sachan, R.; Rahman, M.; Rub, R.A.; Patel, D.K.; Sharma, K.; GahtorI, P.; Al-Abbasi, F.A.; Alhayyani, S.; Anwar, F.; et al. Chemopreventive effects of Melastoma malabathricum L. extract in mammary tumor model via inhibition of oxidative stress and inflammatory cytokines. Biomed. Pharmacother. 2021, 137, 111298. [Google Scholar] [CrossRef]
- Cheung, E.C.; Vousden, K.H. The role of ROS in tumour development and progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef]
- Alemany-Cosme, A.E.; González, S.E.; Moret, I.; Mateos, B.; Iborra, M.; Nos, P.; Sandoval, J.; Beltrán, B. Oxidative Stress in the Pathogenesis of Crohn’s Disease and the Interconnection with Immunological Response, Microbiota, External Environmental Factors, and Epigenetics. Antioxidants 2021, 10, 64. [Google Scholar] [CrossRef]
- Sarkar, B.K.; Akter, R.; Das, J.; Das, A.; Modak, P.; Halder, S.; Sarkar, A.P.; Kundu, S.K. Diabetes mellitus: A comprehensive review. J. Pharmacogn. Phytochem. 2019, 8, 2362–2371. [Google Scholar]
- Fiorelli, S.; Porro, B.; Cosentino, N.; DI Minno, A.; Manega, C.M.; Fabbiocchi, F.; Niccoli, G.; Fracassi, F.; Barbieri, S.; Marenzi, G.; et al. Activation of Nrf2/HO-1 Pathway and Human Atherosclerotic Plaque Vulnerability: An In Vitro and In Vivo Study. Cells 2019, 8, 356. [Google Scholar] [CrossRef]
- Diaz, A.; Treviño, S.; Pulido-Fernandez, G.; Martínez-Muñoz, E.; Cervantes, N.; Espinosa, B.; Rojas, K.; Pérez-Severiano, F.; Montes, S.; Rubio-Osornio, M.; et al. Epicatechin Reduces Spatial Memory Deficit Caused by Amyloid-β25–35 Toxicity Modifying the Heat Shock Proteins in the CA1 Region in the Hippocampus of Rats. Antioxidants 2019, 8, 113. [Google Scholar] [CrossRef]
- Correia, A.S.; Cardoso, A.; Vale, N. Oxidative Stress in Depression: The Link with the Stress Response, Neuroinflammation, Serotonin, Neurogenesis and Synaptic Plasticity. Antioxidants 2023, 12, 470. [Google Scholar] [CrossRef]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef]
- Yatoo, M.I.; Gopalakrishnan, A.; Saxena, A.; Parray, O.R.; Tufani, N.A.; Chakraborty, S.; Iqbal, H.M.N. Anti-Inflammatory Drugs and Herbs with Special Emphasis on Herbal Medicines for Countering Inflammatory Diseases and Disorders—A Review. Recent Pat. Inflamm. Allergy Drug Discov. 2018, 12, 39–58. [Google Scholar] [CrossRef]
- Chandran, R.; Abrahamse, H. Identifying Plant-Based Natural Medicine against Oxidative Stress and Neurodegenerative Disorders. Oxidative Med. Cell. Longev. 2020, 2020, 8648742. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Kishimoto, Y.; Sasaki, M.; Sato, A.; Kamiya, T.; Kondo, K.; Iida, K. Terminalia bellirica (Gaertn.) Roxb. Extract and Gallic Acid Attenuate LPS-Induced Inflammation and Oxidative Stress via MAPK/NF-κB and Akt/AMPK/Nrf2 Pathways. Oxidative Med. Cell. Longev. 2018, 2018, 9364364. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Z.; Zhao, H.; Wang, X.; Pang, J.; Li, Q.; Yang, Y.; Ling, W. Anthocyanin supplementation improves anti-oxidative and anti-inflammatory capacity in a dose-response manner in subjects with dyslipidemia. Redox Biol. 2020, 32, 2213–2317. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Wang, Y.; Jin, J.; Yang, Z.; Guo, R.; Li, X.; Yang, L.; Li, Z. Resveratrol Treats UVB-Induced Photoaging by Anti-MMP Expression, through Anti-Inflammatory, Antioxidant, and Antiapoptotic Properties, and Treats Photoaging by Upregulating VEGF-B Expression. Oxidative Med. Cell. Longev. 2022, 2022, 6037303. [Google Scholar] [CrossRef]
- Banerjee, J.; Das, A.; Sinha, M.; Saha, S. Biological Efficacy of Medicinal Plant Extracts in Preventing Oxidative Damage. Oxidative Med. Cell. Longev. 2018, 2018, 7904349. [Google Scholar] [CrossRef] [PubMed]
- Proestos, C. The Benefits of Plant Extracts for Human Health. Foods 2020, 9, 1653. [Google Scholar] [CrossRef]
- Isah, T.; Omar, S.; Mujib, A.; Sharma, M.P.; Rajassekharan, P.; Zafar, N.; Frukh, A. Secondary metabolism of pharmaceuticals in the plant in vitro cultures: Strategies, approaches, and limitations to achieving higher yield. Plant Cell Tissue Organ Cult. 2018, 132, 239–265. [Google Scholar] [CrossRef]
- Heinrich, M.; Mah, J.; Amirkia, V. Alkaloids Used as Medicines: Structural Phytochemistry Meets Biodiversity-An Update and Forward Look. Molecules 2021, 26, 1836. [Google Scholar] [CrossRef]
- Tirloni, C.A.S.; Silva, A.O.; Palozi, R.A.C.; Vasconcelos, P.C.P.; Souza, R.I.C.; Dos Santos, A.C.; De Almeida, V.P.; Budel, J.M.; De Souza, L.M.; Gasparotto, J.A. Biological Characterization of an Edible Species from Brazilian Biodiversity: From Pharmacognostic Data to Ethnopharmacological Investigation. J. Med. Food 2018, 21, 1276–1287. [Google Scholar] [CrossRef]
- Fraga, L.N.; Oliveira, A.K.S.; Aragão, B.P.; Souza, D.A.; Dos Santos, E.W.P.; Melo, J.A.; Silva, A.M.O.; Junior, A.W.; Corrêa, C.B.; Wartha, E.R.S.A.; et al. Mass spectrometry characterization, antioxidant activity, and cytotoxicity of the peel and pulp extracts of Pitomba. Food Chem. 2021, 340, 127929. [Google Scholar] [CrossRef] [PubMed]
- Bieski, I.G.C.; Rios Santos, F.; De Oliveira, R.M.; Espinosa, M.M.; Macedo, M.; Albuquerque, U.P.; de Oliveira Martins, D.T. Etnofarmacologia de Plantas Medicinais da Região do Pantanal (Mato Grosso, Brasil). Evid.-Based Complement. Altern. Med. 2012, 2012, 272749. [Google Scholar] [CrossRef]
- Vásquez, S.P.F.; De Mendonça, M.S.; Do Noda, S.N. Etnobotânica de plantas medicinais em comunidades ribeirinhas do Município de Manacapuru, Amazonas, Brasil. Acta Amaz. 2014, 44, 457–472. [Google Scholar] [CrossRef]
- Vieira, F.A.; Gusmão, E. Uso de giberelinas na emergência de plântulas de Talisia esculenta (A. St. Hil.) Radlk. Rev. Científica Eletrônica Eng. Florest. 2006, 4, 1–10. [Google Scholar]
- Santos, T.C.; Júnior, J.E.N.; Prata, A.P.N. Frutos da Caatinga de Sergipe utilizados na alimentação humana. Sci. Plena 2012, 8, 1–7. [Google Scholar]
- Costanzo, G.; Vitale, E.; Iesce, M.R.; Naviglio, D.; Amoresano, A.; Fontanarosa, C.; Spinelli, M.; Ciaravolo, M.; Arena, C. Antioxidant Properties of Pulp, Peel and Seeds of Phlegrean Mandarin (Citrus reticulata Blanco) at Different Stages of Fruit Ripening. Antioxidants 2022, 11, 187. [Google Scholar] [CrossRef]
- Waller, S.B.; Peter, C.M.; Hoffmann, J.F.; Cleff, M.B.; Faria De, R.O.; Zani, J.L. Jabuticaba [Plinia peruviana (Poir.) Govaerts]: A Brazilian fruit with a promising application against itraconazole-susceptible and -resistant Sporothrix brasiliensis. Nat. Prod. Res. 2021, 35, 5988–5992. [Google Scholar] [CrossRef] [PubMed]
- Castangia, I.; Manca, M.L.; Allaw, M.; Hellström, J.; Granato, D.; Manconi, M. Jabuticaba (Myrciaria jaboticaba) Peel as a Sustainable Source of Anthocyanins and Ellagitannins Delivered by Phospholipid Vesicles for Alleviating Oxidative Stress in Human Keratinocytes. Molecules 2021, 26, 6697. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.P.A.; Justino, A.B.; Tavernelli, N.; Saraiva, A.L.; Franco, R.R.; De Souza, A.V.; Silva, H.C.G.; De Moura, F.B.R.; Botelho, F.V.; Espindola, F.S. Antioxidant compounds from Annona crassiflora fruit peel reduce lipid levels and oxidative damage and maintain the glutathione defense in hepatic tissue of Triton WR-1339-induced hyperlipidemic mice. Biomed. Pharmacother. 2021, 142, 112049. [Google Scholar] [CrossRef]
- Russo, M.; Fanali, C.; Tripodo, G.; Dugo, P.; Muleo, R.; Dugo, L.; De Gara, L.; Mondello, L. Analysis of phenolic compounds in different parts of pomegranate (Punica granatum) fruit by HPLC-PDA-ESI/MS and evaluation of their antioxidant activity: Application to different Italian varieties. Anal. Bioanal. Chem. 2018, 410, 3507–3520. [Google Scholar] [CrossRef]
- Huo, D.; Dai, J.; Yuan, S.; Cheng, X.; Pan, Y.; Wang, L.; Wang, R. Eco-friendly simultaneous extraction of pectins and phenolics from passion fruit (Passiflora edulis Sims) peel: Process optimization, physicochemical properties, and antioxidant activity. Int. J. Biol. Macromol. 2023, 243, 125229. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea and health: Studies in humans. Curr. Pharm. Des. 2013, 19, 6141–6147. [Google Scholar] [CrossRef] [PubMed]
- Poswal, F.S.; Russell, G.; Mackonochie, M.; Maclennan, E.; Adukwu, E.C.; Rolfe, V. Herbal Teas and their Health Benefits: A Scoping Review. Plant Foods Hum. Nutr. 2019, 74, 266–276. [Google Scholar] [CrossRef]
- Becker, M.M.; Nunes, G.S.; Ribeiro, D.B.; Silva, F.E.P.S.; Catanantea, G.; Martya, J.L. Determination of the Antioxidant Capacity of Red Fruits by Miniaturized Spectrophotometry Assays. J. Braz. Chem. Soc. 2019, 30, 1108–1114. [Google Scholar] [CrossRef]
- Chaves, J.O.; De Souza, M.C.; Da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.D.F.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids From Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef]
- Sajkowska-Kozielewicz, J.J.; Kozielewicz, P.; Barnes, N.M.; Wawer, I.; Paradowska, K. Antioxidant, Cytotoxic, and Antiproliferative Activities and Total Polyphenol Contents of the Extracts of Geissospermum reticulatum Bark. Oxidative Med. Cell. Longev. 2016, 2016, 2573580. [Google Scholar] [CrossRef]
- Pádua, B.C.; Silva, L.D.; Rossoni Júnior, J.V.; Humberto, J.L.; Chaves, M.M.; Silva, M.E.; Pedrosa, M.L.; Costa, D.C. Antioxidant properties of Baccharis trimera in the neutrophils of Fisher rats. J. Ethnopharmacol. 2010, 129, 381–386. [Google Scholar] [CrossRef]
- Aquino-Martins, V.G.Q.; De Melo, L.F.M.; Silva, L.M.P.; Targino, L.T.R.; Queiroz, M.F.; Viana, R.L.S.; Zucolotto, S.M.; Andrade, V.S.; Rocha, H.A.O.; Scortecci, K.C. Antioxidante In Vitro, Anti-Biofilme e Atividades de Proteção Solar do Extrato de Polpa de Melocactus zehntneri (Britton & Rose). Antioxidantes 2019, 8, 439. [Google Scholar] [CrossRef]
- Rodrigues-Souza, I.; Pessatti, J.B.K.; Da Silva, L.R.; De Lima Bellan, D.; De Souza, I.R.; Cestari, M.M.; De Assis, H.C.S.; Rocha, H.A.O.; Simas, F.F.; Da Silva Trindade, E.; et al. Protective potential of sulfated polysaccharides from tropical seaweeds against alkylating- and oxidizing-induced genotoxicity. Int. J. Biol. Macromol. 2022, 211, 524–534. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Moghadam, S.E.; Moridi Farimani, M.; Soroury, S.; Ebrahimi, S.N.; Jabbarzadeh, E.; Hypermongone, C. Accelerates Wound Healing through the Modulation of Inflammatory Factors and Promotion of Fibroblast Migration. Molecules 2022, 24, 2022. [Google Scholar] [CrossRef]
- Pijuan, J.; Barceló, C.; Moreno, D.F.; Maiques, O.; Sisó, P.; Marti, R.M.; Macià, A.; Panosa, A. In vitro Cell Migration, Invasion, and Adhesion Assays: From Cell Imaging to Data Analysis. Front. Cell Dev. Biol. 2019, 7, 107. [Google Scholar] [CrossRef]
- Presa, F.B.; Marques, M.L.M.; Viana, R.L.S.; Nobre, L.T.D.B.; Costa, L.S.; Rocha, H.A.O. The Protective Role of Sulfated Polysaccharides from Green Seaweed Udotea flabellum in Cells Exposed to Oxidative Damage. Mar. Drugs 2018, 16, 135. [Google Scholar] [CrossRef]
- Silva, T.F.; Cavalcanti Filho, J.R.N.; Barreto Fonsêca, M.M.L.; Santos, N.M.D.; Da Silva, A.C.B.; Zagmignan, A.; Abreu, A.G.; Da Silva, A.P.S.; Lima, V.L.D.M.; Silva, N.H.D.; et al. Products Derived from Buchenavia tetraphylla Leaves Have In Vitro Antioxidant Activity and Protect Tenebrio molitor Larvae against Escherichia coli-Induced Injury. Pharmaceuticals 2020, 13, 46. [Google Scholar] [CrossRef]
- Jorjão, A.L.; De Oliveira, F.E.; Leão, M.V.P.; Jorge, A.O.C.; De Oliveira, L.D. Effect of Lactobacillus rhamnosus on the response of Galleria mellonella against Staphylococcus aureus and Escherichia coli infections. Arch. Microbiol. 2018, 200, 383–389. [Google Scholar] [CrossRef]
- Athukorala, Y.; Kim, K.N.; Jeon, Y.J. Antiproliferative and antioxidant properties of an enzymatic hydrolysate from brown alga, Ecklonia cava. Food Chem. Toxicol. 2006, 44, 1065–1074. [Google Scholar] [CrossRef]
- Sousa, E.O.; Miranda, C.M.B.A.; Nobre, C.B.; Boligon, A.A.; Athayde, M.L.; Costa, J.G.M. Phytochemical analysis and antioxidant activities of Lantana camara and Lantana montevidensis extracts. Ind. Crops Prod. 2015, 70, 7–15. [Google Scholar] [CrossRef]
- De Melo, L.F.M.; Gomes, D.L.; Da Silva, L.F.; Silva, L.M.P.; Machado, M.L.; Cadavid, C.O.M.; Zucolotto, S.M.; Oliveira, R.P.; Dos Santos, D.Y.A.C.; Rocha, H.A.O.; et al. Coccoloba alnifolia Leaf Extract as a Potential Antioxidant Molecule Using In Vitro and In Vivo Assays. Oxidative Med. Cell. Longev. 2020, 2020, 3928706. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.L.D.S.; Ribeiro, A.R.C.; De Melo, L.F.M.; Da Silva, L.F.; Fidelis, G.P.; Silva, L.M.P.; Caland, R.B.O.; Cadavid, C.O.M.; Aragão, C.F.S.; Zucolotto, S.M.; et al. Antioxidant Activities of Commiphora leptophloeos (Mart.) J. B. Gillett (Burseraceae) Leaf Extracts Using In Vitro and In Vivo Assays. Oxidative Med. Cell. Longev. 2021, 2021, 3043720. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Sudhakar, S.; Singh, R.P. In Vitro Methods of Assay of Antioxidants: An Overview. Food Rev. Int. 2008, 24, 392–415. [Google Scholar] [CrossRef]
- Halliwell, B.; Whiteman, M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br. J. Pharmacol. 2004, 142, 231–255. [Google Scholar] [CrossRef]
- Mendonça, J.D.S.; Guimarães, R.C.A.; Zorgetto-Pinheiro, V.A.; Fernandes, C.D.P.; Marcelino, G.; Bogo, D.; Freitas, K.C.; Hiane, P.A.; de Pádua, M.E.S.; Vilela, M.L.B.; et al. Natural Antioxidant Evaluation: A Review of Detection Methods. Molecules 2022, 27, 3563. [Google Scholar] [CrossRef]
- Senoner, T.; Dichtl, W. Oxidative stress in cardiovascular diseases: Still a Therapeutic target? Nutrients 2019, 9, 2090. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef] [PubMed]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.-F.; et al. Production of Plant Secondary Metabolites: Examples, Tips and Suggestions for Biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef]
- Koch, W.; Zagórska, J.; Marzec, Z.; Kukula-Koch, W. Applications of Tea (Camellia sinensis) and its Active Constituents in Cosmetics. Molecules 2019, 24, 4277. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Ibarz, R.; Fernandes, J.-M.; Pinheiro, A.C.; Botelho, C.; Rocha, C.M.R.; Teixeira, J.A.; Martín-Belloso, O. Extrato Polifenólico da Casca de Pinheiro Encapsulado durante a Digestão Gastrointestinal: Bioacessibilidade, Bioatividade e Prevenção do Estresse Oxidativo. Foods 2021, 10, 328. [Google Scholar] [CrossRef]
- Sellami, M.; Slimeni, O.; Pokrywka, A.; Kuvačić, G.D.; Hayes, L.; Milic, M.; Padulo, J. Herbal medicine for sports: A review. J. Int. Soc. Sports Nutr. 2018, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, M.; Wawrzyniak, D.; Rolle, K.; Chomczyński, P.; Oziewicz, S.; Jurga, S.; Barciszewski, J. Let food be your medicine: Nutraceutical properties of lycopene. Food Funct. 2019, 10, 3090–3102. [Google Scholar] [CrossRef]
- Alharbi, K.S.; Almalki, W.H.; Makeen, H.A.; Albratty, M.; Meraya, A.M.; Nagraik, R.; Sharma, A.; Kumar, D.; Chellappan, D.K.; Singh, S.K.; et al. Role of Medicinal plant-derived Nutraceuticals as a potential target for the treatment of breast cancer. J. Food Biochem. 2022, 46, e14387. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Melo-Silveira, R.F.; Viana, R.L.S.; Sabry, D.A.; Da Silva, R.A.; Machado, D.; Nascimento, A.K.L.; Scortecci, K.C.; Ferreira-Halder, C.V.; Sassaki, G.L.; Rocha, H.A.O. Antiproliferative xylan from corn cobs induces apoptosis in tumor cells. Carbohydr. Polym. 2019, 210, 245–253. [Google Scholar] [CrossRef]
- Chukwuma, C.I.; Mashele, S.S.; Akuru, E.A. Evaluation of the in vitro ⍺-amylase inhibitory, antiglycation, and antioxidant properties of Punica granatum L. (pomegranate) fruit peel acetone extract and its effect on glucose uptake and oxidative stress in hepatocytes. J. Food Biochem. 2020, 44, e13175. [Google Scholar] [CrossRef]
- Ali, A.M.; Gabbar, M.A.; Abdel-Twab, S.M.; Fahmy, E.M.; Ebaid, H.; Alhazza, I.M.; Ahmed, O.M. Antidiabetic Potency, Antioxidant Effects, and Mode of Actions of Citrus reticulata Fruit Peel Hydroethanolic Extract, Hesperidin, and Quercetin in Nicotinamide/Streptozotocin-Induced Wistar Diabetic Rats. Oxidative Med. Cell. Longev. 2020, 2020, 1730492. [Google Scholar] [CrossRef]
- Abdelghffar, E.A.; Obaid, W.A.; Elgamal, A.M.; Daoud, R.; Sobeh, M.; El Raey, M.A. Pea (Pisum sativum) peel extract attenuates DOX-induced oxidative myocardial injury. Biomed. Pharmacother. 2021, 143, 112120. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Alam, M.B.; Ju, M.K.; Kwon, K.R.; Huh, Y.S.; Han, Y.K.; Lee, S.H. Antioxidant mechanism of polyphenol-rich Nymphaea nouchali leaf extract protecting DNA damage and attenuating oxidative stress-induced cell death via Nrf2-mediated heme-oxygenase-1 induction coupled with ERK/p38 signaling pathway. Biomed. Pharmacother. 2018, 103, 1397–1407. [Google Scholar] [CrossRef]
- Jantapaso, H.; Mittraparp-Arthorn, P. Phytochemical Composition and Bioactivities of Aqueous Extract of Rambutan (Nephelium lappaceum L. cv. Rong Rian) Peel. Antioxidants 2022, 11, 956. [Google Scholar] [CrossRef] [PubMed]
- Boonpisuttinant, K.; Srisuttee, R.; Yen Khong, H.; Chutoprapat, R.; Ruksiriwanich, W.; Udompong, S.; Chompoo, W.; Boonbai, R.; Rakkaew, R.; Sangsee, J.; et al. In vitro anti-ageing activities of ethanolic extracts from Pink rambutan (Nephelium lappaceum Linn.) for skin applications. Saudi Pharm. J. 2023, 31, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Brendler, T.; Brinckmann, J.; Dentali, S.; Gafner, S.; Giancaspro, G.; Johnson, H.; Kababick, J.; Ma, C.; Oketch-Rabah, H.; et al. Understanding plant to extract ratios in botanical extracts. Front. Pharmacol. 2022, 13, 981978. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, M.C.; Orellana, P.J.C.; Hesami, G.; Jafarzadeh, S.; Lorenzo, J.M.; Domínguez, R.; Moreno, A.; Hadidi, M. Spectrophotometric Methods for Measurement of Antioxidant Activity in Food and Pharmaceuticals. Antioxidants 2022, 11, 2213. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.H.U.; Saleem, U.; Ahmad, B.; Rashid, M. Phytochemical and toxicological evaluation of Zephyranthes citrina. Front. Pharmacol. 2022, 13, 1007310. [Google Scholar] [CrossRef]
- Thiesen, L.C.; Baccarin, T.; Fischer-Muller, A.F.; Meyre-Silva, C.; Couto, A.G.; Bresolin, T.M.; Santin, J.R. Photochemoprotective effects against UVA and UVB irradiation and photosafety assessment of Litchi chinensis leaves extract. J. Photochem. Photobiol. B 2017, 167, 200–207. [Google Scholar] [CrossRef]
- Liu, H.; Guo, H.; Jian, Z.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Copper Induces Oxidative Stress and Apoptosis in the Mouse Liver. Oxidative Med. Cell. Longev. 2020, 2020, 1359164. [Google Scholar] [CrossRef]
- Yang, F.; Pei, R.; Zhang, Z.; Liao, J.; Yu, W.; Qiao, N.; Han, Q.; Li, Y.; Hu, L.; Guo, J.; et al. Copper induces oxidative stress and apoptosis through mitochondria-mediated pathway in chicken hepatocytes. Toxicol. Vitr. 2019, 54, 310–316. [Google Scholar] [CrossRef]
- Huo, H.; Wang, S.; Bai, Y.; Liao, J.; Li, X.; Zhang, H.; Han, Q.; Hu, L.; Pan, J.; Li, Y.; et al. Copper exposure induces mitochondrial dynamic disorder and oxidative stress via mitochondrial unfolded protein response in pig fundic gland. Ecotoxicol. Environ. Saf. 2021, 223, 112587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, N.; Xue, M.; Zhang, M.; Liu, W.; Xu, C.; Fã, Y.; Meng, Y.; Zhang, Q.; Zhou, Y. Anti-Inflammatory and Antioxidant Properties of β-Sitosterol in Copper Sulfate-Induced Inflammation in Zebrafish (Danio rerio). Antioxidants 2023, 12, 391. [Google Scholar] [CrossRef]
- Shen, J.; Griffiths, P.T.; Campbell, S.J.; Utinger, B.; Kalberer, M.; Paulson, S.E. Oxidação de ascorbato por ferro, cobre e espécies reativas de oxigênio: Revisão, desenvolvimento de modelo e derivação de constantes de taxa chave. Sci. Rep. 2021, 11, 7417. [Google Scholar] [CrossRef]
- Falcone, E.; Stellato, F.; Vileno, B.; Bouraguba, M.; Lebrun, V.; Ilbert, M.; Morante, S.; Faller, P. Revisiting the pro-oxidant activity of copper: Interplay of ascorbate, cysteine, and glutathione. Metallomics 2023, 15, mfad040. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Ushio-Fukai, M.; Kaplan, J.H. Copper transporters and copper chaperones: Roles in cardiovascular physiology and disease. Am. J. Physiol. Cell Physiol. 2018, 315, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Balsano, C.; Porcu, C.; Sideri, S. Is copper a new target to counteract the progression of chronic diseases? Metallomics 2018, 10, 1712–1722. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Y.; Shi, H.; Peng, Y.; Fan, X.; Li, C. The molecular mechanisms of copper metabolism and its roles in human diseases. Pflügers Arch.-Eur. J. Physiol. 2020, 472, 1415–1429. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Balendra, V.; Obaid, A.A.; Esposto, J.; Tikhonova, M.A.; Gautam, N.K.; Poeggeler, B. Copper-mediated β-amyloid toxicity and its chelation therapy in Alzheimer’s disease. Metallomics 2022, 14, mfac018. [Google Scholar] [CrossRef]
- Jiang, R.; Sui, Y.; Hong, J.; Niimi, M.; Yan, Q.; Shi, Z.; Yao, J. The Combined Administration of Vitamin C and Copper Induces a Systemic Oxidative Stress and Kidney Injury. Biomolecules 2023, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Morris, H.; Cronin, M. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed]
- Lan, A.P.; Chen, J.; Chai, Z.F.; Hu, Y. The neurotoxicity of iron, copper and cobalt in Parkinson’s disease through ROS-mediated mechanisms. Biometals 2016, 29, 665–678. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Dhanya, R.; Kartha, C.C. Quercetin improves oxidative stress-induced pancreatic beta cell alterations via mTOR-signaling. Mol. Cell. Biochem. 2021, 476, 3879–38871. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.M.; Barreto, G.; Galeano, P.; Romero, J.I.; Holubiec, M.I.; Badorrey, M.S.; Capani, F.; Alvarez, L.D. Paullinia cupana Mart. var. Sorbilis protects human dopaminergic neuroblastoma SH-SY5Y cell line against rotenone-induced cytotoxicity. Hum. Exp. Toxicol. 2011, 30, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Ma, Q.; Guo, Y.; Sun, L. Protective effects of rambutan (Nephelium lappaceum) peel phenolics on H2O2-induced oxidative damages in HepG2 cells and d-galactose-induced aging mice. Food Chem. Toxicol. 2017, 108, 554–562. [Google Scholar] [CrossRef] [PubMed]
- De Souza, P.C.; Caloni, C.C.; Wilson, D.; Almeida, R.S. An Invertebrate Host to Study Fungal Infections, Mycotoxins and Antifungal Drugs: Tenebrio molitor. J. Fungi 2018, 4, 125. [Google Scholar] [CrossRef] [PubMed]
- Maurer, E.; Hörtnagl, C.; Lackner, M.; Grässle, D.; Naschberger, V.; Moser, P.; Segal, E.; Semis, M.; Lass-Flörl, C.; Binder, U. Galleria mellonella as a model system to study virulence potential of mucormycetes and evaluation of antifungal treatment. J. Med. Mycol. 2019, 57, 351–362. [Google Scholar] [CrossRef]
- Lozoya-Pérez, N.E.; García-Carnero, L.C.; Martínez-Álvarez, J.A.; Martínez-Duncker, I.; Mora-Montes, H.M. Tenebrio molitor as an Alternative Model to Analyze the Sporothrix Species Virulence. Infect. Drug Resist. 2021, 14, 2059–2072. [Google Scholar] [CrossRef]
- Fornari, G.; Gomes, R.R.; Degenhardt-Goldbach, J.; Dos Santos, S.S.; De Almeida, S.R.; Dos Santos, G.D.; Muro, M.D.; Bona, C.; Scola, R.H.; Trindade, E.S.; et al. A Model for Trans-Kingdom Pathogenicity in Fonsecaea Agents of Human Chromoblastomycosis. Front. Microbiol. 2018, 9, 2211. [Google Scholar] [CrossRef]
- Machado, G.R.M.; Neiva Lavorato, S.N.; Lopes, G.; Vainstein, M.H.; Teixeira, M.L.; Alves, R.J.; De Andrade, S.F.; Fuentefria, A.M. A chloroacetamide derivative as a potent candidate for fusariosis treatment. Braz. J. Microbiol. 2022, 53, 1289–1295. [Google Scholar] [CrossRef]
- Azevedo, S.S.; Mesquita, G.P.; More, Y.C.M.S.; Costa, G.D.E.; Da Silva, L.C.N.; Zagmignan, A. Evaluation of antioxidating activity of tea maked in free fairs and industrialized tea in San Luís-Maranhão. Braz. Res. Soc. Dev. 2020, 9, e06985320. [Google Scholar] [CrossRef]
- Brai, A.; Poggialini, F.; Vagaggini, C.; Pasqualini, C.; Simoni, S.; Francardi, V.; Dreassi, E. Tenebrio molitor as a Simple and Cheap Preclinical Pharmacokinetic and Toxicity Model. Int. J. Mol. Sci. 2023, 24, 2296. [Google Scholar] [CrossRef]
- Coskun, M.; Kayis, T.; Yilmaz, M.; Dursun, O.; Emre, I. Copper and zinc impact on stress biomarkers and growth parameters in a model organism, Galleria mellonella larvae. Biometals 2021, 34, 1263–1273. [Google Scholar] [CrossRef]
- Mese, Y.; Tuncsoy, B.; Ozalp, P. Effects of Cu, Zn and their mixtures on bioaccumulation and antioxidant enzyme activities in Galleria mellonella L. (Lepidoptera: Pyralidae). Ecotoxicology 2022, 31, 649–656. [Google Scholar] [CrossRef]
- Tuncsoy, S.B.; Tuncsoy, M.; Gomes, T.; Souza, V.; Teixeira, M.R.; Bebianno, M.J.; Ozalp, P. E Oxide Nanoparticles on Tissue Accumulation and Antioxidant Enzymes of Galleria mellonella L. Bull. Environ. Contam. Toxicol. 2019, 102, 341–346. [Google Scholar] [CrossRef]
- Fernandez, M.T.; Mira, M.L.; Florêncio, M.H.; Jennings, K.R. Iron and copper chelation by flavonoids: An electrospray mass spectrometry study. J. Inorg. Biochem. 2002, 92, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef] [PubMed]
- Lakey-Beitia, J.; Burillo, A.M.; La Penna, G.; Hegde, M.L.; Rao, K.S. Polyphenols as Potential Metal Chelation Compounds against Alzheimer’s Disease. J. Alzheimers Dis. 2021, 82, S335–S357. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Wang, T.; Zhou, M.; Xue, J.; Luo, Y. In Vitro Antioxidant-Activity Evaluation of Gallic-Acid-Grafted Chitosan Conjugate Synthesized by Free-Radical-Induced Grafting Method. J. Agric. Food Chem. 2016, 64, 5893–5900. [Google Scholar] [CrossRef]
- Truong, V.L.; Ko, S.Y.; Jun, M.; Jeong, W.S. Quercitrin from Toona sinensis (Juss.) M.Roem. Attenuates Acetaminophen-Induced Acute Liver Toxicity in HepG2 Cells and Mice through Induction of Antioxidant Machinery and Inhibition of Inflammation. Nutrients 2016, 8, 431. [Google Scholar] [CrossRef]
- Sachetto, A.T.A.; Rosa, J.G.; Santoro, M.L. Rutin (quercetin-3-rutinoside) modulates the hemostatic disturbances and redox imbalance induced by Bothrops jararaca snake venom in mice. PLoS Neglected Trop. Dis. 2018, 12, e0006774. [Google Scholar] [CrossRef]
- Choi, S.S.; Park, H.R.; Lee, K.A. A Comparative Study of Rutin and Rutin Glycoside: Antioxidant Activity, Anti-Inflammatory Effect, Effect on Platelet Aggregation and Blood Coagulation. Antioxidants 2021, 10, 1696. [Google Scholar] [CrossRef]
- Yang, K.; Deng, X.; Jian, S.; Zhang, M.; Wen, C.; Xin, Z.; Zhang, L.; Tong, A.; Ye, S.; Liao, P.; et al. Gallic Acid Alleviates Gut Dysfunction and Boosts Immune and Antioxidant Activities in Puppies Under Environmental Stress Based on Microbiome-Metabolomics Analysis. Front. Immunol. 2022, 12, 813890. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.P.; Bataglion, G.A.; Da Silva, F.M.; de Almeida, R.A.; Paz, W.H.P.; Nobre, T.A.; Marinho, J.V.N.; Salvador, M.J.; Fidelis, C.H.V.; Acho, L.D.R.; et al. Phenolic and aroma compositions of pitomba fruit (Talisia esculenta Radlk.) assessed by LC–MS/MS and HS-SPME/GC–MS. Food Res. Int. 2016, 83, 87–94. [Google Scholar] [CrossRef]
- Rakariyatham, K.; Liu, X.; Liu, Z.; Wu, S.; Shahidi, F.; Zhou, D.; Zhu, B. Improvement of Phenolic Contents and Antioxidant Activities of Longan (Dimocarpus longan) Peel Extracts by Enzymatic Treatment. Waste Biomass Valorization 2020, 11, 3987–4002. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva Cordeiro, M.L.; de Queiroz Aquino-Martins, V.G.; da Silva, A.P.; Naliato, G.F.S.; Silveira, E.R.; Theodoro, R.C.; da Santos, D.Y.A.C.; Rocha, H.A.O.; Scortecci, K.C. Exploring the Antioxidant Potential of Talisia esculenta Using In Vitro and In Vivo Approaches. Nutrients 2023, 15, 3855. https://doi.org/10.3390/nu15173855
da Silva Cordeiro ML, de Queiroz Aquino-Martins VG, da Silva AP, Naliato GFS, Silveira ER, Theodoro RC, da Santos DYAC, Rocha HAO, Scortecci KC. Exploring the Antioxidant Potential of Talisia esculenta Using In Vitro and In Vivo Approaches. Nutrients. 2023; 15(17):3855. https://doi.org/10.3390/nu15173855
Chicago/Turabian Styleda Silva Cordeiro, Maria Lúcia, Verônica Giuliani de Queiroz Aquino-Martins, Ariana Pereira da Silva, Georggia Fatima Silva Naliato, Elielson Rodrigo Silveira, Raquel Cordeiro Theodoro, Deborah Yara Alves Cursino da Santos, Hugo Alexandre Oliveira Rocha, and Katia Castanho Scortecci. 2023. "Exploring the Antioxidant Potential of Talisia esculenta Using In Vitro and In Vivo Approaches" Nutrients 15, no. 17: 3855. https://doi.org/10.3390/nu15173855
APA Styleda Silva Cordeiro, M. L., de Queiroz Aquino-Martins, V. G., da Silva, A. P., Naliato, G. F. S., Silveira, E. R., Theodoro, R. C., da Santos, D. Y. A. C., Rocha, H. A. O., & Scortecci, K. C. (2023). Exploring the Antioxidant Potential of Talisia esculenta Using In Vitro and In Vivo Approaches. Nutrients, 15(17), 3855. https://doi.org/10.3390/nu15173855








