Recent Advances in Nutraceuticals for the Treatment of Sarcopenic Obesity
Abstract
1. Introduction
2. Definition and Diagnosis
| Study | Sarcopenia Diagnosis Method | Measurement (Cut-Off Points) | Obesity Diagnosis Method (Cut-Off Points) |
|---|---|---|---|
| Baumgartner et al. [8] | ASM divided by height squared | DXA (men < 7.26 kg/m2; women < 5.45 kg/m2) | Body fat- men > 27%; women > 38% |
| Newman et al. [10] | ALM divided by height squared | DXA (men < 7.23 kg/m2; women < 5.67 kg/m2) | BMI ≥ 30 kg/m2 |
| Cruz-Jentoft [11], EWGSOP | ASM divided by height squared | DXA (men ≤ 7.26 kg/m2; women ≤ 5.50 kg/m2) (Rosetta study) DXA (men ≤ 7.25 kg/m2; women ≤ 5.67 kg/m2) (Health ABC study) DXA (men ≤ 7.23 kg/m2; women ≤ 5.67 kg/m2) (Health ABC study) BIA (men: severe ≤ 8.50 kg/m2, moderate 8.51–10.75 kg/m2. women: severe ≤ 5.75 kg/m2; moderate 5.76–6.75 kg/m2) (NHANES III study) | NA |
| Residuals | DXA (ALM (fat mass divided by height), men: −2.29; women: −1.73) | ||
| SMI divided by height squared | BIA (men ≤ 8.87 kg/m2; women ≤ 6.42 kg/m2) | ||
| Muscle strength | Handgrip strength (men < 30 kg; women < 20 kg) | ||
| Muscle strength based on BMI category | Handgrip strength (men: BMI ≤ 24: ≤ 29 kg, BMI 24.1–26: ≤ 30 kg BMI 26.1–28: ≤ 30 kg, BMI > 28: ≤ 32 kg; women: BMI ≤ 23: ≤ 17 kg, BMI 23.1–26: ≤ 17.3 kg BMI 26.1–29: ≤ 18 kg, BMI > 29: ≤ 21 kg) | ||
| Physical performance | SPPB (≤8-point score) Gait speed over 6 m (<1 m/s) (Health ABC study) Gait speed over 6 m (<1.175 m/s) (Health ABC study) Gait speed over 4 m (<0.8 m/s) (InCHIANTI study) | ||
| LK Chen [14], AWGS | ASM divided by height squared | DXA (men < 7.0 kg/m2; women < 5.4 kg/m2) BIA (men < 7.0 kg/m2; women < 5.7 kg/m2) | NA |
| Strength | Handgrip strength (men < 26 kg; women < 18 kg) | ||
| Physical performance | Gait speed over 6 m (< 0.8 m/s) | ||
| Cruz-Jentoft [15], EWGSOP2 | ASM | DXA/BIA (men < 20 kg; women < 15 kg) | NA |
| ASM divided by height squared | DXA/BIA (men < 7.0 kg/m2; women < 5.5 kg/m2) | ||
| Strength | Grip Strength (men: < 27 kg; women < 16 kg) Chair stand > 15 s for five rises | ||
| Physical performance | Gait speed (≤ 0.8 m/s) SPPB (≤ 8-point score) TUG (≥ 20 s) 400 m walk test (non-completion or ≥ 6 min for completion) |
3. Pathophysiology and Complications
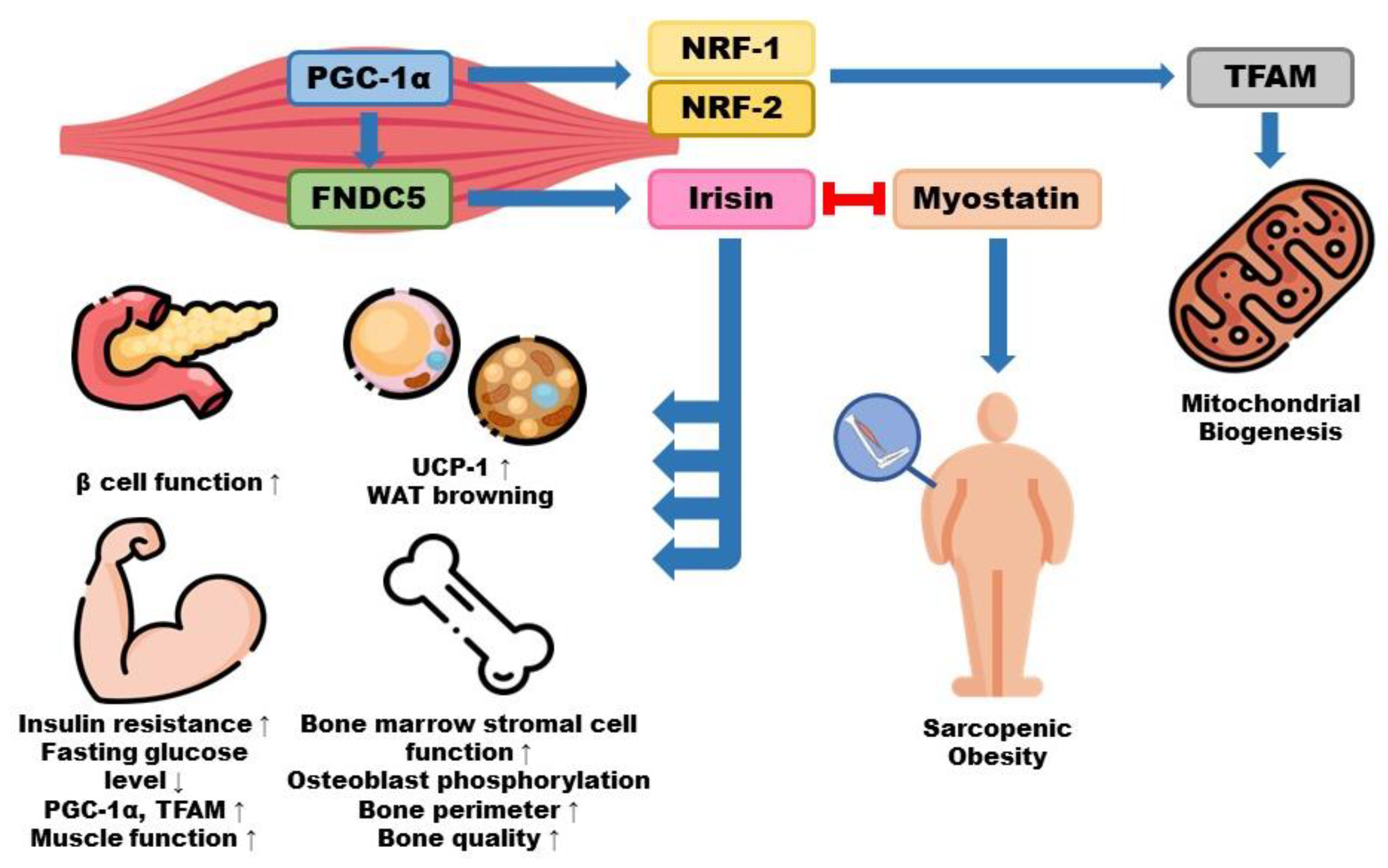
4. Irisin and Its Role in Sarcopenic Obesity
4.1. Pathology and Implications of Irisin for Sarcopenia
4.2. Pathophysiology and Implications of Irisin for Obesity
5. Approaches for the Treatment and Management
5.1. Lifestyle Intervention
| Reference | Intervention (Exposure, Dose, Duration) | Condition | Subjects (Sample Size, Gender, Age) | Markers | |
|---|---|---|---|---|---|
| Medeiros et al. [94] | Cholecalciferol 2000 IU 60 days | Twins Not on vitamin supplementation | CG n = 45 Same sex Age: 18–45 | SG n = 45 Same sex Age: 18–45 | Total body fat ↓ Gynoid lean mass ↑ VDR mRNA expression ↑ |
| Labudzynskyi et al. [96] | Vitamin D3 800 IU/kg 6 weeks | T1DM | Negative CG mice C56B1/J6 n = 8 21 ± 3 g | SG mice C56B1/J6 n = 8 21 ± 3 g | IL-6 mRNA expression ↓ |
| Skinner et al. [107] | Testosterone replacement treatment | Hypogonadism | Placebo n = 1168 Age: ≥ 45 | SG n = 1213 Age: ≥ 45 | Fat free mass ↑ Total body strength ↑ |
| Wilson et al. [113] | Ketogenic diet, western diet 10 weeks | - | n = 25 Age: college-aged | Serum testosterone level ↑ in KD | |
| Rønning et al. [98] | MK-4 1 µM, 10 µM, 20 µM, 50 µM 6 days | Bovine primary skeletal muscle cells cultivated in 2% FBS with Ultroser G serum | CG n = 3000 cells/well | MK-4 10 µM n = 3000 cells/well | Cell proliferation ↑ Gap closure ↑ |
| Knapen et al. [100] | MK-7 180 mcg/day 3 years | Postmenopausal women | SG n = 107 Female Age: 55–65 | Placebo n = 107 Female Age: 55–65 | Adiponectin ↑ Abdominal fat ↓ Visceral adipose tissue area ↓ |
| Félix-Soriano et al. [117] | DHA fish oil 15% HFD 3 times/day 12 months | Fed HFD for 4 months | HFD mice n = 10 Age: 6 months | HFD + DHA mice n = 6 Age: 6 months | Visceral white adipose tissue ↓ Subcutaneous white adipose tissue ↓ Body weight ↓ TFAM ↑ Beige adipose tissue markers ↑ |
| LeMieux et al. [121] | EPA 10% HFD 11 weeks | - | HFD mice n = 8–10 | HFD + EPA mice n = 8–10 | Body weight ↓ Adiposity ↓ Adipocyte size ↓ |
| Osella et al. [66] | Low glycemic index diet 6 months | Metabolic syndrome | CG n = 80 | SG n = 55 | Serum irisin concentration ↑ Fat free mass ↑ |
| Estell et al. [71] | Irisin 2–20 ng/mL 4 h–7 days | Osteoblasts | CG | SG | Osteoblasts/well ↑ |
| Zafar et al. [69] | Low glycemic index diet ≥1 week | Insulin resistance, T1DM, T2DM | n = 54 | Glycated hemoglobin ↓ Fasting glucose ↓ BMI ↓ Total cholesterol ↓ LDL ↓ | |
5.2. Emerging Therapy
5.2.1. Efficacy of Nutraceuticals
| Reference | Substance (Compound) | Intervention (Exposure, Dose, Duration) | Condition | Subjects (Sample Size, Gender, Mean Age) | Markers | |||
|---|---|---|---|---|---|---|---|---|
| Daneshi-Maskooni et al. [149] | Green Cardamom | 2 cardamom 500 mg capsules 3 times/day 3 months | Overweight or obese, NAFLD | Placebo n = 44 Age: 30–60 | Supplement group n = 43 Age: 30–60 | TNF-α ↓ IL-6 ↓ ALT ↓ Degree of fatty liver ↓ | ||
| Daneshi-Maskooni et al. [150] | 2 cardamom 500 mg capsules 3 times/day 3 months | Overweight or obese, NAFLD | Placebo n = 44 Age: 30–60 | Supplement group n = 43 Age: 30–60 | Irisin ↑ HDL-c ↑ TG ↓ LDL-c ↓ HOMA-IR ↓ Degree of fatty liver ↓ | |||
| Oliveira et al. [152] | Green Cardamom | α-terpineol | α-terpineol 25, 50, 100 mg/kg 180 min | Hypernoinception induced by carrageenan | Male Swiss mice n = 6 per group Age: 2–3 months Mass: 28–32 g | TNF-α ↓ Prostaglandin E₂ ↓ | ||
| Sousa et al. [153] | A-terpineol 25, 50, 100 mg/kg 6 weeks | Obese, high-fat and hypercaloric diet | Sqrague-Dawley rats n = 6 per group Age: 21 days Mass: ≃150 g | TNF-α ↓ IL-1β ↓ Weight gain ↓ Degree of fatty liver ↓ | ||||
| Nascimento et al. [154] | Linalool | Linalool 25 mg/kg Alternate days for 27 days | Hyperalgesia, injected with 20 μL pH 4 acidic saline | Male Swiss mice Mass: 25–30 g | Non-inflammatory pain ↓ | |||
| Soundharrajan et al. [155] | Limonene | Limonene 2.5, 5, 10 μM 6 days | Seeded 5 × 10⁴ cells/well, 10% FBS in DMEM, 37 °C, 5% CO₂ | C2C12 skeletal cell 80–90% confluency | Calcium deposition ↑ Myogenin ↑ MyoD ↑ p38 MAPK signalling pathway ↑ | |||
| Santos et al. [156] | Limonene 5% 96 h | Gastrocnemius muscle injured by 0.459 kg metal bar press with 0.811 J | Male Wistar rats | Thiobarbituric acid reactive substances ↓ Superoxide dimutase ↓ | ||||
| Muslce injured group n = 6 Mass: 250–280 g | Supplemented group n = 6 Mass: 250–280 g | |||||||
| Silva et al. [138] | Green Tea | GTE 500 mg/day 15 days | Exerecise-induced muscle soreness | Placebo n = 10 | Supplemented group n = 10 | Muscle recovery ↑ | ||
| Bagheri et al. [139] | GTE 500 mg/day 8 weeks | Under endurance training: Circuit training, fast walking, jogging 3 times/week | Placebo n = 15 | Supplemented group n = 15 | IL-6 ↓ Adiponectin ↑ Irisin ↑ Body weight ↓ BMI ↓ Body fat percentage ↓ Visceral fat area ↓ | |||
| Chen et al. [140] | Green Tea | EGCG | EGCG 856.8 mg/day 12 weeks | BMI ≥ 27 kg/m2, waist circumference ≥ 80 cm | Placebo n = 39 Age: 20–60 | Supplemented group n = 38 Age: 20–60 | Body weight ↓ BMI ↓ Waist circumference ↓ TC ↓ LDL-c ↓ Ghrelin ↓ Adiponectin ↑ | |
| Meador et al. [143] | EGCG 200 mg/kg 8 weeks | Sarcopenia | Sprague-Dawley rats | IGF-1 ↑ | ||||
| Control group Age: 20 months | Supplemented group Age: 20 months | |||||||
| Mafi et al. [147] | Epicatechin | Epicatechin along with resistance training | Sarcopenia | Placebo n = 15 Age: 68.63 ± 2.86 | Supplemented group n = 15 Age: 68.63 ± 2.86 | Myostain ↓ | ||
| Soleimani et al. [158] | Garlic | 2 garlic powder 400 mg tablets/day 15 weeks | NAFLD | Placebo n = 45 Age: 20–79 | Supplemented group n = 45 Age: 20–70 | Body weight ↓ Body fat mass ↓ | ||
| Sangouni et al. [159] | Garlic | 4 garlic powder 400 mg tablets/day 12 weeks | NAFLD | Control group n = 43 Age: ≥ 18 | Supplemented group n = 45 Age: ≥ 18 | Waist circumference ↓ Body fat percent ↓ Fasting glucose level ↓ Insulin resistance ↓ Skeleta muscle mass ↑ | ||
| Gupta et al. [161] | Garlic | SAC | SAC 200 μM 48 h | Atrophic effect by H₂O₂ DMEM, FBS, 2% horse serum, 1 μg/mL ciprofloxacin, 1.25 μg/mL amphotericin B | C2C12 muscle cell 80–90% confluency | TWEAK ↓ IL-6 ↓ Myostatin ↓ Muscle denervation ↓ | ||
| Dutt et al. [162] | SAC 0.01 mM 72 h | Treated with TNF-α 100 ng/mL Seeded 2 × 10⁶ cells/well, DMEM with 20% FBS, 5 μg/mL ciprofloxacin, 2.5 μg/mL amphotericin B, 37 °C, 5% CO₂ | C2C12 muscle cell 70–80% confluency | TNF-α ↓ IL-6 ↓ IL-1β ↓ TWEAK ↓ | ||||
| Chang et al. [127] | Oligonol | 200 mg/kg oligonol 8 weeks | Senescence-accelerated | SAMP8 Mice | phosphorylation of AKT/mTOR/p70sk6 ↑ MuRF-1/MAFbx ↓ PGC-1α/Tfam ↓ Mfn2/Opa1 ↓ cytochrome c ↓ | |||
| Receno et al. [136] | Curcumin | 0.2% curcumin 4 months | Aged | F344xBN rats ad libitum control (CON; n = 18) 0.2% curcumin (CUR; n = 18) pair-fed (PAIR; n = 18) Age: 32 months | Food intake ↓ plantaris mass ↑ force production ↑ nuclear fraction levels of Nrf2 ↑ oxidative macromolecule damage ↓ | |||
5.2.2. Dietary Interventions to Modulate Irisin Secretion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jura, M.; Kozak, L.P. Obesity and related consequences to ageing. Age 2016, 38, 23. [Google Scholar] [CrossRef] [PubMed]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Boutari, C.; Mantzoros, C.S. A 2022 update on the epidemiology of obesity and a call to action: As its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 2022, 133, 155217. [Google Scholar] [CrossRef]
- Silveira, E.A.; da Silva Filho, R.R.; Spexoto, M.C.B.; Haghighatdoost, F.; Sarrafzadegan, N.; de Oliveira, C. The Role of Sarcopenic Obesity in Cancer and Cardiovascular Disease: A Synthesis of the Evidence on Pathophysiological Aspects and Clinical Implications. Int. J. Mol. Sci. 2021, 22, 4339. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Mei, F.; Shang, Y.; Hu, K.; Chen, F.; Zhao, L.; Ma, B. Global prevalence of sarcopenic obesity in older adults: A systematic review and meta-analysis. Clin. Nutr. 2021, 40, 4633–4641. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Margioris, A.N. Sarcopenic obesity. Hormones 2018, 17, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.; Goodpaster, B.; Nevitt, M.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B. Sarcopenia: Alternative definitions and associations with lower extremity function. J. Am. Geriatr. Soc. 2003, 51, 1602–1609. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- World Health Organization Regional Office for the Western Pacific; Regional Office for the Western Pacific. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment; Health Communications Australia: Sydney, Australia, 2000. [Google Scholar]
- Després, J.P. Body fat distribution and risk of cardiovascular disease: An update. Circulation 2012, 126, 1301–1313. [Google Scholar] [CrossRef]
- Garvey, W.T.; Mechanick, J.I.; Brett, E.M.; Garber, A.J.; Hurley, D.L.; Jastreboff, A.M.; Nadolsky, K.; Pessah-Pollack, R.; Plodkowski, R. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for Medical Care of Patients with Obesity. Endocr. Pract. 2016, 22 (Suppl. S3), 1–203. [Google Scholar] [CrossRef]
- Cleasby, M.E.; Jamieson, P.M.; Atherton, P.J. Insulin resistance and sarcopenia: Mechanistic links between common co-morbidities. J. Endocrinol. 2016, 229, R67–R81. [Google Scholar] [CrossRef]
- Stenholm, S.; Harris, T.B.; Rantanen, T.; Visser, M.; Kritchevsky, S.B.; Ferrucci, L. Sarcopenic obesity: Definition, cause and consequences. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 693–700. [Google Scholar] [CrossRef]
- Kim, T.N.; Choi, K.M. Sarcopenia: Definition, epidemiology, and pathophysiology. J. Bone Metab. 2013, 20, 1–10. [Google Scholar] [CrossRef]
- de Luca, C.; Olefsky, J.M. Inflammation and insulin resistance. FEBS Lett. 2008, 582, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, M.; Maruoka, S.; Katayose, S. Inverse relationship between plasma adiponectin and leptin concentrations in normal-weight and obese women. Eur. J. Endocrinol. 2002, 147, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Ceglia, L. Vitamin D and its role in skeletal muscle. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 628–633. [Google Scholar] [CrossRef]
- Maalouf, G.E.; El Khoury, D. Exercise-Induced Irisin, the Fat Browning Myokine, as a Potential Anticancer Agent. J. Obes. 2019, 2019, 6561726. [Google Scholar] [CrossRef]
- Machida, S.; Booth, F.W. Insulin-like growth factor 1 and muscle growth: Implication for satellite cell proliferation. Proc. Nutr. Soc. 2004, 63, 337–340. [Google Scholar] [CrossRef]
- Aguirre, G.A.; De Ita, J.R.; de la Garza, R.G.; Castilla-Cortazar, I. Insulin-like growth factor-1 deficiency and metabolic syndrome. J. Transl. Med. 2016, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Maltais, M.L.; Desroches, J.; Dionne, I.J. Changes in muscle mass and strength after menopause. J. Musculoskelet. Neuronal Interact. 2009, 9, 186–197. [Google Scholar]
- McCarthy, M.; Raval, A.P. The peri-menopause in a woman’s life: A systemic inflammatory phase that enables later neurodegenerative disease. J. Neuroinflamm. 2020, 17, 317. [Google Scholar] [CrossRef]
- Vingren, J.L.; Kraemer, W.J.; Ratamess, N.A.; Anderson, J.M.; Volek, J.S.; Maresh, C.M. Testosterone physiology in resistance exercise and training: The up-stream regulatory elements. Sports Med. 2010, 40, 1037–1053. [Google Scholar] [CrossRef]
- Trendelenburg, A.U.; Meyer, A.; Rohner, D.; Boyle, J.; Hatakeyama, S.; Glass, D.J. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am. J. Physiol. Cell Physiol. 2009, 296, C1258–C1270. [Google Scholar] [CrossRef]
- Hitachi, K.; Nakatani, M.; Tsuchida, K. Myostatin signaling regulates Akt activity via the regulation of miR-486 expression. Int. J. Biochem. Cell Biol. 2014, 47, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Amor, M.; Itariu, B.K.; Moreno-Viedma, V.; Keindl, M.; Jurets, A.; Prager, G.; Langer, F.; Grablowitz, V.; Zeyda, M.; Stulnig, T.M. Serum Myostatin is Upregulated in Obesity and Correlates with Insulin Resistance in Humans. Exp. Clin. Endocrinol. Diabetes 2019, 127, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.; Little, J.P.; Stokl, A.J.; Hettinga, B.P.; Akhtar, M.; Tarnopolsky, M.A. Exercise increases mitochondrial PGC-1alpha content and promotes nuclear-mitochondrial cross-talk to coordinate mitochondrial biogenesis. J. Biol. Chem. 2011, 286, 10605–10617. [Google Scholar] [CrossRef]
- Seo, D.Y.; Kwak, H.B.; Lee, S.R.; Cho, Y.S.; Song, I.S.; Kim, N.; Bang, H.S.; Rhee, B.D.; Ko, K.S.; Park, B.J.; et al. Effects of aged garlic extract and endurance exercise on skeletal muscle FNDC-5 and circulating irisin in high-fat-diet rat models. Nutr. Res. Pract. 2014, 8, 177–182. [Google Scholar] [CrossRef]
- Jandova, T.; Buendia-Romero, A.; Polanska, H.; Hola, V.; Rihova, M.; Vetrovsky, T.; Courel-Ibanez, J.; Steffl, M. Long-Term Effect of Exercise on Irisin Blood Levels-Systematic Review and Meta-Analysis. Healthcare 2021, 9, 1438. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fontana, B.; Reyes-Garcia, R.; Morales-Santana, S.; Avila-Rubio, V.; Munoz-Garach, A.; Rozas-Moreno, P.; Munoz-Torres, M. Relationship between myostatin and irisin in type 2 diabetes mellitus: A compensatory mechanism to an unfavourable metabolic state? Endocrine 2016, 52, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Cauley, J.A. Estrogen and bone health in men and women. Steroids 2015, 99, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Szulc, P.; Beck, T.J.; Marchand, F.; Delmas, P.D. Low skeletal muscle mass is associated with poor structural parameters of bone and impaired balance in elderly men—The MINOS study. J. Bone Miner. Res. 2005, 20, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Daly, R.M.; Sanders, K.M.; Ebeling, P.R. Fall and Fracture Risk in Sarcopenia and Dynapenia with and without Obesity: The Role of Lifestyle Interventions. Curr. Osteoporos. Rep. 2015, 13, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Feng, J.; Zhang, G.; Yuan, X.; Li, F.; Yang, T.; Hao, S.; Huang, D.; Hsue, C.; Lou, Q. Visceral adipose tissue is more strongly associated with insulin resistance than subcutaneous adipose tissue in Chinese subjects with pre-diabetes. Curr. Med. Res. Opin. 2018, 34, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Eagon, J.C.; Trujillo, M.E.; Scherer, P.E.; Klein, S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 2007, 56, 1010–1013. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, N.; Wang, Y.; Li, H. Association between sarcopenia and osteoarthritis: A protocol for meta-analysis. PLoS ONE 2022, 17, e0272284. [Google Scholar] [CrossRef]
- Pegreffi, F.; Balestra, A.; De Lucia, O.; Smith, L.; Barbagallo, M.; Veronese, N. Prevalence of Sarcopenia in Knee Osteoarthritis: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 1532. [Google Scholar] [CrossRef] [PubMed]
- Pickering, M.E.; Chapurlat, R. Where Two Common Conditions of Aging Meet: Osteoarthritis and Sarcopenia. Calcif. Tissue Int. 2020, 107, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Monroe, D.G. Regulation of Bone Metabolism by Sex Steroids. Cold Spring Harb. Perspect. Med. 2018, 8, a031211. [Google Scholar] [CrossRef] [PubMed]
- Vasarhelyi, E.M.; MacDonald, S.J. The influence of obesity on total joint arthroplasty. J. Bone Jt. Surg. Br. 2012, 94, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Xu, Y. Association of sarcopenic obesity with the risk of all-cause mortality: A meta-analysis of prospective cohort studies. Geriatr. Gerontol. Int. 2016, 16, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Barazzoni, R.; Bischoff, S.C.; Boirie, Y.; Busetto, L.; Cederholm, T.; Dicker, D.; Toplak, H.; Van Gossum, A.; Yumuk, V.; Vettor, R. Sarcopenic obesity: Time to meet the challenge. Clin. Nutr. 2018, 37, 1787–1793. [Google Scholar] [CrossRef]
- Flegal, K.M.; Kit, B.K.; Orpana, H.; Graubard, B.I. Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA 2013, 309, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kim, J.H.; Yoon, J.W.; Kang, S.M.; Choi, S.H.; Park, Y.J.; Kim, K.W.; Lim, J.Y.; Park, K.S.; Jang, H.C. Sarcopenic obesity: Prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA). Diabetes Care 2010, 33, 1652–1654. [Google Scholar] [CrossRef]
- Baek, S.J.; Nam, G.E.; Han, K.D.; Choi, S.W.; Jung, S.W.; Bok, A.R.; Kim, Y.H.; Lee, K.S.; Han, B.D.; Kim, D.H. Sarcopenia and sarcopenic obesity and their association with dyslipidemia in Korean elderly men: The 2008–2010 Korea National Health and Nutrition Examination Survey. J. Endocrinol. Investig. 2014, 37, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.N.; Park, M.S.; Lim, K.I.; Choi, H.Y.; Yang, S.J.; Yoo, H.J.; Kang, H.J.; Song, W.; Choi, H.; Baik, S.H.; et al. Relationships between sarcopenic obesity and insulin resistance, inflammation, and vitamin D status: The Korean Sarcopenic Obesity Study. Clin. Endocrinol. 2013, 78, 525–532. [Google Scholar] [CrossRef]
- Waseem, R.; Shamsi, A.; Mohammad, T.; Hassan, M.I.; Kazim, S.N.; Chaudhary, A.A.; Rudayni, H.A.; Al-Zharani, M.; Ahmad, F.; Islam, A. FNDC5/Irisin: Physiology and Pathophysiology. Molecules 2022, 27, 1118. [Google Scholar] [CrossRef]
- Rashid, F.A.; Abbas, H.J.; Naser, N.A.; Addai Ali, H. Effect of Long-Term Moderate Physical Exercise on Irisin between Normal Weight and Obese Men. Sci. World J. 2020, 2020, 1897027. [Google Scholar] [CrossRef]
- Ulupinar, S.; Ozbay, S.; Gencoglu, C.; Altinkaynak, K.; Sebin, E.; Oymak, B. Exercise in the cold causes greater irisin release but may not be enough for adropin. Chin. J. Physiol. 2021, 64, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Jornayvaz, F.R.; Shulman, G.I. Regulation of mitochondrial biogenesis. Essays Biochem. 2010, 47, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, R.A.; Gannon, N.P.; Barberena, M.A.; Garcia-Smith, R.; Bisoffi, M.; Mermier, C.M.; Conn, C.A.; Trujillo, K.A. Characterization of the metabolic effects of irisin on skeletal muscle in vitro. Diabetes Obes. Metab. 2014, 16, 711–718. [Google Scholar] [CrossRef]
- Norheim, F.; Langleite, T.M.; Hjorth, M.; Holen, T.; Kielland, A.; Stadheim, H.K.; Gulseth, H.L.; Birkeland, K.I.; Jensen, J.; Drevon, C.A. The effects of acute and chronic exercise on PGC-1alpha, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014, 281, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Ando, D.; Goto, K.; Kiuchi, M.; Yamakita, M.; Koyama, K. High-intensity exercise causes greater irisin response compared with low-intensity exercise under similar energy consumption. Tohoku J. Exp. Med. 2014, 233, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Lavi, G.; Horwitz, A.; Einstein, O.; Zipori, R.; Gross, O.; Birk, R. Fndc5/irisin is regulated by myogenesis stage, irisin, muscle type and training. Am. J. Transl. Res. 2022, 14, 7063–7079. [Google Scholar] [PubMed]
- Colaianni, G.; Cinti, S.; Colucci, S.; Grano, M. Irisin and musculoskeletal health. Ann. N. Y. Acad. Sci. 2017, 1402, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Dincer, F.; Mesfum, E.; Mantzoros, C.S. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int. J. Obes. 2014, 38, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, Y.; Kurose, S.; Shinno, H.; Thi Thu, H.C.; Takao, N.; Tsutsumi, H.; Hasegawa, T.; Nakajima, T.; Kimura, Y. Effects of Body Weight Reduction on Serum Irisin and Metabolic Parameters in Obese Subjects. Diabetes Metab. J. 2016, 40, 386–395. [Google Scholar] [CrossRef]
- Osella, A.R.; Colaianni, G.; Correale, M.; Pesole, P.L.; Bruno, I.; Buongiorno, C.; Deflorio, V.; Leone, C.M.; Colucci, S.C.; Grano, M.; et al. Irisin Serum Levels in Metabolic Syndrome Patients Treated with Three Different Diets: A Post-Hoc Analysis from a Randomized Controlled Clinical Trial. Nutrients 2018, 10, 844. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Jeong, D.Y.; Kim, J.G.; Lee, S. The Acute Effects of Swimming Exercise on PGC-1alpha-FNDC5/Irisin-UCP1 Expression in Male C57BL/6J Mice. Metabolites 2021, 11, 111. [Google Scholar] [CrossRef]
- Ko, B.J.; Park, K.H.; Shin, S.; Zaichenko, L.; Davis, C.R.; Crowell, J.A.; Joung, H.; Mantzoros, C.S. Diet quality and diet patterns in relation to circulating cardiometabolic biomarkers. Clin. Nutr. 2016, 35, 484–490. [Google Scholar] [CrossRef]
- Zafar, M.I.; Mills, K.E.; Zheng, J.; Regmi, A.; Hu, S.Q.; Gou, L.; Chen, L.L. Low-glycemic index diets as an intervention for diabetes: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2019, 110, 891–902. [Google Scholar] [CrossRef]
- Reginster, J.Y.; Beaudart, C.; Buckinx, F.; Bruyere, O. Osteoporosis and sarcopenia: Two diseases or one? Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Estell, E.G.; Le, P.T.; Vegting, Y.; Kim, H.; Wrann, C.; Bouxsein, M.L.; Nagano, K.; Baron, R.; Spiegelman, B.M.; Rosen, C.J. Irisin directly stimulates osteoclastogenesis and bone resorption in vitro and in vivo. eLife 2020, 9, e58172. [Google Scholar] [CrossRef]
- Lewiecki, E.M. Role of sclerostin in bone and cartilage and its potential as a therapeutic target in bone diseases. Ther. Adv. Musculoskelet. Dis. 2014, 6, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Sanesi, L.; Storlino, G.; Brunetti, G.; Colucci, S.; Grano, M. Irisin and Bone: From Preclinical Studies to the Evaluation of Its Circulating Levels in Different Populations of Human Subjects. Cells 2019, 8, 451. [Google Scholar] [CrossRef]
- Ulualan, G.; Kiraz, Z.K.; Kirel, B. Relation of serum irisin levels to obesity and non-alcoholic fatty liver disease. Turk. J. Pediatr. 2022, 64, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Oguz, A.; Sahin, M.; Tuzun, D.; Kurutas, E.B.; Ulgen, C.; Bozkus, O.; Gul, K. Irisin is a predictor of sarcopenic obesity in type 2 diabetes mellitus: A cross-sectional study. Medicine 2021, 100, e26529. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.G.; Cook, D.A.; Clark, M.M.; Bardia, A.; Levine, J.A. Treatment of obesity. Mayo Clin. Proc. 2007, 82, 93–101; quiz 101–102. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.; Stoenchev, K.; Ashrafian, H.; Teare, J. Current treatments for obesity. Clin. Med. 2019, 19, 205–212. [Google Scholar] [CrossRef]
- Bernal Rivas, C.; Llamunao Tropa, A.; Reyes Barria, A.; Halabi, D.; Pavicic, F.; Ehrenfeld, P.; Martinez Huenchullan, S. Effects of exercise on irisin in subjects with overweight or obesity. A systematic review of clinical studies. Nutr. Hosp. 2022, 39, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Mahgoub, M.O.; D’Souza, C.; Al Darmaki, R.; Baniyas, M.; Adeghate, E. An update on the role of irisin in the regulation of endocrine and metabolic functions. Peptides 2018, 104, 15–23. [Google Scholar] [CrossRef]
- Choi, S.S.; Kim, E.S.; Jung, J.E.; Marciano, D.P.; Jo, A.; Koo, J.Y.; Choi, S.Y.; Yang, Y.R.; Jang, H.J.; Kim, E.K.; et al. PPARgamma Antagonist Gleevec Improves Insulin Sensitivity and Promotes the Browning of White Adipose Tissue. Diabetes 2016, 65, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Anastasilakis, A.D.; Efstathiadou, Z.A.; Makras, P.; Perakakis, N.; Kountouras, J.; Mantzoros, C.S. Irisin in metabolic diseases. Endocrine 2018, 59, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Reiner, M.; Niermann, C.; Jekauc, D.; Woll, A. Long-term health benefits of physical activity--a systematic review of longitudinal studies. BMC Public. Health 2013, 13, 813. [Google Scholar] [CrossRef]
- Vissers, D.; Hens, W.; Taeymans, J.; Baeyens, J.P.; Poortmans, J.; Van Gaal, L. The effect of exercise on visceral adipose tissue in overweight adults: A systematic review and meta-analysis. PLoS ONE 2013, 8, e56415. [Google Scholar] [CrossRef]
- Montero-Fernández, N.; Serra-Rexach, J.A. Role of exercise on sarcopenia in the elderly. Eur. J. Phys. Rehabil. Med. 2013, 49, 131–143. [Google Scholar]
- Johnston, A.P.; De Lisio, M.; Parise, G. Resistance training, sarcopenia, and the mitochondrial theory of aging. Appl. Physiol. Nutr. Metab. 2008, 33, 191–199. [Google Scholar] [CrossRef]
- Harber, M.P.; Konopka, A.R.; Undem, M.K.; Hinkley, J.M.; Minchev, K.; Kaminsky, L.A.; Trappe, T.A.; Trappe, S. Aerobic exercise training induces skeletal muscle hypertrophy and age-dependent adaptations in myofiber function in young and older men. J. Appl. Physiol. 2012, 113, 1495–1504. [Google Scholar] [CrossRef]
- Wilkinson, S.B.; Phillips, S.M.; Atherton, P.J.; Patel, R.; Yarasheski, K.E.; Tarnopolsky, M.A.; Rennie, M.J. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J. Physiol. 2008, 586, 3701–3717. [Google Scholar] [CrossRef]
- Scalzo, R.L.; Peltonen, G.L.; Binns, S.E.; Shankaran, M.; Giordano, G.R.; Hartley, D.A.; Klochak, A.L.; Lonac, M.C.; Paris, H.L.; Szallar, S.E.; et al. Greater muscle protein synthesis and mitochondrial biogenesis in males compared with females during sprint interval training. FASEB J. 2014, 28, 2705–2714. [Google Scholar] [CrossRef]
- Bouchonville, M.F.; Villareal, D.T. Sarcopenic obesity: How do we treat it? Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 412–419. [Google Scholar] [CrossRef]
- Cipriani, C.; Pepe, J.; Piemonte, S.; Colangelo, L.; Cilli, M.; Minisola, S. Vitamin d and its relationship with obesity and muscle. Int. J. Endocrinol. 2014, 2014, 841248. [Google Scholar] [CrossRef]
- Di Filippo, L.; De Lorenzo, R.; Giustina, A.; Rovere-Querini, P.; Conte, C. Vitamin D in Osteosarcopenic Obesity. Nutrients 2022, 14, 1816. [Google Scholar] [CrossRef]
- de Brito Galvao, J.F.; Nagode, L.A.; Schenck, P.A.; Chew, D.J. Calcitriol, calcidiol, parathyroid hormone, and fibroblast growth factor-23 interactions in chronic kidney disease. J. Vet. Emerg. Crit. Care 2013, 23, 134–162. [Google Scholar] [CrossRef] [PubMed]
- Khundmiri, S.J.; Murray, R.D.; Lederer, E. PTH and Vitamin D. Compr. Physiol. 2016, 6, 561–601. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, J.F.P.; de Oliveira Borges, M.V.; Soares, A.A.; Dos Santos, J.C.; de Oliveira, A.B.B.; da Costa, C.H.B.; Cruz, M.S.; Bortolin, R.H.; de Freitas, R.C.C.; Dantas, P.M.S.; et al. The impact of vitamin D supplementation on VDR gene expression and body composition in monozygotic twins: Randomized controlled trial. Sci. Rep. 2020, 10, 11943. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D Deficiency: Effects on Oxidative Stress, Epigenetics, Gene Regulation, and Aging. Biology 2019, 8, 30. [Google Scholar] [CrossRef]
- Labudzynskyi, D.; Shymanskyy, I.; Veliky, M. Role of vitamin D3 in regulation of interleukin-6 and osteopontin expression in liver of diabetic mice. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2916–2919. [Google Scholar]
- Weber, P. Vitamin K and bone health. Nutrition 2001, 17, 880–887. [Google Scholar] [CrossRef]
- Ronning, S.B.; Pedersen, M.E.; Berg, R.S.; Kirkhus, B.; Rodbotten, R. Vitamin K2 improves proliferation and migration of bovine skeletal muscle cells in vitro. PLoS ONE 2018, 13, e0195432. [Google Scholar] [CrossRef]
- Ferri, E.; Marzetti, E.; Calvani, R.; Picca, A.; Cesari, M.; Arosio, B. Role of Age-Related Mitochondrial Dysfunction in Sarcopenia. Int. J. Mol. Sci. 2020, 21, 5236. [Google Scholar] [CrossRef]
- Knapen, M.H.J.; Jardon, K.M.; Vermeer, C. Vitamin K-induced effects on body fat and weight: Results from a 3-year vitamin K2 intervention study. Eur. J. Clin. Nutr. 2018, 72, 136–141. [Google Scholar] [CrossRef]
- Tieland, M.; Trouwborst, I.; Clark, B.C. Skeletal muscle performance and ageing. J. Cachexia Sarcopenia Muscle 2018, 9, 3–19. [Google Scholar] [CrossRef]
- Kirk, B.; Zanker, J.; Duque, G. Osteosarcopenia: Epidemiology, diagnosis, and treatment-facts and numbers. J. Cachexia Sarcopenia Muscle 2020, 11, 609–618. [Google Scholar] [CrossRef]
- Coletti, C.; Acosta, G.F.; Keslacy, S.; Coletti, D. Exercise-mediated reinnervation of skeletal muscle in elderly people: An update. Eur. J. Transl. Myol. 2022, 32, 10416. [Google Scholar] [CrossRef] [PubMed]
- van Dronkelaar, C.; van Velzen, A.; Abdelrazek, M.; van der Steen, A.; Weijs, P.J.M.; Tieland, M. Minerals and Sarcopenia; The Role of Calcium, Iron, Magnesium, Phosphorus, Potassium, Selenium, Sodium, and Zinc on Muscle Mass, Muscle Strength, and Physical Performance in Older Adults: A Systematic Review. J. Am. Med. Dir. Assoc. 2018, 19, 6–11.e13. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, S.K.; Papadimitriou, K.; Voulgaridou, G.; Georgaki, E.; Tsotidou, E.; Zantidou, O.; Papandreou, D. Exercise and Nutrition Impact on Osteoporosis and Sarcopenia—The Incidence of Osteosarcopenia: A Narrative Review. Nutrients 2021, 13, 4499. [Google Scholar] [CrossRef]
- Jin, W.S.; Choi, E.J.; Lee, S.Y.; Bae, E.J.; Lee, T.H.; Park, J. Relationships among Obesity, Sarcopenia, and Osteoarthritis in the Elderly. J. Obes. Metab. Syndr. 2017, 26, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Skinner, J.W.; Otzel, D.M.; Bowser, A.; Nargi, D.; Agarwal, S.; Peterson, M.D.; Zou, B.; Borst, S.E.; Yarrow, J.F. Muscular responses to testosterone replacement vary by administration route: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2018, 9, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Snyder, P.J.; Peachey, H.; Hannoush, P.; Berlin, J.A.; Loh, L.; Holmes, J.H.; Dlewati, A.; Staley, J.; Santanna, J.; Kapoor, S.C.; et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J. Clin. Endocrinol. Metab. 1999, 84, 1966–1972. [Google Scholar] [CrossRef] [PubMed]
- Griggs, R.C.; Kingston, W.; Jozefowicz, R.F.; Herr, B.E.; Forbes, G.; Halliday, D. Effect of testosterone on muscle mass and muscle protein synthesis. J. Appl. Physiol. 1989, 66, 498–503. [Google Scholar] [CrossRef]
- Herbst, K.L.; Bhasin, S. Testosterone action on skeletal muscle. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 271–277. [Google Scholar] [CrossRef]
- Zamir, A.; Ben-Zeev, T.; Hoffman, J.R. Manipulation of Dietary Intake on Changes in Circulating Testosterone Concentrations. Nutrients 2021, 13, 3375. [Google Scholar] [CrossRef]
- Kuchakulla, M.; Narasimman, M.; Soni, Y.; Leong, J.Y.; Patel, P.; Ramasamy, R. A systematic review and evidence-based analysis of ingredients in popular male testosterone and erectile dysfunction supplements. Int. J. Impot. Res. 2021, 33, 311–317. [Google Scholar] [CrossRef]
- Wilson, J.M.; Lowery, R.P.; Roberts, M.D.; Sharp, M.H.; Joy, J.M.; Shields, K.A.; Partl, J.M.; Volek, J.S.; D’Agostino, D.P. Effects of Ketogenic Dieting on Body Composition, Strength, Power, and Hormonal Profiles in Resistance Training Men. J. Strength. Cond. Res. 2020, 34, 3463–3474. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. JPEN J. Parenter. Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef] [PubMed]
- Roche, H.M. Unsaturated fatty acids. Proc. Nutr. Soc. 1999, 58, 397–401. [Google Scholar] [CrossRef]
- Soni, N.; Ross, A.B.; Scheers, N.; Nookaew, I.; Gabrielsson, B.G.; Sandberg, A.S. The Omega-3 Fatty Acids EPA and DHA, as a Part of a Murine High-Fat Diet, Reduced Lipid Accumulation in Brown and White Adipose Tissues. Int. J. Mol. Sci. 2019, 20, 5895. [Google Scholar] [CrossRef] [PubMed]
- Felix-Soriano, E.; Sainz, N.; Fernandez-Galilea, M.; Gil-Iturbe, E.; Celay, J.; Martinez-Climent, J.A.; Moreno-Aliaga, M.J. Chronic docosahexaenoic acid supplementation improves metabolic plasticity in subcutaneous adipose tissue of aged obese female mice. J. Nutr. Biochem. 2023, 111, 109153. [Google Scholar] [CrossRef]
- Lee, M.S.; Shin, Y.; Moon, S.; Kim, S.; Kim, Y. Effects of Eicosapentaenoic Acid and Docosahexaenoic Acid on Mitochondrial DNA Replication and PGC-1alpha Gene Expression in C(2)C(12) Muscle Cells. Prev. Nutr. Food Sci. 2016, 21, 317–322. [Google Scholar] [CrossRef]
- Ochi, E.; Tsuchiya, Y. Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA) in Muscle Damage and Function. Nutrients 2018, 10, 552. [Google Scholar] [CrossRef]
- Castillero, E.; Martin, A.I.; Lopez-Menduina, M.; Villanua, M.A.; Lopez-Calderon, A. Eicosapentaenoic acid attenuates arthritis-induced muscle wasting acting on atrogin-1 and on myogenic regulatory factors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R1322–R1331. [Google Scholar] [CrossRef]
- LeMieux, M.J.; Kalupahana, N.S.; Scoggin, S.; Moustaid-Moussa, N. Eicosapentaenoic acid reduces adipocyte hypertrophy and inflammation in diet-induced obese mice in an adiposity-independent manner. J. Nutr. 2015, 145, 411–417. [Google Scholar] [CrossRef]
- Thach, T.T.; Wu, C.; Hwang, K.Y.; Lee, S.J. Azelaic Acid Induces Mitochondrial Biogenesis in Skeletal Muscle by Activation of Olfactory Receptor 544. Front. Physiol. 2020, 11, 329. [Google Scholar] [CrossRef]
- Salucci, S.; Falcieri, E. Polyphenols and their potential role in preventing skeletal muscle atrophy. Nutr. Res. 2020, 74, 10–22. [Google Scholar] [CrossRef]
- Noh, J.S.; Park, C.H.; Yokozawa, T. Treatment with oligonol, a low-molecular polyphenol derived from lychee fruit, attenuates diabetes-induced hepatic damage through regulation of oxidative stress and lipid metabolism. Br. J. Nutr. 2011, 106, 1013–1022. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, Y.; Im, J.A.; Lee, H. Oligonol suppresses lipid accumulation and improves insulin resistance in a palmitate-induced in HepG2 hepatocytes as a cellular steatosis model. BMC Complement. Altern. Med. 2015, 15, 185. [Google Scholar] [CrossRef]
- Liu, H.W.; Chen, Y.J.; Chang, Y.C.; Chang, S.J. Oligonol, a Low-Molecular Weight Polyphenol Derived from Lychee, Alleviates Muscle Loss in Diabetes by Suppressing Atrogin-1 and MuRF1. Nutrients 2017, 9, 1040. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chen, Y.T.; Liu, H.W.; Chan, Y.C.; Liu, M.Y.; Hu, S.H.; Tseng, W.T.; Wu, H.L.; Wang, M.F.; Chang, S.J. Oligonol Alleviates Sarcopenia by Regulation of Signaling Pathways Involved in Protein Turnover and Mitochondrial Quality. Mol. Nutr. Food Res. 2019, 63, e1801102. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Bahijri, S.M.; Ajabnoor, G.; Hegazy, G.A.; Alsheikh, L.; Moumena, M.Z.; Bashanfar, B.M.; Alzahrani, A.H. Supplementation with Oligonol, Prevents Weight Gain and Improves Lipid Profile in Overweight and Obese Saudi Females. Curr. Nutr. Food Sci. 2018, 14, 164–170. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, H.; Kim, J.M.; Lee, B.J.; Kim, I.J.; Pak, K.; Jeon, Y.K.; Kim, K. Effect of oligonol, a lychee-derived polyphenol, on skeletal muscle in ovariectomized rats by regulating body composition, protein turnover, and mitochondrial quality signaling. Food Sci. Nutr. 2022, 10, 1184–1194. [Google Scholar] [CrossRef]
- Bagherniya, M.; Mahdavi, A.; Shokri-Mashhadi, N.; Banach, M.; Von Haehling, S.; Johnston, T.P.; Sahebkar, A. The beneficial therapeutic effects of plant-derived natural products for the treatment of sarcopenia. J. Cachexia Sarcopenia Muscle 2022, 13, 2772–2790. [Google Scholar] [CrossRef]
- Safdar, A.; de Beer, J.; Tarnopolsky, M.A. Dysfunctional Nrf2-Keap1 redox signaling in skeletal muscle of the sedentary old. Free Radic. Biol. Med. 2010, 49, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Balogun, E.; Hoque, M.; Gong, P.; Killeen, E.; Green, C.J.; Foresti, R.; Alam, J.; Motterlini, R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003, 371, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Xu, C.; Hu, R.; Jain, M.R.; Gopalkrishnan, A.; Nair, S.; Huang, M.T.; Chan, J.Y.; Kong, A.N. Modulation of nuclear factor E2-related factor 2-mediated gene expression in mice liver and small intestine by cancer chemopreventive agent curcumin. Mol. Cancer Ther. 2006, 5, 39–51. [Google Scholar] [CrossRef]
- Sahin, K.; Pala, R.; Tuzcu, M.; Ozdemir, O.; Orhan, C.; Sahin, N.; Juturu, V. Curcumin prevents muscle damage by regulating NF-kappaB and Nrf2 pathways and improves performance: An in vivo model. J. Inflamm. Res. 2016, 9, 147–154. [Google Scholar] [CrossRef]
- Receno, C.N.; Liang, C.; Korol, D.L.; Atalay, M.; Heffernan, K.S.; Brutsaert, T.D.; DeRuisseau, K.C. Effects of Prolonged Dietary Curcumin Exposure on Skeletal Muscle Biochemical and Functional Responses of Aged Male Rats. Int. J. Mol. Sci. 2019, 20, 1178. [Google Scholar] [CrossRef] [PubMed]
- Alway, S.E.; Bennett, B.T.; Wilson, J.C.; Sperringer, J.; Mohamed, J.S.; Edens, N.K.; Pereira, S.L. Green tea extract attenuates muscle loss and improves muscle function during disuse, but fails to improve muscle recovery following unloading in aged rats. J. Appl. Physiol. 2015, 118, 319–330. [Google Scholar] [CrossRef] [PubMed]
- da Silva, W.; Machado, A.S.; Souza, M.A.; Mello-Carpes, P.B.; Carpes, F.P. Effect of green tea extract supplementation on exercise-induced delayed onset muscle soreness and muscular damage. Physiol. Behav. 2018, 194, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, R.; Rashidlamir, A.; Ashtary-Larky, D.; Wong, A.; Grubbs, B.; Motevalli, M.S.; Baker, J.S.; Laher, I.; Zouhal, H. Effects of green tea extract supplementation and endurance training on irisin, pro-inflammatory cytokines, and adiponectin concentrations in overweight middle-aged men. Eur. J. Appl. Physiol. 2020, 120, 915–923. [Google Scholar] [CrossRef]
- Chen, I.J.; Liu, C.Y.; Chiu, J.P.; Hsu, C.H. Therapeutic effect of high-dose green tea extract on weight reduction: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2016, 35, 592–599. [Google Scholar] [CrossRef]
- Ueda-Wakagi, M.; Hayashibara, K.; Nagano, T.; Ikeda, M.; Yuan, S.; Ueda, S.; Shirai, Y.; Yoshida, K.I.; Ashida, H. Epigallocatechin gallate induces GLUT4 translocation in skeletal muscle through both PI3K- and AMPK-dependent pathways. Food Funct. 2018, 9, 4223–4233. [Google Scholar] [CrossRef]
- Casanova, E.; Salvado, J.; Crescenti, A.; Gibert-Ramos, A. Epigallocatechin Gallate Modulates Muscle Homeostasis in Type 2 Diabetes and Obesity by Targeting Energetic and Redox Pathways: A Narrative Review. Int. J. Mol. Sci. 2019, 20, 532. [Google Scholar] [CrossRef] [PubMed]
- Meador, B.M.; Mirza, K.A.; Tian, M.; Skelding, M.B.; Reaves, L.A.; Edens, N.K.; Tisdale, M.J.; Pereira, S.L. The Green Tea Polyphenol Epigallocatechin-3-Gallate (EGCg) Attenuates Skeletal Muscle Atrophy in a Rat Model of Sarcopenia. J. Frailty Aging 2015, 4, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Luk, H.Y.; Appell, C.; Chyu, M.C.; Chen, C.H.; Wang, C.Y.; Yang, R.S.; Shen, C.L. Impacts of Green Tea on Joint and Skeletal Muscle Health: Prospects of Translational Nutrition. Antioxidants 2020, 9, 1050. [Google Scholar] [CrossRef]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [PubMed]
- Sabarathinam, S.; Rajappan Chandra, S.K.; Satheesh, S. Network pharmacology based pharmacokinetic assessment and evaluation of the therapeutic potential of catechin derivatives as a potential myostatin inhibitor: A special view on Sarcopenic Obesity. Nat. Prod. Res. 2023, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mafi, F.; Biglari, S.; Ghardashi Afousi, A.; Gaeini, A.A. Improvement in Skeletal Muscle Strength and Plasma Levels of Follistatin and Myostatin Induced by an 8-Week Resistance Training and Epicatechin Supplementation in Sarcopenic Older Adults. J. Aging Phys. Act. 2019, 27, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Yahyazadeh, R.; Ghasemzadeh Rahbardar, M.; Razavi, B.M.; Karimi, G.; Hosseinzadeh, H. The effect of Elettaria cardamomum (cardamom) on the metabolic syndrome: Narrative review. Iran. J. Basic. Med. Sci. 2021, 24, 1462–1469. [Google Scholar] [CrossRef]
- Daneshi-Maskooni, M.; Keshavarz, S.A.; Qorbani, M.; Mansouri, S.; Alavian, S.M.; Badri-Fariman, M.; Jazayeri-Tehrani, S.A.; Sotoudeh, G. Green cardamom supplementation improves serum irisin, glucose indices, and lipid profiles in overweight or obese non-alcoholic fatty liver disease patients: A double-blind randomized placebo-controlled clinical trial. BMC Complement. Altern. Med. 2019, 19, 59. [Google Scholar] [CrossRef]
- Daneshi-Maskooni, M.; Keshavarz, S.A.; Mansouri, S.; Qorbani, M.; Alavian, S.M.; Badri-Fariman, M.; Jazayeri-Tehrani, S.A.; Sotoudeh, G. The effects of green cardamom on blood glucose indices, lipids, inflammatory factors, paraxonase-1, sirtuin-1, and irisin in patients with nonalcoholic fatty liver disease and obesity: Study protocol for a randomized controlled trial. Trials 2017, 18, 260. [Google Scholar] [CrossRef]
- Held, S.; Schieberle, P.; Somoza, V. Characterization of alpha-terpineol as an anti-inflammatory component of orange juice by in vitro studies using oral buccal cells. J. Agric. Food Chem. 2007, 55, 8040–8046. [Google Scholar] [CrossRef]
- de Oliveira, M.G.; Marques, R.B.; de Santana, M.F.; Santos, A.B.; Brito, F.A.; Barreto, E.O.; De Sousa, D.P.; Almeida, F.R.; Badaue-Passos, D., Jr.; Antoniolli, A.R.; et al. alpha-terpineol reduces mechanical hypernociception and inflammatory response. Basic. Clin. Pharmacol. Toxicol. 2012, 111, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Sousa, G.M.; Cazarin, C.B.B.; Marostica Junior, M.R.; Lamas, C.A.; Quitete, V.; Pastore, G.M.; Bicas, J.L. The effect of alpha-terpineol enantiomers on biomarkers of rats fed a high-fat diet. Heliyon 2020, 6, e03752. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, S.S.; Camargo, E.A.; DeSantana, J.M.; Araujo, A.A.; Menezes, P.P.; Lucca-Junior, W.; Albuquerque-Junior, R.L.; Bonjardim, L.R.; Quintans-Junior, L.J. Linalool and linalool complexed in beta-cyclodextrin produce anti-hyperalgesic activity and increase Fos protein expression in animal model for fibromyalgia. Naunyn Schmiedebergs Arch. Pharmacol. 2014, 387, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Soundharrajan, I.; Kim, D.H.; Srisesharam, S.; Kuppusamy, P.; Sivanesan, R.; Choi, K.C. Limonene promotes osteoblast differentiation and 2-deoxy-d-glucose uptake through p38MAPK and Akt signaling pathways in C2C12 skeletal muscle cells. Phytomedicine 2018, 45, 41–48. [Google Scholar] [CrossRef]
- Santos, M.M.B.; Filho, L.F.S.; De Souza, J.B.; Filho, J.; Mesquita, T.R.R.; Santos, M.S.; De Vasconcelos, C.M.L.; Lauton-Santos, S.; De Oliveira, E.D. Topical application of (S)-(-)-limonene is as effective as phonophoresis for improving oxidative parameters of injured skeletal muscle in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 2293–2300. [Google Scholar] [CrossRef]
- Tesfaye, A. Revealing the Therapeutic Uses of Garlic (Allium sativum) and Its Potential for Drug Discovery. Sci. World J. 2021, 2021, 8817288. [Google Scholar] [CrossRef]
- Soleimani, D.; Paknahad, Z.; Askari, G.; Iraj, B.; Feizi, A. Effect of garlic powder consumption on body composition in patients with nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. Adv. Biomed. Res. 2016, 5, 2. [Google Scholar] [CrossRef]
- Sangouni, A.A.; Mohammad Hosseini Azar, M.R.; Alizadeh, M. Effects of garlic powder supplementation on insulin resistance, oxidative stress, and body composition in patients with non-alcoholic fatty liver disease: A randomized controlled clinical trial. Complement. Ther. Med. 2020, 51, 102428. [Google Scholar] [CrossRef]
- Avula, P.R.; Asdaq, S.M.; Asad, M. Effect of aged garlic extract and s-allyl cysteine and their interaction with atenolol during isoproterenol induced myocardial toxicity in rats. Indian J. Pharmacol. 2014, 46, 94–99. [Google Scholar] [CrossRef]
- Gupta, P.; Dutt, V.; Kaur, N.; Kalra, P.; Gupta, S.; Dua, A.; Dabur, R.; Saini, V.; Mittal, A. S-allyl cysteine: A potential compound against skeletal muscle atrophy. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129676. [Google Scholar] [CrossRef]
- Dutt, V.; Saini, V.; Gupta, P.; Kaur, N.; Bala, M.; Gujar, R.; Grewal, A.; Gupta, S.; Dua, A.; Mittal, A. S-allyl cysteine inhibits TNFalpha-induced skeletal muscle wasting through suppressing proteolysis and expression of inflammatory molecules. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 895–906. [Google Scholar] [CrossRef] [PubMed]
- de Macedo, S.M.; Lelis, D.F.; Mendes, K.L.; Fraga, C.A.C.; Brandi, I.V.; Feltenberger, J.D.; Farias, L.C.; Guimaraes, A.L.S.; de Paula, A.M.B.; Santos, S.H.S. Effects of Dietary Macronutrient Composition on FNDC5 and Irisin in Mice Skeletal Muscle. Metab. Syndr. Relat. Disord. 2017, 15, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.I.; Mills, K.E.; Zheng, J.; Peng, M.M.; Ye, X.; Chen, L.L. Low glycaemic index diets as an intervention for obesity: A systematic review and meta-analysis. Obes. Rev. 2019, 20, 290–315. [Google Scholar] [CrossRef] [PubMed]

| Reference | Intervention (Exposure, Dose, Duration, Follow-Up) | Condition | Subjects (Sample Size, Gender, Age) | Results | |||
|---|---|---|---|---|---|---|---|
| Norheim et al. [60] | Endurance and strength training 12 weeks | Inactive, hyperglycaemic, overweight, pre-diabetic | Normal weight n = 13 Age: 40–65 | Obese group n = 13 Age: 40–65 | Irisin ↑ PGC-1α ↑ FNDC5 ↑ | ||
| Tsuchiya et al. [61] | High-intensity exercise 40 min | Healthy, sedentary | n = 6 Age: 22.5 ± 1.1 Height: 174.8 ± 2.8 cm Weight: 67.1 ± 2.2 kg BMI: 22.1 ± 1.1 | Irisin ↑ LDH ↓ | |||
| Vaughan et al. [59] | Irisin 5 nM 24 h | 5 × 10⁵ cells/well, DMEM containing 4500 mg/L glucose, 10% FBS, 100 U/mL penicillin/streptomycin, 37 °C, 5% CO₂ | C2C12 muscle cell | Mitochondrial content ↑ NRF-1 ↑ TFAM ↑ GLUT4 ↑ UCP-3 ↑ | |||
| Huh et al., (2014) [64] | Irisin 10 nM, 50 nM 12 days | Muscle collected from healthy subjects | 3T3L1 adipocyte | Irisin ↑ FNDC5 ↑ IGF-1 ↑ PGC-1α ↑ UCP-1 ↑ | |||
| Rashid et al. [56] | Moderate exercise 6 months | Obesity | Normal group n = 30 Age: 20–43 BMI: <25 kg/m2 | Obese group n = 30 Age: 20–43 BMI: ≥30 kg/m2 | Irisin ↑ BMI ↓ Waist circumference ↓ Fasting glucose level ↓ Fasting insulin level ↓ HOMA-IR ↓ HOMA-B2 ↓ | ||
| Fukushima et al. [65] | Diet, exercise, behavioral therapy 6 months | Obesity | n = 22 5 males, 17 females Age: 46.1 ± 16.0 BMI: 36.9 ± 5.0 kg/m2 | Irisin ↑ BMI ↓ Body fat percentage ↓ Subcutaneous fat area ↓ Triglycerides ↓ HOMA-IR ↓ Fasting glucose level ↓ | |||
| Osella et al. [66] | Low glycaemic index diet, Mediterranean diet, Low glycaemic index Mediterranean diet 24 weeks | Metabolic syndrome | Control group n = 80 | LGID group n = 55 | MD group n = 51 | LGIMD group n = 45 | Irisin ↑ Saturated fatty acids ↓ Fat free mass ↑ BMI ↓ |
| Cho et al. [67] | Swimming 90 min | Acclimitized to swimming | C57BL/6J | FNDC5 ↑ | |||
| Control group n = 10 Age: 14–16 weeks | Swimming exercise group n = 10 Age: 14–16 weeks | ||||||
| Mâcedo et al. [68] | High protein diet 60 days | - | Standard diet mice n = 7 | High protein diet mice n = 7 | Brown adipose tissue ↑ | ||
| Zafar et al. [69] | Low glycemic index diet ≥1 week | Insulin resistance, T1DM, T2DM | n = 54 | Glycated hemoglobin ↓ Fasting glucose ↓ BMI ↓ Total cholesterol ↓ LDL ↓ | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-C.; Ki, S.-W.; Kim, H.; Kang, S.; Kim, H.; Go, G.-w. Recent Advances in Nutraceuticals for the Treatment of Sarcopenic Obesity. Nutrients 2023, 15, 3854. https://doi.org/10.3390/nu15173854
Kim Y-C, Ki S-W, Kim H, Kang S, Kim H, Go G-w. Recent Advances in Nutraceuticals for the Treatment of Sarcopenic Obesity. Nutrients. 2023; 15(17):3854. https://doi.org/10.3390/nu15173854
Chicago/Turabian StyleKim, Young-Chan, Sang-Woo Ki, Hannah Kim, Sumin Kang, Hayoon Kim, and Gwang-woong Go. 2023. "Recent Advances in Nutraceuticals for the Treatment of Sarcopenic Obesity" Nutrients 15, no. 17: 3854. https://doi.org/10.3390/nu15173854
APA StyleKim, Y.-C., Ki, S.-W., Kim, H., Kang, S., Kim, H., & Go, G.-w. (2023). Recent Advances in Nutraceuticals for the Treatment of Sarcopenic Obesity. Nutrients, 15(17), 3854. https://doi.org/10.3390/nu15173854







